Abstract
In resistance training squats are often used to strengthen the muscles of the lower extremities and core muscles. There are two common forms of squats that use a barbell for loading, the back squat and the front squat. The technique and loading of each squat differ markedly. However, the energetic demands on the muscle between the two forms are not well understood. The purpose of this study was to investigate the difference in energy demands between front and back squats by measuring the change in skeletal muscle oxygen saturation (SmO2) through the use of near infrared spectroscopy (NIRS).
Methods
Eleven resistance trained individuals, (5 female, 6 male) with an average age of 23.7 ± 1.4, completed 3 sets of 15 repetitions at 70% of their 1-RM weight for both back and front squats. Skeletal muscle oxygen saturation (SmO2) of the vastus lateralis was measured using a wireless NIRS device.
Results
The ΔSmO2 was not significantly different between back and front squats but was different between sets 1–3 (44.76 ± 3.24% vs. 55.19 ± 2.75% vs. 56.30 ± 2.63%), main effect p ≤ 0.0001. The recovery of SmO2 was significantly different between back (42.5 ± 3.4 sec) and front squats (30.9 ± 2.8 sec), main effect p ≤ 0.05.
Conclusions
The findings of this study suggest that the energetic demands placed on the vastus lateralis during both front and back squats are similar with a slower recovery of energetics in the back squat.
Keywords: Muscle oxygenation, strength training, energetics, NIRS, SmO2
INTRODUCTION
In the field of strength and conditioning, squats are a frequently used exercise as they engage several large muscle groups of the lower extremities and core muscles. There are two classical forms of squats that use a barbell for weight loading, the back squat, which is extensively used and front squat, which is less frequently used. There are slight variations in the form needed for each squat and several studies have examined the difference in kinematics and muscle activation between these two squat variations (3,8,19,23,25). The decision to select back or front squats has some important factors, including sport specificity, previous injuries, personal preference.
The front squat has been shown to produce lower compressive but similar shear forces on the knee joint when compared to the back squat (8). Additionally, front squats were shown to produce less lumbar stress than the back squat. From these findings, Gullett et al. concluded that front squats may be better suited for people with ACL and meniscal tears and may better promote long-term joint health. Part of the reason the front squat produces less compressive force may be because less weight can be lifted in a front squat as opposed to a back squat. In their study, Gullett et al. found the mean 1-RM for a front squat to be approximately 19 kg less than a back squat (8).
However, Stuart et al. observed that despite the difference in weight lifted, overall muscle activation of several lower extremity muscles in front squats was equal to that of back squats (23). Yavuz et al. also found similar findings to Stuart but did observe slightly higher EMG activity in the vastus medialis (VM) during front squats (25). The higher activation in the VM was not reported in (8) but the difference may be due to the variance in loads between the two studies 100% 1-RM vs 70% 1-RM.
While it appears that the muscle activation between the front and back squats is very similar, the energy demands of each squat form are less understood. Several attempts have been made to measure the energy demands of resistance exercise at the whole body level including accelerometry (22), whole body oxygen consumption (12), as well as within individual muscles using magnetic resonance spectroscopy (MRS) (10,14,20), and near infrared spectroscopy (NIRS) (1,11,20).
The use of NIRS provides a non-invasive method to examine the energetic demand of a muscle. While resistance training is largely dependent on the phospho-creatine (PCr) system (24), there is a direct link between the depletion and re-synthesis of the PCr pool and the muscle oxygenation (SmO2) (15,20). For example, McCully et al. found good agreement in simultaneous measurements of PCr through MRS and muscle oxygenation through NIRS during both submaximal and maximal exercise (15). Therefore, the measurements of SmO2 during a resistance training session can provide valuable insight into the energetic demands of the muscle.
Adaptations to strength training are based on many factors, including the mechanical stretch as well as the energetic demands placed on the muscle (2,5). Understanding how different exercise can impact these factors can help in deciding which exercise should be performed. Additionally, this information can be helpful in determining how rest intervals should be potentially adjusted (21). Because there is a direct positive relationship between the muscle mass recruited and the energy expenditure of the exercise (6,12) it could therefore be presumed that front and back squats should produce a similar energy demand, however, this remains unknown. Therefore, the purpose of this study was to investigate the difference in energy demands of the vastus lateralis between front and back squats using near infrared spectroscopy (NIRS). We hypothesized that the energy demand of front and back squats would be similar as measured by skeletal muscle oxygen saturation (SmO2). This information can clarify which exercise may elicit the greatest energy demand and aid in determining recovery periods in between sets.
METHODS
Participants
All procedures and materials were approved by the Institutional Review Board of Sam Houston State University and all participants provided written informed consent. Additionally, all processes related to this manuscript adhere to the ethical guidelines set forth by Navalta et al. (17). Participants were recruited from the university power lifting club and community and were required to have a minimum of 6-months of current weight training experience (≥ 3 days/week) and be free of any injuries. Eleven participants between the ages of 18–35 completed the study.
Equipment
The measurement of SmO2 was done using a wireless near infrared spectroscopy device (NIRD) (Moxy 3, Firmware 1.1). This NIRD broadcasts wireless via an ANT+ and was collected using PerfPro on a nearby laptop. The NIRD has an LED emitter and two sensors placed 12.5 mm and 25 mm from the LED. The scattered light that reaches the sensors is used to report the ratio of the oxyhemoglobin concentration to the total hemoglobin concentration and reports the percentage as SmO2. The NIRD has been shown in multiple studies as a valid device that compares well with other systems (4,7,16).
Protocol
Participants were required two attend sessions of assessment to determine the 1-RM and two sessions of testing in a randomized cross-over design. Participants were asked to not engage in any strenuous activity 24 hours before each session. The order of testing (front vs back squat) was randomized for each participant. For the 1-RM assessment, participants began with a 5-minute cycling warm-up on a stationary bike and six stretches. Once the warm-up was completed, participants performed 3–5 practice repetitions of their designated lift with the bar weight only. Parallel squat starting position was 0° knee flexion and stopping position was at 90° knee flexion (femur parallel to ground) measured with a goniometer placed at the knee joint. For consistency of parallel squats, a line of tape was set at 90°knee flexion height for participants to touch at the end of the descent phase of a squat and mark beginning of the ascent phase of a squat. If the participant could not feel the tape when squatting, then one spotter would verbally notify participant of when the tape was touched. Participants then performed weighted squats, progressing to their 1-repetition max weight. After completion of their 1- repetition max, participants engaged in a 5-minute cycling cool down on a stationary bike. Participants then scheduled their testing session to be performed between 48 to 96 hours after completion of assessment session.
For the testing session, a previously validated (4,7,16)wireless near infrared spectroscopy device (NIRD) (Moxy 3, Firmware 1.1) was placed over the left vastus lateralis (VL) using adhesive tape and a dark flex wrap. The site of placement was based on the recommended EMG placement as reported by (18), which is also in line with the manufacture’s recommendation, approximately 94 mm superior to the patella, along a line from the from the superior lateral side of the patella extending to the anterior superior iliac spine. NIRD sensors were set to a sample every 0.5 seconds with no data smoothing. Participants’ parallel squat height was re-measured and set for testing session. Participants then performed an identical warm-up as the assessment session before testing. Once the warm-up was complete, participants performed a warm-up set of 3 to 5 repetitions of their body weight.
Next, participants performed 3 sets of 15 repetitions loaded at 70% weight of their one-repetition max weight with a 2 to 3-minute rest (sitting) in-between sets (Figure 1). Participants were required to complete each set within 60 seconds with no pauses. After the final set, participants rested for 5-minutes and then performed a 5-minute cool down on a stationary bicycle. Participants then were scheduled for the second assessment and testing session. The second assessment and testing session are methodically conducted the same, as described above except for the other designated lift (front or back squat).
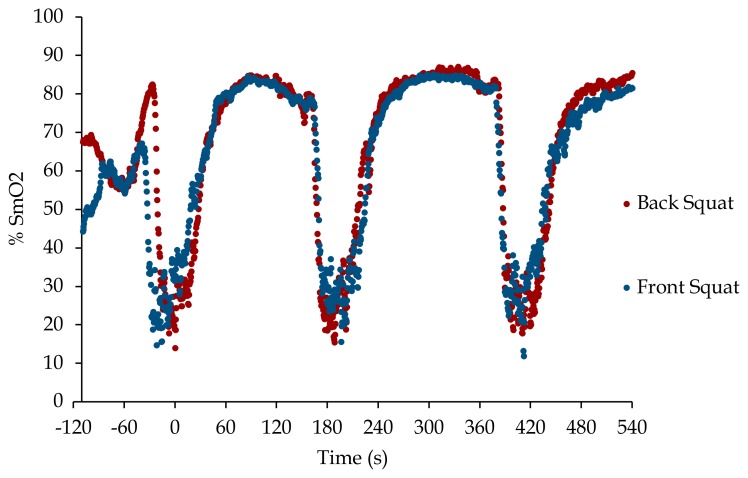
Figure 1.
SmO2 results from a representative participant (#10) during the back squat (black dots) and front squat (blue dots) protocols. Data are smoothed as a 4 sample (2 sec) rolling average. t = 0 marks the completion of the 1st set.
Statistical Analysis
Descriptive statistics are presented as mean ± standard error (SE). Data from the NIRD was collected using PerfPro Studio (version 5.81.10) (Hartware Technologies, Rockford, MI) and exported into Microsoft Excel spreadsheets. SmO2 values were smoothed by calculating a running average over two seconds (4 samplings). The maximum and minimum SmO2 was determined immediately prior to and during each set and the difference calculated and reported as ΔSmO2. The recovery of SmO2 is reported as the time taken after each set to regain 75% of the ΔSmO2. Data were then analyzed using GraphPad Prism (version 8.4.1) (GraphPad Software, San Diego, CA) using a mixed effects model Two-Way ANOVA with the Geisser-Greenhouse correction. Post-hoc analysis was done using the Sidak correction for multiple comparisons between sets. Differences in 1-RM were analyzed by a paired t-test. Statistical significance was set at p ≤ .05. Data are presented as means ± the standard error.
RESULTS
A total of 11 participants (5 female, 6 male) with an average age of 23.7 ± 1.4 participated in the study. The average 1-RM weight was significantly lower in front squats 102.4 ± 9.3 kg vs back squats 123.9 ± 12.6 kg, p ≤ 0.005 (Figure 2). Three participants reached muscle failure after 10 repetitions during the third set of front squats. Due to signal dropouts between the wireless NIRD and the recording software, data was unusable from two participants in set 1 of back and front squats, two participants in set 2 of front squats, and one participant in set 3 of front squats. Therefore, a mixed effects model Two-Way ANOVA was used to accommodate the missing paired data.
Figure 2.
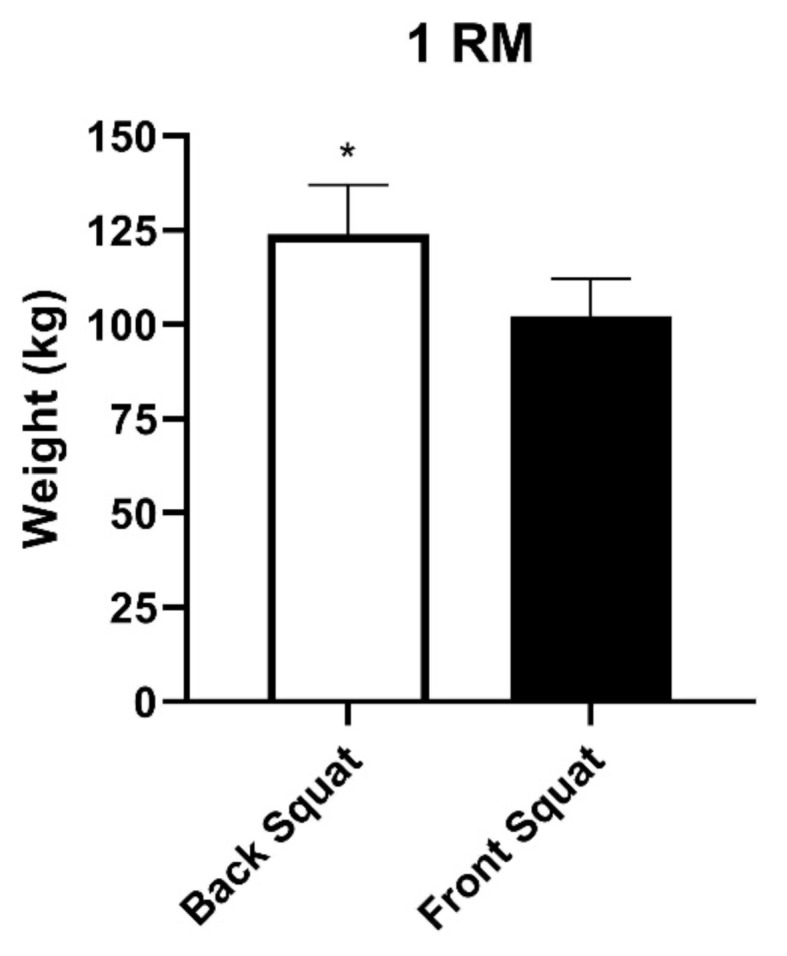
Mean 1-RM of each squat type. (Mean ± SE). * p ≤ 0.005
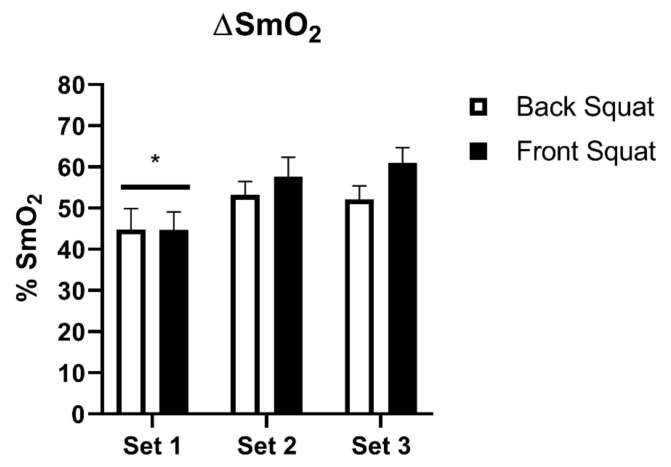
A mixed model Two-Way ANOVA showed that there was no significant difference between back and front squats in ΔSmO2 (50.43 ± 2.24% vs. 54.69 ± 2.75%) (Figure 3). There was a main effect of set (p ≤ 0.0001) on ΔSmO2 (44.76 ± 3.24% set 1 vs. 55.19 ± 2.75% set 2 vs. 56.30 ± 2.63% set 3). Multiple comparisons showed the first set to be significantly different than the second (−10.43 mean difference, 95% CI of difference [−13.68 to −7.18]) and third sets (−11.54 mean difference, 95% CI of difference [−16.63 to −6.46]) (p ≤ 0.0001) (Figure 3).
Figure 3.
The change in %SmO2 (ΔSmO2) for each squat and set. (Mean ± SE). p ≤ 0.0001 main effect of set. *p ≤ 0.001 sets 2 & 3. n = 8, 10, 10 for sets 1, 2, & 3 respectively in back squats. n = 8, 8, & 9 for sets 1, 2, & 3 respectively in front squats.
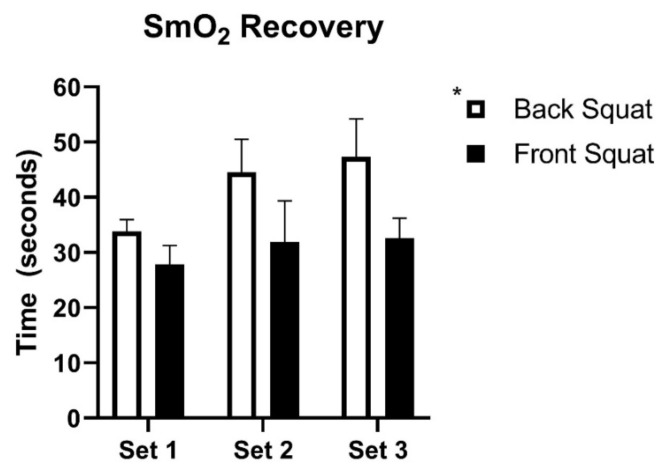
The time to recover 75% of ΔSmO2 was significantly different between back (42.5 ± 3.4 sec) and front (30.9 ± 2.8 sec) squats, main effect p ≤ 0.05, 95%CI of difference [2.127 to 18.06] (Figure 4). There was no main effect of set on SmO2 recovery. Additionally, the absolute minimum SmO2 during each set did not differ between the squat forms or between sets (Table 1). However, the maximum SmO2 prior to each set was significantly different across sets (p ≤ 0.001 main effect) and there was a significant set*squat interaction effect, p ≤ 0.05 (Table 1). Sidak post-hoc comparison showed set 1 to be significantly different from set 2 in the back squat (p ≤ 0.01). In the front squats, set 1 was significantly different from sets 2 & 3 (p ≤ 0.001).
Figure 4.
Time to recover 75% of ΔSmO2 between front and back squats. (Mean ± SE). *p ≤ 0.05 main effect between squat types. n = 8, 10, 10 for sets 1, 2, & 3 respectively in back squats. n = 8, 8, & 9 for sets 1, 2, & 3 respectively in front squats.
Table 1.
Minimum and maximum SmO2 values for each set. (mean ± SE).
| Squat | Set 1 | Set 2 | Set 3 | |
|---|---|---|---|---|
| Minimum (%SmO2) | Back | 22.4 ± 2.9 | 24.7 ± 2.3 | 22.2 ± 3.5 |
| Front | 23.2 ± 4.8 | 24.7 ± 4.3 | 22.2 ± 3.5 | |
| Maximum (%SmO2) | Back | 68.6 ± 2.6* | 77.9 ± 1.5 | 76.3 ± 2.3 |
| Front | 67.9 ± 2.0** | 82.3 ± 0.8 | 83.1 ± 0.8 |
Main effect of set p ≤ 0.001.
p ≤ 0.01 Different from back set 2.
p ≤ 0.001 Different from front set 2 & 3. n = 8, 10, 10 for sets 1, 2, & 3 respectively in back squats. n = 8, 8, & 9 for sets 1, 2, & 3 respectively in front squats.
The rest interval was not strictly controlled during the data collection. To rule out any influence of varying rest intervals on the results the actual rest intervals were calculated and analyzed using a Two-Way ANOVA. The average rest interval for all back squats (150.7 ± 2.0 sec) was not significantly different from front squats (144.6 ± 3.9 sec). Additionally, there were no significant differences between the first (148.3 ± 3.0 sec) or second (147.0 ± 3.4 sec) sets (data not shown).
DISCUSSION
The major finding from this study is that there is no difference in the ΔSmO2 of the VL between back and front squats. However, the ΔSmO2 did increase in the second and third sets (Figure 3). There was a significant difference in the time it took to recover 75% of the ΔSmO2 between the back and front squats (Figure 4). These findings provide some useful insight into the overall energy demands of the back and front squats and suggest that the back squats may require more time to recover in-between sets.
In this study, the ΔSmO2 variable indicates the magnitude of strain placed on the PCr system during the exercise bout. Larger demands on the PCr system through differing intensities of exercise have been shown have a greater decrease in the muscle hemoglobin saturation (15). However, in this study participants were required to lift 70% of their 1-RM for each lift for 15 repetitions, so the intensities were effectively normalized between the two squat types. Therefore, it would seem logical that the strain on the PCr system and hence ΔSmO2 would be similar between the two squats. We did see an increase in the ΔSmO2 over successive sets which is likely due to the hyperemic response and overshoot of SmO2 following the first set (15). This response is shown by the increased maximal SmO2 values at the start of the second and third sets (Table 1). Further supporting the lack of a difference observed in the current study are studies showing that activation of the VL is not different between back and front squats during a 1-RM (25), at 70% of 1-RM (8), as well as with an absolute load of 50 lbs. (23). This is an important consideration for those who have knee injuries that may be exacerbated by higher compressive forces. Since the front squat produces lower compressive forces (8) and in the current study appear to produce a similar energetic demand, those with knee injuries may be better selecting front squats over back.
The measurement of PCr recovery is dependent on several factors, such as mitochondrial oxidative capacity, pH, and blood flow, and it is often used as a measure of aerobic capacity (14). However, practically it can also be used to determine appropriate rest periods in between exercise bouts. For example, in the review by de Salles et al. (21) several considerations for appropriate rest intervals are discussed across several strength training methods, including complete re-synthesis of PCr. While we did not directly measure PCr recovery kinetics, the use of SmO2 has good agreement with PCr kinetics (15,20) and the time to recover 75% of the ΔSmO2 indicates how quickly PCr is recovering. Our finding that there are significant differences in the ΔSmO2 recovery between back and front squats (Figure 4) provide some insight into considerations for appropriate rest intervals. On average back squats took 11.9 seconds longer to recover 75% of the ΔSmO2 than front squats. While the reasons for this slower recovery are not immediately clear, it may be in part due to the higher weight lifted during the back squats (Figure 1).
The choice of which exercises to perform as part of a strength training program has many considerations. Specificity of the exercise to the desired outcome is critical to ensuring maximum performance benefits. Additionally, considerations need to be made to accommodate previous injuries and reduce risk for future injuries. Personal preference also has a major influence on which exercises are performed. Anecdotally, many of the current participants preferred the back squat because more weight can be lifted.
This study did have some limitations which are worth noting. One important point is that the rest interval was 2–3 minutes in between sets and was not tightly controlled. However, there were no significant differences in actual rest intervals between squat types or sets (data not shown). Squats are a complex movement involving multiple joints and muscle groups. In this study we only able to examine the differences experienced by a single muscle (vastus lateralis) during back and front squats. Additional studies would be necessary to get a more complete picture of the energy demands of all major muscle groups involved in squats.
Overall, the findings of this study suggest that the energetic demands of both front and back squats are similar and support the previous findings (8,23,25) that muscle activation is also similar. While specificity should play a critical factor in selecting appropriate exercises, front squats may stress the muscles to a similar extent as back squats despite the reduced load.
REFERENCES
- 1.Cettolo V, Ferrari M, Biasini Vi, Quaresima V. Vastus lateralis O2 desaturation in response to fast and short maximal contraction. Med Sci Sport Exerc. 2007;39(11) doi: 10.1249/mss.0b013e3181453476. [DOI] [PubMed] [Google Scholar]
- 2.Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med. 2007;37(9):737–63. doi: 10.2165/00007256-200737090-00001. [DOI] [PubMed] [Google Scholar]
- 3.Contreras B, Vigotsky A, Schoenfeld B, Beardsley C, Cronin J. A comparison of gluteus maximus, biceps femoris, and vastus lateralis EMG amplitude in the parallel, full, and front squat variations in resistance trained females. Int J Sport Nutr Exerc Metab. 2011;32(1):1–44. doi: 10.1123/jab.2015-0113. [DOI] [PubMed] [Google Scholar]
- 4.Crum EM, O’Connor WJ, Van Loo L, Valckx M, Stannard SR. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur J Sport Sci. 2017;17(8):1037–43. doi: 10.1080/17461391.2017.1330899. [DOI] [PubMed] [Google Scholar]
- 5.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17(2) doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Farinatti P, Neto AGC, Amorim PRS. Oxygen consumption and substrate utilization during and after resistance exercises performed with different muscle mass. Int J Exerc Sci. 2016;9(1):77–88. doi: 10.70252/AKBM3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmann A, Schmitz R, Erlacher D. Near-infrared spectroscopy-derived muscle oxygen saturation on a 0% to 100% scale: Reliability and validity of the Moxy Monitor. J Biomed Opt. 2019;24(11):115001. doi: 10.1117/1.JBO.24.11.115001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gullett JC, Tillman MD, Gutierrez GM, Chow JW. A biomechanical comparison of back and front squats in healthy trained individuals. J Strength Cond Res. 2009;23(1):284–92. doi: 10.1519/JSC.0b013e31818546bb. [DOI] [PubMed] [Google Scholar]
- 9.Hanada A, Okita K, Yonezawa K, Ohtsubo M, Kohya T, Murakami T, Nishijima H, Tamura M, Kitabatake A. Dissociation between muscle metabolism and oxygen kinetics during recovery from exercise in patients with chronic heart failure. Heart. 2000;83(2):161–6. doi: 10.1136/heart.83.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haseler LJ, Hogan MC, Richardson RS, Luke J, Hogan MC, Russell S. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol. 2019;86(6):2013–8. doi: 10.1152/jappl.1999.86.6.2013. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman JR, Im J, Rundell KW, Kang J, Nioka S, Speiring BA, Kime R, Chance B. Effect of muscle oxygenation during resistance exercise on anabolic hormone response. Med Sci Sports Exerc. 2003;35(11):1929–34. doi: 10.1249/01.MSS.0000093613.30362.DF. [DOI] [PubMed] [Google Scholar]
- 12.Jamurtas A, Koutedakis Y, Paschalis V, Tofas T, Yfanti C, Tsiokanos A, Koukoulis G, Kouretas D, Loupos D. The effects of a single bout of exercise on resting energy expenditure and respiratory exchange ratio. Eur J Appl Physiol. 2004;92(4–5):393–8. doi: 10.1007/s00421-004-1156-8. [DOI] [PubMed] [Google Scholar]
- 13.Kime R, Hamaoka T, Sako T, Murakami M, Homma T, Katsumura T, Chance B. Delayed reoxygenation after maximal isometric handgrip exercise in high oxidative capacity muscle. Eur J Appl Physiol. 2003;89(1):34–41. doi: 10.1007/s00421-002-0757-3. [DOI] [PubMed] [Google Scholar]
- 14.McCully K, Posner J. Measuring exercise-induced adaptations and injury with magnetic resonance spectroscopy. Int J Sports Med. 1992;13(Suppl 1):S147–9. doi: 10.1055/s-2007-1024621. [DOI] [PubMed] [Google Scholar]
- 15.McCully KK, Iotti S, Kendrick K, Wang Z, Posner JD, Leigh J, Chance B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol. 1994;77(1):5–10. doi: 10.1152/jappl.1994.77.1.5. [DOI] [PubMed] [Google Scholar]
- 16.McManus CJ, Collison J, Cooper CE. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J Biomed Opt. 2018;23(01):015007. doi: 10.1117/1.JBO.23.1.015007. [DOI] [PubMed] [Google Scholar]
- 17.Navalta JW, Stone WJ, Lyons TS. Ethical Issues Relating to Scientific Discovery in Exercise Science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rainoldi A, Melchiorri G, Caruso I. A method for positioning electrodes during surface EMG recordings in lower limb muscles. J Neurosci Methods. 2004;134(1):37–43. doi: 10.1016/j.jneumeth.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Russell PJ, Phillips SJ. A preliminary comparison of front and back squat exercises. Res Q Exerc Sport. 1989;60(3):201–8. doi: 10.1080/02701367.1989.10607441. [DOI] [PubMed] [Google Scholar]
- 20.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol. 2013;115(12):1757–66. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Salles BF, Simao R, Miranda F, Novaes J, da S, Lemos A, Willardson JM. Rest interval between sets in strength training. Sports Med. 2009;39(9):765–77. doi: 10.2165/11315230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Stec MJ, Rawson ES. Estimation of resistance exercise energy expenditure using triaxial accelerometry. J Strength Cond Res. 2012;26(5):1413–22. doi: 10.1519/JSC.0b013e318248d7b4. [DOI] [PubMed] [Google Scholar]
- 23.Stuart M, Meglan D, Lutz G, Growney E, An K. Comparison of intersegmental tibiofemoral joint forces and muscle activity during various closed kinetic chain exercises. Am J Sports Med. 1996;24(6):792–9. doi: 10.1177/036354659602400615. [DOI] [PubMed] [Google Scholar]
- 24.Tesch Pa, Colliander EB, Kaiser P. Muscle metabolism during intense, heavy-resistance exercise. Eur J Appl Physiol. 1986;55(4):362–6. doi: 10.1007/BF00422734. [DOI] [PubMed] [Google Scholar]
- 25.Yavuz HU, Erdağ D, Amca AM, Aritan S. Kinematic and EMG activities during front and back squat variations in maximum loads. J Sports Sci. 2015;33(10):1058–66. doi: 10.1080/02640414.2014.984240. [DOI] [PubMed] [Google Scholar]