Abstract
Objective:
This study estimates the national prevalence of depression and anxiety among children with epilepsy and determines which demographic variables and comorbidities increase the risk of these psychopathologies. We also compare the rates of depression and anxiety in pediatric epilepsy to those of other chronic health conditions in childhood.
Methods:
We used the 2009–2010 National Survey of Children with Special Health Care Needs to identify children with epilepsy with and without depression and anxiety. We assessed demographic factors and comorbidities associated with depression and anxiety using weighted multivariable logistic regressions. The rates of psychiatric comorbidity in children with chronic conditions other than epilepsy were also determined.
Results:
The final sample included 1,042 children over the age of five with epilepsy. After applying the sampling weights, we estimated that 283,000 children between 5 and 17 years of age have epilepsy in the United States. Among these children, 25 percent have depression and/or anxiety. This figure was not significantly different from the rates seen among children with asthma (16.5 percent) or allergies (21.6 percent) but was significantly lower than the rate seen among children with migraines (43.2 percent). In our analyses of children with epilepsy, low- income children (regardless of race) and children whose needs for specialist care were unmet (relative to those whose needs were met) were more likely to have depression. Low-income black children were less likely to have anxiety than high-income white children. Gender, age, and epilepsy severity were unrelated to depression or anxiety.
Conclusions:
One in four U.S. children with epilepsy has depression and/or anxiety. Therefore, physicians should consider the various factors that are related to depression and anxiety in children with epilepsy so that at-risk children can be screened and managed appropriately.
Keywords: pediatric, epilepsy, depression, anxiety, demographic, risk factors
1. Introduction
Six of every 1,000 children in the United States have epilepsy [1]. These children are more likely than children without epilepsy to have depression or anxiety [1–3]. Such psychiatric comorbidities are associated with reduced quality of life and suicidality [2–4]. For some individuals, psychiatric comorbidities contribute more to health-related quality of life than epilepsy-related factors such as seizure frequency and the side effects of anti-epileptic seizure drugs (AEDs) [5,6].
Several factors are known to be associated with comorbid depression or anxiety in pediatric epilepsy, including psychosocial factors such as family relationship satisfaction (lower satisfaction = more depressive symptoms) [7] and treatment factors such as AED polytherapy (AED polytherapy = increased risk of anxiety disorders) [2]. However, there are additional factors that are understudied or poorly understood. These include demographic factors such as age, race, ethnicity, health insurance type, and income level as well as comorbidities such as migraines and autism-spectrum disorder [2,8]. Understanding these factors may have clinical relevance, in that they may help clinicians effectively determine who is most at risk for developing depression and anxiety, and therefore intervene appropriately.
Here, we use a large national survey to estimate the prevalence of depression and anxiety among children with epilepsy and to characterize the demographic factors and comorbidities associated with the presence of depression and anxiety. We also compare the rates of depression and anxiety in pediatric epilepsy to that of other chronic health conditions, in order to place epilepsy comorbidities in the larger context of child health.
2. Material and methods
2.1. Study Design / Data Source
The National Survey of Children with Special Health Care Needs (NS-CSHCN), conducted periodically by the Child and Adolescent Health Measurement Initiative, is a cross-sectional national survey of the health information of children who have serious or chronic conditions that require increased health services compared to healthy peers [9]. We utilized the 2009–2010 NS- CSHCN, which was the most current version available at the time of this study. We opted to use the NS-CSHCN over a clinical sample because the survey includes weights from the complex survey design, which allows for national estimates of prevalence. In addition, the use of a national survey with a large sample size increases external validity and statistical power to a greater extent than a smaller clinical sample would when examining the risk factors associated with depression and anxiety.
The data set was obtained online from the Data Resource Center for Child and Adolescent Health. The Institutional Review Board (IRB) at Weill Cornell Medicine determined the study as exempt from review (IRB #1803019036A003).
2.2. Sample
The sample of children in the NS-CSHCN was gathered via a telephone survey of households in the United States from July 2009 to March 2011 [9]. Households were called using both list- assisted and independent random-digit-dial. A household was deemed eligible for participation in the study if it had at least one child aged 0 to 17 that had special health care needs, as determined by a validated screener answered by the parents [10]. If there were multiple children with special health care needs in the home, then one was randomly selected to be the focus of the study [11].
We utilized three subsets of the survey data in our analyses. In our analysis of pediatric epilepsy, we included all children with a current diagnosis of epilepsy. In our analysis of all children with special health care needs, we included all children in the data set. For our analysis of children with chronic health conditions other than epilepsy, we excluded children with a current diagnosis of epilepsy. Children younger than five years were excluded from all three subsets of the data, as is typically done in studies of comorbid depression and anxiety [12,13]. We also excluded children who had an unknown depression or anxiety status from all three subsets of the data.
A participant’s response to a question was coded as missing and excluded from analyses if the participant answered “I don’t know” or “refuse to answer.” We explored missing data for each variable. We handled missing data by performing Little’s MCAR test to determine if data was missing completely at random [14]. If so, we planned to use a complete case analysis; else we planned to do multiple imputation analysis of the missing data.
2.3. Measurement
2.3.1. Epilepsy Diagnosis
The diagnosis of epilepsy was assessed by two survey items answered by the parent/guardian. One asked “Has a doctor or other medical provider had ever told you that [the child] had epilepsy or seizure disorder?” and a second asked “Does [the child] currently have epilepsy or seizure disorder?” [9]. The parent/guardian needed to respond “yes” to both questions for the child to be deemed to have epilepsy in our analyses.
2.3.2. Psychiatric Comorbidities
The outcomes of interest were the presence of depression and the presence of anxiety. Two questions determined whether the child currently had depression: one asked “Has a doctor or other medical provider ever told you that [the child] had depression?” and a second asked “Does [the child] currently have depression?” [9]. The parent/guardian needed to respond “yes” to both questions for the child to be deemed to have depression in our analyses. Two other questions determined whether the child currently had anxiety: one asked “Has a doctor or other medical provider ever told you that [the child] had anxiety problems?” and a second asked “Does [the child] currently have anxiety problems?” [9]. The parent/guardian needed to respond “yes” to both questions for the child to be deemed to have anxiety in our analyses.
2.3.3. Demographic characteristics
We grouped the sample into young children (ages 5–11) and adolescents (12–17). Children were grouped into one of three categories for race (white only, black only, other race) and two categories for ethnicity (Hispanic, non-Hispanic). Each child’s home state or district was grouped into one of four regions (Northeast, Midwest, South, West) in accordance with the U.S. Census Bureau [15].
The highest education level of the parents was also noted (less than high school, high school, more than high school). Insurance was categorized into three groups—private or other comprehensive insurance only, public insurance (with or without private insurance), or uninsured.
Children were also grouped into three groups based on whether the parent/guardian believed their child needed a specialist in the past year, and if so, whether those needs were met (specialist not needed, specialist needed and needs met, specialist needed but needs unmet).
Children were divided into two groups based on the income of their household—those below 100 percent of the federal poverty line (“low-income”) were compared to those above 100 percent of the federal poverty line (“high-income”). In addition, given previous research demonstrating an interaction between income and race in relation to psychiatric disorders, we searched for an interaction between race and income in relation to depression or anxiety [16,17]. We planned to stratify the race factor by income if the interaction was significant, thereby creating a six-level race/income variable (low-income blacks, high-income blacks, low-income other race, high- income other race, low-income whites, high-income whites).
2.3.4. Epilepsy Severity
Parents/guardians were asked whether they would rate their child’s epilepsy as mild, moderate, or severe. “Epilepsy severity” is a validated measure for use in surveys and corresponds roughly with seizure frequency [18].
2.3.5. Comorbidities
In addition to depression and anxiety, we examined current diagnoses of other psychiatric and neurologic disorders including attention deficit disorder or attention deficit hyperactive disorder (ADD/ADHD), autism spectrum disorder (ASD), behavior disorder (i.e., any behavioral or conduct disorders including oppositional defiant disorder and conduct disorder), migraines or frequent headaches, and concussion. We also examined current diagnoses of two common medical conditions—asthma and allergies.
2.4. Analysis
We estimated the national prevalence of depression and anxiety in the pediatric epilepsy population using the sampling weights of the data set [19]. Error was calculated by multiplying the standard error by 1.96, yielding a 95 percent confidence interval.
Weighted bivariate analyses were performed between each independent variable and each of the two outcomes of interest: the presence of depression and the presence of anxiety among children with epilepsy.
We determined what factors were related to depression and anxiety among children with epilepsy in two weighted multivariable logistic regressions. We included all the variables from the bivariate analyses in the multivariate regressions regardless of significance for two reasons. First, many of these variables have been underexplored in previous research. Second, we wanted to generate unbiased estimates of depression and anxiety [20]. We examined for multicollinearity to ensure the variables were not strongly intercorrelated.
To determine whether the burden of depression and anxiety in epilepsy is different from that of other chronic conditions, we evaluated the odds of having depression and/or anxiety in children with epilepsy relative to children with special health care needs other than epilepsy, while controlling for demographic variables. We also compared the odds of having depression and/or anxiety in children with epilepsy relative to children with asthma, allergies, and migraines, while controlling for demographic variables. Given that the focus of the current study was on pediatric epilepsy, the demographic variables associated with other chronic health conditions are not reported here.
2.5. Statistical Software
We utilized R software environment (version 3.5.1) [21] and the packages “survey” [22], “usdm” [23], and “BaylorEdPsych” [14] to analyze the survey data.
3. Results
3.1. National Estimates of Depression and Anxiety among Children with Epilepsy
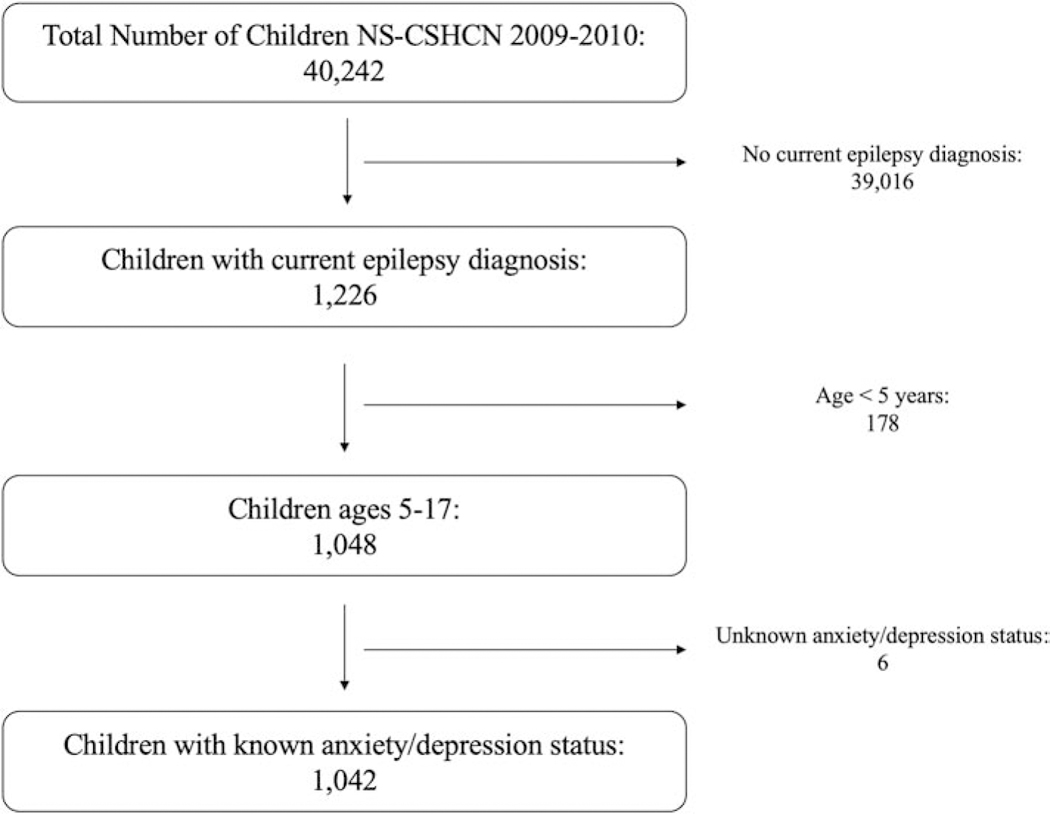
There were 40,242 total respondents in the 2009–2010 NS-CSHCN. 1,226 children had epilepsy. After excluding 178 children who were under age five and six children who had unknown depression or anxiety status, 1,042 children remained as the final sample for the analysis of pediatric epilepsy (Figure 1). These included 799 children without either depression or anxiety, 21 with depression but no anxiety, 125 with anxiety but no depression, and 97 with both anxiety and depression. The demographic data for the sample are shown in Supplemental Table S1.
Figure 1.
Breakdown of the pediatric epilepsy sample used in the study. After excluding children without epilepsy, children under the age of 5, and children with unknown depression/anxiety status, 1,042 children remained as the final sample for the study. NS-CSHCN = National Survey of Children with Special Health Care Needs.
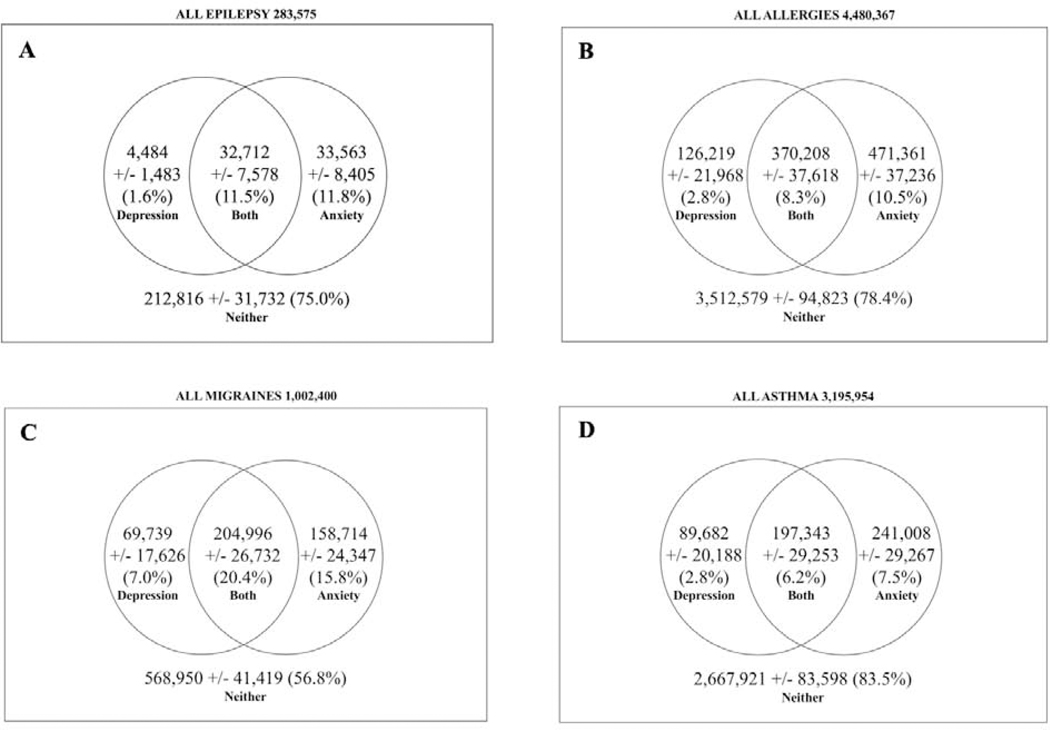
Applying the sampling weights to the 1,042 children indicated there were 283,575 [95% confidence interval +/− 33,732] children aged 5–17 with epilepsy in the United States, which represented 3.1% of all children aged 5–17 with special health care needs and 0.53% of all children aged 5–17 in the United States as per 2010 census reports [24]. Among the 283,575 children with epilepsy, there were 4,483 [+/− 1,483] with current depression but no current anxiety, 33,563 [+/− 8,405] with current anxiety but no current depression, and 32,712 [+/− 7,578] with both current anxiety and current depression (Figure 2a). These findings indicate that 25% of children aged 5–17 with epilepsy have depression and/or anxiety. More specifically, the weighted prevalence rates were 1.6% for depression alone, 11.8% for anxiety alone, and 11.5% for both. The demographic characteristics of the population are described in Table 1.
Figure 2.
Rates of depression and anxiety among children with epilepsy and other chronic conditions. The population rates of depression, anxiety, both, or neither are depicted among U.S. children with current A) epilepsy, B) allergies but no epilepsy, C) migraines but no epilepsy, D) asthma but no epilepsy.
Table 1:
Demographic analysis of the population of children with epilepsy in the U.S.
| Variables | No depression or anxiety (N = 212,816) | Depression, but no anxiety (N = 4,484) | Anxiety, but no depression (N = 33,563) | Both depression and anxiety (N = 32,712) |
|---|---|---|---|---|
| Age† (years) | 11.17 (3.63) | 11.43 (3.87) | 11.56 (3.60) | 12.92 (3.41) |
| Adolescents (12–17) | 97,499 (45.8) | 2,495 (55.6) | 17,634 (52.5) | 20,086 (61.4) |
| Young children (5–11) | 115,317 (54.2) | 1,989 (44.4) | 15,929 (47.5) | 12,626 (38.6) |
| Gender | ||||
| Male | 126,107 (59.3) | 3,052 (68.1) | 22,586 (67.3) | 20,432 (62.5) |
| Female | 86,651 (40.7) | 1,432 (31.9) | 10,977 (32.7) | 12,280 (37.5) |
| Region | ||||
| Northeast | 32,581 (15.3) | 504 (11.2) | 6,544 (19.5) | 4,800 (14.7) |
| Midwest | 47,184 (22.2) | 908 (20.2) | 6,598 (19.7) | 9,213 (28.2) |
| West | 34,285 (16.1) | 699 (15.6) | 8,652 (25.8) | 5,187 (15.9) |
| South | 98,766 (46.4) | 2,373 (52.9) | 11,770 (35.1) | 13,513 (41.3) |
| Race/Income | ||||
| Low-income black | 19,870 (9.5) | 1,097 (24.5) | 768 (2.4) | 2,719 (8.3) |
| High-income black | 30,525 (14.6) | 0 (0) | 1,499 (4.7) | 2,531 (7.7) |
| Low-income other race | 13,261 (6.3) | 403 (9.0) | 2,120 (6.6) | 4,441 (13.6) |
| High-income other race | 27,597 (13.2) | 504 (11.2) | 1,831 (5.7) | 1,309 (4.0) |
| Low-income white | 19,625 (9.4) | 716 (16.0) | 2,346 (7.3) | 12,324 (37.7) |
| High-income white | 98,698 (47.1) | 1,764 (39.3) | 23,395 (73.2) | 9,345 (28.6) |
| Ethnicity | ||||
| Hispanic | 31,977 (15.2) | 571 (12.7) | 6,458 (20.2) | 5,380 (16.5) |
| Non-Hispanic | 178,354 (84.8) | 3,913 (87.3) | 25,503 (79.8) | 27,288 (83.5) |
| Insurance | ||||
| Public | 125,135 (58.8) | 3,321 (74.1) | 18,262 (54.4) | 27,874 (85.2) |
| Uninsured | 2,315 (1.1) | 19 (0.4) | 0 (0) | 414 (1.3) |
| Private or other comprehensive | 85,366 (40.1) | 1,144 (25.5) | 15,301 (45.6) | 4,424 (13.5) |
| Highest parent education level | ||||
| Less than high school | 18,134 (8.5) | 520 (11.6) | 1,054 (3.1) | 5,354 (16.4) |
| High school | 60,445 (28.4) | 2,110 (47.1) | 8,098 (24.1) | 8,155 (24.9) |
| More than high school | 134,238 (63.1) | 1,854 (41.3) | 24,410 (72.7) | 19,203 (58.7) |
| Epilepsy Severity | ||||
| Moderate | 67,478 (31.7) | 1,882 (42.0) | 8,182 (25.4) | 6,803 (20.9) |
| Severe | 39,767 (18.7) | 680 (15.2) | 8,428 (26.1) | 5,972 (18.3) |
| Mild | 105,300 (49.5) | 1,922 (42.9) | 15,625 (48.5) | 19,776 (60.8) |
| Specialist Needs | ||||
| Specialist not needed | 41,631 (19.6) | 814 (18.2) | 3,009 (9.0) | 7,419 (23.1) |
| Specialist needed and needs unmet | 5,060 (2.4) | 115 (2.6) | 742 (2.2) | 5,336 (16.6) |
| Specialist needed and needs met | 166,112 (78.1) | 3,555 (79.3) | 29,811 (88.8) | 19,391 (60.3) |
| ADD/ADH | 37,995 (18.1) | 2,982 (66.5) | 16,670 (49.8) | 21,630 (66.1) |
| Behavior Disorder | 26,921 (12.7) | 1,645 (36.7) | 15,634 (46.6) | 23,778 (73.2) |
| ASD | 24,799 (11.8) | 238 (5.3) | 15,706 (46.8) | 13,260 (40.5) |
| Migraines/Frequent Headaches | 30,766 (14.5) | 2,072 (46.2) | 7,676 (25.1) | 15,709 (48.0) |
| Concussion | 27,552 (13.0) | 702 (17.6) | 3,560 (10.6) | 3,379 (10.4) |
| Depression | 0 (0) | 4,484 (100) | 0 (0) | 32,712 (100) |
| Anxiety | 0 (0) | 0 (0) | 33,563 (100) | 32,712 (100) |
| Asthma | 32,879 (15.4) | 2,211 (49.3) | 12,834 (38.2) | 13,288 (40.6) |
| Allergies | 79,512 (37.6) | 2,219 (49.5) | 17,549 (52.3) | 22,850 (69.9) |
Population estimates are depicted as estimate N (response %).
Percentages are out of the total number of respondents for that factor.
FPL = federal poverty level.
These estimates are illustrated as mean (standard deviation).
3.2. Interaction Between Race and Income
To identify a potential interaction between race and income, we performed several weighted logistic regressions of race on depression and anxiety. We compared the effect of race in individuals below 100 percent of the federal poverty level (FPL; low income extreme) to the effect of race in individuals above 400 percent of the FPL (high income extreme). Black race was positively related to depression in those over 400 percent of the FPL (odds ratio [OR] = 14.4, p = 0.02) but negatively related to depression in those below 100 percent of the FPL (OR = 0.31, p = 0.02). The difference in effect size between income groups was statistically significant (p = 0.002) in a t-test analysis of the two log odds. Black race was negatively associated with anxiety in those under 100 percent FPL (OR = 0.23, p = 0.004) but unrelated to anxiety in those over 400 percent FPL (OR = 2.31, p = 0.35). The difference in effect size between income groups was statistically significant (p = 0.02). Given these findings, we analyzed race and income as one stratified race/income variable for all analyses in this study.
3.3. Correlates of Depression in Pediatric Epilepsy
In bivariate analyses, depression was associated with public insurance (relative to private or other insurance), low-income other race and low-income white race (relative to high-income white race), and unmet needs for specialist care (relative to those whose needs for specialist care were met). Several comorbidities were also related to depression, including ADD/ADHD, anxiety, behavior disorder, ASD, migraines or frequent headaches, asthma, and allergies (Supplemental Table 2).
In the multivariable weighted analysis, low-income children of all races were more likely to have depression relative to high-income white children. Children with unmet needs for specialist care had increased odds of depression relative to children whose needs for specialist care were met. Anxiety, behavior disorder, ADD/ADHD, and migraines or frequent headaches were associated with depression. Public insurance and more severe epilepsy were not associated with depression (Table 2).
Table 2:
Results of weighted multivariable logistic regressions on depression and anxiety among children with epilepsy.
| Depression | Anxiety | ||||
|---|---|---|---|---|---|
| Category | Factor | OR [CI] | p-value | OR [CI] | p-value |
| Age | Adolescent | 1.58 [0.73–3.40] | 0.25 | 1.07 [0.58–1.97] | 0.83 |
| Child | ref | --- | ref | --- | |
| Region | West | 1.77 [0.60–5.21] | 0.30 | 0.77 [0.34–1.75] | 0.53 |
| Midwest | 1.44 [0.54–3.84] | 0.47 | 0.70 [0.30–1.63] | 0.41 | |
| Northeast | 0.74 [0.24–2.32] | 0.61 | 1.14 [0.50–2.60] | 0.76 | |
| South | ref | --- | ref | --- | |
| Gender | Male | 0.59 [0.26–1.32] | 0.20 | 0.66 [0.36–1.20] | 0.18 |
| Female | ref | --- | ref | --- | |
| Ethnicity | Hispanic | 0.91 [0.29–2.92] | 0.88 | 0.60 [0.22–1.65] | 0.32 |
| Non-Hispanic | ref | --- | ref | --- | |
| Race / Income | Low-income black | 5.60 [1.19–26.3] | 0.03* | 0.04 [0.01–0.46] | 0.002** |
| High-income black | 1.80 [0.42–7.72] | 0.52 | 0.35 [0.11–1.18] | 0.09 | |
| Low-income other | 7.00 [1.21–40.4] | 0.03* | 1.47 [0.27–8.14] | 0.66 | |
| High-income other | 1.22 [0.27–5.44] | 0.59 | 0.54 [0.20–1.45] | 0.22 | |
| Low-income white | 8.04 [2.56–25.3] | <0.001*** | 0.75 [0.30–1.85] | 0.54 | |
| High-income white | ref | --- | ref | --- | |
| Highest Parent Education | Less than high school | 1.25 [0.34–4.65] | 0.74 | 0.71 [0.25–2.01] | 0.52 |
| High school | 1.47 [0.48–4.50] | 0.50 | 0.48 [0.20–1.14] | 0.10 | |
| More than high school | ref | --- | ref | --- | |
| Insurance | Public | 0.82 [0.26–2.59] | 0.74 | 1.24 [0.61–2.53] | 0.56 |
| Uninsured | 3.54 [0.61–20.7] | 0.16 | 0.42 [0.10–1.76] | 0.24 | |
| Private or other comprehensive | ref | --- | ref | --- | |
| Specialist Needs | No specialist needs | 1.38 [0.51–3.68] | 0.53 | 0.96 [0.35–2.61] | 0.94 |
| Specialist needed and needs unmet | 3.91 [1.40–10.9] | 0.009** | 0.88 [0.22–3.53] | 0.86 | |
| Specialist needed and needs met | ref | --- | ref | --- | |
| Epilepsy Severity | Moderate | 1.14 [0.43–2.99] | 0.80 | 0.99 [0.51–1.95] | 0.99 |
| Severe | 0.95 [0.41–2.24] | 0.91 | 0.82 [0.37–1.81] | 0.62 | |
| Mild | ref | --- | ref | --- | |
| Comorbidity | Behavior disorder | 4.57 [1.99–10.5] | <0.001*** | 6.05 [2.77–13.2] | <0.001*** |
| ASD | 0.86 [0.32–2.28] | 0.76 | 2.60 [1.27–5.35] | 0.009** | |
| ADD/ADHD | 3.23 [1.52–6.83] | 0.002** | 1.40 [0.72–2.75] | 0.32 | |
| Depression | --- | --- | 29.1 [11.8–72.0] | <0.001*** | |
| Anxiety | 30.5 [11.8–79.0] | <0.001*** | --- | --- | |
| Migraines/frequent headaches | 3.97 [1.65–9.54] | 0.002** | 1.80 [0.94–3.44] | 0.08 | |
| Concussion | 0.82 [0.28–2.40] | 0.71 | 0.90 [0.35–2.35] | 0.83 | |
| Asthma | 0.63 [0.21–1.90] | 0.42 | 2.48 [1.21–5.06] | 0.01* | |
| Allergies | 2.24 [0.92–5.43] | 0.07 | 2.74 [1.47–5.11] | 0.002** | |
OR = odds ratio, CI = 95% confidence interval, ref = reference level for that variable. Significance levels of .05, .01, and .001 are indicated by *, **, and *** respectively.
3.4. Correlates of Anxiety in Pediatric Epilepsy
In bivariate analyses, anxiety was associated with low-income white race (relative to high- income white race), unmet needs for specialist care (relative to those whose needs for specialist care were met), ADD/ADHD, depression, behavior disorder, ASD, migraines or frequent headaches, asthma, and allergies. High-income other race (relative to high-income white race) was negatively associated with anxiety (Supplemental Table 2).
In the multivariable weighted analysis, low-income black children were less likely to have anxiety relative to high-income white children. Children with behavior disorder, depression, asthma, and allergies were more likely to have anxiety. More severe epilepsy was not associated with anxiety (Table 2).
3.5. Missingness and Multicollinearity
No variable had more than 1.5 percent missing values. Little’s MCAR test was non-significant (p = 0.29), indicating that a complete-case analysis was unbiased.
All of the predictor variables were examined for multicollinearity using the Variance Inflation Factor (VIF). None had a VIF value greater than 1.5, and therefore all were retained in the regression models.
3.6. Rates of Depression and Anxiety among Other Children with Special Health Care Needs
There were 33,786 respondents with a child aged 5–17 who did not have epilepsy. Applying the sampling weights indicated that there are 9,086,851 [+/− 148,241] children aged 5−17 with a special health care need other than epilepsy in the United States. Among those with known depression/anxiety status, 22.1% have depression and/or anxiety (i.e. 3.5% with depression alone, 10.7% with anxiety alone, and 7.9% with both). In a regression of all children with special health care needs, having epilepsy did not increase the odds (OR = 0.89 [0.68–1.15], p = 0.37) of having depression and/or anxiety while controlling for age, region, gender, ethnicity, race/income, highest parent education, insurance, and needs for specialist care.
We also examined three specific chronic conditions among children aged 5–17: allergies, migraines, and asthma. Of all children aged 5–17 with special health care needs, 49.7% had allergies, 11.1% had migraines, and 31.4% had asthma. When comparing our estimates to 2010 United States census figures, we estimate that of all children aged 5–17 in the United States, 8.3% had allergies, 1.9% had migraines, and 5.9% had asthma [24]. The rates of depression and/or anxiety among children with these conditions are depicted in Figures 2b, 2c, and 2d. Relative to all children with epilepsy, estimated rates of depression and/or anxiety were not significantly different among children with asthma (16.5%, p = 0.36) or allergies (21.6%, p = 0.31) but were significantly higher among children with migraines (43.2%, p < 0.001) while controlling for age, region, gender, ethnicity, race/income, highest parent education, insurance, and needs for specialist care.
4. Discussion
4.1. Summary of Findings
Our estimates indicate 283,000 children between 5 and 17 years of age have epilepsy in the United States, representing 3.1 percent of all children with special health care needs and 0.53 percent of all children in the United States. One in four children with epilepsy has depression and/or anxiety. This figure is comparable to the overall rate seen among children with other special health care needs. In two multivariable regressions, children from households of poverty (regardless of race) and children whose needs for specialist care were unmet were more likely to have depression. Low-income black children were less likely to have anxiety. Several comorbidities were also associated with having depression or anxiety – those with ADD/ADHD, anxiety, migraines, or a behavior disorder were more likely to have depression, while those with asthma, allergies, ASD, depression, or a behavior disorder were more likely to have anxiety. Gender, age, and epilepsy severity were not significantly associated with depression or anxiety.
4.2. Prevalence of Depression and Anxiety in Children with Epilepsy
When comparing the 283,575 children with epilepsy to United States census data from 2010, we estimate that 0.53 percent of children aged 5–17 in the United States have epilepsy, which is consistent with previous estimations of 0.6 percent in the general pediatric population [1,24]. Among children with special health care needs, 3.1 percent have epilepsy, which is about five times greater than estimates of prevalence in the general pediatric population [1]. This is likely because the survey was administered only to parents/guardians of children with special health care needs and thus the data represents a sicker population of children compared to the general population.
Our results show that among children with epilepsy in the United States, 13.1 percent of have depression and 23.3 percent have anxiety. These prevalence figures are consistent with those reported in a study of the 2007 National Survey of Children’s Health [1]. In that study, among children with epilepsy, depression was present in 8 percent and anxiety in 17 percent. Many other studies have estimated the prevalence of depression and anxiety among children with epilepsy but estimates vary widely between studies—rates of depression range from 8 to 33 percent, while rates of anxiety range from 5 to 48.5 percent [1,3,12,25–28]. The broad ranges of estimates are likely due to varying methodologies and different study samples.
It is important to note that 11.5 percent of all children with epilepsy had both depression and anxiety. This finding suggests that when one of the two disorders is present, there is a high likelihood the child has the other. This is unsurprising given the two disorders can have overlapping symptoms [29]. We found that anxiety was more prevalent in children with depression than depression was in children with anxiety – 88 percent of children with depression had anxiety whereas 49 percent of children with anxiety had depression. This signifies that anxiety often occurs alone in children with epilepsy but that depression is usually comorbid with anxiety, which is consistent with previous research on pediatric epilepsy [4].
4.3. Depression and Anxiety in Children with Other Special Health Care Needs
Among all children with special health care needs, having epilepsy did not increase the odds of having depression and/or anxiety while controlling for demographic variables. Interpreted differently, the rate of depression and/or anxiety among all children with epilepsy was not significantly different from that of children with other special health care needs. This finding supports past research demonstrating that chronic health conditions in general are associated with increased risk of depression and/or anxiety and add that epilepsy is not unique in its high psychiatric comorbidity [30]. This idea is further supported by our finding that the rate of depression and/or anxiety among children with epilepsy was comparable to the rates seen among children with asthma or allergies without epilepsy. It is important to acknowledge that for each chronic illness there may be specific risk factors which make individuals more or less susceptible to depression or anxiety.
It is noteworthy that our estimated prevalence rates of allergies, asthma, and migraines among children with special needs are higher than that of the general population [31–32]. This overestimation is likely due to the fact that this study focused on children with special health care needs, which represents a sick portion of the population.
Our estimated prevalence rates of allergies and asthma in the general pediatric population are consistent with previous reports [31]. However, our estimate of migraine prevalence in the general pediatric population was lower than that seen in the literature, which may be attributed to differences in the ages included for each study [32].
4.4. Novel Associations in Pediatric Epilepsy
Income has received little attention in studies of depression among children with epilepsy [2]. Our study indicates that poverty is associated with depression regardless of race among children with epilepsy. This relationship between poverty and depression is also seen in the general population and is explained by many potential factors including low self-esteem and poor social support [33].
Children with unmet needs for specialist care were more likely to have depression. Though there is little research on the role of unmet specialist needs in depression, one possible interpretation is that the stress of being unable to access appropriate medical care can contribute to depression [33]. Or, it may be that inadequate or insufficient interactions with a specialist contribute to a feeling of loss of control over the disease, which could perpetuate feelings of depression [34]. We also found that low-income black children with epilepsy had lower odds of anxiety relative to high-income white children with epilepsy. There are several potential interpretations of this finding. Given research showing that black race and low socioeconomic status (SES) are associated with lower mental health service utilization, one possible explanation is that low- income blacks are less likely to visit the doctor for anxiety than other groups [35,36]. This interpretation mirrors the findings of general pediatric health studies, which demonstrate that black children have lower rates of asthma specialist visits relative to non-minorities [37]. It is also possible that, due to stigma, low-income black parents are less likely than high-income white parents to report that their child has an anxiety disorder. This idea is supported by research by Dirks and colleagues [38] showing that discrepancies in parent and child estimates of the child’s anxiety symptoms were greater in African Americans than in non-Hispanic whites. Underreporting may be exacerbated in low-income blacks given the increased personal stigma experienced by individuals from lower SES backgrounds [39].
Inherent racial biases within the healthcare system may also contribute to disparities in anxiety diagnosis. It is possible that, due to implicit bias, providers are less likely to diagnose or treat black children for anxiety, as has been observed in the adult primary care setting [40,41]. In addition, the widely known criteria for recognizing anxiety may not be universal given that many are based on population research, which has historically focused on the majority population [42]. Research in panic disorder indicates that the Brief Panic Disorder Screen is less reliable and less valid in African Americans than it is in Caucasians [43]. Thus, it is possible that anxiety diagnoses may be missed to a greater extent in black children than in white children. A deeper understanding of this finding is warranted in future research.
The strongest predictor of depression was anxiety while the strongest predictor of anxiety was depression. This finding is most likely due to the high comorbidity between depression and anxiety, which is seen not only among children with epilepsy [4] but also in the general population [44]. Other comorbidities associated with depression and anxiety included migraines or frequent headaches, ADD/ADHD, ASD, behavior disorder, allergies, and asthma. These associations were unsurprising given that these disorders increase the risk of depression and anxiety in individuals without epilepsy [45–50].
4.5. Confirmation of Previous Findings in Pediatric Epilepsy
The absence of a relationship between gender and depression or anxiety aligns with many previous studies of pediatric epilepsy [2,8]. This finding is interesting because in the general pediatric population, girls are more likely to have a mood or anxiety disorder than boys [51]. Future research might investigate why this trend is not seen in pediatric epilepsy.
In this study age was unrelated to depression or anxiety. Some studies suggest depression is more common in older children with epilepsy [52,53]. However, Dunn and colleagues [26] did not find any relationship between age and depressive symptoms. Reports on age and anxiety have also been inconsistent—while some studies find adolescents at higher risk, others suggest younger children are more at risk [2,8]. Our findings suggest that depression and anxiety are equally prevalent across age groups of pediatric epilepsy.
Interestingly, we did not find an association between epilepsy severity and the prevalence of internalizing affective disorders. Some studies have found increased rates of depression in children with more severe epilepsy or more frequent seizures whereas other studies have not confirmed such relationships [2,54]. Our findings support the notion that epilepsy severity does not impact the risk of psychopathology.
4.6. Clinical Implications
The finding that one in four children with epilepsy has depression and/or anxiety reinforces the importance of screening children with epilepsy for psychopathology [55]. Moreover, the lack of an age predilection for depression or anxiety highlights the need that all children with epilepsy be screened for depression and/or anxiety, not just adolescents as is recommended in the general pediatric population by the U.S. Preventative Services Task Force [56]. Physicians can use instruments such as the emotional component of the Strengths and Difficulties Questionnaire (SDQ) [57] or the Revised Child Anxiety and Depression Scale (RCADS) [58] to screen for depression and anxiety. Recommendations for the treatment of depression and anxiety in children with epilepsy are discussed elsewhere [2].
An understanding of the demographic risk factors for depression and anxiety also has clinical implications. Given that depression and anxiety symptoms are major determinants of health-related quality of life among children with epilepsy, early identification and treatment of psychiatric comorbidities can vastly improve the well-being of such children [5]. In addition, it has been shown that the presence of internalizing psychiatric disorders, including depression and anxiety, is associated with lack of seizure remission five years after the onset of childhood epilepsy [59]. Thus, knowing who is most at risk for psychiatric comorbidities allows physicians to identify and manage these psychiatric comorbidities as early as possible and thereby maximize the probability of seizure remission. Physicians may also be able to prevent suicide attempts among children with epilepsy through early identification and treatment of psychiatric disorders, as the risk of suicidal ideation increases with each additional psychiatric diagnosis [60]. This concept is especially important to consider given that 11.5 percent of children with epilepsy had both depression and anxiety.
4.7. Limitations
Several limitations merit discussion. First, the NS-CSHCN relies on parental-report. A diagnosis of epilepsy could not be verified from medical records as has been done in previous studies [4,12]. We were also unable to interview the children to determine whether they currently have a diagnosis of depression or anxiety, as others have done [25,28,53]. In fact, given the ambiguous wording of the anxiety survey items, it may be that some parents reported the presence of anxiety symptoms rather than a diagnosis of anxiety, which would inflate estimates of psychopathology. This is further complicated by evidence that parents may overestimate internalizing behaviors due to projection of their own emotional concerns into the response [61], meaning some parents may have reported depression and anxiety that was not truly present in their children. In contrast, another study found that parents underestimate the severity of anxiety symptoms in their children [38]. However, these studies focused on rating scales of psychiatric behaviors and symptoms rather than asking parents to recall whether the child had a specific diagnosis made by a medical professional. For the more straightforward question of presence / absence of a condition, parents have acceptable levels of recall for their child’s medical and psychiatric diagnoses [62–64].
We recognize that our findings rely on parental report, rather than clinical assessment, for the diagnosis of depression or anxiety. We note, however, that our study replicated several well- described associations in the literature, including the finding that gender is not related to psychopathology among children with epilepsy, which strengthens confidence in these associations [2,8]. We also found several risk factors previously understudied in the literature, including income and unmet needs for specialist care. Future studies should use clinical samples of children with epilepsy to confirm our novel findings.
Another limitation was that we did not have sufficient data to determine the role of other important epilepsy-related variables, such as the use of AEDs, which can contribute to depression and anxiety and potentially interact with demographic factors [8]. Other variables such as family relationship satisfaction [7] and feelings of stigma [25] are also associated with depression and anxiety but were not measured in the NS-CSHCN. Future work may be valuable to further specify the relative importance of demographic, psychosocial, and epilepsy-related variables in determining the risk for depression and anxiety.
4.8. Conclusion
One in four U.S. children with epilepsy has depression and/or anxiety. This high rate of psychiatric comorbidity is similar to that seen in children with other special health care needs. The experience of depression and anxiety can lead to reduced quality of life [2,4,5], suicidality [2,4], and potentially lower rates of seizure remission among children with epilepsy [58]. Therefore, physicians should consider the various factors that are related to depression and anxiety in children with epilepsy, including income, race, needs for specialist care, and various comorbidities, so that at-risk children can be screened and managed appropriately.
Supplementary Material
Highlights.
Approximately 0.53 percent of US children aged 5–17 have epilepsy
One in four children with epilepsy across the US has depression and/or anxiety
This high comorbidity rate is comparable in children with other chronic conditions
Income, race, and needs for specialist care are associated with psychopathology
Gender, age, and epilepsy severity are unrelated to the risk of psychopathology
Acknowledgements
This work was supported by a grant (UL1-TR-002384) from the Clinical and Translational Science Center at Weill Cornell Medicine, which is funded by the National Center for Advancing Translational Sciences (NCATS). This source had no involvement in the creation, analysis, or interpretation of the research. The authors thank Meghan Joline for statistical assistance.
Declarations of Interest
ZG receives research funding for the Centers for Disease Control and Prevention, the Pediatric Epilepsy Research Foundation, the Epilepsy Research Fund, the Epilepsy Foundation, and from Weill Cornell Medicine. ZG also performs medical legal consulting work. The other authors have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics 2012;129:256–64. 10.1542/peds.2010-1371. [DOI] [PubMed] [Google Scholar]
- [2].Ekinci O, Titus JB, Rodopman AA, Berkem M, Trevathan E. Depression and anxiety in children and adolescents with epilepsy: Prevalence, risk factors, and treatment. Epilepsy Behav 2009;14:8–18. 10.1016/j.yebeh.2008.08.015. [DOI] [PubMed] [Google Scholar]
- [3].Ettinger AB, Weisbrot DM, Nolan EE, Gadow KD, Vitale SA, Andriola MR et al. Symptoms of depression and anxiety in pediatric epilepsy patients. Epilepsia 1998;39:595–9. 10.1111/j.1528-1157.1998.tb01427.x. [DOI] [PubMed] [Google Scholar]
- [4].Caplan R, Siddarth P, Gurbani S, Hanson R, Sankar R, Shields WD. Depression and anxiety disorders in pediatric epilepsy. Epilepsia 2005;46:720–30. 10.1111/j.1528-1167.2005.43604.x. [DOI] [PubMed] [Google Scholar]
- [5].Stevanovic D, Jancic J, Lakic A. The impact of depression and anxiety disorder symptoms on the health-related quality of life of children and adolescents with epilepsy. Epilepsia 2011;52:e75–8. 10.1111/j.1528-1167.2011.03133.x. [DOI] [PubMed] [Google Scholar]
- [6].Johnson EK, Jones JE, Seidenberg M, Hermann BP. The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia 2004;45:544–50. 10.1111/j.0013-9580.2004.47003.x. [DOI] [PubMed] [Google Scholar]
- [7].Rodenburg R, Marie Meijer A, Dekovic M, Aldenkamp AP. Family predictors of psychopathology in children with epilepsy. Epilepsia 2006;47:601–14. 10.1111/j.1528-1167.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- [8].Reilly C, Agnew R, Neville BG. Depression and anxiety in childhood epilepsy: A review. Seizure 2011;20:589–97. 10.1016/j.seizure.2011.06.004. [DOI] [PubMed] [Google Scholar]
- [9].[dataset] Child and Adolescent Health Measurement Initiative (CAHMI). National Survey of Children with Special Health Care Needs Indicator Data Set, Data Resource Center for Child and Adolescent Health 2009–2010, www.childhealthdata.org [accessed 13 December 2017].
- [10].Bethell C, Read D, Neff J, Blumberg SJ, Stein RE, Sharp V et al. Comparison of the Children With Special Health Care Needs Screener to the Questionnaire for Identifying Children With Chronic Conditions—Revised. Ambul Pediatr 2002;2:49–57. . [DOI] [PubMed] [Google Scholar]
- [11].Bramlett MD, Blumberg SJ, Ormson AE, George JM, Williams KL, Frasier AM et al. Design and operation of the National Survey of Children with Special Health Care Needs, 2009–2010. National Center for Health Statistics. Vital Health Stat 2014;1:1–271. [PubMed] [Google Scholar]
- [12].Berg AT, Caplan R, Hesdorffer DC. Psychiatric and neurodevelopmental disorders in childhood-onset epilepsy. Epilepsy Behav 2011;20:550–5. 10.1016/j.yebeh.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Davies S, Heyman I, Goodman R. A population survey of mental health problems in children with epilepsy. Dev Med Child Neurol 2003;45:292–5. 10.1017/s0012162203000550. [DOI] [PubMed] [Google Scholar]
- [14].Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 1988;83:1198–202. 10.2307/2290157. [DOI] [Google Scholar]
- [15].U.S. Census Bureau. Census regions and divisions of the United States, https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf [accessed 25 October 25 2018].
- [16].Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: Findings from the National Health and Nutrition Examination Survey III. Am J Public Health 2005;95:998–1000. 10.2105/AJPH.2004.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cockerham WC. A test of the relationship between race, socioeconomic status, and psychological distress. Soc Sci Med 1990;31:1321–6. 10.1016/0277-9536(90)90071-Y. [DOI] [PubMed] [Google Scholar]
- [18].Chan CJ, Zou G, Wiebe S, Speechley KN. Global assessment of the severity of epilepsy (GASE) scale in children: Validity, reliability, responsiveness. Epilepsia 2015;56:1950–6. 10.1111/epi.13216. [DOI] [PubMed] [Google Scholar]
- [19].Lumley T Complex surveys: A guide to analysis using R. Hoboken: John Wiley & Sons, Inc; 2010. [Google Scholar]
- [20].Wang H, Peng J, Wang B, Lu X, Zheng JZ, Wang K et al. Inconsistency between univariate and multiple logistic regressions. Shanghai Arch Psychiatry 2017;29:124–8. 10.11919/j.issn.1002-0829.217031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, http://www.R-project.org/ [accessed 25 October 2018]. [Google Scholar]
- [22].Lumley T Analysis of complex survey samples. J Stat Softw 2004;9:1–19. 10.18637/jss.v009.i08. [DOI] [Google Scholar]
- [23].Naimi B, Hamm NAS, Groen TA, Skidmore AK, Toxopeus AG. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014;37:191–203. 10.1111/j.1600-0587.2013.00205.x. [DOI] [Google Scholar]
- [24].Howden L, Meyer J. Age and Sex Composition: 2010; https://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf; 2011. [accessed 31 Oct 2019].
- [25].Adewuya AO, Ola BA. Prevalence of and risk factors for anxiety and depressive disorders in Nigerian adolescents with epilepsy. Epilepsy Behav 2005;6:342–7. 10.1016/j.yebeh.2004.12.011. [DOI] [PubMed] [Google Scholar]
- [26].Dunn DW, Austin JK, Huster GA. Symptoms of depression in adolescents with epilepsy. J Am Acad Child Adolesc Psychiatry 1999;38:1132–8. 10.1097/00004583-199909000-00017. [DOI] [PubMed] [Google Scholar]
- [27].Plioplys S Depression in children and adolescents with epilepsy. Epilepsy Behav 2003;4:39–45. 10.1016/j.yebeh.2003.08.016. [DOI] [PubMed] [Google Scholar]
- [28].Alwash RH, Hussein MJ, Matloub FF. Symptoms of anxiety and depression among adolescents with seizures in Irbid, Northern Jordan. Seizure 2000;9:412–6. 10.1053/seiz.2000.0427. [DOI] [PubMed] [Google Scholar]
- [29].Kwon O-Y, Park S-P. Depression and anxiety in people with epilepsy. J Clin Neurol 2014;10:175–88. 10.3988/jcn.2014.10.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Clarke DM, Currie KC. Depression, anxiety and their relationship with chronic diseases: a review of the epidemiology, risk and treatment evidence. Med J Aust 2009;190:S54–60. [DOI] [PubMed] [Google Scholar]
- [31].Bloom B, Jones L, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 2013:10:1–81. [PubMed] [Google Scholar]
- [32].Abu-Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: a systematic review of population‐based studies. Dev Med Child Neurol 2010:52:1088–97. 10.1111/j.1469-8749.2010.03793.x. [DOI] [PubMed] [Google Scholar]
- [33].Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res 2002;53:891–5. 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- [34].Christensen AJ, Turner CW, Smith TW, Holman JM Jr, Gregory MC. Health locus of control and depression in end-stage renal disease. J Consult Clin Psychol 1991;59:419–24. 10.1037//0022-006x.59.3.419. [DOI] [PubMed] [Google Scholar]
- [35].Padgett DK, Patrick C, Burns BJ, Schlesinger HJ. Ethnicity and the use of outpatient mental health services in a national insured population. Am J Public Health 1994;84:222–6. 10.2105/ajph.84.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Steele LS, Glazier RH, Lin E. Inequity in mental health care under Canadian universal health coverage. Psychiatr Serv 2006;57:317–24. 10.1176/appi.ps.57.3.317. [DOI] [PubMed] [Google Scholar]
- [37].Stewart KA, Higgins PC, McLaughlin CG, Williams TV, Granger E, Croghan TW. Differences in Prevalence, Treatment, and Outcomes of Asthma Among a Diverse Population of Children With Equal Access to Care: Findings From a Study in the Military Health System. Arch Pediatr Adolesc Med 2010;164:720–6. 10.1001/archpediatrics.2010.100. [DOI] [PubMed] [Google Scholar]
- [38].Dirks MA, Weersing VR, Warnick E, Gonzalez A, Alton M, Dauser C et al. Parent and youth report of youth anxiety: evidence for measurement invariance. J Child Psychol Psychiatry 2013;55:284–91. 10.1111/jcpp.12159. [DOI] [PubMed] [Google Scholar]
- [39].Eisenberg D, Downs MF, Golberstein E, Zivin K. Stigma and help seeking for mental health among college students. Med Care Res Rev 2009;66:522–41. 10.1177/1077558709335173. [DOI] [PubMed] [Google Scholar]
- [40].Fitzgerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18 10.1186/s12910-017-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stockdale SE, Lagomasino IT, Siddique J, McGuire T, Miranda J. Racial and ethnic disparities in detection and treatment of depression and anxiety among psychiatric and primary health care visits, 1995–2005. Med Care 2008;46:668–77. 10.1097/mlr.0b013e3181789496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Smedley BD, Stith AY, Nelson AR. Unequal treatment: confronting racial and ethnic disparities in healthcare. Washington, D.C.: The National Academies Press; 2003. [PubMed] [Google Scholar]
- [43].Johnson MR, Hartzema AG, Mills TL, De Leon JM, Yang M, Frueh C et al. Ethnic differences in the reliability and validity of a panic disorder screen. Ethnicity Health 2007;12:283–96. 10.1080/13557850701235069. [DOI] [PubMed] [Google Scholar]
- [44].Hirschfield RMA. The comorbidity of major depression and anxiety disorders. Prim Care Companion J Clin Psychiatry 2001;3:244–54. 10.4088/pcc.v03n0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kim JA, Szatmari P, Bryson SE, Streiner DL, Wilson FJ. The prevalence of anxiety and mood problems among children with autism and asperger syndrome. Autism 2000;4:117–32. https://10.1177/1362361300004002002. [Google Scholar]
- [46].Zwart JA, Dyb G, Hagen K, Ødegård KJ, Dahl AA, Bovim AA et al. Depression and anxiety disorders associated with headache frequency. The Nord-Trøndelag Health Study. Eur J Neurol 2003;10:147–52. https://10.1046/j.1468-1331.2003.00551.x. [DOI] [PubMed] [Google Scholar]
- [47].Schatz DB, Rostain AL. ADHD with comorbid anxiety. J Atten Disord 2006;10:141–9. 10.1177/1087054706286698. [DOI] [PubMed] [Google Scholar]
- [48].Newcomer PL, Barenbaum E, Pearson N. Depression and anxiety in children and adolescents with learning disabilities, conduct disorders, and no disabilities. J Emot Behav Disord 1995;3:27–39. 10.1177/106342669500300104. [DOI] [Google Scholar]
- [49].Zielinski TA, Brown ES, Nejtek VA, Khan DA, Moore JJ, Rush AJ. Depression in asthma: Prevalence and clinical implications. Prim Care Companion J Clin Psychiatry 2000;02:153–8. 10.4088/pcc.v02n0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Patten SB, Williams JV. Self-reported allergies and their relationship to several axis I disorders in a community sample. Int J Psychiatry Med 2007;37:11–22. 10.2190/l811-0738-10ng-7157. [DOI] [PubMed] [Google Scholar]
- [51].Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L et al. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 2010;49:980–9. 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Oguz A, Kurul S, Dirik E, Eylül D. Relationship of epilepsy-related factors to anxiety and depression scores in epileptic children. J Child Neurol 2002;17:37–40. 10.1177/088307380201700109. [DOI] [PubMed] [Google Scholar]
- [53].Thome-Souza S, Kuczynski E, Assumpção F Jr, Rzezak P, Fuentes D, Fiore L et al. Which factors may play a pivotal role on determining the type of psychiatric disorder in children and adolescents with epilepsy? Epilepsy Behav 2004;5:988–94. 10.1016/j.yebeh.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [54].Plioplys S, Dunn DW, Caplan R. 10-year research update review: Psychiatric problems in children with epilepsy. J Am Acad Child Adolesc Psychiatry 2007;46:1389–402. 10.1097/chi.0b013e31815597fc. [DOI] [PubMed] [Google Scholar]
- [55].Wilson SJ, Baxendale S, Barr W, Hamed S, Langfitt J, Samson S et al. Indications and expectations for neuropsychological assessment in routine epilepsy care: Report of the ILAE Neuropsychology Task Force, Diagnostic Methods Commission, 2013–2017. Epilepsia 2015;56:674–81. 10.1111/epi.12962. [DOI] [PubMed] [Google Scholar]
- [56].Siu AL, US Presentative Services Task Force. Screening for depression in children and adolescents: U.S. Preventive Services Task Force recommendation statement. Pediatrics 2016;137:e20154467. 10.1542/peds.2015-4467. [DOI] [PubMed] [Google Scholar]
- [57].Silva TB, Osório FL, Loureiro SR. SDQ: Discriminative validity and diagnostic potential. Front Psychol 2015;6:811 10.3389/fpsyg.2015.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chorpita BF, Moffitt CE, Gray J Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behav Res Ther 2005;43:309–22. 10.1016/j.brat.2004.02.004. [DOI] [PubMed] [Google Scholar]
- [59].Berg AT, Caplan R, Hesdorffer DC. Psychiatric and neurodevelopmental disorders in childhood-onset epilepsy. Epilepsy Behav 2011;20:550–5. 10.1016/j.yebeh.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jones JE, Siddarth P, Gurbani S, Shields WD, Caplan R. Screening for suicidal ideation in children with epilepsy. Epilepsy Behav 2013;29:521–6. 10.1016/j.yebeh.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Eom S, Caplan R, Berg AT. Behavioral problems and childhood epilepsy: Parent vs child perspectives. J Pediatr 2016;179:233–9. 10.1016/j.jpeds.2016.08.096. [DOI] [PubMed] [Google Scholar]
- [62].Pless C, Pless I. How well they remember: the accuracy of parent reports. Arch Pediatr Adolesc Med 1995;149:553–8. 10.1001/archpedi.1995.02170180083016. [DOI] [PubMed] [Google Scholar]
- [63].Ghandour R, Sherman L, Vladutiu C, Ali M, Lynch S, Bitsko R, et al. Prevalence and Treatment of Depression, Anxiety, and Conduct Problems in US Children. J Pediatr 2019;206:256–67. 10.1016/j.jpeds.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kentgen L, Klein R, Mannuzza S, Davies M. Test-retest reliability of maternal reports of lifetime mental disorders in their children. J Abnorm Child Psychol 1997;25:389–98. 10.1023/a:1025785008050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.