Abstract
Objectives
Performance in running-based sport depends on the ability to perform repetitive high intensity muscle contractions. Previous studies have shown that capsaicin analog (CAP) (i.e. Capsiate) supplementation may improve this performance. The purpose of this study was to investigate the acute effect of CAP supplementation on short (400 m) and middle distance (3000 m) running time-trial performance, maximum heart rate (HR), and rate of perceived exertion (RPE).
Methods
Twelve physically active men completed four randomized, double-blind trials: CAP condition (12 mg) or a placebo condition. Forty-five minutes after supplementation, the participants performed a 400- or 3000-meter running time trial. Time (in seconds) was recorded. HR was analyzed at rest and immediately post-exercise, and RPE was collected immediately after exercise.
Results
For both the 400 m time-trial (CAP = 66.4 + 4.2 sec vs Placebo = 67.1 + 4.8 sec, p = 0.046) and the 3000 m time-trial (CAP = 893.9 ± 46.8 sec vs Placebo = 915.2 ± 67.6 sec, p = 0.015), the time in seconds was significantly less in the CAP compared to placebo conditions. There were no statistically significant differences for HR and RPE in any condition.
Conclusion
In summary, acute CAP supplementation improved 400 m and 3000 m running time-trial performance in a distance-dependent way but without modifying the HR and RPE.
Keywords: Capsinoids, endurance, aerobic exercise
INTRODUCTION
Capsaicin (8-methyl-N-vanillyl-trans-6-nonenamide), dihydrocapsaicin, nordihydrocapsaicin, homocapsaicin, and homodihydrocapsaicin constitute the capsaicinoids, which are bioactive phytochemicals found primarily in chili peppers, cayenne pepper, red pepper and other spicy foods. These phytochemicals are responsible for the fruit’s ‘pungent’ heat sensation (15). Their concentrations typically range from 0.1 mg/g in chili pepper to 2.5 mg/g in red pepper and 60 mg/g in oleoresin red pepper. Additional capsaicin exists among the natural capsinoids (capsiate, dihydrocapsiate and nordihidrocapsiate), which are found in non-pungent Capsicums (Capsicum annuum L. or Capsicum frutescens L.), such as the pepper cultivar CH-19 sweet, were found to contain 0.22–20 mg total capsinoids/g of dry weight (19). They are structurally identical to the pungent constituents of Capsicum, i.e, capsaicin, dihydrocapsaicin, and nordihydrocapsaicin, respectively, though their ergogenic or therapeutic properties have not been as heavily investigated. The mean consumption of Capsicum spices in different cultures was reported to be 2.5 g/person/day in India, 5 g/person/day in Thailand (17) and 20 g/person per day in Mexico (12). Assuming a content of capsaicinoids in these spices of about 1%, the daily intake of capsaicinoids in these countries has been estimated to be 25–200 mg/person/day (4).
Capsaicin and capsaicin analog, such as Capsiate (CAP) act through the Transient Receptor Potential Channel Vanilloid type-1 (TRPV1), a transmembrane channel expressed in many tissues, including the brain stem, mid-brain, hypothalamus, and limbic system centrally, and the gastrointestinal tract and adipose tissue peripherally (7). There is evidence that capsinoids possesses anti-obesity properties via reductions in appetite and energy intake (14). Although the mechanisms are not totally understood, it seems that it occurs through interactions with appetite-related hormones, such as an increase of GLP-1 (anorexigenic hormones) (22), as well as increased energy expenditure via activation of the sympathetic nervous system (7).
Regarding the potential ergogenic effect of CAP, most studies have been conducted with rodents (8, 9, 23). Faraut et al. (8) investigated the effect of 14 days of CAP administration and verified an enhancement of aerobic ATP production attributable to UCP3 downregulation in rodent skeletal muscle. Yashiro et al. (23) demonstrated that 2 weeks of CAP resulted in a reduced oxidative cost of contraction in exercising mouse skeletal muscle. In addition, Kim et al. (10) demonstrated that 10 mg of capsaicin supplementation increased swimming endurance capacity (time to exhaustion), and the authors postulated that the increases in endurance performance were explained in part by an increase in plasma free fatty acids as a result of higher epinephrine release, thereby generating a glycogen sparing effect. Other potential mechanisms whereby capsaicin may improve performance could be related to TRVP1 activation in skeletal muscle, and subsequent increases in the release of calcium by the sarcoplasmic reticulum (13), inducing an enhanced interaction between actin-myosin filaments and greater force production (11).
The ergogenic effects and mechanisms of CAP in humans are less known. Recently, a single dose of 12 mg CAP supplementation improved 1500-m running time-trial performance by approximately five seconds and decreased the rating of perceived exertion (RPE) in physically active adults (6). Conversely, Opheim and Rankin (18) investigated the effect of 28.5 mg of cayenne pepper for 7 days on repeated-sprint exercise (15 × 30-m all-out sprints with 35-s rest intervals) in healthy athletes, however, the authors did not find significant benefits. Discrepancies between studies that may explain these divergent results include dose, the form of the pepper extract used (CAP vs. cayenne powder), duration (acute vs. chronic), and metabolic characteristics of the exercises employed.
Considering that CAP enhances aerobic ATP production and decreases the oxidative cost of contraction in rodent skeletal muscle (23), it could be an ergogenic strategy to improve middle distance running time-trial performance (3000 m). Additionally, because capsaicin-mediated activation of the TRVP1 in skeletal muscle induces a larger calcium release and promotes an enhanced interaction between actin-myosin filaments and greater force production (11), it could also be efficient during exercises recruiting more type II fibers, such as a 400 m running. Therefore, the purpose of this study was to investigate the acute effect of capsaicin analog supplementation (Capsiate) on short (400 m) and middle distance (3000 m) running time-trial performance, maximum heart rate, and rating of perceived exertion in physically active adults and to compare the magnitudes of changes between distances.
METHODS
Participants
Twelve physically active men were recruited (age = 28.6±5.4 y). The inclusion criteria for participation in the study were: between 18 and 35 years old; no participation in regimented running training during the previous six months; and not taken any supplements to improve performance during the 6 months prior to the tests. However, the participants practiced regular exercise, such as resistance training and jogging (less than three times a week and less than one hour per day). During the study, all participants were instructed not to consume any dietary supplements or ergogenic aids, as well as not to make any nutritional changes in their regular diet before each test. The study was approved by the Ethics Research Group of the Federal University of Piauí, Teresina, PI, Brazil (Protocol number: 3.169.545) and the research was conducted according to the 2013 Revision of the Declaration of Helsinki and all the participants signed an informed consent form about the purpose of the study and the possible risks. This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (16).
Protocol
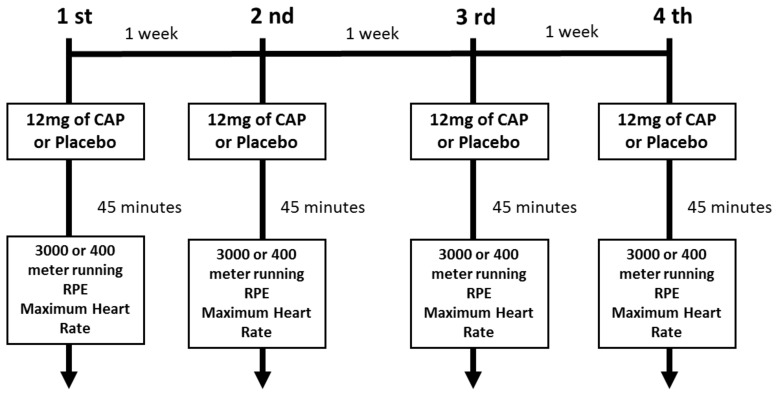
This study used a randomized, double-blind, crossover design. Subjects completed six visits: on the first visit, measurements of anthropometrics and body composition were performed, and subjects completed one familiarization session on a treadmill and outdoor track. On the second visit, participants completed the maximal aerobic speed test. On the following four experimental trials, each participant consumed either the placebo or CAP in a counterbalanced design and then completed either a 400- or 3000-meter run performed at the same time of day (6:30 to 9 AM), separated by one week, as illustrated in Figure 1. The time in seconds, maximum heart rate, and RPE was collected after exercise.
Figure 1.
Experimental Design.
Anthropometric and Body Composition Assessment: Subject stature (height) was measured with a Sanny brand stadiometer (Sanny, São Paulo, SP, Brazil) with precision of 0.1 cm and maximum length of 2.20 meters. Body weight was measured on an electronic scale (Filizola PL 50; Filizola, Ltda., Brazil), with an accuracy of 0.1 kg. Fat mass and fat free mass in kilograms were assessed using bioelectrical impedance analysis and accompanying software (InBody S10, Gangnam-gu, Seoul, Korea). Participants wore light clothing, they were positioned in a supine position, and remained still throughout the examination.
Maximal Aerobic Speed Test: Participants performed an incremental test on a treadmill (Evoque Jet-9, TRG Fitness, Blumenau, SC, Brazil) to volitional exhaustion. Initial treadmill velocity was set at 8.0 km.h−1 and increased by 1 km.h−1 per 2-min stage until the participant could no longer continue. Maximal velocity reached in the test was defined as the maximal aerobic speed (MAS). When the subject was not able to finish the last stage, speed was expressed according to the time of permanence in the last stage, determined as the following: MAS = velocity of penultimate stage + [(time, in seconds, remained at the last stage multiplied by 1 km.h−1)/60s].
Supplementation Protocol: Volunteers were instructed not to consume any spicy foods or add any spice to meals during the study, as well as not consume tea, coffee, alcoholic beverages, or stimulant beverages for a period of 12 hours before each test. Questionnaires were distributed to all participants to record food and fluid intake for 24 hours prior to each trial. Participants were instructed to eat their breakfast by eating healthy foods and replicate the same dietary intake as the first day in the subsequent trials. To ensure that food intake was similar between experimental trials, all food intakes were analyzed for total consumption of kilocalorie and macronutrients (Software - Dietpro version 52.8). The software used the database of the Brazilian food composition table (TACO) to calculate food consumption.
In the exercise test sessions, each participant randomly consumed 2 capsules of Placebo (starch) or CAP with 6 mg per capsule, which were identical to ensure a double-blind design, 45 minutes before each experimental test. This time was selected because capsaicin reaches maximum concentrations within 45 minutes after ingestion, the half-life of capsaicin is approximately 25 minutes and complete plasma clearance occurs approximately 105 minutes after ingestion (3). The product is standardized in 40% to 50% of Capsinoids (Capsiate) and the final product contained 50% in each 6 mg (Capsicum annuum L.) from India (Purifarma-Gemini Pharmaceutical Industry Ltda, Anapolis, GO, Brazil). The correction factor in assay calculation was used by Pharma Nostra (Campinas, Brazil) to guarantee 100% of capsinoids in each capsule, therefore, 12 mg total Capsiate was administrated. Finally, this dose was selected because the same product and dose improved performance in our previous studies with humans and none of the participants reported “hot” sensations or gastrointestinal discomforts (5, 6).
Short and Middle-Distance Running Time-Trial Performance: Initially, participants completed one familiarization session to become acquainted with outdoor track. After seven days, participants performed a randomized short (400 m) or middle (3000 m) distance run on an outdoor track at Federal University of Piauí-PI, Brazil, separated by seven days. Prior to each testing session, participants completed a 5-minute warm-up (stretching and jogging). Participants were tested one at a time and were instructed to cover the distance in the shortest possible time, which was recorded using a polar stopwatch (V800, Polar Electro, Kempele, Finland). Participants were instructed to wear the same kind of clothes (light shorts, light t-shirts, and running shoes) in each test. All tests were carried out on the same day at the same hour (6:30 to 9 AM), and all tests were conducted when the following climate values were presented: temperature (23–26°C), humidity (70–80%), pressure (1014–1016 mb), and wind speed (11–15 km.h−1). Four fitness professionals supervised all testing sessions and the volunteers maintained current activity but abstained from exercise the day before each trial.
Heart Rate and Rating of Perceived Exertion: To measure resting heart rate, volunteers were instructed to remain in silence, awake, resting, spontaneously breathing, and lying down comfortably for five minutes using a polar stopwatch (V800, Polar Electro, Kempele, Finland). These procedures were performed before each trial and data were recorded in beats per minute (beats.min−1). The maximum heart rate was recorded also immediately post-exercise.
The rating of perceived exertion (RPE) was measured after each exercise session using the OMNI-Walk/Run Scale ratings of perceived exertion (0–10 points) immediately after running time-trial.
Statistical Analysis
First, we performed a power analysis of this study based on the observation from a previous study that verified the effects of nitrate supplementation on exercise performance at moderate and very-high simulated altitudes (21). The authors reported a mean difference of 64.8 sec for 3 km time-trial and a standard deviation of 48.3 (19). When we used a type I error of 0.05 and 12 subjects, according to PS software (version 3.1.2, Dupont and Plummer, http://biostat.mc.vanderbilt.edu/wiki/Main/PowerSampleSize), the power analysis computed was 0.98.
Next, we verified via GraphPad software that one of the values was a significant outlier, thus this participant from the 3000 m condition was not included at final analysis. The data normality was verified using the Shapiro-Wilk test. Data are presented as means ± standard deviations, and the comparison of kilocalorie and macronutrients between trials were analyzed via a repeated measured ANOVA. The effect of CAP on the time-trial performance, maximum heart rate and rate of perceived exertion during short (400 m) and middle distance (3000 m) running were analyzed via a paired t test using the Statistical Package for Social Sciences 17.0 (SPSS Inc. Chicago, IL USA). Effect size was calculated via Cohen’s d, which describes the difference between the means normalized to the pooled standard deviation (SD) of the two groups, whereby a value of > 0.20 was considered small, > 0.50 moderate, and > 0.80 large. The 95% confidence intervals are also reported (CI 95%).
RESULTS
Table 1 presents the mean and standard deviation values for general sample characteristics and Table 2 shows the comparison of the dietary intake and macronutrient distribution 24 h prior to each trial. There were no statistically significant differences between conditions in both total and relative to body weight dietary intake and macronutrient distribution.
Table 1.
General characteristics of the sample.
| Variables | Mean ± SD (n = 12) |
|---|---|
| Age (years) | 28.6 ± 5.4 |
| Height (cm) | 175.0 ± 5.4 |
| Weight (kg) | 76.1 ± 8.4 |
| Fat mass (kg) | 12.6 ± 6.7 |
| Fat free mass (kg) | 63.6 ± 6.4 |
| MAS (km.h−1) | 14.6 ± 0.7 |
| RPE | 10 ± 0.5 |
| Maximum heart rate (bpm) | 189 ± 10 |
Note: MAS= maximal aerobic speed (km.h−1). RPE= rating of perceived exertion.
Table 2.
Comparison on the dietary intake and macronutrient distribution between trials.
| 24 h Dietary Intake | Trial 1 | Trial 2 | Trial 3 | Trial 4 | p |
|---|---|---|---|---|---|
| CHO (g) | 261.5 ± 118.3 | 276.9 ± 109.2 | 291.0 ± 122.6 | 283.6 ± 115.8 | 0.860 |
| PRO (g) | 111.8 ± 52.8 | 134.3 ± 67.2 | 110.1 ± 49.6 | 114.0 ± 53.4 | 0.645 |
| FAT (g) | 59.0 ± 25.3 | 70.7 ± 21.9 | 63.7 ± 31.0 | 66.5 ± 38.7 | 0.712 |
| Total Intake (kcal) | 2169 ± 691 | 2365 ± 567 | 2264 ± 691 | 2197 ± 898 | 0.849 |
| CHO (g/kg) | 3.4 ± 1.6 | 3.7 ± 1.6 | 3.8 ± 1.5 | 3.7 ± 1.4 | 0.895 |
| PRO (g/kg) | 1.5 ± 0.6 | 1.7 ± 0.8 | 1.4 ± 0.6 | 1.5 ± 0.7 | 0.698 |
| FAT (g/kg) | 0.8 ± 0.3 | 0.9 ± 0.2 | 0.8 ± 0.4 | 0.9 ± 0.5 | 0.759 |
| Total Intake (kcal/kg) | 28.5 ± 8.2 | 31.2 ± 6.8 | 29.6 ± 8.0 | 28.8 ± 11.4 | 0.839 |
Note: Total Intake (kcal) = Dietary Intake relative to body weight, CHO= carbohydrate; FAT= lipids; PRO= protein.
We also compared the first and second attempt of each trial to verify the influence of the learning effect and there were no significant differences between first and second attempts in both 400 m (p = 0.906) and 3000 m (p = 0.896) trials.
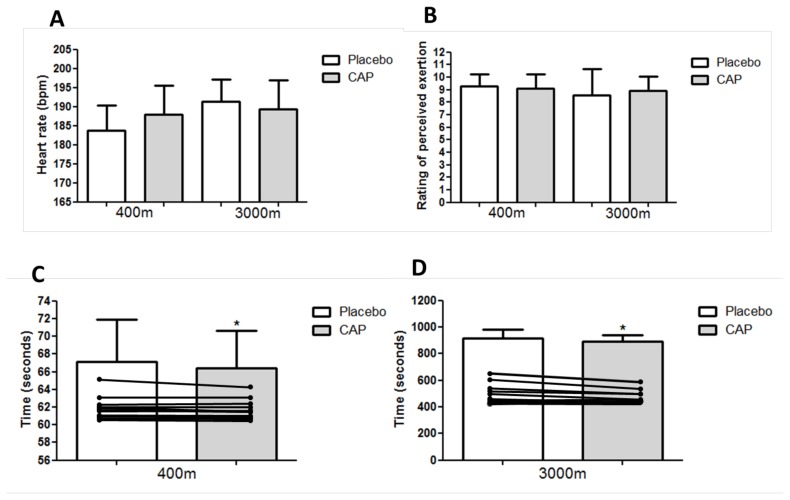
For the 3000 m time-trial, the time in seconds was significantly less in the CAP compared to placebo condition (CAP = 893.9 ± 46.8 sec vs Placebo = 915.2 ± 67.6 sec, t = 2.939, p = 0.015) and there was a large effect size [d = 1.30, Δ% = −2.2 ± 2.5 (CI 95%= −3.7 to −0.6)].
In the 400 m time trial, the time in seconds was significantly less in the CAP compared to placebo condition (CAP = 66.4 ± 4.2 sec vs Placebo = 67.1 ± 4.8 sec, t = 2.250, p = 0.046) and the effect was very small [d = 0.16, Δ% = −1.0 ± 1.4% (CI 95%= −1.9 to −0.1)].
For the maximum heart rate (bpm) in the 3000 m (CAP = 189.3 ± 7.7 vs Placebo = 191.3 ± 5.9 bpm, t = 1.044, p = 0.319) and 400 m (CAP = 7.4 ± 7.6 vs Placebo= 183.7 ± 6.6 bpm, t = −1.717, p = 0.114) there were no statistically significant difference between conditions. The effect size was small for 3000 m [d = 0.30, Δ% = −1.0 ± 3.5% (CI 95%= −3.2 to 1.2)] and for 400 m there was a moderate effect [d = 0.52, Δ% = 2.4 ± 4.9% (CI 95%= −0.7 to 5.6)]. Figure 2 shows the differences in performance for the CAP and placebo condition.
Figure 2.
Comparison between Capsaicin analog supplementation and placebo conditions on the performance. A = Maximum heart rate (bpm); B = Rating of perceived exertion (RPE); C = 400 m time-trial (seconds) and individual 400 m time-trial; D = 3000 m time-trial (seconds) and individual 3000 m time-trial; CAP= Capsaicin analog supplementation (CAP= Capsiate); *= statistically significant difference between CAP and placebo conditions.
There was no statistically significant difference between conditions in RPE for the 3000 m time-trial (CAP = 8.9 ± 1.1 vs Placebo = 8.6 ± 2.0, t = −0.804, p = 0.438) and the 400 m time-trial (CAP = 9.1 ± 1.1 vs Placebo = 9.2 ± 1.0, t = 0.518, p = 0.615). The effect sizes were small for both 3000 m [d= 0.20, Δ%= 9.6 ± 30.5% (CI 95% = −9.8 to 29.0)] and 400 m [d = 0.10, Δ% = −1.4 ± 12.1% (CI 95% = −9.0 to 6.3)].
DISCUSSION
This is the first study to analyze the effects of Capsiate (CAP) supplementation on running time-trial performance with two different distances. The main finding of this study was that acute CAP supplementation improved both 400 m and 3000 m running time-trial performances without modifying maximum heart rate or RPE in physically active adults.
Currently, there are only two other studies investigating the effects of CAP supplementation on running performance. Recently, our group analyzed the effect of CAP supplementation (12 mg) on RPE, blood lactate concentrations, and performance during middle distance running (1500m) in physically active adults. The results demonstrated that CAP supplementation was effective to increase middle distance running performance (6). We also found that CAP supplementation increased the volume of work performed during four sets of back squat to fatigue with 70% of one-repetition maximum, and also a lower RPE (5). In contrast, Opheim and Rankin (18) did not find any significant time reduction after 15 × 30-m all-out sprints with 35-s rest intervals.
Collectively, these findings suggest that exercise type is an important factor with regard to the potential ergogenic benefits of CAP supplementation on exercise performance. The present study used the duration of time trials around ~1 min (400 m) and ~13–17 min (3000 m). Regarding our previous study, the duration of time-trial was around ~5–7 min (1500 m) (6) and approximately 45 seconds per set (70% 1RM squats to failure) (5). In contrast, the duration per sprint (30 m) in Opheim and Rankin (18) ranged from approximately 4.5 to 4.85 seconds between sprint 1 and 15, respectively. Considering the results of the present study, de Freitas et al. (6), and de Freitas et al. (5), it seems that CAP supplementation may have an ergogenic role according to the exercise duration, benefitting exercises that rely heavily on glycolysis. This study showed that CAP supplementation decreased time by 0.7 seconds during the 400 m running time-trial; however, the reduction was most meaningful in the 3000 m running time-trial (21 sec). Interestingly, our previous study observed that CAP reduced ~5 seconds in 1500 m running time-trial following CAP supplementation.
Results from animal studies suggest that the benefits in running performance may be partially due to an increase in plasma free fatty acids and glycogen sparing brought about by increased epinephrine concentrations (10). Regarding the greater effect of CAP supplementation on middle distance running (3000m), we hypothesize that during the 3000 m trial there was greater glycogen depletion, which leads to a reduction in calcium release by the sarcoplasmic reticulum, and consequentially lowers muscle force production capacity. This would suggest that CAP supplementation is most effective during exercise protocols that rely heavily on glycolysis and reduces muscle glycogen, as CAP intake is associated with a glycogen sparing effect (9, 10). However, more studies are needed to substantiate this hypothesis in humans, and future research is necessary to investigate the ergogenic potential of CAP on longer duration endurance activities.
Muscular fatigue is one of the limiting factors that affects running performance, as a result of lower calcium release by the sarcoplasmic reticulum, leading to reduced force production by skeletal muscle (1). The literature has shown that accumulation of hydrogen ions, inorganic phosphate, and glycogen depletion during exercise may reduce sarcoplasmic reticulum function, which leads to impairment in contraction efficiency and performance (20). Activation of TRPV1 in the skeletal muscle results in increased calcium release by the sarcoplasmic reticulum and potentially increases the interaction of actin-myosin filaments, resulting in greater force production (11). Thus, we believe that the greater myofiber force generation induced by CAP on the TRPV1 activation could enhance running economy. Supporting this hypothesis, Kazuya et al. (9) investigated the effects of acute CAP supplementation at a low (10 mg/kg body wt) and a high (100 mg/kg) dose on gastrocnemius muscle function and energetics during 6 min of repeated fatiguing isometric contractions in mice. This study found that CAP reduced the ATP cost during exercise with the higher dose also increasing force-generating capabilities in skeletal muscle. The lower energy cost of exercises suggests that CAP supplementation improves exercise economy. Our study showed that CAP increased running performance without modifying parameters of internal load (maximum heart rate and RPE). Based on these findings, acute CAP supplementation may also improve running performance by enhancing movement economy, but further studies are necessary to measure the ATP costs of exercising with CAP supplementation.
This study may be applied by coaches and trainers looking to improve middle distance (3000 m) running time-trial performance in physically active adults. In addition, some participants responded positively to the CAP while others did not, thus more studies should investigate whether single polynucleotide polymorphisms in the TRVP1 gene explain the individual responses (2). Despite the importance of this study, some limitations need to be mentioned. First, aerobic metabolism and metabolites (i.e. lactate) were not measured; second, we were unable to measure hydrogen ions, inorganic phosphate, glycogen depletion, calcium release, and catecholamine response. In addition, further studies are necessary to measure the metabolic and energy system contributions in humans. It is necessary to highlight that the administered form and dose of CAP in the present study was well-tolerated and none of the subjects reported gastrointestinal distress.
Conclusions: In summary, acute capsaicin analog supplementation (Capsiate) improved 400 m and 3000 m running time-trial performance but without modifying the maximum heart rate or RPE in physically active adults. Furthermore, the present study showed a small improvement only in the performance during 3000 m time-trial, suggesting that CAP works better in middle distance rather than short distance (400 m) performances.
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol. 2008;104(1):296–305. doi: 10.1152/japplphysiol.00908.2007. [DOI] [PubMed] [Google Scholar]
- 2.Binder A, May D, Baron R, Maier C, Tölle TR, Rolf-Detlef T, et al. Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PloS One. 2011;6(3):e17387. doi: 10.1371/journal.pone.0017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaiyasit K, Khovidhunkit W, Wittayalertpanya S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J Med Assoc Thai. 2009;92(1):108–113. [PubMed] [Google Scholar]
- 4.Council of Europe. Committee of experts on flavouring substances. Datasheet on Capsaicin. 2002. Available at: https://ec.europa.eu/food/sites/food/files/safety/docs/fs_food-improvement-agents_flavourings-out120.pdf.
- 5.de Freitas MC, Cholewa JM, Freire RV, Carmo BA, Bottan J, Bratfich M, et al. Acute capsaicin supplementation improves resistance training performance in trained men. J Strength Cond Res. 2018;32(8):2227–2232. doi: 10.1519/JSC.0000000000002109. [DOI] [PubMed] [Google Scholar]
- 6.de Freitas MC, Cholewa JM, Gobbo LA, de Oliveira JVNS, Lira FS, Rossi FE. Acute capsaicin supplementation improves 1,500-m running time-trial performance and rate of perceived exertion in physically active adults. J Strength Cond Res. 2018;32(2):572–577. doi: 10.1519/JSC.0000000000002329. [DOI] [PubMed] [Google Scholar]
- 7.Edwards JG. TRPV1 in the central nervous system: synaptic plasticity, function, and pharmacological implications. Prog Drug Res. 2014;68:77–104. doi: 10.1007/978-3-0348-0828-6_3. [DOI] [PubMed] [Google Scholar]
- 8.Faraut B, Giannesini B, Matarazzo V, Le Fur Y, Rougon G, Cozzone PJ, et al. Capsiate administration results in an uncoupling protein-3 downregulation, an enhanced muscle oxidative capacity and a decreased abdominal fat content in vivo. Int J Obes. 2009;33(12):1348–1355. doi: 10.1038/ijo.2009.182. [DOI] [PubMed] [Google Scholar]
- 9.Kazuya Y, Tonson A, Pecchi E, Dalmasso C, Vilmen C, Le Fur Y, et al. A single intake of capsiate improves mechanical performance and bioenergetics efficiency in contracting mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2014;306(10):e1110–1119. doi: 10.1152/ajpendo.00520.2013. [DOI] [PubMed] [Google Scholar]
- 10.Kim KM, Kawada T, Ishihara K, Inoue K, Fushiki T. Increase in swimming endurance capacity of mice by capsaicin-induced adrenal catecholamine secretion. Biosci Biotechnol Biochem. 1997;61(10):1718–1723. doi: 10.1271/bbb.61.1718. [DOI] [PubMed] [Google Scholar]
- 11.Linari M, Brunello E, Reconditi M, Fusi L, Caremani M, Narayanan T, et al. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature. 2015;528(7581):276–279. doi: 10.1038/nature15727. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Carrillo L, Avila HM, Dubrow R. Chili pepper consumption and gastric cancer in Mexico: A case-control study. Am J Epidemiol. 1994;139:263–2711. doi: 10.1093/oxfordjournals.aje.a116993. [DOI] [PubMed] [Google Scholar]
- 13.Lotteau S, Ducreux S, Romestaing C, Legrand C, Van Coppenolle F. Characterization of functional TRPV1 channels in the sarcoplasmic reticulum of mouse skeletal muscle. PloS One. 2013;8(3):e58673. doi: 10.1371/journal.pone.0058673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludy MJ, Mattes RD. The effects of hedonically acceptable red pepper doses on thermogenesis and appetite. Physiol Behav. 2011;102:251–258. doi: 10.1016/j.physbeh.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludy MJ, Moore GE, Mattes RD. The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans. Chem Senses. 2012;37(2):103–121. doi: 10.1093/chemse/bjr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monsereenusorn Y. Subchronic toxicity studies of capsaicin and capsicum in rats. Res Commun Chem Pathol Pharmacol. 1983;41:95–110. [PubMed] [Google Scholar]
- 18.Opheim MN, Rankin JW. Effect of capsaicin supplementation on repeated sprinting performance. J Strength Cond Res. 2012;26(2):319–326. doi: 10.1519/JSC.0b013e3182429ae5. [DOI] [PubMed] [Google Scholar]
- 19.Parrish M. Liquid chromatographic method of determining capsaicinoids in capsicums and their extractives: collaborative study. J Assoc Off Anal Chem. 1996;79:738–745. [Google Scholar]
- 20.Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R502–516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- 21.Shannon OM, Duckworth L, Barlow MJ, Deighton K, Matu J, Williams EL, et al. Effects of dietary nitrate supplementation on physiological responses, cognitive function, and exercise performance at moderate and very-high simulated altitude. Front Physiol. 2017;8:401. doi: 10.3389/fphys.2017.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smeets A, Westerterp-Plantenga M. The acute effects of a lunch containing capsaicin on energy and substrate utilisation, hormones, and satiety. Eur J Nutr. 2009;48(4):229–234. doi: 10.1007/s00394-009-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yashiro K, Tonson A, Pecchi É, Vilmen C, Le Fur Y, Bernard M, et al. Capsiate supplementation reduces oxidative cost of contraction in exercising mouse skeletal muscle in vivo. PloS one. 2015;10(6):e0128016. doi: 10.1371/journal.pone.0128016. [DOI] [PMC free article] [PubMed] [Google Scholar]