Abstract
Purpose:
The purpose of this study is to evaluate demographic and clinical factors associated with self-reported dysphagia after oral endotracheal intubation and mechanical ventilation in patients with acute lung injury (ALI).
Materials and methods:
This is a prospective cohort study of 132 ALI patients who had received mechanical ventilation via oral endotracheal tube.
Results:
The primary outcome was binary, whether clinically important symptoms of dysphagia at hospital discharge were reported by patients, using the Sydney Swallowing Questionnaire score 200 or more. Of 132 patients, 29% reported clinically important symptoms of dysphagia. Of 18 relevant demographic and clinical variables, only 2 were found to be independently associated with clinically important symptoms of dysphagia in a multivariable logistic regression model: upper gastrointestinal comorbidity (odds ratio, 2.82; 95% confidence interval, 1.09–7.26) and duration of oral endotracheal intubation (odds ratio, 1.79; [95% confidence interval, 1.15–2.79] per day for first 6 days, after which additional days of intubation were not associated with a further increase in the odds of dysphagia).
Conclusions:
In ALI survivors, patient-reported, postexubation dysphagia at hospital discharge was significantly associated with upper gastrointestinal comorbidity and a longer duration of oral endotracheal intubation during the first 6 days of intubation.
Keywords: Deglutition, Dysphagia, Intubation, Mechanical ventilation, Acute lung injury
1. Introduction
There are an estimated 5.7 million intensive care unit (ICU) admissions in the United States annually [1], with at least one-third requiring intubation with mechanical ventilation [2,3]. The number of adults requiring mechanical ventilation is growing, most rapidly for individuals more than 65 years old, with an expected 80% increase from 2000 to 2026 [4,5].
With the introduction of an oral endotracheal tube, laryngeal injury [6,7] and altered laryngeal sensation [8–11] frequently occur and may result in impaired swallowing [12]. Postextubation swallowing disorders (ie, dysphagia) have been reported in 14% to 83% of adult patients undergoing prolonged mechanical ventilation [13–17].
Dysphagia can have significant sequelae, including aspiration leading to lung injury and death [18–22]. Clinical studies of dysphagia after extubation have largely evaluated the presence of aspiration alone and are frequently limited by small sample sizes and heterogeneous patient groups [23,24]. Acute lung injury (ALI) is an archetype of critical illness [25], with patients having a high severity of illness, prolonged mechanical ventilation, and ICU-acquired muscle weakness, all of which may put patients at high risk for postextubation dysphagia. The aim of this study was to evaluate the association between the duration of oral endotracheal intubation and patient-reported dysphagia at hospital discharge in mechanically ventilated ICU patients with ALI.
2. Materials and methods
2.1. Study population
This evaluation was conducted as part of a prospective, multisite cohort study [26] evaluating consecutive mechanically ventilated patients with ALI, as defined by the American-European Consensus Conference criteria [27]. Eligible patients were recruited from 13 ICUs at 4 teaching hospitals in Baltimore, MD. Key patient exclusion criteria for this prospective cohort study were (1) more than 5 days of mechanical ventilation before ALI, (2) preexisting cognitive impairment or communication/language barrier, (3) transfer into a study site ICU with preexisting ALI of more than 24-hour duration, (4) limitations in advancing ICU care at the time of study eligibility (eg, no use of vasopressors), and (5) preexisting illness with a life expectancy of less than 6 months. To avoid including patients with primary neurologic disease or head trauma, neurologic specialty ICUs at participating hospitals were excluded from the study. In addition, because of this evaluation’s focus on dysphagia symptoms after oral endotracheal intubation, for purposes of this analysis, we excluded study patients who (1) had a tracheostomy or nasal endotracheal tube during their ICU stay, (2) had a history of prior tracheostomy, (3) were not consented, not eating by mouth or not capable of completing the Sydney Swallowing Questionnaire (SSQ) (eg, due to physical or cognitive impairment) at hospital discharge, or (4) discharged directly to another acute care hospital (ie, discharge from study site hospital did not represent the ultimate timing of acute care hospital discharge). All institutional review boards at participating sites approved this study, and written informed consent was obtained from each study participant or their substitute decision maker.
2.2. Primary outcome
The primary outcome measure for this evaluation was self-reported, clinically important dysphagia symptoms at hospital discharge. Dysphagia symptoms were assessed using the SSQ. The SSQ is a patient-reported, 17-item, validated symptom inventory used to assess severity of dysphagia symptoms [28]. The SSQ primarily uses a visual analog scale, with items scored 0 to 100 and the total SSQ score ranging from 0 to 1700. The SSQ was scored in the same manner as the original validation study [28], with higher scores representing increased patient-perceived difficulty with swallowing. Scores 200 or more are considered indicative of clinically important dysphagia [28], which was the primary binary outcome used in this evaluation.
2.3. Primary exposure
The primary exposure measure was duration of incident oral endotracheal intubation, measured in days. Patients extubated for less than 48 hours before being reintubated were considered to be continuously intubated from the initial placement of the oral endotracheal tube until extubation for 48 continuous hours or more [29].
2.4. Covariates
Patient and ICU variables evaluated for their potential association with dysphagia in this study were selected based on the existing literature and investigators’ prior knowledge in this field. The following patient characteristics were considered: age, sex, race, and body mass index (BMI). Body mass index was categorized according to the standard criteria [30] to assist with clinical interpretation. Overall comorbidity burden (as measured by the Charlson Comorbidity Index [31]) was evaluated. We also evaluated preexisting neurologic comorbid disease (defined as stroke and any other neurologic disease [eg, transient ischemic attack, Parkinson disease, multiple sclerosis, and dementia]) and comorbid upper gastrointestinal disease (defined to include peptic ulcer, hiatal hernia, and gastroesophageal reflux disease). The following variables related to patients’ critical illness were also included in this evaluation: ICU admitting diagnosis category, severity of illness at ICU admission (Acute Physiology and Chronic Health Evaluation [APACHE] II score [32]), organ dysfunction at ALI onset (Sequential Organ Failure Assessment [SOFA] [33]), reintubation, and ICU length of stay.
2.5. Statistical analysis
Descriptive statistics were reported using median and interquartile range (IQR) for continuous data and proportions for categorical data. A Wilcoxon rank sum test was used to test for a significant difference in the time between extubation and completion of SSQ at hospital discharge for patients with vs without dysphagia. To confirm the appropriateness of modeling the odds of dysphagia as a linear function of each continuous variable, we examined a locally weighted smoother scatterplot [34–36] of the predicted odds vs the variable. Of all continuous variables, only the primary exposure variable demonstrated a potentially nonlinear relationship with the primary outcome, with a linear increase observed during the first 6 days of oral endotracheal intubation, followed by a plateau with minimal change thereafter (Fig. 1).
Fig. 1.
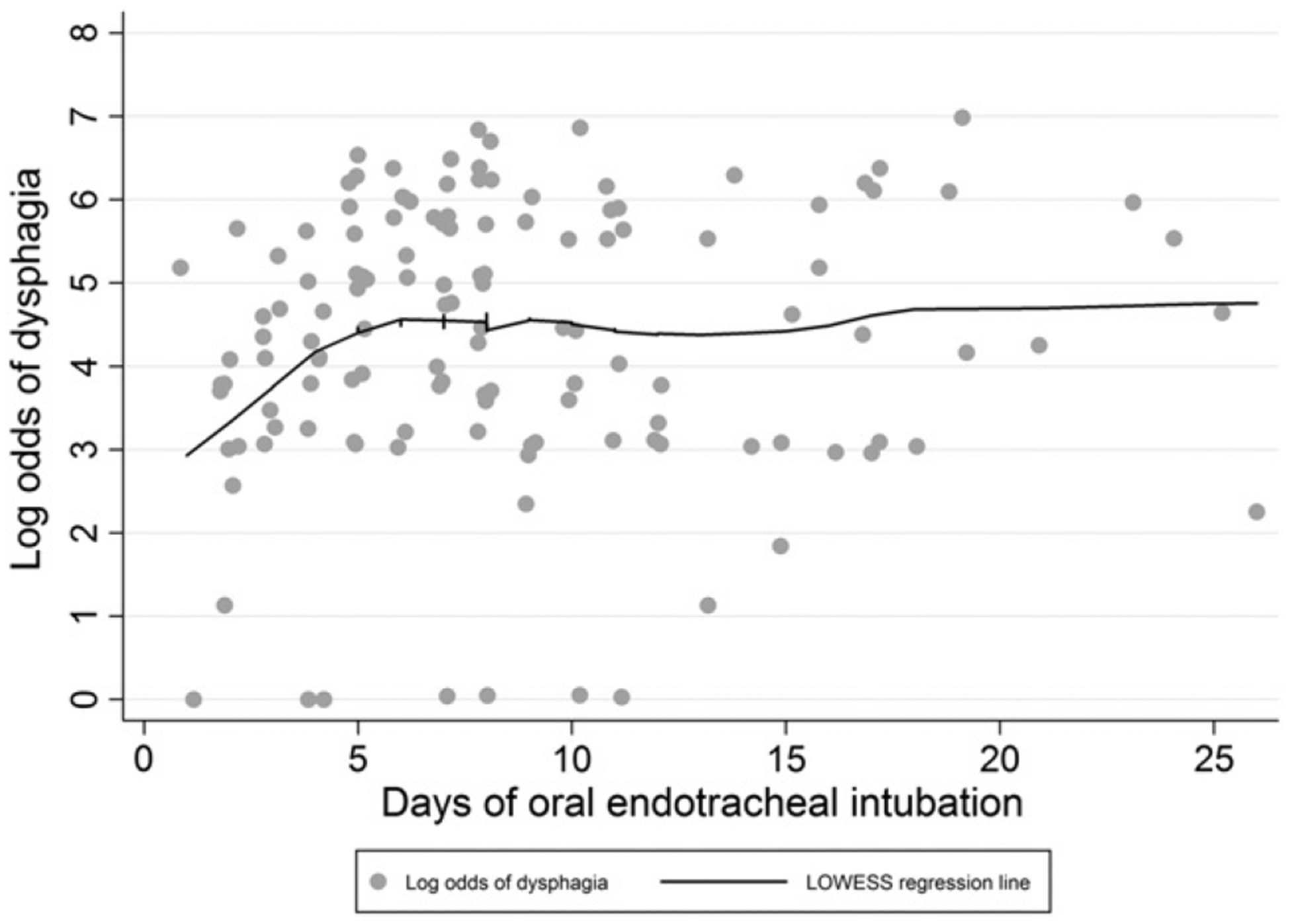
Log odds of dysphagia (ie, SSQ score, ≥200) vs duration of mechanical ventilation with an oral endotracheal tube.
The associations of individual variables with the primary outcome (ie, binary indicator of dysphagia) were evaluated using logistic regression, with associations presented as odds ratios (OR). To prevent overfitting the multivariable logistic regression model, we limited the number of variables in this model to a ratio of 1 variable per 10 outcomes [37,38]. Individual covariates were included in the multivariable logistic model if they exhibited a bivariable association with the primary outcome with a P < .10. To address the nonlinear association of mechanical ventilation duration with the primary outcome in regression analyses, the duration of intubation was modeled using a linear spline with a “knot” at 6 days; thus, permitting different linear associations between the duration of intubation and the primary outcome before and after the designated “knot” [36,39].
As a secondary analysis, we evaluated the association of individual variables with the continuous SSQ score using linear regression, with associations presented as relative medians (RM). Because the distribution of SSQ scores was right skewed, we used the log-transformed SSQ score as the outcome variable for this model. As in the logistic regression model, duration of intubation was modeled using a linear spline with a “knot” at 6 days, and individual covariates were included in the final multivariable model if they exhibited bivariable associations with the outcome with a P < .10.
There were no missing data for all covariates considered in the final multivariable logistic and linear regression models. Variance inflation factors [40,41] were used to confirm the lack of multicollinearity in both multivariable models. A post hoc sensitivity analysis excluding patients with upper gastrointestinal comorbidities was conducted with no material change in the results. Model fit was confirmed using a Hosmer-Lemeshow test [42] for the logistic regression model. Cook’s distance and dfbeta statistics were used to determine influential points for the linear regression model. A 2-sided P < .05 was considered statistically significant. All statistical analyses were completed using Stata statistical software, version 12.1 (Stata Corporation, College Station, TX).
3. Results
The prospective cohort study enrolled a total of 520 ALI patients, with 51% (n = 144) of the 283 hospital survivors being eligible for this evaluation and 132 (92%) of these eligible patients having complete SSQ data for analysis (Fig. 2). For these 132 patients, median (IQR) age was 48 (40, 56) years, with 52% male and 58% white race (Table 1). A minority of patients had neurologic (14%) or upper gastrointestinal (18%) comorbidities. The median (IQR) APACHE II and SOFA scores were 23 (19, 28) and 8 (5, 10), respectively; and the median (IQR) durations of oral endotracheal intubation, ICU stay, and hospital stay were 8 (5, 11), 12 (8, 16), and 21 (14, 30) days, respectively.
Fig. 2.
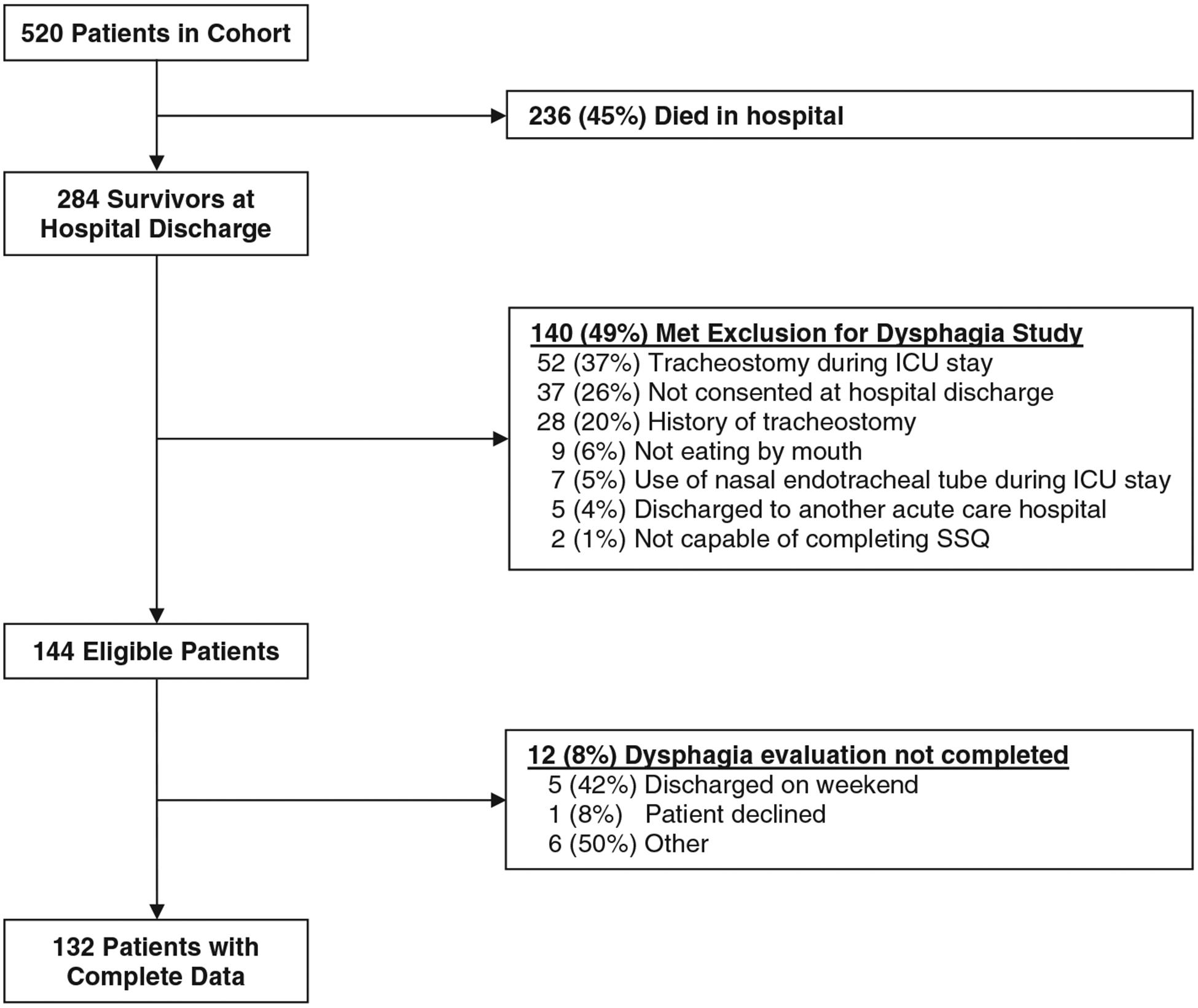
Study flow diagram.
Table 1.
Acute lung injury patient characteristics, by dysphagia status
| Total (N = 132) | No dysphagia (n = 87) | Dysphagiaa (n = 45) | |
|---|---|---|---|
| Demographics | |||
| Age, median (IQR) years | 48 (40, 56) | 48 (38, 57) | 45 (40, 53) |
| Male, no. (%) | 69 (52) | 46 (53) | 23 (51) |
| White, no. (%) | 77 (58) | 53 (61) | 24 (53) |
| Baseline health status before admission | |||
| Charlson Comorbidity Index, median (IQR) score | 1 (0, 3) | 1 (0, 3) | 2 (0, 4) |
| Neurologic diseaseb, no. (%) | 19 (14) | 10 (11) | 9 (20) |
| Upper gastrointestinal diseasec, no. (%) | 24 (18) | 11 (13) | 13 (29) |
| BMId | |||
| Underweight (<18.5 kg/m2) | 5 (4) | 3 (4) | 2 (5) |
| Normal (18.5–24.9 kg/m2) | 31 (26) | 22 (28) | 9 (23) |
| Overweight (25–29.9 kg/m2) | 42 (36) | 30 (38) | 12 (31) |
| Obese (≥30 kg/m2) | 40 (34) | 24 (30) | 16 (41) |
| ICU admission diagnosise, no. (%) | |||
| Respiratory (including pneumonia) | 77 (58) | 51 (59) | 26 (58) |
| Nonpulmonary sepsis and infectious disease | 20 (15) | 12 (14) | 8 (18) |
| Upper gastrointestinal | 11 (8) | 7 (8) | 4 (9) |
| Trauma | 6 (5) | 4 (5) | 2 (4) |
| Cardiovascular | 5 (4) | 4 (5) | 1 (2) |
| Other | 13 (10) | 9 (10) | 4 (9) |
| ICU factors | |||
| APACHE II score at ICU admission, median (IQR) | 23 (19, 28) | 24 (20, 28) | 22 (17, 26) |
| SOFA score at ALI onset, median (IQR) | 8 (5, 10) | 7 (5, 10) | 8 (6, 10) |
| Ever reintubated, no. (%) | 23 (17) | 16 (18) | 7 (16) |
| Duration of orotracheal intubation, median (IQR) days | 8 (5, 11) | 7 (4, 11) | 8 (5, 11) |
| ICU length of stay, median (IQR) days | 12 (8, 16) | 11 (7, 15) | 13 (8, 18) |
Sydney Swallowing Questionnaire score ≥200 or more is considered indicative of clinically important dysphagia [28].
Includes stroke and any other neurologic disease (eg, transient ischemic attack, Parkinson disease, multiple sclerosis, and dementia).
Includes peptic ulcer, hiatal hernia, and gastroesophageal reflux disease.
Body mass index was not available for 14 patients.
Percentages may not add to 100% due to rounding.
The median (IQR) SSQ score was 82 (25, 285) with 45 (34%) patients having clinically important symptoms of dysphagia (ie, SSQ score, ≥200). The median (IQR) time between extubation and hospital discharge was 11 (7, 19) days for all patients, with no significant difference comparing those with vs without dysphagia (P = .849).
For the logistic regression analysis, duration of oral endotracheal intubation and upper gastrointestinal comorbidity exhibited unadjusted associations with dysphagia of P < .10 and were included in the multivariable model (Table 2). Based on this multivariable model, duration of oral endotracheal intubation was significantly associated with dysphagia with an OR (95% CI) of 1.79 (1.15–2.79; P = .010) for each day of intubation up to 6 days. Odds of dysphagia did not change with increasing duration of intubation beyond day 6 (OR = 0.98, 95% CI = 0.90–1.07; P = .724). Upper gastrointestinal comorbidity was statistically significant with an OR (95% CI) of 2.82 (1.09–7.26; P = 0.032).
Table 2.
Factors associated with dysphagia in ALI patients with oral endotracheal intubation
| Bivariable association | Multivariable model | |||
|---|---|---|---|---|
| OR (95% CI) | Pa | OR (95% CI) | Pa | |
| Primary exposure | ||||
| Orotracheal intubation ≤6 days, per day | 1.81 (1.17, 2.80) | .008 | 1.79 (1.15, 2.79) | .010 |
| Orotracheal intubation >6 days, per day | 0.98 (0.90, 1.06) | .598 | 0.98 (0.90, 1.07) | .724 |
| Demographics | ||||
| Age | 1.00 (0.97, 1.02) | .827 | ||
| Male | 0.93 (0.45, 1.91) | .848 | ||
| White | 0.73 (0.35, 1.52) | .403 | ||
| Baseline health status before admission | ||||
| Charlson Comorbidity Index | 1.08 (0.93, 1.24) | .304 | ||
| Neurologic diseaseb | 1.93 (0.72, 5.15) | .192 | ||
| Upper gastrointestinal diseasec | 2.81 (1.14, 6.92) | .025 | 2.82 (1.09, 7.26) | .032 |
| BMI | ||||
| Normal (18.5–24.9 kg/m2) | (Reference) | |||
| Underweight (<18.5 kg/m2) | 1.63 (0.23, 11.45) | .624 | ||
| Overweight (25–29.9kg/m2) | 0.98 (0.35, 2.72) | .966 | ||
| Obese (≥30 kg/m2) | 1.62 (0.60, 4.43) | .339 | ||
| ICU admission diagnosis | ||||
| Respiratory (including pneumonia) | (Reference) | |||
| Nonpulmonary sepsis and infectious disease | 1.31 (0.48, 3.60) | .603 | ||
| Upper gastrointestinal | 1.12 (0.30, 4.18) | .865 | ||
| Trauma | 0.98 (0.17, 5.71) | .983 | ||
| Cardiovascular | 0.49 (0.05, 4.61) | .533 | ||
| Other | 0.87 (0.25, 3.10) | .832 | ||
| ICU factors | ||||
| APACHE II score at ICU admission | 0.98 (0.93, 1.03) | .439 | ||
| SOFA score at ALI onset | 1.10 (0.98, 1.22) | .105 | ||
| Ever reintubated | 0.82 (0.31, 2.16) | .684 | ||
| ICU length of stay | 1.04 (0.99, 1.10) | .110 | ||
P calculated using simple and multiple logistic regression analysis for bivariable and multivariable results, respectively. Covariates were included in the multivariable logistic model based on a bivariable association at P < .10.
Includes stroke and any other neurologic disease.
Includes ulcer, hernia, and reflux.
For the linear regression analysis, 4 variables exhibited unadjusted associations of P < .10 and were included in the multivariable model: duration of intubation, upper gastrointestinal comorbidity, Charlson Comorbidity Index, and SOFA score at hospital admission. These variables were included in a multivariable model. In this multivariable model, duration of oral endotracheal intubation was significantly associated with the log-transformed SSQ score with an RM (95% CI) of 1.31 (.04–1.63; P = .020) for each day of intubation up to 6 days and no significant association (RM = 0.98, 95% CI = 0.92–1.05; P = .553). Upper gastrointestinal comorbidity was also statistically significant (RM = 2.16, 95% CI = 1.05–4.46; P = .037), with neither Charlson Comorbidity Index nor SOFA significant in the multivariable model.
4. Discussion
In this multisite prospective cohort study of ALI patients with oral endotracheal intubation, we found that 34% of patients reported clinically important symptoms of dysphagia and that preexisting upper gastrointestinal comorbidity and the duration of oral endotracheal intubation during the first 6 days of intubation were independently associated with dysphagia. After endotracheal intubation for 24 hours or more, there is a range of dysphagia prevalence estimates, with most studies reporting more than 20% based on clinical and/or instrumental evaluations across multiple patient populations (eg, medical, surgical, cardiac, and trauma) [13–17]. A recent study found that, even with less than 48 hours of intubation, 84% of patients had at least mild dysphagia [43]. The 34% prevalence in our study may be conservative due to use of a patient-reported survey of dysphagia and the relatively later timing of evaluation (ie, hospital discharge vs shortly after extubation) [15,16,43,44].
The literature has conflicting results regarding the association of the duration of endotracheal intubation and dysphagia. This lack of agreement among prior studies is likely due to variable methods for evaluating dysphagia, heterogeneous patient samples, analyses not adjusting for confounding, and small sample sizes [23]. Only 3 studies used regression analysis to adjust for confounding. One study prospectively evaluated patients who were intubated for more than 24 hours, using electromyography to measure initiation of the pharyngeal swallow and showed no association of intubation duration with swallow initiation [9]. A second study retrospectively reviewed patient medical records and found a positive association of duration of short-term (ie, intraoperative) oral endotracheal intubation with dysphagia in cardiac surgery patients undergoing intraoperative transesophageal echocardiography [45]. The third study prospectively recruited patients who were intubated for more than 10 days, using a fiberoptic endoscopy to evaluate swallowing function and demonstrated that reduced muscle strength, a penetration-aspiration scale [46] score more than 1, and duration of mechanical ventilation are associated with symptomatic aspiration [47]. It is difficult to directly compare our data with these studies because of differences in study design and patient populations. Greater investigation in this field is needed to have a larger foundation of epidemiological data.
Laryngeal injury begins within the first day of intubation, including edema, tissue damage, and voice dysfunction [6,7], each of which is a risk factor for postextubation dysphagia [17,48–50]. With endotracheal intubation for longer than 48 hours, laryngeal injury can lead to permanent vocal fold damage, vocal fold paralysis, and dysphagia resulting in aspiration [8,51–53]. Our finding of daily increased odds of dysphagia and severity of swallowing dysfunction during the first 6 days of oral endotracheal intubation suggests a critical period during which reduction in intubation duration (eg, through measures such as reduced sedation and daily spontaneous breathing trials [54–56]) may reduce dysphagia risk. Consistent with other studies, we did not find an association of age or sex with dysphagia [9,15–17,44,45].
We found that patients with upper gastrointestinal comorbidities have a 3-fold increased odds of having clinical important dysphagia symptoms. This finding is not surprising given the overlap of symptoms associated with globus pharyngeus and gastroesophageal reflux and dysphagia in the questions of the SSQ [28,57–60]. Of note, we did not find an association between patients with neurologic comorbidities and clinically important dysphagia symptoms. This finding is not consistent with prior research that showed 93% of patients with neurologic impairments had dysphagia after extubation and that longer durations of intubation were independently associated with moderate-severe dysphagia; however, that study focused exclusively on patients with primary diagnoses of neurologic disorders, an exclusion criterion for the present study [24].
4.1. Limitations
This study has several potential limitations. First, due to the nature of critical illness and the emergent need for mechanical ventilation, it is not possible to evaluate patients for dysphagia before intubation. Consequently, only the prevalence, rather than incidence, of dysphagia could be estimated in this study. Second, our study used the SSQ, a patient-reported measure, instead of a clinical or instrumental assessment of dysphagia. Hence, physiologic aspects of swallowing were not assessed. Third, we exclusively studied ALI patients recruited from 4 teaching hospitals in Baltimore, and a substantial proportion of ALI survivors were not eligible for this analysis (as per a priori eligibility criteria), which may limit the generalizability of the study findings. However, our relatively large and homogenous sample (compared with prior studies in this field) is a strength of the present study. Given the relative ease of administration of the SSQ, we encourage further research in other ICU populations, including studies using instrumental assessment of swallow physiology, to consider evaluating dysphagia symptoms using the SSQ.
5. Conclusions
Our multisite, prospective cohort study suggests that approximately one-third of orally intubated ALI patients had clinically important symptoms of dysphagia at hospital discharge. Preexisting upper gastrointestinal comorbidity was independently associated with dysphagia along with the duration of oral endotracheal intubation through the first 6 days of intubation. Our results may help focus attention on the risk of dysphagia after oral endotracheal intubation and encourage further research aimed at reducing complications associated with the duration of endotracheal intubation and its effects on swallowing.
Acknowledgments
The authors thank the patients who participated in the study and the research assistants and coordinators who assisted with data collection and management for the study, including Nardos Belayneh, Rachel Bell, Kim Boucher, Abdulla Damluji, Carinda Feild, Thelma Harrington, Praveen Kondreddi, Frances Magliacane, Stacey Murray, Kim Nguyen, Susanne Prassl, Arabela Sampaio, Kristin Sepulveda, Shabana Shahid, Faisal Siddiqi, Michelle Silas, and Jennifer Titus.
This research was supported by the National Institutes of Health (P050HL73994 and 5KL2RR025006).
Footnotes
Research completed at Johns Hopkins Hospital, Johns Hopkins Bayview Medical Center, University of Maryland Medical Center, and Baltimore VA Medical Center.
References
- [1].Wunsch H, Angus DC, Harrison DA, et al. Variation in critical care services across North America and Western Europe. Crit Care Med 2008;36:2787–93 [e2781–2789]. [DOI] [PubMed] [Google Scholar]
- [2].Higgins TL, Kramer AA, Nathanson BH, et al. Prospective validation of the intensive care unit admission Mortality Probability Model (MPM0-III). Crit Care Med 2009;37:1619–23. [DOI] [PubMed] [Google Scholar]
- [3].Angus DC, Shorr AF, White A, et al. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med 2006;34:1016–24. [DOI] [PubMed] [Google Scholar]
- [4].Needham DM, Bronskill SE, Calinawan JR, et al. Projected incidence of mechanical ventilation in Ontario to 2026: preparing for the aging baby boomers. Crit Care Med 2005;33:574–9. [DOI] [PubMed] [Google Scholar]
- [5].Zilberberg MD, de Wit M, Pirone JR, et al. Growth in adult prolonged acute mechanical ventilation: implications for healthcare delivery. Crit Care Med 2008;36:1451–5. [DOI] [PubMed] [Google Scholar]
- [6].Massard G, Rouge C, Dabbagh A, et al. Tracheobronchial lacerations after intubation and tracheostomy. Ann Thorac Surg 1996;61:1483–7. [DOI] [PubMed] [Google Scholar]
- [7].Mencke T, Echternach M, Kleinschmidt S, et al. Laryngeal morbidity and quality of tracheal intubation: a randomized controlled trial. Anesthesiology 2003;98:1049–56. [DOI] [PubMed] [Google Scholar]
- [8].Burgess III GE, Cooper JR Jr, Marino RJ, et al. Laryngeal competence after tracheal extubation. Anesthesiology 1979;51:73–7. [DOI] [PubMed] [Google Scholar]
- [9].de Larminat V, Montravers P, Dureuil B, et al. Alteration in swallowing reflex after extubation in intensive care unit patients. Crit Care Med 1995;23:486–90. [DOI] [PubMed] [Google Scholar]
- [10].Sasaki C, Fukuda H, Kirchner J. Laryngeal abductor activity in response to varying ventilatory resistance. Trans Am Acad Ophthalmol Otolaryngol 1973;77:ORL403. [PubMed] [Google Scholar]
- [11].Sasaki CT, Suzuki M, Horiuchi M, et al. The effect of tracheostomy on the laryngeal closure reflex. Laryngoscope 1977;87:1428–33. [DOI] [PubMed] [Google Scholar]
- [12].Weymuller EA Jr. Laryngeal injury from prolonged endotracheal intubation. Laryngoscope 1988;98:1–15. [DOI] [PubMed] [Google Scholar]
- [13].Tolep K, Getch CL, Criner GJ. Swallowing dysfunction in patients receiving prolonged mechanical ventilation. Chest 1996;109:167–72. [DOI] [PubMed] [Google Scholar]
- [14].Barquist E, Brown M, Cohn S, et al. Postextubation fiberoptic endoscopic evaluation of swallowing after prolonged endotracheal intubation: a randomized, prospective trial. Crit Care Med 2001;29:1710–3. [DOI] [PubMed] [Google Scholar]
- [15].Ajemian MS, Nirmul GB, Anderson MT, et al. Routine fiberoptic endoscopic evaluation of swallowing following prolonged intubation: implications for management. Arch Surg 2001;136:434–7. [DOI] [PubMed] [Google Scholar]
- [16].El Solh A, Okada M, Bhat A, et al. Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med 2003;29:1451–5. [DOI] [PubMed] [Google Scholar]
- [17].Barker J, Martino R, Reichardt B, et al. Incidence and impact of dysphagia in patients receiving prolonged endotracheal intubation after cardiac surgery. Can J Surg 2009;52:119–24. [PMC free article] [PubMed] [Google Scholar]
- [18].Martin BJ, Corlew MM, Wood H, et al. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia 1994;9:1–6. [DOI] [PubMed] [Google Scholar]
- [19].Schmidt J, Holas M, Halvorson K, et al. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia 1994;9:7–11. [DOI] [PubMed] [Google Scholar]
- [20].Langmore SE, Terpenning MS, Schork A, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia 1998;13:69–81. [DOI] [PubMed] [Google Scholar]
- [21].Martin-Harris B, McMahon SJ, Haynes R. Aspiration and dysphagia: pathophysiology and outcome. Phonoscope 1998;1:123–32. [Google Scholar]
- [22].Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest 2003;124:328–36. [DOI] [PubMed] [Google Scholar]
- [23].Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation. Chest 2010;137:665–73. [DOI] [PubMed] [Google Scholar]
- [24].Macht M, King CJ, Wimbish T, et al. Post-extubation dysphagia is associated with longer hospitalization in survivors of critical illness with neurologic impairment. Crit Care 2013;17:R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Herridge MS, Angus DC. Acute lung injury—affecting many lives. N Engl J Med 2005;353:1736–8. [DOI] [PubMed] [Google Scholar]
- [26].Needham DM, Dowdy DW, Mendez-Tellez PA, et al. Studying outcomes of intensive care unit survivors: measuring exposures and outcomes. Intensive Care Med 2005;31:1153–60. [DOI] [PubMed] [Google Scholar]
- [27].Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–24. [DOI] [PubMed] [Google Scholar]
- [28].Wallace KL, Middleton S, Cook IJ. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology 2000;118:678–87. [DOI] [PubMed] [Google Scholar]
- [29].Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159–68. [DOI] [PubMed] [Google Scholar]
- [30].Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med 1998;158:1855–67. [DOI] [PubMed] [Google Scholar]
- [31].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [32].Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- [33].Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- [34].Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979;74:829–36. [Google Scholar]
- [35].Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc 1988;83:596–610. [Google Scholar]
- [36].Vittinghoff E, Glidden DV, Shiboski SC, et al. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. 2nd ed New York, NY: Springer; 2012. [Google Scholar]
- [37].Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Ann Intern Med 1993;118:201–10. [DOI] [PubMed] [Google Scholar]
- [38].Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–9. [DOI] [PubMed] [Google Scholar]
- [39].Harrell FE. Regression modeling strategies: with applications to linear models, losgistic regression, and survival analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- [40].Stine RA. Graphical interpretation of variance inflation factors. Am Stat 1995;49:53–6. [Google Scholar]
- [41].Acock AC. A gentle introduction to Stata (ed revised 3rd). College Station, TX: Stata Press; 2012. [Google Scholar]
- [42].Hosmer DW, Lemeshow S. Applied logistic regression. New York, NY: Wiley; 2000. [Google Scholar]
- [43].Macht M, Wimbish T, Clark BJ, et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care 2011;15:R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Leder SB, Cohn SM, Moller BA. Fiberoptic endoscopic documentation of the high incidence of aspiration following extubation in critically ill trauma patients. Dysphagia 1998;13:208–12. [DOI] [PubMed] [Google Scholar]
- [45].Rousou JA, Tighe DA, Garb JL, et al. Risk of dysphagia after transesophageal echocardiography during cardiac operations. Ann Thorac Surg 2000;69:486–9. [DOI] [PubMed] [Google Scholar]
- [46].Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia 1996;11:93–8. [DOI] [PubMed] [Google Scholar]
- [47].Mirzakhani H, Williams JN, Mello J, et al. Muscle weakness predicts pharyngeal dysfunction and symptomatic aspiration in long-term ventilated patients. Anesthesiology 2013;119:389–97. [DOI] [PubMed] [Google Scholar]
- [48].Hedden M, Ersoz CJ, Donnelly WH, et al. Laryngotracheal damage after prolonged use of orotracheal tubes in adults. JAMA 1969;207:703–8. [PubMed] [Google Scholar]
- [49].Whited RE. A prospective study of laryngotracheal sequelae in long-term intubation. Laryngoscope 1984;94:367–77. [DOI] [PubMed] [Google Scholar]
- [50].Tadié JM, Behm E, Lecuyer L, et al. Post-intubation laryngeal injuries and extubation failure: a fiberoptic endoscopic study. Intensive Care Med 2010;36:991–8. [DOI] [PubMed] [Google Scholar]
- [51].Dubick MN, Wright BD. Comparison of laryngeal pathology following long‐term oral and nasal endotracheal intubations. Anesth Analg 1978;57:663–8. [PubMed] [Google Scholar]
- [52].Brandwein M, Abramson AL, Shikowitz MJ. Bilateral vocal cord paralysis following endotracheal intubation. Arch Otolaryngol Head Neck Surg 1986;112:877–82. [DOI] [PubMed] [Google Scholar]
- [53].Bishop MJ, Weymuller EA, Fink BR. Laryngeal effects of prolonged intubation. Anesth Analg 1984;63:335–42. [PubMed] [Google Scholar]
- [54].Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126–34. [DOI] [PubMed] [Google Scholar]
- [55].Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471–7. [DOI] [PubMed] [Google Scholar]
- [56].Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet 2010;375:475–80. [DOI] [PubMed] [Google Scholar]
- [57].Chen CL, Szczesniak MM, Cook IJ. Evidence for oesophageal visceral hypersensitivity and aberrant symptom referral in patients with globus. Neurogastroenterol Motil 2009;21:e1142–96. [DOI] [PubMed] [Google Scholar]
- [58].Han MS, Lee H, Jo JH, et al. Transition zone defect associated with the response to proton pump inhibitor treatment in patients with globus sensation. J Gastroenterol Hepatol 2013;28:954–62. [DOI] [PubMed] [Google Scholar]
- [59].Moser G, Wenzel-Abatzi TA, Stelzeneder M, et al. Globus sensation: pharyngoe-sophageal function, psychometric and psychiatric findings, and follow-up in 88 patients. Arch Intern Med 1998;158:1365–73. [DOI] [PubMed] [Google Scholar]
- [60].Ravich WJ, Wilson RS, Jones B, et al. Psychogenic dysphagia and globus: reevaluation of 23 patients. Dysphagia 1989;4:35–8. [DOI] [PubMed] [Google Scholar]


