Abstract
Maximal accumulated oxygen deficit (MAOD) provides a measure of anaerobic capacity. However, its measurement is a time-consuming process. The purpose of this study was to evaluate a measure of anaerobic capacity that avoids contentious assumptions and demands of the MAOD method. Twelve women and eight men volunteered for the study and completed cycle ergometer tests that resulted in exhaustion after ~4 min and ~8 min. In each test, anaerobic capacity was determined as (i) the MAOD and (ii) the sum of the phosphocreatine and glycolytic contributions (PCr+glycolysis). MAOD was determined by subtraction of the accumulated oxygen uptake from the total oxygen cost. Phosphocreatine and glycolytic contributions were calculated from post-exercise VO2 and blood lactate responses. MAOD in the 4-min and 8-min tests (79.1 ± 7.6 mL·kg−1 and 79.6 ± 7.4 mL·kg−1) and PCr+glycolysis in these tests (80.0 ± 7.3 mL·kg−1 and 79.0 ± 6.9 mL·kg−1) were correlated (r ≥ 0.91) and not significantly different. These results support the use of postexercise measures to quantify the phosphocreatine and glycolytic contributions and to provide an alternative to MAOD for measurement of anaerobic capacity.
Keywords: Energy demand, creatine phosphate, cycling
INTRODUCTION
Maximal accumulated oxygen deficit (MAOD), calculated as the difference between the estimated oxygen cost of exercise and the accumulated oxygen uptake, is an accepted measure of anaerobic capacity (22). In 2010, Bertuzzi and colleagues (4) proposed that the time-consuming procedure of determining oxygen deficit could be replaced by performance of a single bout of exercise, based on the postulation that the fast phase of the post-exercise VO2 response profile reflects phosphocreatine (PCr) contribution (12, 19) and that the peak blood lactate concentration is quantitatively related to glycolysis contribution (19). They summed the estimates of PCr contribution and the glycolytic contribution to generate a measure of total anaerobic contribution. Researchers have reported that oxygen deficit and this PCr+glycolysis measure did not differ, and were highly correlated, for cycling (4, 20, 24), running (25), table tennis (26), and even intermittent exercise (23). Many studies have used the PCr+glycolysis method and it has been applied to a number of exercise modes and activities, such as, for example, rock climbing (3), taekwondo (18), and rowing (6).
Despite widespread use of the PCr+glycolysis measure, we believe that its validity warrants further investigation, in part because most of the validation studies have reported values in absolute terms (mL) and not relative to body weight and, in part, because only one validation study has included women (14). Therefore, the purpose of this study was to compare measures of MAOD and PCr+glycolysis from exhaustive severe intensity cycling exercise performed by men and women. The hypothesis was that the PCr+glycolysis measure would provide a valid measure of anaerobic capacity. This is a companion paper to a paper titled “The increase in oxygen demand during severe intensity exercise must be included in calculation of oxygen deficit” in which we established the accuracy of the criterion MAOD measures used in the present study.
METHODS
Participants
Participants were twelve women (mean ± SD, age 21 ± 1 y, height 168 ± 7 cm, and mass 71.2 ± 9.7 kg) and eight men (age 22 ± 2 y, height 178 ± 9 cm, and mass 80.0 ± 13.5 kg). All participants were involved in recreational sport or fitness activities, but not organized sport activities. This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (21). This study was approved by the Institutional Review Board for the Protection of Human Subjects and conducted in accordance with the latest Declaration of Helsinki. All participants provided written informed consent after the procedures, risks, and benefits of the study had been explained to them.
Protocol
Each participant made six visits to the Applied Physiology Laboratory, at the same time of day (± 1 h). All tests were performed under similar conditions in a temperature-controlled laboratory (20 °C to 22 °C; ~50% relative humidity), with no distractions. Testing sessions were separated by at least 48 hours and were completed within a 21-day period. Participants did not alter their usual exercise, diet, or sleep habits over the course of the study.
In the first visit to the laboratory, the participants completed paperwork, and, in the second and third visits, they performed exhaustive incremental tests. Detailed information about paperwork, obtaining informed consent, screening, familiarization, and performance of incremental tests is provided in our companion paper. Briefly, two exhaustive incremental cycle ergometer tests were performed, with work rates that were individually selected and slightly different from one test to the next. VO2max was determined, as well as steady state VO2 values at each submaximal stage.
In the fourth, fifth, and sixth sessions, participants performed constant power tests. Detailed information about these tests is provided in our companion paper. Briefly, data were obtained from two tests that were performed at work rates that were individually selected to result in exhaustion after ~4 min and after ~8 min. These durations were selected because exercise that leads to exhaustion in 4 min or 8 min is of an intensity appropriate for attainment of a maximal anaerobic contribution (13, 15). VO2peak was determined, as well as data needed to calculate the MAOD. When each test was terminated, the participant remained seated on the ergometer for 7 min, for collection of the data needed to estimate the PCr and glycolytic contributions. We continued to measure VO2 and we obtained blood samples during the 7-min recovery.
As detailed in our companion paper, we used regression analysis on SPSS v22 (IBM, Armonk, NY, USA) and data from the two incremental tests to describe the linear relationship between oxygen uptake (VO2) and work rate, which was then used to extrapolate the individual’s oxygen demand (in mL·kg−1·min−1) at the work rates that would be used in the constant power tests. The total oxygen cost (mL·kg−1) for each individual during each test was calculated as oxygen demand (in mL·kg−1·min−1) × duration (min) + excess oxygen cost (mL·kg−1), where the excess oxygen cost is equal to the excess oxygen uptake attributable to the slow component. Oxygen deficit (in this case, the maximal value for oxygen deficit, MAOD) is the difference between the total oxygen cost and the total accumulated oxygen uptake.
Determination of the PCr+glycolysis contribution in constant power tests involved estimation of the phosphocreatine contribution from the post-exercise VO2 response and estimation of the glycolytic contribution from the post-exercise blood lactate response. For each test, the postexercise breath-by-breath VO2 data were reduced to rolling 5-breath averages. Parameters of the post-exercise VO2 response profile were determined using nonlinear regression on KaleidaGraph 4.50 (Reading, PA, USA), by fitting responses to the following model:
VO2(t) is the value for VO2 at time = t; Abaseline is the baseline VO2; Afast and Aslow are the asymptotic amplitudes for the two exponential terms; taufast and tauslow are the respective time constants; and TD is the common time delay. The area under the curve of the fast phase of the post-exercise exercise VO2 response, which included the TD, was calculated using KaleidaGraph. This area is considered to quantifiably represent the PCr contribution (10).
Blood lactate concentration peaks 4 min to 6 min post-exercise (9). Therefore, exactly 4 min, 5 min, and 6 min after each exercise test, two blood samples were obtained from a warmed cleaned fingertip using single-use, sterile, disposable lancets. Samples were analyzed using identical Accusport Accutrend Lactate Analyzers (Hawthorne, New York, USA). This analyzer has been validated against bench chemistry reference methods (7). The highest value was recorded as the peak post-exercise blood lactate concentration. As part of the familiarization for each participant, resting fingerstick blood samples were obtained and analyzed for blood lactate concentration: mean values were 1.0 ± 0.1 mM (range 0.9 to 1.2 mM). The glycolysis contribution was calculated according to Margaria and colleagues (19), assuming a resting blood lactate concentration of 1.0 mM for each participant:
Assuming a resting value of 1.0 mM, rather than individually sampling prior to each exercise test, may introduce an error no greater than 0.7 mL·kg−1 in any particular estimate of glycolytic contribution, and would have no effect on the overall mean.
The estimate of the PCr contribution and the estimate of the glycolytic contribution were summed to obtain the PCr+glycolysis measure of anaerobic capacity.
Statistical analyses
All statistical analyses were performed using SPSS. To address the appropriateness of the PCr+glycolysis method, the values for anaerobic capacity were compared using a two-way analysis of variance (ANOVA), with repeated measures across method (PCr+glycolysis vs MAOD) and test duration (4 min vs 8 min). Initially, the analysis was carried out with a three-way (method × duration × gender) ANOVA. However, there was no evidence of a significant interaction effect involving gender for any variable. Therefore, data were collapsed across gender and the two-way ANOVA was used.
In addition, correlations between the measures of anaerobic capacity were determined and Bland-Altman (5) comparisons of values were performed using MAOD, the gold standard measure of anaerobic capacity, on the x-axis (17). The significance level for all analyses was set at p < 0.05. Values are presented as means ± SD.
RESULTS
Mean VO2max was 44.9 ± 6.7 mL·kg−1·min−1. Actual durations for 4-min and 8-min constant power tests were 246 ± 22 s (4.1 ± 0.4 min) and 490 ± 24 s (8.2 ± 0.4 min).
Post-exercise VO2 responses and blood lactate concentrations that were used in the calculation of PCr+glycolysis are presented in Table 1. The VO2 responses during exercise, which were used to calculate the values of MAOD in the 4-min and 8-min tests, are presented in our companion paper. Comparisons of those MAOD values and the PCr+glycolysis values are in Table 2. Results of the two-way ANOVA revealed no significant effects of method or duration. There were strong correlations between the MAOD and PCr+glycolysis values determined in the 4-min tests (r = 0.93, p < 0.01) and in the 8-min tests (r = 0.91, p < 0.01).
Table 1.
Mean (± SD) values (with the 95% confidence intervals) for post-exercise responses and estimates of PCr and glycolytic contributions.
| 4-min test | 8-min test | |
|---|---|---|
| TD (s) | 2 ± 2 | 2 ± 1 |
| (95% C.I.) | (1, 3) | (2, 2) |
| SEE TD (s) | 1 ± 1 | 1 ± 1 |
| taufast (s) | 40 ± 7 | 41 ± 6 |
| (95% C.I.) | (37, 43) | (38, 44) |
| SEE taufast (s) | 4 ± 3 | 4 ± 2 |
| Afast (mL·kg−1·min−1) | 35.9 ± 5.4 | 35.2 ± 5.6 |
| (95% C.I.) | (33.5, 38.3) | (32.7, 37.7) |
| SEE Afast (mL·kg−1·min−1) | 3.9 ± 2.1 | 3.7 ± 3.2 |
| tauslow (s) | 632 ± 53 | 704 ± 61 |
| (95% C.I.) | (609, 655) | (677, 731) |
| SEE tauslow (s) | 47 ± 13 | 53 ± 21 |
| Aslow (mL·kg−1·min−1) | 4.7 ± 4.1 | 6.5 ± 4.7 |
| (95% C.I.) | (2.9, 6.5) | (3.7, 9.3) |
| SEE Aslow (mL·kg−1·min−1) | 1.7 ± 0.9 | 2.2 ± 1.4 |
| baseline VO2 (mL·kg−1·min−1) | 4.0 ± 0.3 | 4.0 ± 0.3 |
| (95% C.I.) | (3.9, 4.1) | (3.9, 4.1) |
| PCr (mL·kg−1) | 25.4 ± 5.3 | 25.2 ± 4.6 |
| (95% C.I.) | (23.1, 27.7) | (23.2, 27.2) |
| blood lactate concentration (mM) | 17.6 ± 2.1 | 17.0 ± 2.0 |
| (95% C.I.) | (16.7, 18.5) | (16.5, 18.2) |
| glycolysis (mL·kg−1) | 54.6 ± 6.9 | 53.8 ± 6.5 |
| (95% C.I.) | (50.4, 56.4) | (50.1, 55.7) |
| PCr+glycolysis (mL·kg−1) | 80.0 ± 7.3 | 79.0 ± 6.9 |
| (95% C.I.) | (76.8, 83.2) | (76.0, 82.8) |
Table 2.
Mean (± SD) values (with the 95% confidence intervals) for MAOD and PCr+glycolysis in 4-min and 8-min tests.
| 4-min test | 8-min test | |
|---|---|---|
| MAOD (mL·kg−1) | 79.1 ± 7.6 | 79.6 ± 7.4 |
| (95% C.I.) | (75.8, 82.4) | (76.4. 82.8) |
| PCr+glycolysis (mL·kg−1) | 80.0 ± 7.3 | 79.0 ± 6.9 |
| (95% C.I.) | (76.8, 83.2) | (76.0, 82.0) |
When values were collapsed across the two durations, the correlation between MAOD and PCr+glycolysis was 0.92 (p < 0.01; n = 40). Regression analysis revealed that MAOD could be predicted from PCr+glycolysis:
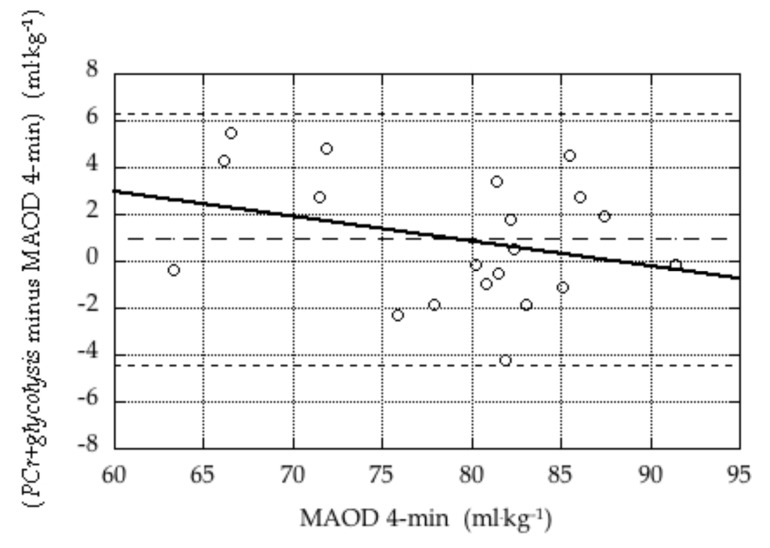
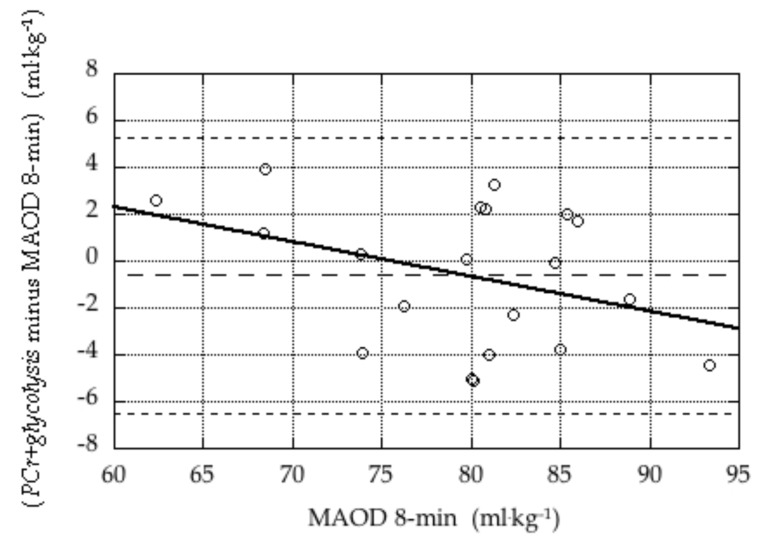
Bland-Altman plots illustrating the validity of the PCr+glycolysis measure in the 4-min tests and the 8-min tests are presented in Figures 1 and 2.
Figure 1.
Bland-Altman plot of difference estimates of anaerobic capacity during the 4-min tests (PCr+glycolysis minus MAOD 4-min) versus the criterion measure (MAOD 4-min). The three dashed lines indicate the mean difference (bias; 0.9 ± 2.7 mL·kg−1) and the mean ± 2 × SD. The solid line represents the linear regression of the difference against the criterion (difference = 9.4 – 0.11 × mean), which was not statistically significant (p = 0.27).
Figure 2.
Bland-Altman plot of difference in estimates of anaerobic capacity during the 8-min tests (PCr+glycolysis minus MAOD 8-min) versus the criterion measure (MAOD 8-min). The three dashed lines indicate the mean difference (bias; −0.6 ± 3.0 mL·kg−1) and the mean ± 2 × SD. The solid line represents the linear regression of the difference against the criterion (difference = 11.2 – 0.15 × mean), which was not statistically significant (p = 0.20).
DISCUSSION
The important result in the present study is that the PCr+glycolysis measure provides a valid estimate of anaerobic capacity in exhaustive severe intensity cycling exercise. PCr+glycolysis was validated against MAOD, using results from exhaustive exercise tests performed at two different work rates that were clearly within the severe intensity domain. Similar values for both MAOD and PCr+glycolysis were obtained at the two work rates. These results demonstrate that PCr+glycolysis provides an attractive alternative to MAOD because it does not require performance of multiple moderate intensity trials to estimate oxygen demand (in fact it does not require any assumptions about oxygen demand) and it gives information about the two components of the anaerobic contribution.
Since the work of Margaria (19), it has been accepted that post-exercise blood lactate concentration is related to the glycolytic contribution during the exercise and that the postexercise VO2 response is related to the rate of replenishment of PCr following the exercise. Although prior studies had detailed a method by which PCr and glycolytic contributions could be concurrently and independently quantified (e.g., 2, 3), it was the work of Bertuzzi and colleagues (4), who first validated the PCr+glycolysis measure, that stimulated its adoption.
Using data from cycle ergometer tests, Bertuzzi and colleagues (4) reported that the mean oxygen deficit in the exercise (39 mL·kg−1) and the sum of the PCr (9 mL·kg−1) and glycolysis measures (30 mL·kg−1) did not differ and were highly correlated (r = 0.78, p = 0.01) in their nine male subjects, although, notably, when expressed in units of mL O2 and not when expressed relative to body weight (we report their findings here in mL·kg−1 to allow comparisons between studies). Urso and colleagues (24) also had nine male subjects perform exhaustive cycle ergometer tests and reported a significant relationship between the measure of glycolytic contribution and the difference between MAOD and the measure of PCr contribution, which suggests a meaningful relationship between MAOD and PCr+glycolysis, although this was not directly evaluated. More recently, Miyagi and colleagues (20) calculated MAOD and PCr+glycolysis values for fourteen men performing exhaustive cycle ergometer exercise across a range of cycling work rates. At 115% of the work rate associated with VO2max, the values were correlated when expressed in absolute terms (r = 0.68, p < 0.01) and when expressed relative to lower leg lean mass (r = 0.55, p < 0.05), but not when expressed relative to body mass (r = 0.27) or lean mass (r = 0.45). The mean values were (17 mL·kg−1 + 30 mL·kg−1 = 47 mL·kg−1) for PCr+glycolysis and 47 mL·kg−1 for MAOD.
PCr+glycolysis has also been validated against MAOD in running. Zagatto and colleagues (25) reported that, for 15 men completing tests of ~2. min to ~5 min duration, for which the anaerobic contribution should be maximal, the PCr+glycolysis measures (mean, 22 mL·kg−1 + 30 mL·kg−1) were correlated with the traditional measure of MAOD obtained in the ~3 min test (mean, 55 mL·kg−1), although, again, significantly correlated only using absolute units of mL. In addition, they demonstrated the robust nature of the PCr+glycolysis measure, which was unaffected across a range of running speeds that elicited exhaustion in ~1 min to ~5 min. Hill and colleagues (14) had 30 university students (13 women and 17 men) run for 3 min, 7 min, and to exhaustion (~10.3 min) at individually selected speeds. AOD (MAOD in the exhaustive tests) and PCr+glycolysis were calculated using the same methods as in the present study, and reported in mL·kg−1. The PCr+glycolysis values consistently underestimated the AOD values (by ~3 mL·kg−1), but the values were very highly correlated across the three exercise durations. Panissi and colleagues (23) evaluated PCr+glycolysis for use in intermittent exercise, specifically a session of high intensity interval training performed by thirteen men. They found no difference between the mean values of MAOD and PCr+glycolysis but did not provide information about correlations between the values as the study was not primarily designed to test the validity of the PCr+glycolysis method.
Conceptually, our procedures were the same as in previous validation studies (4, 20, 24, 25). There were only small methodological differences. First, in determining the MAOD, our calculations take into consideration that the oxygen demand is increasing during exercise (see our companion paper); their calculations assume a constant oxygen demand during exercise. Correcting the total oxygen cost for the loss of efficiency observed during exercise generated values for MAOD that were ~8 mL·kg−1 larger for tests of ~4 min duration and ~37 mL·kg−1 for 8-min tests. This methodological difference would have little impact on results of previous validation studies, as exercise durations were only 154 ± 38 s (4), 157 ± 28 s (22) 189 ± 40 s (16), 161 ± 40 s (21) and 10 × 60 s for the intermittent exercise (23), and so correcting the oxygen cost for the loss of efficiency would have added no more than perhaps 5 mL·kg−1 to the MAOD estimates in those studies. Second, we did not attempt to adjust our MAOD values to account for the use of stored oxygen; in these other validation studies, calculated values of oxygen deficit estimates were reduced 10%. Certainly, corrections for the use of stored oxygen have been used by a variety of researchers, although the magnitude of the correction is usually smaller than 10% of the oxygen deficit, and not considered to be a function of the overall anaerobic contribution, such as the 2.3 mL·kg−1 calculated by Barstow and colleagues (1). Third, in estimating the glycolytic contribution from the blood lactate concentration, we used the conversion factor of 3.3 mL·kg−1 per mM increase in blood lactate concentration (19). Other studies validating the PCr+glycolysis method used 3.0 mL·kg−1 per mM, which generates 10% lower estimates of glycolytic contribution. In addition, lactate concentration values in the present study may have been underestimated, compared to values that would have been obtained using a Yellow Springs analyzer (YSI Inc., Yellow Springs, OH, USA) (8), which was used in the other validation studies. Finally, for the PCr contribution, when we calculated the area under the curve of the fast phase of the post-exercise VO2 response, we included the area during the time delay. In other studies, this area was not calculated or included, and PCr contribution was calculated as taufast × Afast (e.g., 4, 20, 24, 25), and this could lead to up to ~10% lower values for PCr contribution in those studies. The procedural differences resulted in mean values for both MAOD and PCr+glycolysis values in the present study being higher than would have been found using the exact methods of the Bertuzzi et al. (4) and most subsequent validation studies. Importantly, correlations between MAOD and PCr+glycolysis values in the present study (with values expressed relative to body mass) were greater than correlations in other validation studies, most of which relied on comparisons of values expressed in absolute units. Recently, using procedures identical to those in the present study, we have reported correlations of between 0.80 (in 3-min tests) and 0.94 (in exhaustive, ~10.3 min tests) between (M)AOD and PCr+glycolysis, expressed relative to body weight (14). In that study, as in the present study, results of a Bland-Altman analysis confirmed the validity of PCr+glycolysis.
We note that this is only the second validation study that has included women as participants. As in that study (14), ANOVA revealed no interaction effect involving gender, suggesting that the PCr+glycolysis method is equally valid for women as well as for men. A concern with including women with men in the subject pool is that gender differences in MAOD and PCr+glycolysis could widen the range of values and inflate correlation coefficients. Reporting the values on a per-kg basis removes most differences attributable to gender, and inspection of our values reveals less variability in MAOD and PCr+glycolysis values than in other validation studies.
To validate the PCr+glycolysis measure requires the ability to measure AOD, which is dependent upon the ability to estimate the energy demand or oxygen demand. While the estimation of oxygen demand of heavy or severe intensity exercise has long been contentious and a source of controversy (e.g., 11, 22) and continues to be the focus of research study (see our companion paper), it can be accomplished in activities like running, cycling, and rowing, by extrapolation from steady-state responses in moderate intensity exercise. However, this method cannot be applied to activities like rock climbing, martial arts, and surfing, or to most team sports. Quantifying the anaerobic contribution in these sports or measuring the anaerobic capacities of athletes in these sports can be a very useful tool for coaches, athletes, and sport scientists. Thus, the importance of a measure like PCr+glycolysis is obvious, and the importance of carefully validating this measure is clear.
Despite difficulties in validating the PCr+glycolysis measure, it has been used in a variety of activities, including rock climbing (3), martial arts (18), and table tennis test (26). However, it has been validated for only one of these activities. Zagatto and Gobatto (26) reported that PCr+glycolysis in table tennis tests lasting ~2 min, ~3 min, ~5 min, and ~8 min averaged 60 mL·kg−1, which was not different from the oxygen deficit in the exhaustive ~5 min test (mean, 60 mL·kg−1). Across the four tests in this latter study, PCr estimates averaged 43 mL·kg−1, which is far higher than reported in other studies (e.g., 2, 14, 20, 25, the present study) and the glycolysis contribution averaged only 18 mL·kg−1, suggesting that both oxygen deficit and the PCr+glycolysis methods must be evaluated further prior to use in activities other than large muscle activities like cycling and running. In the present study, we have not simply confirmed that PCr+glycolysis provides a valid replacement for MAOD, we have demonstrated a stronger relationship than in previous studies. We attribute this to the methods used in calculating PCr+glycolysis and in calculating MAOD (the latter described in our companion paper).
Not only is the estimation of exercise efficiency a contentious element in the calculation of oxygen deficit (e.g., 11, 22), it is also, in and of itself, an important factor related to sport performance (e.g., 16). While one attraction of the PCr+glycolysis method is that it does not require measurement of or assumptions about exercise efficiency and oxygen demand, we believe that the method can also be used to generate a measure of exercise efficiency. If we add our PCr+glycolysis value to the accumulated oxygen uptake to obtain an estimate of total oxygen cost, divide this by the exercise duration to obtain an estimate of the average oxygen demand, and then divide this average oxygen demand by the work rate (divide by speed, for running), we obtain a meaningful measure of the oxygen demand of exercise (i.e., the mathematical inverse of ‘efficiency’), with units of mL·min−1 per W (or mL·kg−1·min−1 per m•min−1, for running). For other activities, like martial arts or climbing, the effort can only be described, and an actual work rate cannot be educed, so the calculated value might be reported as ‘the oxygen demand (in mL·kg−1·min−1) of a simulated taekwondo competition’ or ‘the oxygen demand (in mL·kg−1·min−1) of a Class 5.10 Grade II climb’. We acknowledge, and caution, that oxygen demand in activities like martial arts competition will vary depending on duration, level of intensity, time spent grappling versus in an upright defensive position, etc.; similarly oxygen demand in a climbing task will vary with the rate of ascent and the exact technical aspects of the climb. Therefore, these results would be interpreted with care and on an individual basis. Thus, using the PCr+glycolysis concept, a measure of efficiency can be determined without the need for performing a series of submaximal steady state trials. For continuous severe intensity activities, these efficiency values are somewhat intensity-specific, as the slow component contribution to the true total oxygen cost is greater with lower intensities and longer durations of exercise. However, the impact will be small, as the (larger) total oxygen cost is divided by (the longer) exercise duration when generating the measure of average oxygen demand.
In conclusion, it has become common practice in several laboratories to replace the cumbersome, time-consuming, and somewhat contentious oxygen deficit measure with the PCr+glycolysis measure. In this study, we found that PCr+glycolysis values from exhaustive 4-min and 8-min tests were correlated with calculated MAODs, and there were no differences between PCr+glycolysis and MAOD values, in a sample of men and women. Results of the present study provide strong support for using the sum of the PCr contribution, obtained from kinetics of the post-exercise VO2 response, and the glycolytic contribution, obtained from peak post-exercise blood lactate concentration, as a measure of total anaerobic contribution, and specifically as a measure of anaerobic capacity. In addition, our data suggest that the methods used to generate PCr+glycolysis, in combination with the measure of accumulated oxygen uptake during the exercise test, can be used to generate values for gross cycling efficiency or running efficiency, without the need for any submaximal steady state exercise bouts.
ACKNOWLEDGEMENTS
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Barstow TJ, Lamarra N, Whipp BJ. Modulation of muscle and pulmonary O2 uptakes by circulatory dynamics during exercise. J Appl Physiol. 1990;68:979–989. doi: 10.1152/jappl.1990.68.3.979. [DOI] [PubMed] [Google Scholar]
- 2.Beneke R, Pollman C, Bleif I, Leithäuser RM, Hüttler M. How anaerobic is the Wingate Anaerobic Test for humans? Eur J Appl Physiol. 2002;87:388–392. doi: 10.1007/s00421-002-0622-4. [DOI] [PubMed] [Google Scholar]
- 3.Bertuzzi RCM, Franchini E, Kokubun E, Kiss MA. Energy system contributions in indoor rock climbing. Eur J Appl Physiol. 2007;101:293–300. doi: 10.1007/s00421-007-0501-0. [DOI] [PubMed] [Google Scholar]
- 4.Bertuzzi RCM, Franchini E, Ugrinowitsch C, Kokubun E, Lima-Silva AE, Pires FO, Nakamura FY, Kiss MAPDM. Predicting MAOD using only a supramaximal exhaustive test. Int J Sports Med. 2010;31:477–481. doi: 10.1055/s-0030-1253375. [DOI] [PubMed] [Google Scholar]
- 5.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet. 1986;8476:307–310. [PubMed] [Google Scholar]
- 6.de Campos Mello F, de Moraes Bertuzzi RC, Grangeiro PM, Franchini E. Energy systems contribution in 2,000 m race simulation: a comparison among rowing ergometers and water. Eur J Appl Physiol. 2009;107:615–619. doi: 10.1007/s00421-009-1172-9. [DOI] [PubMed] [Google Scholar]
- 7.Fell JW, Rayfield JM, Gulbin JP, Gaffney PT. Evaluation of the Accusport lactate analyser. Int J Sports Med. 1998;19:199–204. doi: 10.1055/s-2007-971904. [DOI] [PubMed] [Google Scholar]
- 8.Franchini E, Matsuchingue KA, Colantonio E, Kiss MAPD. Comparação dos analisadores de lactate Accusport e Yellow Springs. R Bras Ci e Mov (Braz J Sci Mov) 2004;12:39–44. [Google Scholar]
- 9.Gass GC, Rogers S, Mitchell R. Blood lactate concentration following maximum exercise in trained subjects. Brit J Sports Med. 1981;15:172–176. doi: 10.1136/bjsm.15.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gladden LB, Welch HG. Efficiency of anaerobic work. J Appl Physiol: Respirat Environ Exerc Physiol. 1978;44(4):564–570. doi: 10.1152/jappl.1978.44.4.564. [DOI] [PubMed] [Google Scholar]
- 11.Green S, Dawson BT. Methodological effects on the VO2-power regression and the accumulated O2 deficit. Med Sci Sports Exerc. 1996;28:392–397. doi: 10.1097/00005768-199603000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Henry FM. Aerobic oxygen consumption and alactic debt in muscular work. J Appl Physiol. 1951;3:427–438. doi: 10.1152/jappl.1951.3.7.427. [DOI] [PubMed] [Google Scholar]
- 13.Hill DW, Davey KM, Stevens EC. Maximal accumulated O2 deficit in running and cycling. Can J Appl Physiol. 2002;27:463–478. doi: 10.1139/h02-025. [DOI] [PubMed] [Google Scholar]
- 14.Hill DW, Riojas AE, McFarlin BK, Vingren JL. An alternative to oxygen deficit as a way to quantify anaerobic contributions in running. J Hum Sport Exerc. 2020 doi: 10.14198/jhse.2020.154.11. in press. [DOI] [Google Scholar]
- 15.Hill DW, Vingren JL. Maximal accumulated oxygen deficit in running and cycling. Appl Physiol Nutr Metab. 2011;36:831–838. doi: 10.1139/h11-108. [DOI] [PubMed] [Google Scholar]
- 16.Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol. 2008;586:35–44. doi: 10.1113/jphysiol.2007.143834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krouwer JS. Why Bland-Altman plots should use X, not (Y+X)/2, when X is a reference method. Stat Med. 2008;27:778–780. doi: 10.1002/sim.3086. [DOI] [PubMed] [Google Scholar]
- 18.Lopes-Silva JP, Da Silva Santos JF, Artiola GG, Loturco I, Abbiss C, Franchini E. Sodium bicarbonate ingestion increases glycolytic contribution and improves performance during simulated taekwondo combat. Eur J Sport Sci. 2018;18(3):431–440. doi: 10.1080/17461391.2018.1424942. [DOI] [PubMed] [Google Scholar]
- 19.Margaria R, Edwards HT, Dill DB. The possible mechanism of contracting and paying the oxygen debt and the role of lactic acid in muscular contraction. Am J Physiol. 1933;106:689–714. [Google Scholar]
- 20.Miyagi WE, de Poli RAB, Papoti M, Bertuzzi R, Zagatto AM. Anaerobic capacity estimated in a single supramaximal test in cycling: validity and reliability analysis. Sci Reports. 2017;7 doi: 10.1038/srep42485. article number 42485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noordhof DA, de Koning JJ, Foster C. The maximal accumulated oxygen deficit method: A valid and reliable measure of anaerobic capacity? Sports Med. 2010;40(4):285–302. doi: 10.2165/11530390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Panissa VLG, Fukuda DH, Caldeira RS, Gerosa-Neto J, Lira FS, Zagatto AM, Franchini E. Is oxygen uptake measurement enough to estimate energy expenditure during high-intensity intermittent exercise? Quantification of anaerobic contribution by different methods. Front Physiol. 2018;9:868. doi: 10.3389/fphys.2018.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urso RR, Silva-Cavalcante MD, Correia-Oliveira CF, Bueno S, Damasceno MV, Lima-Silva AE, Bertuzzi R. Determinação dos metabolismos lático e alático da capacide anaeróbia por meio do consumo de oxigênio (Determination of lactic and alactic metabolisms of the anaerobic capacity using oxygen uptake) Revista Brasileira de Cineantropometria e Desempenho Humano. 2013;15:616–627. [Google Scholar]
- 25.Zagatto AM, Bertuzzi R, Miyagi WE, Padulo J, Papoti M. MAOD determined in a single supramaximal test: a study on the reliability and effects of supramaximal intensities. Int J Sports Med. 2016;37:700–707. doi: 10.1055/s-0042-104413. [DOI] [PubMed] [Google Scholar]
- 26.Zagatto AM, Gobatto CA. Relationship between anaerobic parameters provided from MAOD and critical power model in specific table tennis set. Int J Sports Med. 2012;33:613–620. doi: 10.1055/s-0032-1304648. [DOI] [PubMed] [Google Scholar]