Abstract
Background:
Parenteral amino acid (AA) nutrition administration after premature birth is necessary to ensure adequate growth and neurodevelopment. However, optimizing safety and efficacy remain major challenges. This study investigated effects of intravenous AA administration on plasma AA profiles in premature baboons and infants.
Methods:
Premature baboons were delivered by C-section at 125 days (67% gestation) and chronically ventilated. At 24 hours of life, a parenteral AA protocol comparable to the early and high AA regimens used in premature infants was initiated. Serial plasma AA concentrations were obtained on days of life (DOL) 1, 3, and 7 and compared to concentrations at similar DOL from preterm infants. Fetal baboon (165±2 days; 89% gestation) and term baboon plasma AA concentrations were obtained for comparison.
Results:
Premature baboons receiving early and high parenteral AA supplementation exhibited significant differences in plasma AA concentrations compared to fetuses. In particular, concentrations of leucine, isoleucine, valine, and ornithine were elevated (fold increase: 2.14, 2.03, 1.95, 16.5, respectively; p<0.001) on DOL 3 vs fetuses. These alterations mimicked those found in preterm infants.
Conclusion:
Early and high AA supplementation in extremely premature baboons significantly disrupted plasma AA concentrations. Elevated concentrations of branched-chain AAs and ornithine raise concerns for adverse neurodevelopmental outcomes. These results are consistent with those found in premature human infants and emphasize the need to optimize parenteral AA solutions for the unique metabolic requirements of premature infants. Improved technologies for rapid monitoring of AA concentrations during treatment are essential.
INTRODUCTION
Providing adequate nutrition for extremely low birth weight (ELBW, birth weight <1000g) preterm infants remains extremely challenging. Over 50% of ELBW infants develop extra- uterine growth restriction by discharge1,2, resulting in major detrimental impacts for neurodevelopmental outcomes3,4. Proteins and amino acids (AA) are essential components of growth, development, and metabolic regulation. Two fundamental facts about AA and protein nutrition in fetuses and neonates are generally well supported and accepted: fetal accretion of protein is about 3–4 g·kg−1·day−1 5, and early postnatal loss of protein stores can be prevented or ameliorated by administration of a relatively low dose of 1–1.5 g·kg–1·day−1 of parenteral AA6. The nutritional gap between this rate of AA administration and the rate of fetal accretion of protein has increasingly been suspected to contribute to postnatal growth failure in preterm infants6. Therefore, a growing trend towards more accelerated nutritional support, seeking to mimic the intrauterine state by increasing AA infusion rates to 3–4 g·kg−1·day−1within the first 24– 48 hours of life has emerged (reviewed in7).
However, preterm infants must rely solely on immature metabolic pathways for energy homeostasis, without the metabolic support of the placenta available for the fetus. Therefore, seeking to mimic fetal states may not be achievable in preterm newborns with immature liver, skeletal muscle, and kidney pathways. Initial studies tested early AA supplementation up to 3 g·kg−1·day−1 in preterm infants, however, AA concentrations were measured after short exposures (24 hours)6,8. To date, there have not been convincing data to determine the level of ex-utero AA replacement required to improve growth. Indeed, some evidence suggests there may deleterious effects depending on the level of replacement. Clark et al9 randomized premature neonates to receive a lower (starting at 1.0 g·kg−1·day−1 and advancing to a maximum of 2.5 g·kg−1·day−1) or higher (starting at 1.5 g·kg−1·day−1 and advancing to a maximum of 3.5 g·kg−1·day−1) dose of AA supplementation and found no difference in growth in the higher dose group. However, they found elevated AA concentrations in both groups at 7 days of life (DOL). The increase was more marked in the higher dose group compared to term newborn reference values. In a previous study, we also found high plasma AA concentrations when we randomly assigned ELBW infants to standard (starting at 0.5 g·kg−1·day−1 and advanced to a maximum of 3 g·kg−1·day−1) or high dose (starting at 2.0 g·kg−1·day−1 and advanced to a maximum of 4 g·kg−1·day−1) supplementation regimens and measured serial AA concentrations10. We also found that at two year follow ups, ELBW infants that received early and high AA supplementation during the first week of life exhibited poor overall growth4. In both studies, the patterns of AA concentrations demonstrated high variability with both abnormally high and low values and no evident patterns. Finally, a recent randomized trial in infants <1250g comparing lower vs higher (1–2 vs 3–4 g·kg−1·day−1) AA supplementation found no differences in neurodevelopmental outcomes at 18–24 months, but infants in the high AA group had lower weight, length, and head circumference percentiles and higher serum urea nitrogen (SUN) at 36 weeks corrected gestational age and at hospital discharge 11.
Published data provide no clear pathway to optimizing nutritional management of ELBW infants. Therefore, we undertook a study in premature baboons to investigate their plasma AA concentrations while receiving the same intravenous (IV) AA utilized in human premature infants’ clinical care. To provide context in which to interpret the data, we also compared AA concentrations observed during infusions to values observed in undisturbed control fetal and newborn baboons. Finally, to add new translational information, we compared the patterns of plasma AA concentrations observed in baboons to those observed in human infants. This will allow us to further elucidate which AA metabolic pathways are not fully developed when infants are born preterm, with the ultimate goal of tailoring IV AA solutions to meet the needs of premature infants.
METHODS
Animal Care
All animal experiments were approved by the Institutional Animal Care and Use Committee at the Texas Biomedical Research Institute (TBRI; San Antonio, Texas, USA) and the University of Texas Health Science Center (UTHSCSA; San Antonio, Texas, USA) and were conducted in accordance with accepted standards of humane animal care. In total, 45 baboons were studied. Animals were obtained from the Southwest National Primate Center at the TBRI, or from the University of Oklahoma Health Science Center Primate Center (El Reno, Oklahoma, USA). Infants were delivered from healthy, non-diabetic mothers. Animals included in this study served as shared controls for various on-going studies. All animals born from 2005–2012 for which blood samples were available for analysis were included. Animals were euthanized by exsanguination and pentobarbital.
Preterm Baboons
Fourteen preterm baboons were delivered at 125±2 days gestation (67% gestation; human equivalent ~27 weeks) by Caesarean-section under general anesthesia as previously described in detail12. Mothers were given steroids initiated 48 hours prior to delivery with betamethasone (6mg, intramuscular) every 24 hours (2 doses) for a total of 12 mg. Routine care of preterm animals was performed per protocol as previously described13. Briefly, animals were intubated immediately after birth, administered Surfactant (Survanta®, Abbott Laboratories, Abbott Park, IL, USA), and chronically ventilated for a planned survival of 14 days. Central IV lines were placed for fluid and nutrition management. An IV solution of 5% dextrose was started after birth at a rate of 150 mL·kg−1·day−1. The IV AA were started at 1.75 g·kg−1·day−1and increased to 3.5 g·kg−1·day−1 at 24 hours of life and continued for 14 days. The IV AA solution used was TrophAmine® 10% (B. Braun Laboratories, Bethlehem, PA), with 40 mg·kg−1·day−1 of cysteine hydrochloride. Dopamine or dobutamine were administered as needed to maintain mean arterial pressure > 25 mmHg. Prophylactic antibiotics were administered for 48 hours to mimic human care. Eight preterm animals had AA data available for DOLs 1, 3, and 7, while the remaining six had samples for only DOLs 1 and 3.
Enteral feeds were initiated (via orogastric tube) on DOL 3 if bowel gas pattern was considered normal on radiograph. Similac Special Care 20 infant formula (Abbott Laboratories) was introduced as trophic feed and increased as tolerated to a maximum volume of 40 mL·kg−1·day−1.
Fetal Baboons
Twenty-two fetal baboons were delivered at 165±2 gestational days (89% gestation; human equivalent ~36 weeks) via Caesarean-section and immediately euthanized.
Term Baboons
Five baboons were delivered vaginally and breastfed by their mothers for up to 3 days before transfer to UTHSCSA. Animals were then bottle-fed Similac Advance infant formula (Abbott Laboratories) every 3–4 hours daily until euthanasia at DOL 2–4. Animals were housed in temperature controlled environments and monitored by veterinary staff daily.
AA analysis
Plasma samples for measurements of AA concentrations were obtained on DOLs 1, 3, and 7 if preterm or prior to euthanasia if fetal or term. Concentrations were determined by reverse-phase high-pressure liquid chromatography (HPLC) by use of the Waters PICO tag method10. All animal samples were run in 1 batch. Standards of known concentrations were used daily for recalibration. Concentrations in plasma control samples from a single pool were determined in triplicate after each recalibration. The average intra-assay coefficient was 6%.
Statistical Analysis
Statistical analyses were performed using SPSS (Version 22.0, SSPS Inc., Chicago, Illinois). Data are reported as mean ± standard deviation of the mean, unless otherwise indicated. One- way analysis of variance with Bonferroni post-hoc was performed to determine significant differences between groups within baboon groups. Independent samples t-Tests were performed to determine significant differences between preterm humans and preterm baboons. P-value <0.05 was considered statistically significant. A power calculation was performed based on differences in branched-chained AA (BCAA; Valine (Val), Leucine (Leu), Isoleucine (Ile)) concentrations of preterm baboons on DOL 3 compared with fetal baboons, and a total of 5 animals per group was calculated. All available animal samples were included to perform additional comparisons with ELBW human infants.
Reference Groups
To extrapolate the baboon results to human premature infants, we compared the plasma AA concentrations with AA concentrations of preterm infants enrolled in a previous study at our institution 10.
In this study, AA profiles of preterm humans were obtained from 61 ELBW infants admitted to University Hospital Neonatal Intensive Care Unit, San Antonio, Texas, and recruited prospectively between November 2002 and September 2005. Inclusion criteria were birth weight (BW) <1000 g and age <12 hours of life. A third inclusion criterion of gestational age ≥ 24 weeks was added by the Data Safety Monitoring Board. Exclusion criteria were major congenital anomalies and imminent death. Written informed consent was obtained from infants’ parents before enrollment. The UTHSCSA Institutional Review Board approved the study. Infants with comparable inclusion criteria were not enrolled if their parents refused or withdrew during the enrollment period.
Infants were randomly assigned to receive either a standard AA protocol of 0.5 g·kg−1·day−1 of Aminosyn® PF 10% (Abbott Laboratories, Chicago, Illinois, USA) with 40 mg·kg−1·day−1of cysteine hydrochloride after 24 hours of age and increased to a maximum of 3.0 g·kg−1·day−1, or an early and high AA protocol starting at 2.0 g·kg−1·day−1 within 24 hours of age, and increased to a maximum of 4.0 g·kg−1·day−1.
Plasma samples for AA measurements were obtained on DOLs 1, 3, and 7. AA concentrations were determined using HPLC as previously described for the baboons.
RESULTS
Animal Characteristics
The demographic characteristics of the study animals are summarized in Table 1. There were no sex differences between groups, with the exception of Threonine (Thr) in the term group (P<0.05).
Table 1:
Characteristics of Study Baboons.
| Fetal (n=22) | Preterm (n=14) | Term (n=5) | |
|---|---|---|---|
| Birth weight (g) | 806 ± 110 | 369 ± 47 | 900 ± 114 |
| Gender (M:F) | 10:12 | 5:9 | 1:4 |
| Gestation (% of full term) | 89 | 67 | 92−100 |
Preterm animals received minimal enteral feeding during the study period. Animals began enteral feeds on approximately DOL 6 ± 1. No animals received enteral feedings on DOLs 1 or 3. On DOL 7, the average volume of enteral feeds received was 12.4 ± 8.1 mL·kg−1·day−1. The maximum feed volume tolerated on DOL 7 was 30.9 mL·kg−1·day−1. Preterm animals received a mean protein intake (g·kg−1·day−1) of 1.6 ± 0.3, 3.4 ± 0.3, and 3.2 ± 0.6 on DOLs 1, 3, and 7, respectively, and a mean glucose infusion rate (mg·kg−1·min−1) of 3.5 ± 1.7, 4.3 ± 1.3, and 7.8 ± 2.5 on DOLs 1, 3, and 7, respectively.
SUN
The average SUN of the preterm group on DOL 1 was 20.3 ± 6.7 mg/dL. This value peaked at 45.0 ± 10.6 mg/dL on DOL 3, and then decreased to 31.3 ± 10.4 on DOL 7. The peak SUN values are consistent with those previously recorded for preterm human infants receiving similar doses of AAs (39.9 ± 22.2 mg/dL, P=0.26)10.
Plasma AA Concentrations
The mean (±SD) and 95% CI for the 22 AAs evaluated at each serial time point are shown in Table 2. The significance of the group effect (includes fetal, term, and preterm animals) and the interaction between fetal baboon and preterm baboon, as well as preterm baboon and preterm human are also shown. Significant differences from fetal animals occurred in nearly every AA examined. Because the primary goal of AA therapies in preterm infants has been to mimic the fetal state, we chose to focus our analysis on comparisons between preterm and fetal animals. However, data on term animals are included in the table and graphs to provide further reference points. AAs are classified as essential, conditionally essential for neonates, or non-essential based on Rassin 199414.
Table 2:
Plasma AA concentrations in baboon and human neonates. The mean (±SD) and 95% CI for the 22 AA evaluated on days of life 1, 3, and 7 are shown. The significance of the group effect (includes fetal, term and preterm animals; evaluated by one-way ANOVA) and the interaction between fetal baboon and preterm baboon (evaluated by Bonferroni Post-hoc), as well as preterm baboon and preterm human (evaluated by independent samples T-Test) are also shown. AA, amino acid; DOL, day of life; ELBW, extremely low birth weight; NS, not significant (P >0.05).
| AA | Group | Day of Life (Mean±SD) | Day of Life 1(95% CI) | Day of Life 1Group Effect | Day of Life 1Interaction | Day of Life 3(Mean±SD) | Day of Life 3(95% CI) | Day of Life 3Group Effect | Day of Life 3Interaction | Day of Life 7(Mean±SD) | Day of Life 7(95% CI) | Day of Life 7Group Effect | Day of Life 7Interaction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Val | Preterm Baboon | 174.1±39.2 | (151.4, 196.7) | NS | NS | 328.7±81.4 | (277.0, 380.4) | p<0.001 | p<0.001 | 226.4±106.8 | (137.1±315.7) | NS | NS |
| Fetal Baboon | 168.5±36.6 | (151.9, 185.2) | 168.5±36.6 | (151.9, 185.2) | 168.5±36.6 | (151.9, 185.2) | |||||||
| Term Baboon | 151.8±83.3 | (48.4, 255.3) | 151.8±83.3 | (48.4, 255.3) | 151.8±83.3 | (48.4, 255.3) | |||||||
| ELBW Human | 164.2±66.2 | (148.2, 180.2) | NS | 231.7±125.5 | (201.6, 261.9) | p<0.01 | 233.1±55.9 | (218.9, 247.4) | NS | ||||
| Leu | Preterm Baboon | 100.7±34.4 | (80.8, 120.6) | NS | NS | 231.6±49.3 | (200.2, 262.9) | p<0.001 | p<0.001 | 158.4±82.0 | (89.8, 227.0) | NS | NS |
| Fetal Baboon | 108.4±39.4 | (90.5, 126.4) | 108.4±39.4 | (90.5, 126.4) | 108.4±39.4 | (90.5, 126.4) | |||||||
| Term Baboon | 117.9±63.2 | (39.4, 196.4) | 117.9±63.2 | (39.4, 196.4) | 117.9±63.2 | (39.4, 196.4) | |||||||
| ELBW Human | 107.7±55.0 | (94.4, 121.0) | NS | 183.4±99.1 | (159.6, 207.2) | p<0.05 | 194.8±43.9 | (183.6, 206.1) | NS | ||||
| Ile | Preterm Baboon | 67.1±18.8 | (56.3, 77.9) | NS | NS | 158.2±25.1 | (142.2, 174.1) | p<0.001 | p<0.001 | 87.7±55.0 | (41.8, 133.6) | NS | NS |
| Fetal Baboon | 77.6±24.9 | (66.3, 88.9) | 77.6±24.9 | (66.3, 88.9) | 77.6±24.9 | (66.3, 88.9) | |||||||
| Term Baboon | 63.4±39.1 | (14.8, 112.0) | 63.4±39.1 | (14.8, 112.0) | 63.4±39.1 | (14.8, 112.0) | |||||||
| ELBW Human | 138.8±54.3 | (125.6, 152.0) | p<0.001 | 171.5±66.6 | (155.5, 187.5) | NS | 127.8±38.5 | (118.0, 137.7) | p<0.05 | ||||
| Thr | Preterm Baboon | 219.0±69.2 | (179.0, 258.9) | p<0.001 | p<0.001 | 218.7±78.7 | (168.7, 268.7) | p=0.001 | p=0.001 | 227.7±122.3 | (125.4, 329.9) | p<0.05 | p=0.01 |
| Fetal Baboon | 139.1±39.0 | (121.4, 156.9) | 139.1±39.0 | (121.4, 156.9) | 139.1±39.0 | (121.4, 156.9) | |||||||
| Term Baboon | 156.1±41.6 | (104.4, 207.8) | 156.1±41.6 | (104.4, 207.8) | 156.1±41.6 | (104.4, 207.8) | |||||||
| ELBW Human | 263.6±158.7 | (225.2, 302.0) | NS | 198.7±144.5 | (163.5, 234.0) | NS | 121.4±66.8 | (104.1, 138.6) | p<0.05 | ||||
| Lys | Preterm Baboon | 460.6±121.8 | (390.3, 530.9) | p<0.001 | p=0.058 | 330.6±142.9 | (239.8, 421.4) | p<0.05 | NS | 366.0±105.9 | (277.4, 454.5) | p<0.01 | NS |
| Fetal Baboon | 369.4±106.5 | (321.0, 417.9) | 369.4±106.5 | (321.0, 417.9) | 369.4±106.5 | (321.0, 417.9) | |||||||
| Term Baboon | 191.8±54.9 | (123.6, 260.0) | 191.8±54.9 | (123.6, 260.0) | 191.8±54.9 | (123.6, 260.0) | |||||||
| ELBW Human | 181.2±71.6 | (163.9, 198.5) | p<0.001 | 249.1±124.6 | (177.8, 224.2) | p<0.001 | 182.3±104.0 | (155.4, 209.1) | p=0.001 | ||||
| Trp | Preterm Baboon | 83.9±24.7 | (69.8, 98.0) | p<0.001 | p<0.001 | 78.6±22.2 | (43.3, 50.2) | p<0.001 | p<0.001 | 53.2±33.7 | (25.0, 81.4) | NS | NS |
| Fetal Baboon | 46.7±7.6 | (43.3, 50.2) | 46.7±7.6 | (43.3, 50.2) | 46.7±7.6 | (43.3, 50.2) | |||||||
| Term Baboon | 37.7±22.4 | (9.9, 65.4) | 37.7±22.4 | (9.9, 65.4) | 37.7±22.4 | (9.9, 65.4) | |||||||
| ELBW Human | |||||||||||||
| Met | Preterm Baboon | 52.7±25.4 | (38.0, 67.4) | NS | NS | 51.1±16.9 | (40.3, 61.8) | p<0.05 | NS | 49.0±30.2 | (23.8, 74.2) | NS | NS |
| Fetal Baboon | 45.0±8.4 | (41.2, 48.8) | 45.0±8.4 | (41.2, 48.8) | 45.0±8.4 | (41.2, 48.8) | |||||||
| Term Baboon | 31.2±8.4 | (20.7, 41.6) | 31.2±8.4 | (20.7, 41.6) | 31.2±8.4 | (20.7, 41.6) | |||||||
| ELBW Human | 50.7±27.0 | (44.1, 57.2) | NS | 77.9±25.3 | (71.8, 83.9) | p=0.001 | 60.2±26.9 | (49.1, 59.3) | NS | ||||
| Phe | Preterm Baboon | 89.9±16.4 | (80.5, 99.4) | p<0.01 | NS | 86.0±15.2 | (76.3, 95.6) | p<0.01 | NS | 74.0±24.4 | (53.7, 94.4) | p<0.01 | NS |
| Fetal Baboon | 98.6±27.6 | (86.1, 111.2) | 98.6±27.6 | (86.1, 111.2) | 98.6±27.6 | (86.1, 111.2) | |||||||
| Term Baboon | 58.2±17.2 | (36.8, 79.6) | 58.2±17.2 | (36.8, 79.6) | 58.2±17.2 | (36.8, 79.6) | |||||||
| ELBW Human | 89.3±16.9 | (85.2, 93.4) | NS | 86.7±27.2 | (78.5, 92.0) | NS | 98.6±27.6 | (80.2, 95.9) | NS | ||||
| His | Preterm Baboon | 353.5±157.8 | (262.4, 444.6) | p<0.001 | p<0.001 | 242.2±87.7 | (186.5, 297.9) | p<0.001 | p<0.001 | 227.1±129.9 | (118.5, 335.6) | p=0.01 | p=0.01 |
| Fetal Baboon | 144.4±29.6 | (130.9, 157.8) | 144.4±29.6 | (130.9, 157.8) | 144.4±29.6 | (130.9, 157.8) | |||||||
| Term Baboon | 123.3±18.5 | (100.3, 146.2) | 123.3±18.5 | (100.3, 146.2) | 123.3±18.5 | (100.3, 146.2) | |||||||
| ELBW Human | 81.3±29.6 | (74.1, 88.5) | p<0.001 | 101.8±28.5 | (95.0, 108.7) | p<0.001 | 103.4±20.9 | (98.0, 108.8) | p<0.05 | ||||
| Tyr | Preterm Baboon | 565.9±351.7 | (362.9, 769.0) | p<0.001 | p<0.001 | 215.6±118.1 | (140.5, 290.6) | p<0.001 | p<0.001 | 174.1±154.2 | (45.2, 303.1) | p<0.01 | p<0.01 |
| Fetal Baboon | 63.3±18.3 | (55.0, 71.7) | 63.3±18.3 | (55.0, 71.7) | 63.3±18.3 | (55.0, 71.7) | |||||||
| Term Baboon | 93.2±17.3 | (71.7, 114.7) | 93.2±17.3 | (71.7, 114.7) | 93.2±17.3 | (71.7, 114.7) | |||||||
| ELBW Human | 177.4±117.5 | (149.0, 205.9) | p=0.001 | 124.3±119.2 | (95.7, 152.9) | p<0.05 | 54.2±55.3 | (40.1, 68.4) | NS | ||||
| Arg | Preterm Baboon | 276.1±63.5 | (239.4, 312.7) | p<0.001 | p<0.001 | 265.3±65.0 | (224.0, 306.6) | p<0.001 | p<0.001 | 314.4±108.0 | (224.1, 404.7) | p<0.001 | p<0.001 |
| Fetal Baboon | 93.4±24.3 | (82.4, 104.5) | 93.4±24.3 | (82.4, 104.5) | 93.4±24.3 | (82.4, 104.5) | |||||||
| Term Baboon | 187.3±13.8 | (170.2, 204.4) | 187.3±13.8 | (170.2, 204.4) | 187.3±13.8 | (170.2, 204.4) | |||||||
| ELBW Human | 69.4±41.0 | (59.5, 79.3) | p<0.001 | 93.1±58.3 | (79.1, 107.1) | p<0.001 | 123.6±53.4 | (109.9, 127.3) | p=0.001 | ||||
| Tau | Preterm Baboon | 25.2±13.6 | (−8.6, 58.9) | p<0.001 | p<0.001 | 14.9±6.8 | (−2.0, 31.2) | p<0.001 | p<0.001 | 110.7±108.3 | (−23.8, 245.1) | p<0.05 | p<0.05 |
| Fetal Baboon | 206.4±55.6 | (181.1, 231.7) | 206.4±55.6 | (181.1, 231.7) | 206.4±55.6 | (181.1, 231.7) | |||||||
| Term Baboon | 152.9±97.6 | (31.7, 274.1) | 152.9±97.6 | (31.7, 274.1) | 152.9±97.6 | (31.7, 274.1) | |||||||
| ELBW Human | |||||||||||||
| Gln | Preterm Baboon | 506.5±107.3 | (444.5, 568.4) | p<0.001 | p<0.001 | 392.0±135.0 | (306.2, 477.7) | p<0.001 | p<0.001 | 377.9±193.9 | (215.7, 540.0) | p<0.001 | p<0.001 |
| Fetal Baboon | 736.6±127.3 | (677.0, 796.2) | 736.6±127.3 | (677.0, 796.2) | 736.6±127.3 | (677.0, 796.2) | |||||||
| Term Baboon | 499.7±47.7 | (440.5, 558.9) | 499.7±47.7 | (440.5, 558.9) | 499.7±47.7 | (440.5, 558.9) | |||||||
| ELBW Human | 420.0±182.1 | (376.0, 464.1) | p=0.001 | 406.6±160.2 | (368.1, 445.1) | NS | 438.7±128.2 | (405.9, 471.5) | NS | ||||
| Ser | Preterm Baboon | 223.0±74.1 | (180.2, 265.8) | p=0.001 | p<0.001 | 203.7±52.9 | (170.1, 237.3) | p<0.01 | p=0.001 | 228.2±116.3 | (131.0, 325.4) | p<0.05 | p=0.01 |
| Fetal Baboon | 147.5±32.7 | (132.6, 162.4) | 147.5±32.7 | (132.6, 162.4) | 147.5±32.7 | (132.6, 162.4) | |||||||
| Term Baboon | 160.8±32.5 | (120.5±201.1) | 160.8±32.5 | (120.5±201.1) | 160.8±32.5 | (120.5±201.1) | |||||||
| ELBW Human | 153.4±66.4 | (137.3, 169.5) | p=0.001 | 158.9±72.5 | (141.5. 176.3) | p<0.05 | 196.2±58.5 | (181.3, 211.2) | NS | ||||
| Orn | Preterm Baboon | 235.0±77.7 | (190.1, 279.9) | p<0.001 | p<0.001 | 905.5±305.2 | (711.6, 1099.4) | p<0.001 | p<0.001 | 647.9±515.9 | (216.6, 1079.2) | p<0.001 | p<0.001 |
| Fetal Baboon | 54.9±27.2 | (42.5, 67.3) | 54.9±27.2 | (42.5, 67.3) | 54.9±27.2 | (42.5, 67.3) | |||||||
| Term Baboon | 86.5±51.6 | (22.4, 150.5) | 86.5±51.6 | (22.4, 150.5) | 86.5±51.6 | (22.4, 150.5) | |||||||
| ELBW Human | |||||||||||||
| Glu | Preterm Baboon | 153.1±28.8 | (136.4, 169.7) | p<0.05 | p<0.05 | 133.8±53.4 | (99.9, 167.7) | NS | NS | 139.5±105.6 | (51.2, 227.8) | NS | NS |
| Fetal Baboon | 81.6±99.1 | (36.4, 126.7) | 81.6±99.1 | (36.4, 126.7) | 81.6±99.1 | (36.4, 126.7) | |||||||
| Term Baboon | 83.7±35.4 | (39.7, 127.7) | 83.7±35.4 | (39.7, 127.7) | 83.7±35.4 | (39.7, 127.7) | |||||||
| ELBW Human | 84.7±61.0 | (70.0, 99.5) | p<0.001 | 93.0±65.2 | (77.4, 108.7) | p<0.05 | 120.8±67.4 | (103.6, 138.1) | NS | ||||
| Asp | Preterm Baboon | 13.1±7.0 | (9.0, 17.1) | NS | NS | 22.4±9.6 | (16.3, 28.5) | p<0.001 | p=0.001 | 15.8±6.7 | (10.2, 21.4) | NS | NS |
| Fetal Baboon | 10.1±8.6 | (6.2, 14.0) | 10.1±8.6 | (6.2, 14.0) | 10.1±8.6 | (6.2, 14.0) | |||||||
| Term Baboon | 7.7±1.7 | (5.6, 9.7) | 7.7±1.7 | (5.6, 9.7) | 7.7±1.7 | (5.6, 9.7) | |||||||
| ELBW Human | 8.9±5.8 | (7.4, 10.4) | p=0.023 | 14.5±9.2 | (12.3, 16.7) | p<0.01 | 18.3±7.4 | (16.4, 20.2) | NS | ||||
| Ala | Preterm Baboon | 281.3±73.6 | (238.8, 323.7) | NS | NS | 200.4±126.8 | 119.9, 281.0) | p<0.05 | p<0.01 | 166.0±80.0 | (99.2, 232.9) | p<0.01 | p<0.01 |
| Fetal Baboon | 360.5±147.6 | (293.3, 427.7) | 360.5±147.6 | (293.3, 427.7) | 360.5±147.6 | (293.3, 427.7) | |||||||
| Term Baboon | 371.1±181.3 | (145.9, 596.2) | 371.1±181.3 | (145.9, 596.2) | 371.1±181.3 | (145.9, 596.2) | |||||||
| ELBW Human | 259.9±96.9 | (236.5, 283.4) | NS | 241.6±107.3 | (215.4, 267.7) | NS | 206.1±117.8 | (175.7, 236.6) | NS | ||||
| Asn | Preterm Baboon | 67.1±27.7 | (51.1, 83.1) | NS | NS | 28.1±11.7 | (20.6, 35.5) | p<0.001 | p<0.001 | 28.3±4.2 | (24.8, 31.9) | p<0.001 | p<0.01 |
| Fetal Baboon | 56.0±20.5 | (46.7, 65.4) | 56.0±20.5 | (46.7, 65.4) | 56.0±20.5 | (46.7, 65.4) | |||||||
| Term Baboon | 75.3±15.6 | (55.9, 94.6) | 75.3±15.6 | (55.9, 94.6) | 75.3±15.6 | (55.9, 94.6) | |||||||
| ELBW Human | 46.8±22.6 | (41.3, 52.4) | p<0.01 | 36.7±25.9 | (30.2, 43.2) | NS | 34.7±30.8 | (26.4, 43.1) | NS | ||||
| Gly | Preterm Baboon | 299.8±75.6 | (256.2, 343.5) | p<0.001 | NS | 333.5±166.5 | (227.7, 439.3) | p=0.001 | NS | 563.9±362.0 | (261.3, 866.6) | p<0.05 | NS |
| Fetal Baboon | 393.8±89.1 | (353.2, 434.4) | 393.8±89.1 | (353.2, 434.4) | 393.8±89.1 | (353.2, 434.4) | |||||||
| Term Baboon | 647.6±261.1 | (323.4, 971.8) | 647.6±261.1 | (323.4, 971.8) | 647.6±261.1 | (323.4, 971.8) | |||||||
| ELBW Human | 288.9±124.9 | (258.6, 319.1) | NS | 258.8±97.5 | (235.2, 282.0) | p<0.05 | 326.4±98.6 | (301.1, 351.6) | NS | ||||
| Cit | Preterm Baboon | 35.6±16.0 | (26.4, 44.9) | p<0.001 | NS | 20.4±10.9 | (13.5, 27.3) | p<0.001 | NS | 27.8±17.6 | (13.1, 42.6) | p<0.001 | NS |
| Fetal Baboon | 26.9±8.2 | (23.2, 30.6) | 26.9±8.2 | (23.2, 30.6) | 26.9±8.2 | (23.2, 30.6) | |||||||
| Term Baboon | 54.5±18.7 | (31.2, 77.7) | 54.5±18.7 | (31.22, 77.7) | 54.5±18.7 | (31.22, 77.7) | |||||||
| ELBW Human | |||||||||||||
| β-Ala | Preterm Baboon | 7.6±4.2 | (−2.8, 18.0) | NS | NS | 5.8±1.9 | (1.1, 10.4) | NS | NS | 15.0±8.2 | (4.8, 25.2) | NS | NS |
| Fetal Baboon | 8.0±8.2 | (4.3, 11.7) | 8.0±8.2 | (4.3, 11.7) | 8.0±8.2 | (4.3, 11.7) | |||||||
| Term Baboon | 15.4±4.6 | (9.7, 21.1) | 15.4±4.6 | (9.7, 21.1) | 15.4±4.6 | (9.7, 21.1) | |||||||
| ELBW Human |
Essential AAs
Elevated BCAAs
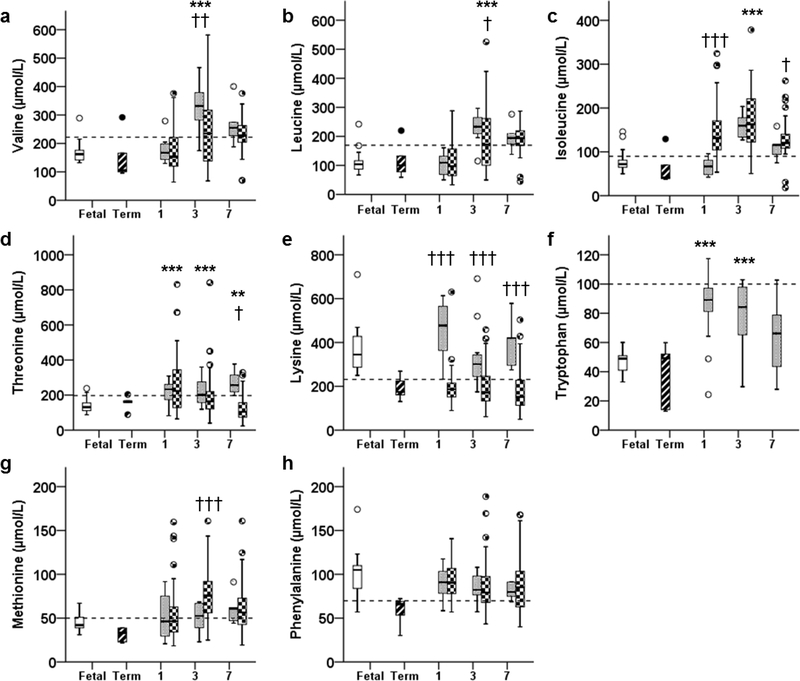
On DOL 1, Val concentrations were similar between fetal and preterm animals (P=1.0, Figure 1A). By DOL 3, Val was higher in preterm animals (P<0.001) and remained 1.3-fold higher in preterm animals on DOL 7 (however, P=0.127). Similarly, Leu concentrations were comparable to fetal concentrations on DOL 1 (P=1.0), but rose on DOL 3 to 2.1-fold higher in preterm animals (P<0.001, Figure 1B). Leu remained 1.5-fold higher in preterm animals on DOL 7 (however, P=0.110). Ile concentrations were also similar to fetal concentrations on DOL 1 (P=0.691), and rose on DOL 3 to 2.0-fold higher than fetal concentrations (P=0.015, Figure 1C). Ile concentrations were similar between preterm and fetal animals by DOL 7 (p=1.0).
Figure 1:
Plasma concentrations of essential amino acids: A) Valine, B) Leucine, C) Isoleucine, D) Threonine, E) Lysine, F) Tryptophan, G) Methionine, and H) Phenylalanine, in fetal (open bar), term (stripped bar) and preterm baboons (gray bar), and ELBW human infants (checkered bar) at days of life 1, 3, and 7 are shown. *P<0.05, ** P <0.01, *** P <0.001; preterm baboon vs fetal baboon. † P <0.05, †† P <0.01, ††† P <0.001; preterm baboon vs ELBW human. Dashed bar indicates the upper 95% percentile for term breastfed infants reported in reference 32.
Other Elevated Essential AAs
Thr was higher in preterm animals throughout the study: 2.1-fold higher at DOL 1 (P<0.001), and approximately 1.6-fold higher on DOL 3 (P=0.001) and 7 (P<0.05, Figure 1D). Lysine (Lys) concentrations tended to be higher in preterm animals on DOL 1 (P=0.058), but fell to fetal concentrations by DOL 3 (P=1.0), and remained stable to DOL 7 (P=1.0, Figure 1E). Tryptophan (Trp) concentrations in preterm animals were almost double those of fetal animals on DOLs 1 and 3 (P<0.001). However, by DOL 7, Trp concentrations were similar between preterm and fetal animals (P=1.0, Figure 1F).
Unaltered Essential AAs
Concentrations of Methionine (Met) and Phenylalanine (Phe) were similar (P=0.554, P=0.491, P=1.0 and P =0.856, P =0.425, P =0.086, respectively) between preterm and fetal animals throughout the study (Figure 1, G and H, respectively).
Conditionally Essential AAs
Elevated Conditionally Essential AAs
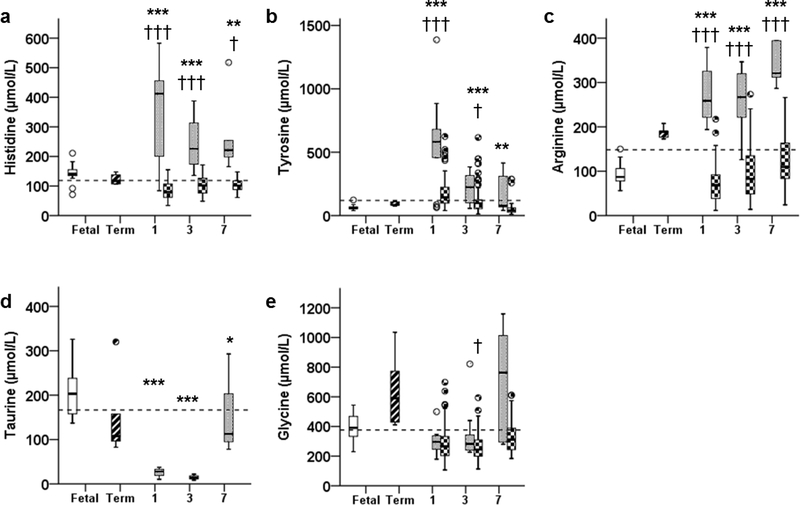
Histidine (His) concentrations were 2.4-fold higher in preterm animals on DOL 1 (P <0.001) and remained elevated on DOL 3 (P <0.001) and 7 (~ 1.6-fold higher; P =0.01, Figure 2A). Tyrosine (Tyr) concentrations were 8.9-fold higher in preterm animals at DOL 1 (P <0.001) and remained higher from DOLs 3 (P <0.001) to 7 (P <0.01, Figure 2B). Contrary to what is found in preterm infants, Arginine (Arg) was higher (P <0.001) in preterm animals on DOLs 1, 3, and 7 and rose up to 3.4-fold higher than fetal concentrations (Figure 2C).
Figure 2:
Plasma concentrations of conditionally essential amino acids: A) Histidine, B) Tyrosine, C) Arginine, D) Taurine, and E) Glycine, in fetal (open bar), term (stripped bar), and preterm baboons (gray bar) and ELBW human infants (checkered bar) at days of life 1, 3, and 7 are shown. * P <0.05, ** P <0.01, *** P <0.001; preterm baboon vs fetal baboon. † P <0.05, †† P <0.01, ††† P <0.001; preterm baboon vs ELBW human. Dashed bar indicates the upper 95% percentile for term breastfed infants reported in reference 32.
Decreased Conditionally Essential AAs
Taurine (Tau) was lower in preterm animals throughout the study. Concentrations were 12% (P <0.001), 7% (P <0.001), and 54% (P <0.05) of fetal concentrations on DOLs 1, 3, and 7 (Figure 2D).
Unaltered Conditionally Essential AAs
Glycine (Gly) concentrations were similar to fetal animals throughout the study period (P =0.076, P =0.776, P =0.176, DOLs 3–7; Figure 2E).
Non-essential AAs
Increased Non-essential AAs
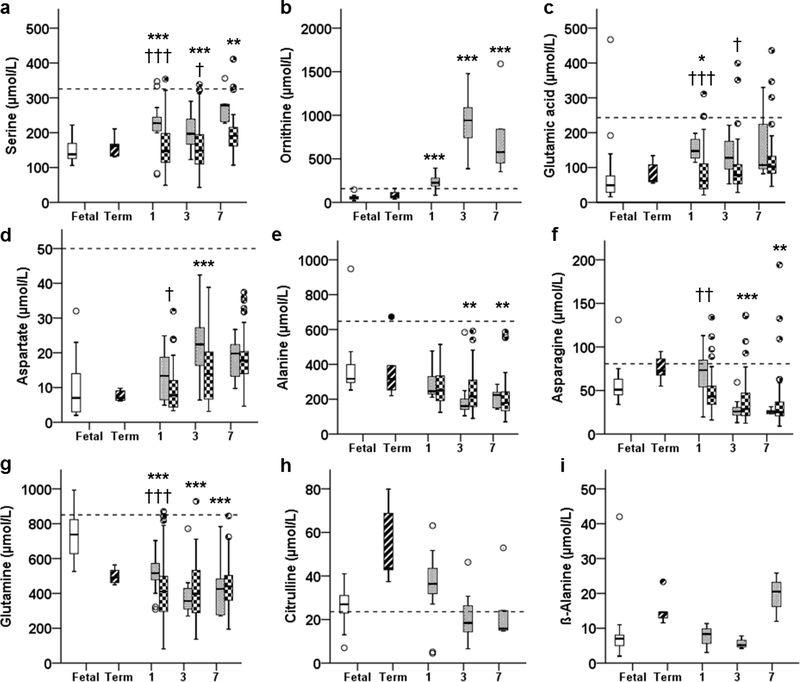
Serine (Ser) concentrations were 1.5-fold higher in preterm animals throughout the study (P <0.001, P <0.01, P =0.01, DOLs 3–7, Figure 3A). Additionally, Ornithine (Orn) concentrations were 4-fold higher in preterm animals on DOL 1, and 16-fold higher on DOL 3, and were 12- fold higher than fetal concentrations on DOL 7 (P <0.001, all days; Figure 3B).
Figure 3:
Plasma concentrations of non-essential amino acids: A) Serine, B) Ornithine, C) Glutamic Acid, D) Aspartate, E) Alanine, F) Asparagine, G) Glutamine, H) Citrulline, and I) β- Alanine, in fetal (open bar), term (stripped bar), and preterm baboons (gray bar) and ELBW human infants (checkered bar) at days of life 1, 3, and 7 are shown. * P <0.05, ** P <0.01, *** P <0.001; preterm baboon vs fetal baboon. † P <0.05, †† P <0.01, ††† P <0.001; preterm baboon vs ELBW human. Dashed bar indicates the upper 95% percentile for term breastfed infants reported in reference 32.
Glutamic Acid (Glu) was higher in preterm animals on DOL 1 (P <0.05), whereas on DOLs 3 and 7 differences were no longer significant (P =0.258, P =0.458, respectively; Figure 3C). Concentrations of Aspartate (Asp) were similar on DOL1 (P =0.791), rose on DOL 3 (2.2-fold higher in preterm animals, P <0.001), then dropped back to fetal levels on DOL 7 (P =0.245, Figure 2D).
Decreased Non-essential AAs
Preterm concentrations of Alanine (Ala) and Asparagine (Asn) were lower than fetal concentrations on DOLs 3 (P <0.05 and P <0.001, respectively) and 7 (P <0.01 and P <0.001, respectively; Figure 3, E and F). Furthermore, Glutamine (Gln) was lower in preterm animals throughout the study (P<0.001) and fell to approximately 50% of fetal levels by DOL 7 (Figure 3G).
Unaltered Non-essential AAs
Concentrations of Citrulline (Cit) (P =0.166, P =0.315, P =1.0) and β-Alanine (β-Ala) (P=1.0, P =1.0, P =0.247) were similar to fetal animals throughout the study (Figure 3, H and I, respectively).
Baboon vs Human Concentrations
We next compared results obtained in preterm baboons receiving AA supplementation to those from ELBW human infants at approximately similar gestational ages and similar DOLs enrolled in an early and high AA supplementation regimen similar to the regimen the preterm baboons received. Comparisons are shown in Table 2 and Figures 1–3. Most of the AA concentrations were similar between preterm human infants and preterm baboons (Met, Phe, Ser, Thr), including those that were significantly lower than fetal concentrations (Gln, Asp). Only four AAs were significantly different between preterm baboons and preterm humans during the first week of life (Arg, Lys, His and Tyr) (Table 2 and Figures 1E and 2, A–C).
DISCUSSION
To prevent failure of energy availability, premature infants require exogenous energy substrates. It is widely accepted that administration of a minimum of 1 – 1.5 g·kg−1·day−1 of parenteral AAs improves nitrogen balance and increases protein synthesis6. However, the effect of parenteral AA administration on serum AA concentrations and protein metabolism remains unclear, and postnatal growth failure continues to be a major problem1,15. Therefore, many studies have attempted to mimic fetal accretion of protein in utero through more accelerated (earlier initiation, higher dosing) AA administration4,9,10,16–20, but AA concentrations are not monitored routinely and long term follow up is lacking. The purpose of this study was to investigate the effects of early and high AA supplementation on plasma AA concentrations using a non-human primate model of prematurity. This approach has the advantage of including a number of fetal and term controls well matched to the study population, with a controlled diet and minimal confounders, to provide context in which to interpret the data. Furthermore, baboons have 97% phylogenetic proximity to humans, exhibit a similar clinical course to human preterm infants, and have been used to study placental AA transport and neonatal parenteral nutrition21,22.
Our results demonstrate that AA concentrations in parenterally supplemented preterm animals, analogous to ELBW infants in their gestational maturity, are significantly different from fetal animals. Among the perturbations, we found relatively persistent elevations in Orn and the BCAAs - Leu, Ile, and Val. These results are consistent with those in human neonates reported by Clark et al9 and our previous clinical study10. It was of particular interest to note that concentrations of BCAAs were almost identical between ELBW infants and preterm baboons. The 3 essential BCAAs- Leu, Ile, and Val- are initially catabolized by common pathways and later diverge into complex pathways. Collectively, branched-chain organic acidurias result from an abnormality of specific enzymes causing specific diseases such as maple syrup urine disease, isovaleric aciduria, propionic aciduria, methylmalonic aciduria and other disorders involving elevations of Leu, Ile and Val23. Presentation of these disorders includes acidosis, neurological involvement (coma, lethargy, hypotonia, and muscle weakness), liver dysfunction, vomiting, and failure to thrive. BCAAs play an important role in neurotransmitter development and urea formation, and prolonged BCAA elevation leads to demyelination, especially of periventricular white matter23,24. We have previously found that concentrations of BCAAs correlate inversely with neurodevelopmental outcome when AA solutions (Aminosyn® -PF 10%) were administered up to 4 g·kg−1·day−1 in ELBW infants during the first week of life 4,24. Finally, in term infants, high protein intake has been shown to increase plasma BCAAs, leading to dysregulation of fatty acid β-oxidation and subsequent fat storage, and contributing to high early weight gain and body fat accumulation25. Collectively, these findings, and the findings of this study, highlight the importance of maintaining normalcy of BCAAs, particularly during critical periods of development. It remains to be determined if these transient abnormalities in preterm baboons translate into long term neurological impairments.
We were surprised to find extremely elevated concentrations of Orn. The hyperornithinemia found in this study is extremely concerning, considering the adverse effects of this condition26. In the neonatal period, the main role of ornithine aminotransferase (OAT) is to maintain the urea cycle by converting Gln to Arg; a deficiency causes late hyperornithinemia leading to gyral degradation and retinal toxicity23. On DOLs 3 and 7, respectively, preterm baboon plasma concentrations of Orn were 16-fold and 12-fold higher than fetal baboons. These levels are as high as those reported in neonates with OAT deficiency (5-fold to 20-fold higher) and might have severe consequences, even if exposed for shorter periods of time than neonates with inherited deficiencies27. Unfortunately, we did not have Orn concentrations in our preterm human infants for comparison. Therefore, further studies in preterm infants need to be conducted as elevation early in life could be contributing to the neurodevelopmental impairments commonly found in preterm infants. Collectively, these results emphasize a need for caution when implementing early and high AA supplementation strategies in ELBW premature infants when their brains are at a critical stage of development.
Abnormally low AA concentrations are also a concern. Gln continues to be shown to be low in preterm animals/humans compared to their fetal levels. Gln plays a role in immune function and participates in metabolic pathways including methylation,28 and although no specific benefit of Gln supplementation has been found29, mimicking fetal levels may provide unknown immediate or later-life benefits. Similarly, Ala, Asn, and Tau are extremely low in premature infants with current AA solutions. Ala and Asn participate in protein biosynthesis and therefore a low level might be expected to impair growth. Furthermore, Tau plays an important role in mitochondrial oxidative phosphorylation and Tau deficiencies may contribute to mitochondrial dysfunction and associated pathologies30.
We also found that, for many AAs, the greatest aberrations in plasma AA concentrations tended to occur on DOL 3. We speculate this is likely due to the maturation of metabolic pathways postnatally, and the postnatal increase in physiologic processes, such as glomerular filtration rates. We have previously demonstrated such patterns of postnatal development in the insulin signaling pathways of premature baboons from birth to 14 days old13. A limitation of this study is that preterm infants/baboons received a small amount of enteral feeds, which is different from the current approach of early enteral feeding initiation. However, it is unlikely that sick preterm infants will be receiving a large enough amount of enteral feeds (>60 ml·kg−1·day−1) by DOL 3 and therefore will likely be receiving 3–4 g·kg−1·day−1 of IV AAs in their parenteral nutrition; this common approach will likely place them at risk of having high BCAA concentrations during DOL 3, but perhaps not on DOL7. Further studies evaluating AA metabolism during the first week of life with higher enteral feeding approaches need to be performed.
The varied compositions of currently available products for parenteral AA supplementation further complicate the issue of optimizing nutritional regimens for preterm infants. Both of the products approved for use in the US (TrophAmine® and Aminosyn® -PF 10%) were formulated according to the needs of term breastfed infants and lead to increased serum BCAAs if administered to neonates born preterm. In a study by Vlaardingerbroek et al, provision of AAs up to 3.6 g·kg−1·day−1 in very-low-birth-weight infants produced lower BCAA levels than in the current study31. This protocol utilized a different AA solution (Primene® 10%, Baxter Healthcare Ltd, Thetford, UK) that is not available in the United States and is significantly different from the AA solutions utilized in this baboon study and our prior human study. Further research into parenteral AA supplements, comparing Primene® 10% to United States- approved AA solutions in premature infants is certainly warranted.
In conclusion, our results demonstrate disruption of plasma AA concentrations in premature baboons treated with early and high AA supplementation compared to fetal controls. When considered in context with currently available clinical studies, these results suggest we may be exposing developing organs at key stages of maturation and differentiation to abnormal AA concentrations by broadly adopting early and high AA supplementation. Furthermore, AA solutions currently available in the United States may not be suitable for the most immature infants, in particular during the first week of life, when metabolic pathways are not fully developed. Large, multicenter studies with extended follow-up periods are needed to better determine safety, dose, and efficacy of AA solutions specifically tailored for premature infants. Finally, newer technology to rapidly measure AA concentrations for clinical practice is needed to monitor patients as AA doses are increased, as infants may have different protein requirements/tolerance depending on gestational age, postnatal age, and severity of illness.
CLINCAL RELEVANCY STATEMENT.
Understanding effects of intravenous AA solutions on protein metabolism in premature infants is critical to developing appropriate nutritional regimens for this vulnerable population of neonates. Here, we demonstrate dysregulation of plasma AA concentrations in premature baboons receiving early and high AA supplementation analogous to that received by human infants. These findings suggest current AA solutions and regimens are not well suited to the metabolic demands of extremely premature infants. Further studies are necessary.
Acknowledgments
Financial Support: Robert Wood Johnson Foundation (C.B.), UTHSCSA CTSA (UL1RR025767, C.B), American Diabetes Association (C.B).
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest relevant to the content of this manuscript.
REFERENCES
- 1.Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003;111(5):986–990. [DOI] [PubMed] [Google Scholar]
- 2.Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth Failure in the Preterm Infant : Can We Catch Up ? Semin Perinatol. 2003;27(4):302–310. [DOI] [PubMed] [Google Scholar]
- 3.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–1261. [DOI] [PubMed] [Google Scholar]
- 4.Blanco CL, Gong AK, Schoolfield J, et al. Impact of early and high amino acid supplementation on ELBW infants at 2 years. J Pediatr Gastroenterol Nutr. 2012;54(5):601–607. doi: 10.1097/MPG.0b013e31824887a0. [DOI] [PubMed] [Google Scholar]
- 5.Zlotkin SH. Intravenous Nitrogen Intake Requirements in Full-Term Newborns Undergoing Surgery. Pediatrics. 1984;73(493–496). [PubMed] [Google Scholar]
- 6.Thureen PJ, Melara D, Fennessey P V, Hay WW. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res. 2003;53(1):24–32. [DOI] [PubMed] [Google Scholar]
- 7.Hay WW. Aggressive nutrition of the preterm infant. Curr Pediatr Rep. 2014;1(4):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera A, Bell EF, Bier DM. Effect of Intravenous Amino Acids on Protein Metabolism of Preterm Infants during the First Three Days of Life. Pediatr Res. 1993;33(2):6–11. [DOI] [PubMed] [Google Scholar]
- 9.Clark RH, Chace DH, Spitzer AR. Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit : a randomized, controlled trial. Pediatr Res. 2007;120(6):1286–1296. doi: 10.1542/peds.2007-0545. [DOI] [PubMed] [Google Scholar]
- 10.Blanco CL, Gong AK, Green BK, Falck A, Schoolfield J, Liechty EA. Early changes in plasma amino acid concentrations during aggressive nutritional therapy in extremely low birth weight infants. J Pediatr. 2011;158(4):543–548.e1. doi: 10.1016/j.jpeds.2010.09.082. [DOI] [PubMed] [Google Scholar]
- 11.Balakrishnan M, Jennings A, Przystac L, et al. Growth and neurodevelopmental outcomes of early, high-dose parenteral amino acid intake in very low birth weight infants: a randomized controlled trial. J Parenter Enter Nutr. 2017;1(1). [DOI] [PubMed] [Google Scholar]
- 12.Li C, Mcdonald TJ, Wu G, Nijland MJ, Nathanielsz PW. Intrauterine growth restriction alters term fetal baboon hypothalamic appetitive peptide balance. Endocrinology. 2013;217:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco CL, McGill-Vargas LL, Gastaldelli A, et al. Peripheral insulin resistance and impaired insulin signaling contribute to abnormal glucose metabolism in preterm baboons. Endocrinology. 2015;156(3):813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rassin DK. Essential and Non-essential Amino Acids in Neonatal Nutrition. In: Protein Metabolism During Infancy. Vol 33; 1994:183–195. [Google Scholar]
- 15.Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104(2):280–289. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim HM, Jeroudi MA, Baier RJ, Dhanireddy R, Krouskop RW. Aggressive early total parental nutrition in low-birth-weight infants. J Perinatol. 2004;24:482–486. doi: 10.1038/sj.jp.7211114. [DOI] [PubMed] [Google Scholar]
- 17.Maggio L, Cota F, Gallini F, Lauriola V, Zecca C, Romagnoli C. Effects of high versus standard early protein intake on growth of extremely low birth weight infants. J Pediatr Gastroenterol Nutr. 2007;44:124–129. [DOI] [PubMed] [Google Scholar]
- 18.Poindexter BB, Langer JC, Dusick AM, Ehrenkranz RA. Early provision of amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. J Pediatr. 2006;148(3):300–305. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Lee BS, Park H, et al. Effect of high vs standard early parenteral amino acid supplementation on the growth outcomes in very low birth weight infants. J Parenter Enter Nutr. 2013;37(3):327–334. [DOI] [PubMed] [Google Scholar]
- 20.Blanco CL, Falk A, Green BK, Cornell JE, Gong AK. Metabolic responses to early and high protein supplementation in a randomized trial evaluating the prevention of hyperkalemia in extremely low birth weight infants. J Pediatr. 2008:535–540. [DOI] [PubMed] [Google Scholar]
- 21.Pantham P, Rosario FJ, Nijland M, et al. Reduced placental amino acid transport in response to maternal nutrient restriction in the baboon. Am J Physiol Regul Integr Comp Physiol. 2015;2:740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stabler SP, Morton RL, Winski SL, Allen RH, White CW. Effects of parenteral cysteine and glutathione feeding in a baboon. Am J Clin Nutr. 2000;72:1548–1557. [DOI] [PubMed] [Google Scholar]
- 23.Baumgartner M, Shih V. Disorders of ornithine metabolism In: Fernandes J, Saudubray J, Van Den Berghe G, Walter J, eds. Inborn Metabolic Diseases Diagnosis and Treatment. 4th ed. Berlin: Springer; 2011:283–291. [Google Scholar]
- 24.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454. [DOI] [PubMed] [Google Scholar]
- 25.Kirchberg FF, Harder U, Weber M, et al. Dietary protein intake affects amino acid and acylcarnitine metabolism in infants aged 6 months. J Clin Endocrinol Metab. 2015;100(1):149–158. [DOI] [PubMed] [Google Scholar]
- 26.Shih VE, Efron ML, Moser HW. Hyperornithinemia, hyperammonemia, and homocitrullinuria. A new disorder of amino acid metabolism associated with myoclonic seizures and mental retardation. Am J Dis Child. 1969;117(1):83–92. [PubMed] [Google Scholar]
- 27.Zubarioglu T, Kiykim E, Cansever MS, Zeybek CA. Ornithine aminotransferase deficiency in differential diagnosis of neonatal hyperammonemia : a case with a novel OAT gene mutation. Indian J Pediatr. 2016;83(July):754–755. [DOI] [PubMed] [Google Scholar]
- 28.Ulrey CL, Liu L, Andrews LG, Tollefsbol TO. The impact of metabolism on DNA methylation. Hum Mol Genet. 2005;14(1):139–147. [DOI] [PubMed] [Google Scholar]
- 29.Poindexter BB, Ehrenkranz RA, Stoll BJ, et al. Parenteral glutamine supplementation does not reduce the risk of mortality or late-onset sepsis in extremely low birth weight infants. Pediatrics. 2004;113(5). [DOI] [PubMed] [Google Scholar]
- 30.Hansen SH, Andersen ML, Cornett C, Gradinaru R, Grunnet N. A role for taurine in mitochondrial function. J Biomed Sci. 2010;17(Suppl 1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlaardingerbroek H, Vermeulen MJ, Rook D, et al. Safety and efficacy of early parenteral lipid and high-dose amino acid administration to very low birth weight infants. J Pediatr. 2013;163(3):638–644.e5. [DOI] [PubMed] [Google Scholar]
- 32.Wu PYK, Edwards N, Storm MC. Plasma amino acid pattern in normal term breast-fed infants. J Pediatr. 1986;109(2):7–9. [DOI] [PubMed] [Google Scholar]