Abstract
Development and maturation of vascular and neuronal tissues occurs simultaneously in utero, and are regulated by significant crosstalk. We report on the development of a 3D tissue system to model neurogenesis and recapitulate developmental signaling conditions. Human umbilical vein endothelial cells (HUVECs) were seeded inside channels within collagen gels to represent nascent vascular networks. Axons extending from chicken dorsal root ganglia (DRGs) grew significantly longer and preferentially towards the HUVEC seeded channels with respect to unloaded channels. To replicate these findings without the vascular component, channels were loaded with brain-derived neurotrophic factor (BDNF), the principle signaling molecule in HUVEC-stimulated axonal growth, and axons likewise were significantly longer and grew preferentially towards the BDNF-loaded channels with respect to controls. This 3D tissue system was then used as an in vitro replicate for peripheral nerve injury, with neural repair observed within 2 weeks. These results demonstrate that our 3D tissue system can model neural network formation, repair after laceration injuries, and can be utilized to further study how these networks form and interact with other tissues, such as skin or skeletal muscle.
Keywords: neurogenesis, neurovascular unit, collagen, tissue engineering, nerve repair
Graphical Abstract
A 3D tissue system is developed to model neurogenesis and understanding the development, maturation, and repair of neural networks. Neural networks are patterned on collagen gels and align along channel dimensions from neurotrophic cues embedded within the gel. A laceration injury model is described to study mechanisms involved in neural repair and regeneration.

1. INTRODUCTION
The development and repair of the peripheral nervous system requires complex signaling events from a variety of cell populations. Development of vascular and neuronal tissues occurs simultaneously in utero,[1] and are regulated by significant crosstalk between cellular signals important in the maturation of each individual network.[2] Analysis of signaling events between these cell types in tissue models of the blood brain barrier demonstrate that this dynamic relationship persists throughout the lifespan of each network.[3] Interestingly, the repair process of injured nerves follows a similar process as that found in embryonic development,[4] suggesting that a deeper understanding of how these tissues develop and mature should enhance our ability to understand, and ultimately direct, peripheral nerve regeneration and repair.
Current efforts to understand peripheral nerve development and repair are largely focused on in vivo studies, which employ critically sized neural defects and utilize cylindrical nerve grafts with increasing complexity to repair the lacerated nerve.[5] There is an increasing need to generate in vitro models capable of replicating the complex signaling environment present in nerve repair either for facile screening of drug compounds, the novel formulation of nerve grafts, or to increase the understanding of neural network development and behavior in tissue regeneration in general.[6] At present, there are at least three types of tissue models being explored in the literature: innervation of existing in vitro tissue systems, organotypic culture models, and cell-derived neural applications.[6] The study of tissue innervation into peripheral tissue systems, such as the skin, involves the inclusion of dorsal root ganglia (DRG) neurons into existing scaffold-based systems. In one such system, neural density and axonal growth were increased with the inclusion of endothelial cells and/or keratinocytes, illustrating the utility of cell-based approaches to understand how neural networks can form in maturing in vitro models.[7] However, depending on the composition of the scaffold, these systems typically require histological processing to visualize innervation of the constructs, which makes identification of metrics such as neural density and axonal growth difficult. Current approaches to modeling nerve repair focus on spinal cord organotypic slice cultures and either pair them with nerve segments,[8] or bare gels composed of extracellular matrix proteins such as collagen or fibrin.[9] In the first study, nerve repair was defined as the ability of axons to extend from the spinal cord explant into nerve segments, mimicking the ability of motor neurons to extend through nerve grafts. A combination of glial-derived neurotrophic factor (GDNF) and insulin-like growth factor 1 (IGF1) enhanced axonal survival and penetration into the grafts,[8] replicating signaling events found near regenerating skeletal muscle to form new neuromuscular junctions via IGF1 signaling.[10] This model focused on describing the importance of Schwann cells already present in the nerve graft on axonal growth and assumed that there would be a robust network of Schwann cells present to aid the repair process, which does not recapitulate the scenario found either in development or in regeneration. Alternatively, fibrin or collagen gels were positioned near the spinal cord explants, allowing for the study of how various extracellular matrix compositions affect axonal growth.[9] These biomaterial systems focus on the ingrowth of axons into acellular constructs, and require additional characterization to indicate how they can be used for neural network development or repair. Cell-derived based applications are varied, with one study of the most direct applications generating neurosphere cultures to showing improvement of 3D over 2D culture practices.[11] Using the hanging drop method, increased cell-cell communication was found in the 3D systems, and the measured length of axonal extensions may be longer than they appear in conventional microscopy techniques.
In the present study, we develop our own tissue model for the study of neurogenesis and ultimately for neural repair to overcome some of the above limitations. The model systems were fabricated in collagen gels, as this material has been used extensively in the culture of functional neural,[12] and vascular,[13] networks. Collagen gels were fabricated with hollow channels, which were either seeded with endothelial cells to stimulate neural network development, brain-derived neurotrophic factor (BDNF)-loaded collagen gels to direct neural growth in an acellular system, or DRG explants to observe neural growth in these environments with additional DRG explants seeded on top of the gels. We hypothesized that hollow channel collagen gels would support targeted axonal growth from DRG explants. We also examined the ability of this system to serve as an injury-based model of neural repair by generating an in vivo relevant laceration injury. The ultimate goal of this approach was to generate a facile model with which to recapitulate developmental cues between neural and vascular networks to study how these systems mature and how we can leverage these systems to direct targeted neural growth in situ for tissue regeneration needs.
2. RESULTS and DISCUSSION
2.1. Endothelialization of Channels Maintains Cobblestone Morphology
To determine the efficacy of hollow channels within 3D collagen gels to support extended culture with multiple cell types, endothelial cells were seeded into hollow channel collagen gels, and cell morphology was observed over time. Single channel collagen constructs were fabricated, coated with fibronectin, and seeded with HUVECs. The channels were fabricated via the removal of Teflon coated steel mandrels 508 μm in diameter from polydimethylsiloxane (PDMS) frames (Figure 1A). This channel size was selected based on previous work in our lab and others in generating hollow channel vascular systems.[13b, 14] This process generated channels with uniform dimensions, with little to no additional topographic features from pulling the mandrels out of the channel. Fibronectin coating enhanced endothelial cell attachment over the first two days of culture (Figure 1 C-D) and facilitated the growth and maintenance of these cells over one week (Figure 1 E-F). There was a change in the diameter of the channels as a result of the difference in the outer diameter of the blunt-end needle (820 μm vs. 508 μm) on the outer edges of the channels (Figure 1 C, F). HUVECs attached and fully coated the entire length of the 1 cm long, 500 μm wide, channel (Figure 1E, inset). Seeding efficiencies were improved by injecting higher numbers of HUVECs (5-10*106 cell mL−1) into the channels, and further improved by injecting HUVECs a second time immediately before inverting the devices to seed the top of the channels. HUVECs maintained their cobblestone morphology throughout seven days of culture, a phenotypic marker of endothelial cell health and maturity. It is well known that flow-induced shear stress improves vascular health and stability,[15] and incorporating flow into similar systems produced angiogenic sprouting from the channels.[13] However, it is important to note that our constructs supported endothelial cell maturity under static conditions for at least one week of culture, suggesting that either the cylindrical shape of the channels or the fibronectin coating maintained the maturity of the HUVECs during this time period.
Figure 1.
Endothelialization of channels and formation of cobblestone morphology. (A) Schematic of experimental design. Single channel collagen gels were fabricated and endothelial cells were seeded within channels. Phalloidin staining of (B) empty channels and channels seeded after (C) one day and (D) two days show robust attachment within the channel. Endothelial cells persisted at least seven days after seeding, and (E) were observed to grow on all sides of the channel (inset). Additionally, the cells appeared to have a cobblestone morphology in the channels, suggesting the formation of mature endothelium. (F) Image of entire channel to confirm endothelial cell seeding efficiency after seven days of culture. Dashed lines indicate channel boundaries. Scale: 500 μm.
2.2. Endothelial Cell Seeded Channels Direct Enhanced Neural Growth in 3D
To demonstrate that endothelial cells (ECs) stimulate axonal growth in 3D constructs and recapitulate developmental signaling conditions, HUVECs were seeded within channels and DRG explants were seeded on top of the constructs (Figure 2A), and the distance between the top of the gels and the channels was approximately 1 mm (Figure 2F). Further, HUVECs maintained their presence and healthy morphology throughout the channel during co-culture with DRGs, as visualized by cross-sectional analysis of HUVEC-seeded channels (Figure 2F). Axons from DRGs that were seeded directly above the EC-seeded channels extended 1,720 ± 274 μm along the length of the channel (Figure 2B). Axons continued to preferentially grow toward EC-seeded channels when DRGs were seeded as far as 2 mm away from these channels (Figure 2E). To confirm the presence of ECs within the channels, ECs were either preloaded with the lipophilic dye DiO (Figure 2C), or stained with phalloidin (Figure 2D). Both dye methods confirmed the presence of ECs within channels that directed guided axonal growth. To observe the morphology of ECs, constructs were bisected through the midline of the channels and stained with phalloidin. In addition to visualizing the morphology of ECs, this view also facilitated the measurement of the thickness of the collagen gels. Regardless of the thickness of the gels, visualization of the cross-section of bisected channels revealed that axons were occasionally observed to be in close proximity to ECs within their channels (Figure 3A). The ECs stained positive for CD31, a cell-cell junction marker indicative of a mature EC network. These data, combined with the cobblestone morphology, suggested that these pseudo-vascular channels can support culture of ECs under static conditions for one week. Further, the co-localization of these neurovascular structures within these constructs mimics physiologic structures found in mammalian anatomy.[16] It is well known that peripheral nervous and vascular systems develop in utero in tandem, resulting in the co-localization of these structures observed.[16]
Figure 2.
Endothelial cell guided neural growth in 3D. (A) Schematic of experimental design. One channel was seeded with endothelial cells and DRGs were seeded in an adjacent channel or on top of the collagen gel. (B,E) Immunostaining of DRGs that were seeded on top of the collagen gel and were observed to grow along or towards channels seeded with ECs. (C,F) Confirmation that channels observed in (B,E) were seeded with ECs. (C) Endothelial cells were preloaded with the lipophilic tracking dye DiO and (F) a construct was bisected along the center line of the channel and stained for phalloidin to reveal a cross-sectional view of the HUVEC-seeded channel. Dashed lines indicate channel boundaries. Scale: 500 μm. (D,G) Quantified histograms of the axonal angle from each DRG shown. In all cases, the 0-180° axis corresponds with the long axis of the images presented in (B,E).
Figure 3.
Endothelial cells direct axonal alignment and enhance axonal growth. (A) Immunostaining of neurons and ECs after seven days in culture. The tissue construct was bisected along the center line of the channel to observe EC morphology in a cross-sectional view. ECs expressed cell junction marker CD31 and axons were observed to grow along these cells. Scale: 100 μm. (B) The Gaussian standard deviation of the axonal histograms from DRGs grown in co-culture with and without ECs. (C) Quantification of axonal length with and without ECs in an adjacent channel. † indicates statistical significance between groups as determined by Student’s t-test (p<0.05, N≥3 independent experiments).
Polar histograms were generated to quantitatively determine axonal alignment with respect to EC seeded channels. In almost all cases, axons preferentially extended along (Figure 2D) and/or towards (Figure 2G) EC-seeded channels. Non-preferential growth would show an even distribution of axons across all angles extended from the explant body. Here, there is an obvious bias in the number of axons growing towards and along the length of EC seeded channels. To quantitatively evaluate this bias in the axonal histograms, we fit the length and angle of each axon to a Gaussian distribution, and computed the standard deviation of this fit. The Gaussian standard deviation of axonal alignment from DRG explants cultured near EC-seeded was significantly decreased (2-fold) from the Gaussian standard deviation of axonal alignment from DRG explants cultured near control, unloaded channels (Figure 3B). This honing of axonal growth towards EC-seeded channels and the formation of these co-localized neuro-vascular structures suggests that the early formation of blood vessels within damaged tissue may be essential to enhancing peripheral nerve regeneration. We previously identified that the confluence of ECs affects their rate of secretion of factors such as brain-derived neurotrophic factor (BDNF),[17] which may be an important factor to consider in peripheral nerve growth. Natural nerve regeneration is traditionally mediated by Schwann cells, which direct axonal growth through soluble and physical cues.[18] While Schwann cells are present in DRG explants,[19] additional Schwann cells have not been added into the bulk or channels of the tissue system, so axons from the explant tissue are likely responding to factors secreted by HUVECs, namely BDNF. These data support the hypothesis that our 3D tissue system can successfully recapitulate signaling conditions found in development, and can be used to study the development of the neurovascular unit in peripheral nerve settings.
To confirm that HUVEC-secreted BDNF was also stimulating axonal growth in 3D, we quantified the length of the longest axon from each DRG cultured with or without ECs. As previously seen in 2D studies,[17] there was a significant increase in axonal length when cultured near EC-loaded channels with respect to empty, control channels (Figure 3C). Taken together, these data demonstrate the ability of EC networks to direct targeted axonal growth in a 3D environment, and that the geometry of these constructs permits the passive diffusion of EC-secreted factors, such as BDNF, through the collagen gel.
2.3. BDNF-Loaded Acellular Channels Align Neural Growth in 3D
To build on our previous studies with EC-driven axonal growth, we next sought to develop acellular constructs also capable of directing aligned neural growth. Here, the hollow channels were injected with low density collagen gels loaded with or without BDNF (Figure 4A). DRGs seeded on these BDNF-loaded systems had numerous axonal extensions sprouting from the explants (Figure 4D). Unloaded (no BDNF) control constructs supported less axonal sprouting from the explants than the BDNF-loaded constructs (Figure 4B). Additionally, the axonal projections were more fasciculated than the dense, branched network supported on the BDNF-loaded constructs, indicating that the released BDNF promoted axonal growth.
Figure 4.
BDNF-loaded channels facilitate targeted axonal growth. (A) Schematic of experimental design to replicate earlier findings without endothelial cells. Single channel devices were constructed and loaded with collagen gels with or without BDNF and DRGs were seeded on top of the collagen gels. (B,D) Immunostaining of neurons cultured near (B) control channels and (D) BDNF-loaded channels. Dashed lines indicate channel boundaries. Scale: 500 μm. (C,E) Quantified histograms of the axonal angle from each DRG shown. In all cases, the 0-180° axis corresponds with the x-axis of the images presented in (B,D). (F) The Gaussian standard deviation of the axonal histograms from DRGs grown on BDNF loaded or unloaded channels. † indicates statistical significance between groups as determined by Student’s t-test (p<0.05, N≥3 independent experiments).
Polar histograms were generated from the angles of the axons extending from the explant to determine alignment with respect to BDNF-loaded or control channels. After four days in culture, axons grew towards and along the axis of the BDNF-loaded channels (Figure 4E), while axons cultured on or near the control channels did not show preferential growth along any axis (Figure 4C). The Gaussian standard deviation of axonal alignment from DRG explants cultured on the BDNF-loaded constructs was significantly decreased (2-fold) from the Gaussian standard deviation of axonal alignment from the DRG explants cultured on control, acellular channels, indicating increased alignment of axons towards BDNF-loaded channels (Figure 4F). Directing targeted alignment of neurons is essential for functional recovery of patients suffering from injuries in the peripheral nervous system.[20] Spontaneous neural regrowth, when it occurs at all, can result in misdirected and/or hyper-innervation, which leads to a permanent loss of function.[20a] Biomaterial-based approaches to address this issue of targeted alignment are usually based on either contact guidance such as with conduits or growth factor gradients.[20c, 21] Further, many of these systems rely on their overall macroscopic shape, i.e. cylindrical constructs, which also limit the directionality of axonal growth of neurons.[22] Here, we demonstrate that patterning simple geometries of BDNF within a collagen gel was sufficient to enhance targeted axonal growth, without the need to constrain overall growth with the use of larger macroscopic constructs.
These BDNF, or other neurotrophic factor, geometries could alternatively be made utilizing microfabrication techniques or 3D bioprinting approaches. For instance, a completely 3D printed nerve guide of a branched nerve pathway has been fabricated previously and, through the incorporation of specific neurotrophic factors in separate branches of the guide, was able to recruit specific nerves (i.e. sensory vs. motor) into the desired branches within the guide.[22] Similar approaches could be employed in future studies to generate more complex geometries within collagen gel constructs to develop more sophisticated neural or vascular networks.[23] Importantly, the directed axonal growth observed in the present study was achieved with a single delivery of BDNF, suggesting that this tissue system can mimic some of the conditions necessary to generate neural networks. The ability to develop neural and vascular systems in vitro should aid in the understanding of how peripheral systems develop and can ultimately inform future tissue engineered strategies for peripheral nerve and vascular regrowth.
To observe the temporal effects of BDNF release from acellular constructs, axonal lengths were measured as early as two days post seeding. Robust axonal sprouting was observed two days after seeding, with polar histograms demonstrating preferentially aligned axonal growth even at this early time point (Figure 5A). Long axonal extensions were present three (Figure 5B) and four (Figure 5C) days after seeding, displaying consistent growth biases towards the BDNF-loaded channels. When cultured for four days on unloaded, control constructs, axons sprouted from the explant with radial symmetry, a characteristic of non-preferential growth (Figure 5D). Axonal growth significantly increased over time on the BDNF-loaded constructs, with an average increase of approximately 400 μm each day in culture (Figure 5E). Axonal growth on control constructs increased approximately 280 μm each day, indicating that BDNF-loaded channels supported an accelerate rate of growth. BDNF-loaded channels supported significantly longer axons than the unloaded, control channels at all time points, and the length of axons cultured on BDNF-loaded channels was qualitatively longer than the length of axons cultured on control channels. The observed increases in axonal growth occurred both when explants were seeded on top of the constructs and when the explants were seeded within adjacent hollow channels, spaced between 1 to 3 mm away from the BDNF-loaded channels (Figure S1). Axons grew along the edges of the channels and seldom entered the bulk, high density collagen gel surrounding the channels. Additionally, the DRG bodies were observed to be slightly smaller in diameter than the channels (405 ± 75 μm). This size allowed for the straightforward injection of DRGs into the channels, and did not require any additional optimization to the construct or channel geometry. In development, peripheral nerves grow as a result of co-signaling events with nascent blood vessel networks.[16] When peripheral nerves are damaged after these networks have been established, regrowth is largely mediated by Schwann cells, which secrete a variety of neurotrophic factors to stimulate axonal growth.[24] However, as shown before with HUVEC-seeded channels, the delivery of exogenous BDNF in the channels appears to dominate Schwann cell-mediated axonal growth, as shown by the accelerated rate of axonal growth on the BDNF-loaded constructs. Taken together, these data demonstrate the utility of this model to generate neural networks, and to accelerate their targeted growth towards specific stimuli.
Figure 5.
BDNF-loaded channels enhance axonal growth. Immunostaining of DRG explants cultured with BDNF-loaded channels after (A) two, (B) three, (C) or four days. (D) DRG explants cultured with unloaded channels (controls) after four days of culture. Insets within each image correspond to the quantified histogram of the axonal angle from each DRG, and share the same x-axis. Dashed lines indicate channel boundaries. Scale: 500 μm. (E) Quantification of axonal length in the presence of BDNF loaded or unloaded channels. * indicates statistical significance between indicated groups as determined by one-way ANOVA and † indicates statistical significance between groups at the same time point as determined by Student’s t-test (p<0.05, N≥3 independent experiments).
2.4. Development of a Model for Peripheral Nerve Injury and Repair
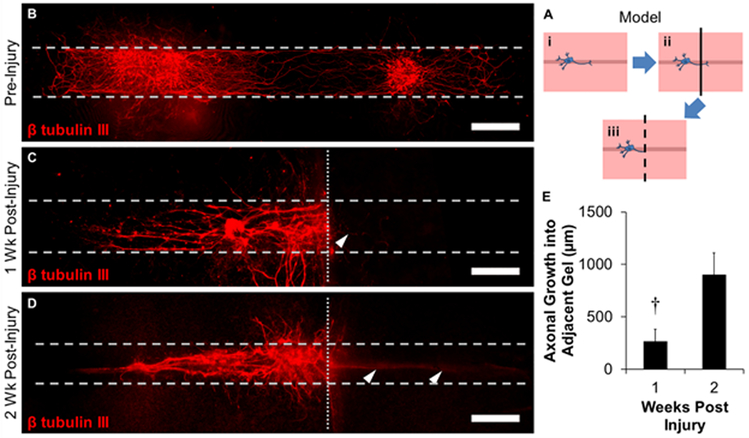
To determine the ability of hollow channel collagen gels to model peripheral nerve injury and repair, a laceration injury protocol was developed to simulate in vivo injuries (Figure 6A). Here, DRG explants were cultured within the hollow channels, as opposed to on top of the gels, for four days to facilitate a robust neural network (Figure 6Ai, B). At this time, the gels were bisected orthogonally to the longitudinal axis of the channels, creating two injured axonal networks (Figure 6Aii), and a new hollow channel construct similarly coated with fibronectin, was affixed to the injured channel (Figure 6Aiii). Several thin axons were observed to grow into the new channels 1 week post-injury, while the rest of the injured network was maintained (Figure 6C). Long axons extended into the hollow channels two weeks post-injury (Figure 6D), demonstrating utility for the model to simulate neural injury and repair. The length of the repaired axons tripled (268 ± 115 μm vs. 903 ± 258 μm) from one to two weeks post injury, respectively (Figure 6E), suggesting that there is an initial lag-phase prior to accelerated axonal growth. While axons were also observed to penetrate into the high density, bulk collagen gel, the overwhelming majority of axons were observed to grow along the channels, particularly the new hollow channels placed adjacent to the injured channels. Neural repair typically begins with the disintegration of existing axonal structures along the myelin sheath in a process called Wallerian degeneration.[25] After this process, new extracellular matrix is deposited by Schwann cells, and this in addition to gradients of neurotrophic factors facilitates axonal growth.[18, 25] To assess the contribution of Schwann cells to the growth of nascent axons within neural networks, several constructs were stained for S100, a Schwann cell marker, and observed numerous S100+ cells present at or before nascent neural formation (Figure S2); supporting the hypothesis that this collagen channel model system can support events found in neural regeneration. Axons present in the bulk space of the construct were also accompanied by S100+ cells, suggesting that Schwann cells are assisting in the formation of these axons. The hollow channels were coated with fibronectin, a component of the provisional wound healing matrix, which supported early axonal sprouting.[26] The inclusion of other basal lamina proteins such as laminin may enhance earlier axonal sprouting into the hollow channel, as this may help shorten the initial lag-phase observed in our model. The lag-phase may also be a result of the axons searching for proper guidance cues, such as surface topography or the fibronectin coating, as the constructs were glued together with collagen gel which may have infiltrated portions of the channels. Regardless, protein hollow tube systems have been extensively used to support neural growth and regeneration, both in vitro and in vivo.[5, 18, 27] While the channels in the present study do not have any specifically designed surface topography, this strategy has been previously used to direct aligned neural growth,[21, 28] and could be applied to the present system in the future to further enhance neural alignment and growth. We did not observe many axons sprouting into the bulk of the collagen gel, rather, we consistently observed sprouting towards and into the channels. This suggests that axons are able to locate the hollow channels and populate them, showing promise for this system to be used to model neural repair.
Figure 6.
Utilization of tissue construct system as an injury model for peripheral nerve repair. (A) Schematic of experimental design. (i) DRGs were seeded within hollow channels and (ii) bisected orthogonally to the longitudinal axis of the channels after 4 days of culture. (iii) Bisected gels were affixed to empty gels with the channels adjacent to one-another and cultured. (B-D) Immunostaining of neurons (B) pre-injury, (C) 1 week post injury, and (D) 2 weeks post injury. Dashed lines indicate channel boundaries and dotted lines indicate the laceration plane. Arrowheads indicate nascent axons infiltrating into new channels. Scale: 500 μm. (E) Quantification of length of repaired axons as measured from the laceration plane. † indicates statistical significance between groups as determined by Student’s t-test (p<0.05, N≥3 independent experiments).
Current approaches to study and understand peripheral nerve repair are almost entirely conducted in in vivo with critically sized defects. While this is certainly the most direct method, there remains an unmet need for a highly reproducible in vitro model to understand these processes prior to in vivo implantation.[29] Tissue models designed to study peripheral nerve growth are limited in that they lack the ability to create neural networks, directly measure the growth of these nascent networks, or accurately model injury conditions to study repair and regeneration. Here, we implanted DRG explants into our hollow channel collagen gels, which supported axonal spreading without the use of additional materials or cell types. Once a neural network was formed, the constructs were subjected to a laceration injury and axons extended into channels coated with extracellular matrix proteins positioned at the laceration interface. To our knowledge, this is the first instance where a peripheral nerve injury has been replicated in vitro, and further, demonstrated that axons regrow into adjacent channels after the injury. The recovering axons in these systems appear to preferentially grow into these open channels as opposed to within the dense collagen gel, suggesting that there are topographical and matrix-based guidance cues present within the hollow fiber tissue system that are facilitating axonal growth. Type I collagen has been shown to be effective for peripheral nerve repair and can facilitate nerve guidance in both hydrogel,[12c, 30] and conduit formulations previously.[31]
This tissue system could be utilized to study reinnervation of tissue systems such as skin or skeletal muscle. The neural source utilized in these studies is DRGs, which are an excellent source of sensory neurons. Further characterization will have to be done to confirm the relevancy of this model for use with motor neurons, which will greatly aid in the understanding and development of future strategies for peripheral nerve regeneration.
3. CONCLUSIONS
A 3D tissue system consisting of hollow channels within collagen gels was developed to support neural network formation and reformation after injury. The tissue system supported EC attachment and growth, showing a healthy morphology and cell-cell junctions after one week in culture under static conditions. The EC network formed within the hollow channels stimulated axonal growth from DRG explants, and further guided the growth of axons to preferentially extend towards and/or along the EC-seeded channel dimensions. By analyzing the angle of all of the axons from the axonal tree of the explants, the alignment of the axons was significantly enhanced along this EC-seeded channel axis with respect to cultures with empty channels. The observation of the close proximity of axonal growth towards EC structures mimics neurovascular structures found throughout the peripheral nervous system, and could become a model to study these interactions in much greater depth. To be able to study the growth of neural networks without ECs, channels were loaded with BDNF, the factor that was previously identified to be responsible for HUVEC-mediated axonal growth.[17] Axonal growth from DRG explants followed a similar behavior in these conditions as when they were cultured with EC-seeded channels, i.e. axons were significantly longer and more aligned towards the axis of the channels loaded with BDNF rather than blank channels. This neural-only network was then subjected to a laceration injury, and affixed to a blank channel to observe if neural regrowth could be observed. Over the course of two weeks post injury, axons extended into the new channels as opposed to the dense collagen gel, indicating that this system could be used to study neural network injury and subsequent repair and regeneration. Composing the tissue system entirely out of collagen provided optical transparency and facilitated imaging without the need of histologic processing. Taken together, these results demonstrate that our 3D tissue system can model neural, and perhaps vascular, network formation, and repair after laceration injuries, and can be utilized to further study how these networks form and interact with other tissues.
4. EXPERIMENTAL SECTION
Fabrication of Hollow Channel Collagen Gels:
A custom polycarbonate mold was created by machining 0.9 cm tall cuboidal posts 1 cm x 1 cm or 1 cm x 0.75 cm (Figure 7A). The outer walls were machined with a 45° angle to facilitate ease of removing constructs from the mold. Polydimethylsiloxane (PDMS; Sylgard 184; Dow Corning, Midland, MI) was mixed at a 10:1 (w/w) ratio of base to curing agent, degassed for at least 1 hr, poured into the polycarbonate mold, and degassed for an additional 30 minutes. After curing at 60°C for at least 8 hrs, the PDMS was peeled from the mold along the inclined walls of the mold, which made a total of six wells. Individual wells were separated with a razor blade (Figure 7B, B’), and autoclaved. After sterilization, 2 pairs of 21G blunt-end needles (McMaster-Carr, Robbinsville, NJ) were affixed on either side of the 1 cm x 1 cm wells for dual channel studies, or 1 pair of 21G blunt-end needles were affixed on either side of the 1 cm x 0.75 cm wells for single channel studies in aseptic conditions (Figure 7C, C’). Teflon coated steel mandrels 508 μm in diameter (McMaster-Carr) were threaded through each pair of needles. To generate the bulk of the 3D tissue constructs, collagen (Corning, Corning, NY) was mixed to a final concentration of 6.5 mg mL−1 with 10X phosphate buffered saline (PBS; ThermoFisher, Waltham, MA), cell culture medium (EGM2 for HUVEC studies, DMEM for DRG-only studies), and sodium hydroxide (NaOH; Sigma-Aldrich, St. Louis, MO) on ice, per manufacturer’s instructions. The mixed collagen solution was pipetted into PDMS wells to cover the steel mandrels with approximately 1–2 mm of solution to a final gel height of 2–3 mm, and incubated at 37°C for 1 hr to induce gelation (Figure 7D, D’).
Figure 7.
Overview of fabrication of 3D constructs for neural-endothelial cell co-cultures. (A) Polydimethylsiloxane (PDMS) was cured in negative templates of co-culture molds, removed and (B) cut into individual wells. Note that each well has a thin bottom to facilitated live imaging as needed. (C) Blunt-end needles were inserted into the PDMS frame and Teflon-coated stainless steel wires were passed through needle pairs. (D) A collagen gel was cast around the needles and once polymerized, the needles were removed to create hollow channels. Channels were coated in various ECM proteins, seeded with either endothelial or neural cells, and (E) removed from the PDMS frames into 24 well plates for culture. In all cases, ‘ panels are representative photographs of their respective schematics.
For HUVEC-seeded and DRG-seeded channels, after gelation the steel mandrels were carefully removed from the molds to minimize the generation of additional topographic features. The channels, with an internal radius of 508 μm and length of 1 cm, were coated with fibronectin (Corning) at a concentration of 50 μg mL−1 mixed in cell culture medium for at least 30 minutes. For acellular studies where BDNF was loaded into the channels, the steel mandrels were carefully removed after incubation at 37°C for 1 hr and the channels were filled with a low density collagen gel that was previously mixed on ice to a final concentration of 2.5 mg mL−1 with or without BDNF, mixed to a final concentration of 100 ng mL−1. This collagen solution was injected into empty channels, taking care to not allow any overflow from the channels. Constructs were incubated at 37°C for 1 hr and subsequently removed from the PDMS frames into 24 well plates filled with the appropriate cell culture medium (Figure 7E, E’).
Cell Culture and Seeding of Constructs:
Human umbilical vein endothelial cells (HUVECs, Lonza, Walkersville, MD) were cultured in complete EGM-2 medium (Lonza) according to the manufacturer’s instructions. Cells were incubated at 37°C with 5% CO2 and maintained using standard cell culture techniques. Routine cell passage was conducted at 80–90% confluence using 0.25% trypsin-EDTA (CellGro). Cells were not used for experiments once they reached passage 11, and were typically between passages 6–10. HUVECs were injected into constructs at a density between 1 – 10 * 106 cell mL−1 by affixing a 1 mL syringe to the 21G needles. After injection, constructs were incubated for 30 minutes, injected with HUVECs a second time, and inverted to coat the upper half of the channels. After an additional 30 minutes the constructs were removed from the PDMS frames and cultured in 24 well plates for 3 days prior to the addition of DRGs. To analyze HUVEC morphology within the channels without the presence of DRGs, constructs were fixed with 4% paraformaldehyde (Boston BioProducts, Ashland, MA) at specified time points.
DRG Isolation and Culture:
Chicken DRGs were isolated from E8 chicken embryos (University of Connecticut, Poultry Farm, CT) in accordance with NIH and Tufts guidelines for embryonated chicken eggs, as previously described.[17] Briefly, under aseptic conditions, tissue from the embryo was dissected away to expose the spinal column. DRGs were removed from each embryo using fine-pointed forceps and surrounding fascia were removed using forceps and scalpel blades. DRGs were cut in half using scalpel blades and cultured in complete Dulbecco’s modified Eagle Medium growth medium (DMEM; 1:1 (v/v) ratio of high glucose DMEM (Gibco BRL, Gaithersburg, MD) and Ham’s F12 (Gibco) supplemented with 4 mM L-glutamine and Ham’s F12 (Gibco) and 10% fetal bovine serum (FBS, Sigma)). For both co-culture studies with HUVECs and seeding onto acellular constructs, 2–4 DRG explant sections were seeded on top and towards the center of the collagen constructs. In some cases, DRGs were also injected into adjacent channels using a 25G needle affixed to a syringe. DRG co-cultures were cultured for 4 days and then fixed with 4% paraformaldehyde for immunostaining. For the injury model, 4–6 DRGs were injected into the central region of the channels and cultured for 4 days prior to injury to facilitate neural network formation. In all cases, similar numbers of DRGs were added to ensure that the density, amount, and volume of the DRGs were the same for each biological replicate within each independent experiment. Data was collected from at least three independent experiments with at least three biological replicates within each experiment.
Development of Injury Model:
DRG-seeded hollow channels were cultured for 4 days to facilitate neural network formation. Collagen gels were fabricated from the same PDMS wells used from the previous seeding to provide a new environment for axons to extend into after injury. To create the injury, the gels were cut in half with surgical scissors, taking care to cut through axons present within the channels. The newly fabricated collagen gels were coated with fibronectin, cut in half at approximately the same location as the DRG-seeded constructs and interfaced with the wounded construct, taking care to line up the two channels. A drop of a 3.5 mg mL−1 collagen gel was applied on top of these constructs to fuse them together. The fused constructs were incubated at 37°C for 1 hr and carefully returned to their 24 well plates. Medium was replaced twice a week and after 1 or 2 weeks the constructs were fixed with 4% paraformaldehyde for immunostaining to characterize axonal ingrowth into the new channel.
Immunocytochemistry:
After fixation with 4% paraformaldehyde and subsequent rinses with PBS, tissue constructs were permeabilized with 0.1% Triton X-100 (Sigma), blocked using 5% bovine serum albumin (BSA; Sigma), and immunostained with primary antibodies against β-tubulin III (1:500, Cat No. T2200; Sigma), CD31 (1:50, Cat No. 555444, BD Biosciences, San Jose, CA), Alexafluor 488 or 594 tagged phalloidin (1:500, A-12379 and A-12381 respectively; ThermoFisher), or co-stained with primary antibodies against β-tubulin III (1:500, Cat No. ab78078; Abcam, Cambridge, MA) and S100 (1:250, Cat No. PA5–16257; Sigma) overnight at 4°C. The following day, samples were washed several times with PBS, incubated with species-matched Alexafluor 488 and 594 secondary antibodies (1:500, A-21202 and A-11072 respectively; ThermoFisher), and then rinsed with PBS. In some cases, constructs were bisected through the length of the channel to visualize the cross-section of the channels.
DRG Axon Length Measurements:
Fluorescent images were obtained using a Keyence BZ-X700 microscope and associated software with a 4X or 10X objective (Keyence, Elmwood Park, NJ). To visualize DRGS or ECs seeded within the channels, the “full focus” feature of the Keyence microscope was utilized, which scanned through the thickness of the constructs and captured the pixels fully in focus for each image. Multiple images were merged together to assemble composite images of each DRG explant (Figure 8A). The brightness and contrast of merged images was adjusted equally throughout the entire image and similarly across all images. Merged images were opened in Image J (NIH) and the image was converted to binary and then subsequently skeletonized to visualize the axonal tree from the DRG explant (Figure 8B). The length and angle with respect to the explant was recorded for each terminal axon. The longest axon of these measurements was compiled for each experimental condition and the angles were binned and plotted using a polar histogram in MATLAB (Figure 8C; Mathworks, Natick, MA). In all cases, the observer was blinded to the treatment group that they were imaging and analyzing. The size of each DRG body was also recorded. Any DRG that did not have an easily defined DRG body, or a body that was smaller than 150 μm was omitted from further analysis.
Figure 8.
Analysis of axonal alignment from DRG explants. (A) Composite images of DRGs and all of their axons were collected, converted to binary, and (B) skeletonized using ImageJ to visualize the axonal tree. The length of each axon and its relative angle from the DRG explant body were recorded and (C) visualized in a polar histogram.
Analysis of Axonal Alignment:
The 2D discrete Fourier transform (DFT) was used to calculate orientation distributions and relative degrees of alignment of axons extending from DRG explants cultured with HUVECs or BDNF-loaded gels seeded in hollow channels using a custom MATLAB script.[32] Composite images of each DRG explant were run through the script and after DFT analysis were converted into polar coordinates and fit against a Gaussian distribution to characterize the degree of alignment of the axons extending from a single DRG explant.
Statistics:
Statistical analyses were performed using a one-way analysis of variance (ANOVA) with p<0.05 indicating significant differences between groups using SigmaPlot 13.0 software (Systat Software, Inc., San Jose, CA). For post hoc analyses, a Holm-Sidak pairwise multiple comparison test was performed to determine significance between experimental groups using an overall significance level of p<0.05. Where indicated, a Student’s t-test was performed where differences between conditions were considered significant at p<0.05. Data are reported as means ± standard errors.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dana M. Cairns, Rosalyn D. Abbott, and Nicolas Rouleau for helpful discussions. This research was funded in part by the NIH (P41-EB002520, R01-AR055993, F32-DE026058), the WM Keck Foundation and the Paul G Allen Foundation (2171).
Footnotes
SUPPORTING INFORMATION
Supporting Information is available from the Wiley Online Library or from the author.
REFERENCES
- [1].Sawada M, Matsumoto M, Sawamoto K, Front Neurosci 2014, 8, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Segura I, De Smet F, Hohensinner PJ, Ruiz de Almodovar C, Carmeliet P, Trends Mol Med 2009, 15, 439. [DOI] [PubMed] [Google Scholar]
- [3].Ehret F, Vogler S, Kempermann G, Stem Cell Res 2015, 15, 514. [DOI] [PubMed] [Google Scholar]
- [4].Padilla F, Marc Mege R, Sobel A, Nicolet M, J Neurosci Res 1999, 58, 270. [PubMed] [Google Scholar]
- [5].Belanger K, Dinis TM, Taourirt S, Vidal G, Kaplan DL, Egles C, Macromol Biosci 2016, 16, 472. [DOI] [PubMed] [Google Scholar]
- [6].Geuna S, Raimondo S, Fregnan F, Haastert-Talini K, Grothe C, Eur J Neurosci 2016, 43, 287. [DOI] [PubMed] [Google Scholar]
- [7].Gingras M, Bergeron J, Dery J, Durham HD, Berthod F, FASEB J 2003, 17, 2124. [DOI] [PubMed] [Google Scholar]
- [8].Vyas A, Li Z, Aspalter M, Feiner J, Hoke A, Zhou C, O’Daly A, Abdullah M, Rohde C, Brushart TM, Experimental neurology 2010, 223, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gerardo-Nava J, Hodde D, Katona I, Bozkurt A, Grehl T, Steinbusch HW, Weis J, Brook GA, Biomaterials 2014, 35, 4288. [DOI] [PubMed] [Google Scholar]
- [10].Caroni P, Schneider C, Kiefer MC, Zapf J, J Cell Biol 1994, 125, 893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kraus D, Boyle V, Leibig N, Stark GB, Penna V, Journal of neuroscience methods 2015, 246, 97. [DOI] [PubMed] [Google Scholar]
- [12] a).Tang-Schomer MD, White JD, Tien LW, Schmitt LI, Valentin TM, Graziano DJ, Hopkins AM, Omenetto FG, Haydon PG, Kaplan DL, Proceedings of the National Academy of Sciences of the United States of America 2014, 111, 13811; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Murphy AR, Laslett A, O’Brien CM, Cameron NR, Acta Biomater 2017, 54, 1; [DOI] [PubMed] [Google Scholar]; c) Schuh C, Day AGE, Redl H, Phillips J, Tissue Eng Part A 2018, DOI: 10.1089/ten.TEA.2017.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13] a).Chrobak KM, Potter DR, Tien J, Microvasc Res 2006, 71, 185; [DOI] [PubMed] [Google Scholar]; b) Nguyen DH, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS, Proceedings of the National Academy of Sciences of the United States of America 2013, 110, 6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rnjak-Kovacina J, Wray LS, Golinski JM, Kaplan DL, Adv Funct Mater 2014, 24, 2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15] a).Wentzel JJ, Chatzizisis YS, Gijsen FJ, Giannoglou GD, Feldman CL, Stone PH, Cardiovasc Res 2012, 96, 234; [DOI] [PubMed] [Google Scholar]; b) Galie PA, Nguyen DH, Choi CK, Cohen DM, Janmey PA, Chen CS, Proceedings of the National Academy of Sciences of the United States of America 2014, 111, 7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].James JM, Mukouyama YS, Semin Cell Dev Biol 2011, 22, 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grasman JM, Kaplan DL, Scientific reports 2017, 7, 4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Faroni A, Mobasseri SA, Kingham PJ, Reid AJ, Adv Drug Deliv Rev 2015, 82–83, 160. [DOI] [PubMed] [Google Scholar]
- [19].a) Wang HB, Mullins ME, Cregg JM, McCarthy CW, Gilbert RJ, Acta Biomater 2010, 6, 2970; [DOI] [PubMed] [Google Scholar]; b) Romano NH, Madl CM, Heilshorn SC, Acta Biomater 2015, 11, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20] a).Allodi I, Udina E, Navarro X, Prog Neurobiol 2012, 98, 16; [DOI] [PubMed] [Google Scholar]; b) Cerri F, Salvatore L, Memon D, Martinelli Boneschi F, Madaghiele M, Brambilla P, Del Carro U, Taveggia C, Riva N, Trimarco A, Lopez ID, Comi G, Pluchino S, Martino G, Sannino A, Quattrini A, Biomaterials 2014, 35, 4035; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Marquardt LM, Ee X, Iyer N, Hunter D, Mackinnon SE, Wood MD, Sakiyama-Elbert SE, Tissue Eng Part A 2015, 21, 2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Srinivasan A, Tahilramani M, Bentley JT, Gore RK, Millard DC, Mukhatyar VJ, Joseph A, Haque AS, Stanley GB, English AW, Bellamkonda RV, Biomaterials 2015, 41, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL, Engel EA, Krick KD, Ju A, Meng F, Enquist LW, Jia X, McAlpine MC, Adv Funct Mater 2015, 25, 6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23] a).Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA, Proceedings of the National Academy of Sciences of the United States of America 2016, 113, 3179; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zheng Z, Wu J, Liu M, Wang H, Li C, Rodriguez MJ, Li G, Wang X, Kaplan DL, Advanced healthcare materials 2018, 7, e1701026. [DOI] [PubMed] [Google Scholar]
- [24].Gordon T, Hand Clin 2016, 32, 103. [DOI] [PubMed] [Google Scholar]
- [25].Pinho AC, Fonseca AC, Serra AC, Santos JD, Coelho JF, Advanced healthcare materials 2016, 5, 2732. [DOI] [PubMed] [Google Scholar]
- [26].Harris GM, Madigan NN, Lancaster KZ, Enquist LW, Windebank AJ, Schwartz J, Schwarzbauer JE, Matrix Biol 2017, 60–61, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27] a).Lin YC, Ramadan M, Hronik-Tupaj M, Kaplan DL, Philips BJ, Sivak W, Rubin JP, Marra KG, Ann Plast Surg 2011, 67, 147; [DOI] [PubMed] [Google Scholar]; b) Sivak WN, White JD, Bliley JM, Tien LW, Liao HT, Kaplan DL, Marra KG, J Tissue Eng Regen Med 2017, 11, 733. [DOI] [PubMed] [Google Scholar]
- [28] a).Simitzi C, Ranella A, Stratakis E, Acta Biomater 2017, 51, 21; [DOI] [PubMed] [Google Scholar]; b) Mukhatyar V, Pai B, Clements I, Srinivasan A, Huber R, Mehta A, Mukhopadaya S, Rudra S, Patel G, Karumbaiah L, Bellamkonda R, Ann Biomed Eng 2014, 42, 1436; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mukhatyar VJ, Salmeron-Sanchez M, Rudra S, Mukhopadaya S, Barker TH, Garcia AJ, Bellamkonda RV, Biomaterials 2011, 32, 3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shrirao AB, Kung FH, Omelchenko A, Schloss RS, Boustany NN, Zahn JD, Yarmush ML, Firestein BL, Biotechnol Bioeng 2018, 115, 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Antman-Passig M, Levy S, Gartenberg C, Schori H, Shefi O, Tissue Eng Part A 2017, 23, 403. [DOI] [PubMed] [Google Scholar]
- [31].van Neerven SGA, Haastert-Talini K, Boecker A, Schriever T, Dabhi C, Claeys K, Deumens R, Brook GA, Weis J, Pallua N, Bozkurt A, J Tissue Eng Regen Med 2017, 11, 3349. [DOI] [PubMed] [Google Scholar]
- [32].Abbott RD, Howe AK, Langevin HM, Iatridis JC, Biochemical and biophysical research communications 2012, 421, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.