Abstract
Environmental changes cause stress, Reactive Oxygen Species and unfolded protein accumulation which hamper synaptic activity and trigger cell death. Heat shock proteins (HSPs) assist protein refolding to maintain proteostasis and cellular integrity. Mechanisms regulating the activity of HSPs include transcription factors and posttranslational modifications that ensure a rapid response. HSPs preserve synaptic function in the nervous system upon environmental insults or pathological factors and contribute to the coupling between environmental cues and neuron control of development. We have performed a biased screening in Drosophila melanogaster searching for synaptogenic modulators among HSPs during development. We explore the role of two small-HSPs (sHSPs), sHSP23 and sHSP26 in synaptogenesis and neuronal activity. Both sHSPs immunoprecipitate together and the equilibrium between both chaperones is required for neuronal development and activity. The molecular mechanism controlling HSP23 and HSP26 accumulation in neurons relies on a novel gene (CG1561), which we name Pinkman (pkm). We propose that sHSPs and Pkm are targets to modulate the impact of stress in neurons and to prevent synapse loss.
Introduction
Synaptic dynamics remodel neuronal circuits under stress conditions [1]. The Heat Shock Protein family (HSPs) is involved in preserving cellular functions such as stress tolerance, protein folding and degradation, cytoskeleton integrity, cell cycle and cell death [2–7]. HSPs are molecular chaperones that represent an intracellular protein quality system to maintain cellular protein homeostasis, preventing aggregation and promoting protein de novo folding or refolding and degradation of misfolded proteins [8]. In addition, HSPs participate in developmental functions in a stress-independent manner [9, 10]. In Drosophila development small Heat Shock proteins (sHsps) have a specific temporal and spatial pattern of expression [10]. In particular, sHsp23 and sHsp26 show high expression levels in CNS during development, suggesting a role in neural development [10].
sHSPs include a large group of proteins represented in all kingdoms of life [11], with a conserved protein binding domain of approximately 80 amino-acid alpha crystallin [12]. These molecular chaperones were initially described as low molecular weight chaperones that associate early with misfolded proteins and facilitate refolding or degradation by other chaperones and co-factors [11] [13]. However, members of the sHSPs have diverse functions beyond the chaperon activity including cytoskeleton assembly [14], the suppression of reactive oxygen species, anti-inflammatory, autophagy, anti-apoptotic and developmental functions (reviewed in [2]). sHSPs represent the most extended subfamily of HSPs, albeit the less conserved [15]. sHSPs have a conserved primary structure divided in three elements required for their function: 1) a variable N-terminal long-sequence related to oligomerization, 2) the conserved α-crystallin domain required for dimmers formation that represents the main hallmark of sHsps family, and 3) a flexible short C-terminal sequence mediating oligomers stability [11, 16]. Posttranslational modifications in sHSPs shift the folding/degradation balance and, in consequence, alter dimer or oligomer formation and function [11, 17]. This chaperone control system modulates critical decisions for the folding or degradation proteins and a failure causes pathological conditions [17].
HSPs protect synaptic function in the nervous system from environmental insults or pathological factors [18–20] (reviewed in [21]), and are also associated to neurodegenerative diseases, aberrant protein-induced neurotoxicity and disease progression [13]. The sHSPs family is involved as a non-canonical role in Drosophila development and other biological processes such as synaptic transmission [22]. However, its implication in synaptic dynamics during development has not been described yet. Synapse number can be altered due to the influence of physiological parameters (aging, hormonal state, exercise) [23–26], pathological (neurodegenerative process) [27, 28] or induced conditions (mutants) [29] which alter cellular components and pathways [30]. The imbalance between the pro- and anti-synaptogenic pathways modulates the number of synapses [30]. The neuromuscular junction (NMJ) of Drosophila melanogaster is a stereotyped structure well established for the study of synapses [31]. Most of the molecules involved in synaptic transmission are conserved between Drosophila and vertebrates thus, this model system is well established for the study of synapses [32].
Here, we study the contribution of two sHsps, sHp23 and sHsp26 in the development of the CNS and synapse modulation. sHsp23 and sHsp26 are expressed in the CNS during the development [10, 33, 34] but their function remains unclear. In addition, we describe the function of CG1561, named Pinkman (Pkm), as a novel putative kinase that interacts with sHSP23 and sHSP26. Pkm regulates expression and protein stability and participates in the establishment of synapse number during development.
Materials and methods
Drosophila strains
Flies were maintained at 25°C in fly food in cycles of 12 hours of light and 12 hours of darkness. The following stocks were used: UAS.LacZ (gift from Dr. Wurz). Fly stocks from the Bloomington Stock Center: Gal4.D42 (BL-8816), UAS.Hsp23 (BL-30541), P{UAS-mLexA-VP16-NFAT}H2/TM3, Sb1 (BL- 66543), P{LexAop-CD8-GFP-2A-CD8-GFP}2; P{UAS-mLexA-VP16-NFAT}H2, P{lexAop-rCD2-GFP}3/TM6B, Tb1 (BL-66542). Fly Stocks from Vienna Stocks Center: Hsp26-GFP-V5-Flag (VDR318685), UAS.Hsp26RNAi and UAS.CG1561RNAi (VDR106503 KK (1), VDR32634 GD (2) and VDR32635 GD (3)). Fly Stocks from the FlyORF Zurich ORFeome Project: UAS.Hsp26 (F000796).
Drosophila dissection and immunostaining
Drosophila third instar larvae were dissected in phosphate-buffered saline (PBS) and fixed with PFA 4% in phosphate-buffered saline (PBS). Then, the samples were washed with PBST (PBS with 0.5% Triton X-100) and blocked with 5% bovine serum albumin (BSA) (Sigma) in PBST. We quantified the total number of active zones per NMJ of third instar larvae. We used the binary system Gal4/UAS (Brand & Perrimon, 1993) to drive all genetic manipulations to motor neurons (D42-Gal4). Actives zones were visualized using a mouse monoclonal antibody nc82 (1:20, DSHB, IA) which identifies the protein Bruchpilot, a presynaptic element. Neuronal membranes were visualized with rabbit anti-HRP (1:300, Jackson ImmunoResearch, West Grove, PA). Fluorescent secondary antibodies were Alexa 488 (goat anti-mouse, 1:500, Molecular Probes, Eugene, OR) and Alexa 568 (goat anti-rabbit, 1:500, Molecular Probes). Larvae were mounted in Vectashield medium (Vector Labs, Burlingame, CA). Synapse quantifications were obtained from the NMJ Drosophila model in muscle fiber 6/7 of the third abdominal segment only to regulate inter-individual data variability.
To localize sHSP23 or sHSP26, third instar larval brain or NMJ were dissected. We use an Hsp26-GFP-V5 fusion construct. sHSP23 was visualized using an anti-Hsp23 (Sigma-Aldrich S 0821) (1:500), and sHSP26 was visualized using anti-V5 (1:50) (Invitrogen 1718556) and anti-GFP mouse (1:50) (Invitrogen A11122). Drosophila brains were mounted in Vectashield with DAPI medium (Vector Labs, Burlingame, CA).
Image acquisition
Confocal Images were acquired at 1024x256 resolution as serial optical sections every 1 μm. We used a 63x objective with a Leica Confocal Microscope TCS SP5 II (Mannheim, Germany). We used IMARIS software (Bitplane, Belfast, UK) to determine the number of mature active zones with the ‘spot counter’ module.
We visualized Hsp23-Hsp26 co-localization and CaLex signal in ventral ganglia cells of third instar larva brains. We acquired brain images at 1024x1024 resolution as serial optical sections every 1μm at 20x objective. We acquired ventral ganglia cells images at 1024x1024 resolution, 63x objective with magnification of 2.5. We processed the images and analyzed them with LAS-AF (Leica Application Suite software).
Antibody generation
To detect sHsp26 protein in western blot we generated (Abmart) a mouse monoclonal antibody against the sHsp26 peptide sequence: GKENGAPNGKDK MSLSTLLSLVDELQEPRSPIYELGLGLHPHSRYVLPLGTQQRRSINGCPCASPICPSSPAGQVLALRREMANRNDIHWPATAHVGKDGFQVCMDVAQFKPSELNVKVVDDSILVEGKHEERQDDHGHIMRHFVRRYKVPDGYKAEQVVSQLSSDGVLTVSIPKPQAVEDKSKERIIQIQQVGPAHLNVKANESEVKGKENGAPNGKDK
Co-Immunoprecipitation
For biochemical assays, 5–10 adult fly heads were lysed in immunoprecipitation lysis buffer (NaCl 150 mM, 0,1% Tween-20 (Polyoxyethylene sorbitane monolaureate), TBS pH 7.5). We incubated Protein A/G agarose beads overnight at 4°C with 2 μl of the indicated antibody or control IgG (1:100), followed by incubation at 4°C for 1 h with supernatants. We washed the beads and resuspended in 1× SDS–PAGE loading buffer for western blot analysis in a 4%–12% gradient SDS-PAGE for the detection of sHSP23 and sHSP26. After electro-blotted onto nitrocellulose 0.45 μM (GE Healthcare) 100V for 1 hour, we blocked the membranes in TBS-Tween-20 buffer with 5% BSA. We incubated the membranes overnight at 4°C in constant agitation with anti-Hsp23 antibody (1:1000) (Sigma-Aldrich S0821), anti-Hsp26 (1:1000) (Abmart) We visualized the antibody-protein interaction by chemoluminescence using IRDye Secondary Antibodies anti-mouse (IRDye 800CW, LI-COR), anti-rabbit (IRDye 680 RD, LI-COR) and developed with Odyssey equipment. We used three RNAi tools to downregulate pkm expression to replicate this condition. pkm RNAi 2 was selected to do the rest of experiments due to the evidences we obtained in the blot assay.
Western blot
5–10 head samples were treated with lysis buffer (TBS1x, 150mMNaCl, IP 50x) and then homogenized and centrifuged 13500 rpm for 5 minutes. After selecting the supernatant we added NuPage 4x (Invitrogen by Thermo Fisher Scientific) and ß-mercaptoethanol 5%. Western blot analysis samples were run in a 4%–12% gradient SDS-PAGE for the detection of sHSP23 and sHSP26. After electro-blotted onto nitrocellulose 0.45 μM (GE Healthcare) 100V for 1 hour, we blocked the membranes in TBS-Tween-20 buffer with 5% BSA. We incubated the membranes overnight at 4°C in constant agitation with anti-Hsp23 antibody (1:1000) (Sigma-Aldrich S0821), anti-Hsp26 (1:1000) (Abmart) We visualized the antibody-protein interaction by chemo luminescence using IRDye Secondary Antibodies anti-mouse (IRDye 800CW, LI-COR), anti-rabbit (IRDye 680 RD, LI-COR) and developed with Odyssey equipment. Tubulin were used as a control.
Gene expression analysis with qPCR
10–15 head tissue samples were treated and homogenized with Trizol (Ambiend for Life techonologies). Chloroform was added and then centrifuged 13000 rpm at 4°C for 15 minutes. After discarding the supernatant, the RNA was treated with Isopropanol and then centrifuged 13000 rpm at 4°C for 10 minutes and washed with 75% Ethanol. RNA pellet was dissolved in DNAase RNAase free water. Then we performed a transcriptase reaction and a qPCR assay using Rp49 gene as a control. Primers for sHsp23, sHsp26, Pinkman and Cat were used: sHsp23 Fv (5′-3′) TGCCCTTCTATGAGCCCTAC, sHsp23 Rv (3′-5′) TCCTTTCCGATTTTCGACAC, sHsp26 Fv (5′-3′) TAGCCATCGGGAACCTTGTA, sHsp26 Rv (3′-5′) GTGGACGACTCCATCTTGGT, pkm Fv (5′-3′) TCGTGCTGGAGGATCTGTCTT, pkm Rv (3′-5′) CCCGGCCAATGATATAGCAT, Catalase Fv (5′-3′) TTCGATGTCACCAAGGTCTG, Catalase Rv (3′-5′) TGCTCCACCTCAGCAAAGTA, rp49 Fv (5′-3′) CCATACAGGCCCAAGATCGT, rp49 Rv (5′-3′) AACCGATGTTGGGCATCAGA.
Statistics
To analyze the data, we used GraphPad Prism 6 GraphPad Software, La Jolla, CA). Data are shown as mean ± SD. Statistical significance was calculated using D´Agostino & Pearson normality test and a Student’s two-tailed t-test with Welch‐correction. In case data were not normal, we performed a Student´s two-tailed t-test with Mann–Whitney-U correction. For multiple comparisons, we used One‐way ANOVA test with Bonferroni post‐test. *p value ≤ .05; ** p value ≤ .01; *** p value ≤ .001; **** p value<0,0001. p value > .05 were not considered significant.
Technical considerations
Each experiment condition has its own control sample to reduce external variables.
Results
Heat shock proteins modify synapses in CNS
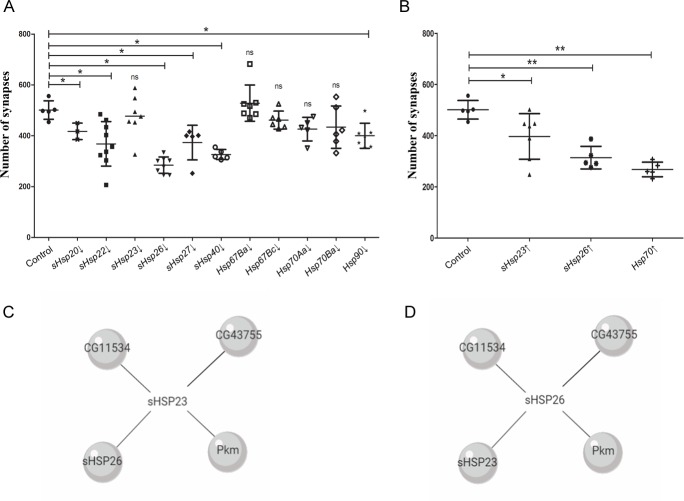
To determine the effect of HSPs in synaptogenesis, we used the UAS/Gal4 Drosophila binary expression system [35] to modify Hsps expression in motor neurons using D42-Gal4 lines. We used UAS-RNAi lines knockdown sHsp20, sHsp22, sHsp23, sHsp26, sHsp27, sHsp40, Hsp67 Ba, sHsp27 Bc, Hsp70 Aa, Hsp70 Ba and Hsp90 (Fig 1A). To visualize the number of active zones in the NMJs we used anti-bruchpilot (brp) antibody. The quantification of the active zones revealed that the knockdown of sHsp20, sHsp22, sHsp26, sHsp27, sHsp40 and Hsp90 during development provoked a reduction in synapse number. In addition, we tested the effect in synapse number of sHsp23, sHsp26 and Hsp70 overexpression (Fig 1B). The results show that the upregulation of sHsp23, sHsp26 or Hsp70 decrease the number of active zones (Fig 1B).
Fig 1. Small heat shock proteins modulate synapses during Drosophila development.
Synapses quantification screening with sHsps genetic tools under D42 driver expression. (A) Synapses modulation were detected by sHsp20 RNAi (sHsp20↓), sHsp22 RNAi (sHsp22↓), sHsp23 (sHsp23↓), sHsp26 RNAi (Hsp26↓), sHsp27 RNAi (Hsp27↓), Hsp40 RNAi (Hsp40↓), Hsp90 RNAi (Hsp90↓), (B) UAS.sHsp23 (Hsp23↑) UAS.sHsp26 (Hsp26↑) and UAS.Hsp70 (Hsp70↑) samples. One‐way ANOVA test with Dunn's multiple comparisons post‐test. *p value ≤ .05; ** p value ≤ .01; *** p value ≤ .001. p value > .05 were not considered significant. Error bars show S.D. (C) Diagram of sHsp23 interactome and (D) diagram of sHsp26 interactome form http://flybi.hms.harvard.edu/results.php.
We focused on the role of two sHSPs, sHSP23 and sHSP26, due to their potential role as non-canonical-sHSPs in the CNS and their unexplored implication in synapses modulation. The upregulation of sHsp23 in presynaptic neurons causes a reduction in synapse number (Fig 1B). In addition, sHsp26 knockdown or upregulation induces a reduction in synapse number (Fig 1A and Fig 1B). Thus, the results suggest that sHSP23 is not required for synapse formation but in excess it is detrimental for the neuron and causes a reduction of synapse number during development. Besides, modification in any direction of sHsp26 expression affects to the correct establishment of synapse number during development, suggesting that sHSP26 fine control is required during development for synapse organization.
According to the interactome (flybase) both chaperones are predicted to physically interact with each other [36] (Fig 1C and Fig 1D). Furthermore, sHSP23 and sHSP26, both interact with: CG11534, CG43755 and Pkm (CG1561) proteins [36] (Fig 1C and Fig 1D).
sHSP23 and sHSP26 colocalize in neurons and interact physically
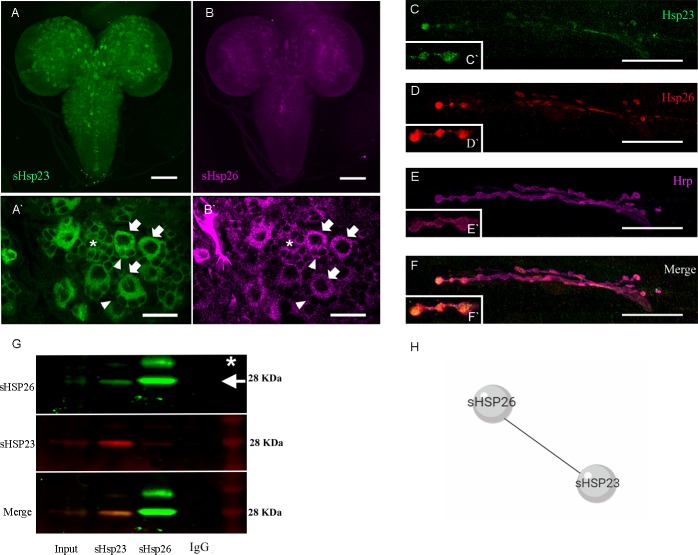
To determine the expression and subcellular localization of sHSP23 and sHSP26 proteins in larval brain we used a green fluorescent reporter tagged form of sHSP26 (HSP26-GFP-V5) and we generated a monoclonal specific antibody against sHSP26 (S1 Fig). We dissected third instar larvae brain and visualized both sHSPs. The data show that sHSP23 and sHSP26 localize in the cytoplasm of CNS cells, in particular in the optic lobes and the central nerve cord (Fig 2A and 2B`). The co-localization of both proteins occurs in neuroblasts and also in ganglion mother cells and differentiated neurons, compatible with a general role in nervous system development.
Fig 2. sHSP23 and sHSP26 colocalize in CNS.
(A-F) Confocal microscopy images of 3rd instar Drosophila larval brain and NMJs. (A) sHSP23 is labeled with anti-GFP antibody driven by D42-Gal4 to visualize its expression in brain regions (magenta). Scale bar size 100 um. (B) sHSP26 is stained with anti-sHSP23 (green). (A`-B`) Magnification images of larval brain. Arrows indicate neuroblast, arrowheads indicate ganglion mother cells and asterisk indicate neurons where sHSP23 and sHSP26 colocalize in the cytoplasm. Scale bar size 100 um. (C-F) sHSP26 is labeled with anti-GFP antibody driven by D42-Gal4 to visualize its expression in NMJ (red), sHSP23 is stained with anti-sHSP23 (green) and neuronal membrane is detected with anti-HRP staining (magenta). Scale bar size 50 um (C`-F`) Magnification images of synaptic boutons in NMJ. (G) Co-Immunoprecipitation assay membrane revealed with sHSP26 (green, arrow) and sHSP23 (red) antibodies in control samples. Fly heads were lysed in immunoprecipitation lysis buffer and incubated with protein A/G agarose beads previously treated with sHSP23 or sHSP26 antibody and IgG antibody as a control. The samples were prepared for western blot analysis. The antibody-protein interaction is visualized by chemoluminescence. Molecular weights are indicated in all the membrane images. * Unknown/unspecific band (H) sHSP23 and sHSP26 interaction diagram.
To further analyze the presence and co-localization of sHSP23 and sHSP26 we analyzed larvae NMJs (Fig 2C–2F`). The confocal images show an accumulation and colocalization of sHSP23 and sHSP26 throughout the NMJ but particularly intense in the synaptic buttons (Fig 2C–2F`). This observation is compatible with a role in synaptic activity as most of the active zones are in the synaptic buttons.
sHSP23 and sHSP26 interact physically
In general, sHSPs proteins exhibit regions susceptible of posttranslational modifications (PTMs) which favor their oligomerization and alter the affinity of interaction by co-chaperones [17, 37]. Since, this mechanism maintains the activity of sHSPs it has been proposed that it regulates their function [17].
The results show that both proteins are localized in the same sub cellular compartments. To determine if both chaperones interact physically, we performed a co-immunoprecipitation assay. We used head protein extracts that were incubated with specific antibodies to specifically immobilize each sHSP in Protein A/G agarose beads. Samples were pre-cleared with untagged beads to avoid unspecified binding. Agarose beads were incubated with HSP23 or HSP26 antibody overnight. Head extract proteins and antibody-bound beads were incubated 1 hour. We revealed the western blot membranes with sHSPs antibodies and the results show that sHSP23 (Fig 2G lane 2) immunoprecipitation also precipitates HSP26, and vice versa (Fig 2G lane 3). Both specific bands are corroborated in the input positive control (Fig 2G lane 1) and the lack of signal in the negative control (Fig 2G lane 4) 22c10 antibody). These results confirm the physical interaction between sHSP23 and sHSP26 (Fig 2H), and it is consistent with their co-localization in the motor neuron buttons.
Pkm interacts with sHSP23 and sHSP26 and modulate synapse number
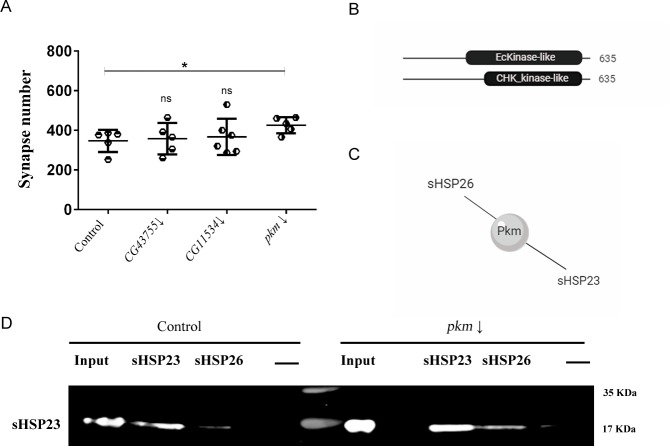
CG11534, CG43755 and Pkm proteins have been postulated that interact with both sHSP23 and sHSP26 (Flybase). They have unknown functions but predicted to have protein kinase like activity (Flybase, http://flybi.hms.harvard.edu/results.php). HSPs posttranslational modifications modulate their function [17] and therefore, we quantified the number of active zones in the NMJ upon knockdown of each candidate gene. The knockdown of pkm in motor neurons increases synapses number while we could not find any significant change for CG11534 and CG43755 knockdown (Fig 3A). In consequence, we focused our study in pkm as a candidate gene to interact with sHsp23 and sHsp26 in nervous system development.
Fig 3. Pkm does not affect to sHSP23-sHSP26 interaction.
(A) Quantification of synapse active zones in the NMJ is shown for the knockdown of all candidate genes genotypes: CG43755 RNAi (CG43755↓), CG11534 RNAi (CG11534↓) and pkm RNAi (pkm↓). One‐way ANOVA test with Bonferroni post‐test* P<0.05. Error bars show S.D. (B) pkm contains a EcKinase like (Ecdysteroid kinase-like) domain between 257–545 aa sequence and a CHK_kinase like (Choline kinase-like) domain between 346–543 aa sequence. (C) Diagram of Pkm interactome. Pkm physically interacts with sHSP23 and sHSP26. (D) Co-immunoprecipitation assay membrane revealed with sHSP23 antibody in control and pkm RNAi samples. Molecular weights are indicated.
pkm is a novel gene that encodes a protein with kinase like domains (Flybase) (Fig 3B). Pkm is reported to physically interact with sHSP23 and sHSP26 [36] (Fig 3C). Posttranslational changes modulate chaperones and co-chaperones interaction and activity [17], thus it is suggested that these mechanisms represent a system to modulate chaperone dynamics. Accordingly, we did immunoprecipitation assays to determine if Pkm was necessary for the sHSP23 and sHSP26 complex formation (Fig 3D). The results revealed that pkm knockdown does not modify the interaction between chaperones, thus pkm expression is dispensable for sHSP23-sHSP26 physical interaction.
Furthermore, we tested if the expression of pkm could modulate the expression of sHsps. To evaluate transcription, we did quantitative PCR (qPCR) experiments of pkm, sHsp23, sHsp26 and catalase (Cat) as a positive control. The results show that pkm RNAi proved effective since its transcription is drastically reduced (Fig 4A). On the other hand, sHsp23, but not sHsp26, expression is largely increased (Fig 4A). In order to confirm the transcriptional results, we analyzed the total protein amount of sHSPs upon pkm knockdown and those of sHSP23 and sHSP26 by Western blot assays (Fig 4B). To silence the expression of pkm we used three RNAi tools to replicate this condition and to reaffirm the regulation changes that we found with the qPCR. The knockdown of pkm with pkm RNAi 2 tool provokes an increase in sHSP23 and sHSP26 proteins (Fig 4C). The data for sHsp23 are consistent with the qPCR assays and suggest that pkm is necessary to restrict sHsp23 expression, while sHsp26 expression is independent of pkm expression but protein accumulations is increased upon pkm knockdown. These data are compatible with sHSP26 posttranslational modifications mediated by Pkm to control protein stability and degradation.
Fig 4. sHSPs amount is regulated by the novel candidate gene pkm.
(A) qPCR assay of pkm RNAi sample measuring mRNA expression fold change of 3rd instar larval of pkm, sHsp23, sHsp26 expression and Cat as positive control, normalized with Rp49 as a control. (B) Western blot assay of control and pkm RNAi (pkm↓) samples stained against sHSP23 and sHSP26. We used three RNAi tools to confirm the protein amount changes under pkm downregulation condition. pkm RNAi 2 was selected due to its efficacy. Tubulin was used as a control. (C) Mean Intensity sHSP23 and sHSP26 signal are shown for control and pkm RNAi (pkm↓) samples. Unpaired T-test Welch´s correction* P<0.05. Error bars show S.D. (D) Quantification of synapse active zones in the NMJ is shown for the combination of sHsp23 and pkm, sHsp26 downregulation under D42 driver expression. (E) Synapse number quantification in NMJs after sHsp26 upregulation and pkm downregulation. Unpaired T-test Mann Whitney post-test * p value<0.05; p value > .05 were not considered significant. Error bars show S.D.
Pkm modulates synapse number
Small chaperones work as dimers or oligomers to modulate their activity [38], therefore sHSPs protein-protein interaction opens a potential activity as a complex. Since sHSPs family is characterized by forming oligomer assemblies based on dimers joined [39], we investigated the coordinated effect of sHSPs and Pkm.
pkm RNAi causes an increase of synapse number in development (Figs 3A, 4D and 4E), moreover sHsp23 upregulation reduces synapse number and knockdown does not change synapse number during development (Fig 1). We combined pkm RNAi and sHsp23 upregulation in neurons and we observed an increase in synapse number sample comparable to pkm RNAi alone, suggesting that the effect of pkm RNAi for synaptogenesis during development is mediated by sHSPs (Fig 4D). These results suggest that the synaptogenic effect of pkm knockdown is not restricted to sHsp23 upregulation (Fig 4A–4C), thus we investigated the contribution of sHsp26 in combination with pkm. Protein quantification experiments show that sHSP26 is accumulated upon pkm RNAi expression but in a lesser extent than sHSP23. To demonstrate that sHSP26 is the limiting factor in sHSP23/26 complex we upregulated sHsp26 together with pkm RNAi. Synapse quantifications show that upregulation of sHsp26 can further increase synapse number in pkm RNAi background (Fig 4E). These results suggest that sHSP26 is a limiting factor for synaptogenesis in development and pkm reduction contributes to stabilize sHSP26 partially but sHsp26 upregulation causes complementary synaptogenesis.
To further determine if sHSPs protein interaction and pkm modulation contribute to synaptogenesis, we altered sHsps expression together and counted synapse number in NMJs. The joint upregulation of sHsp23 and 26 induces an increase in synapse number (Fig 5A). This increase contrasts with the reduction elicited by each sHsp when upregulated separately (compare to Fig 1A). Moreover, the upregulation of both chaperones in combination with pkm knockdown maintains the drastic increase in synapse number and, actually, is of a larger magnitude than the pkm knockdown by itself (Fig 5A). This observation is compatible with a functional interaction of Pkm kinase with at least one of the two sHSP analyzed here.
Fig 5. Pkm activity is restricted by sHsps expression.
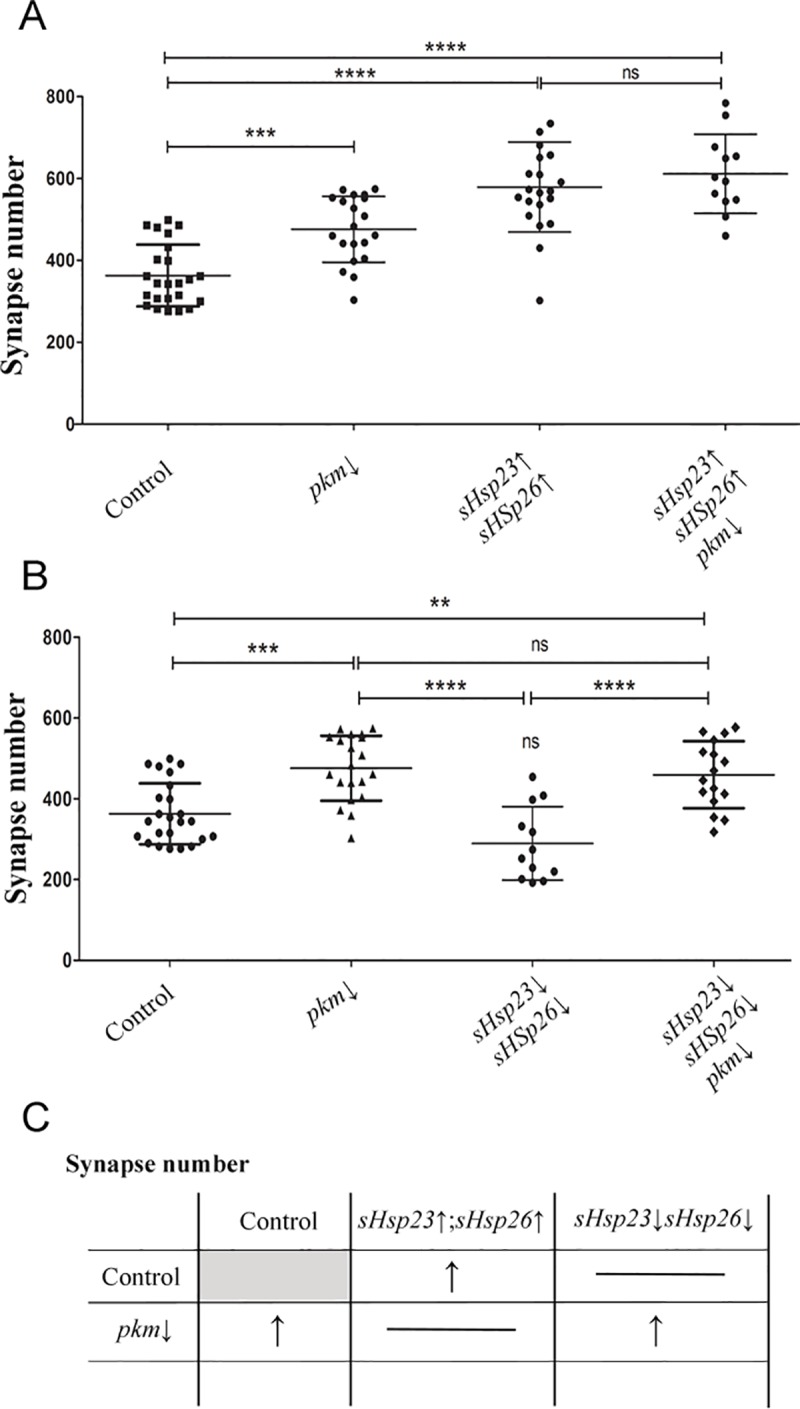
Quantification of synapse active zones in the NMJ is shown for the combination of sHsps expression and pkm downregulation under D42 driver expression: (A) pkm RNAi (pkm↓), UAS.sHsp23; UAS.sHsps26 (sHsp23↑; sHsp26↑), UAS.sHsp23; UAS.sHsps26/pkm RNAi (sHsp23↑; sHsp26↑/pkm↓), (B) UAS.sHsp23 RNAi; UAS.sHsps26 RNAi (sHsp23↓; sHsp26↓), UAS.sHsp23 RNAi; UAS.sHsps26 RNAi /pkm RNAi (sHsp23↓; sHsp26↓/pkm↓). One‐way ANOVA test with Bonferroni post‐test. *p value ≤ .05; ** p value ≤ .01; *** p value ≤ .001. p value > .05 were not considered significant. Error bars show S.D. (C) Summary table for the combination of sHsps expression and pkm downregulation.
Finally, the combined knockdown of both sHsps does not alter synapse number. This result suggests that only the modulation of one single chaperone of this combo produces an imbalance that triggers synapse loss. If both chaperones are reduced in combination, there is no effect what supports the idea that the equilibrium between sHSP23 and sHSP26 is relevant. Moreover, sHsp23, sHsp26 and pkm RNAi co-expression show an increase in synapse number (Fig 5B). Thus, we conclude that the effect of sHsps upregulation surpasses Pkm contribution but, sHsps knockdown is not sufficient to prevent the increase in synapse number caused by pkm knockdown (Fig 5B).
As a result, we suggest that sHSP23 and sHSP26 together form a complex that promotes synapse formation in presynaptic neurons, Pkm is an anti-synaptogenic element in neurons through, but not restricted to, the modulation of sHsp23 and sHsp26 (Fig 5C).
Neuronal activity correlates with synapses changes caused by sHSPs and Pkm
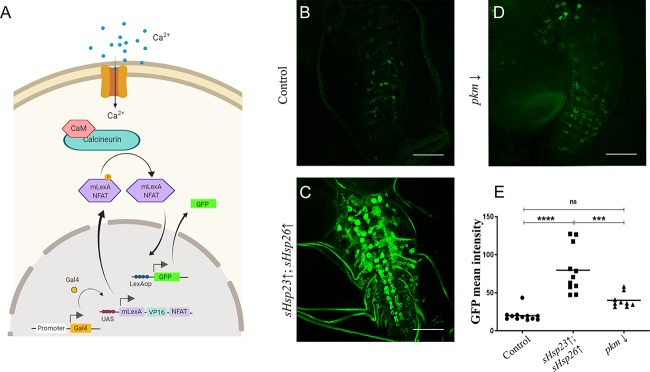
Changes in synapse number are expected to reflect on neuronal activity. To evaluate the cellular effect of the observed synapse number changes, we took advantage of CalexA system (Calcium-dependent nuclear import of LexA) to perform a functional assay in motor neurons [40].
CalexA is a tracing system to label neuronal activity based on calcium/NFAT signaling and the two binary expression systems UAS/Gal4 and LexA/LexAop [41]. We used specific lines to drive a modified NFAT form to motor neurons (D42.Gal4 and P{LexAop-CD8-GFP-2A-CD8-GFP}2; P{UAS-mLexA-VP16-NFAT}H2, P{lexAop-rCD2-GFP}3/TM6B, Tb1). The accumulation of Ca2+ due to the action potentials activates calcineurin which dephosphorylates NFAT, provoking its import into the nucleus. NFAT binds to LexAop sequence and induces the expression of a GFP reporter gene (Fig 6A). Therefore, GFP signal becomes a reporter of neuronal activity.
Fig 6. sHSPs contribute to neuronal activity GFP signal.
(A) CalexA system labels neuronal activity based on calcium/NFAT signaling after a neuronal action potential and the two binary expression systems UAS/Gal4 and LexA/LexAop. The accumulation of calcium activates calcineurin that dephosphorylates NFAT that are imported to the nucleus. NFAT binds to LexAop sequence and induces the expression of GFP reporter gene that correlates with neuronal activity. (B-D) Confocal microscopy images of 3rd instar Drosophila larval ventral nerve cord of (B) control, (C) UAS.sHsp23; UAS.sHsps26 (sHsp23↑; sHsp26↑), (D) pkm RNAi (pkm↓) samples. (E) GFP mean intensity signal quantification is shown for sHsps expression and pkm downregulation under D42 driver expression: UAS.sHsp23; UAS.sHsps26 (sHsp23↑; sHsp26↑), pkm RNAi (pkm↓), One‐way ANOVA test with Bonferroni post‐test.; *** p value = 0.002. **** p value<0,0001;. p value > .05 were not considered significant. Error bars show S.D.
To evaluate neuronal activity and sHsps and pkm expression we measured the signal of the GFP reporter in larva brains (Fig 6B–6E). The sHsp23 and sHsp26 upregulation increases GFP signal in cells of ventral nerve cord (Fig 6C and 6E) which correlates with an increase in the number of synapses (Fig 5A). By contrast, pkm knockdown reduces CalexA reporter signal in motor neurons (Fig 6D and 6E) which correlates with its anti-synaptogenic role in motor neurons. The results indicate that Pkm contribution is not limited to sHSP23 and sHSP26. Here pkm knockdown reproduces the effect of small chaperones upregulation on neuronal activity. Besides, we have shown that pkm RNAi synaptogenic effect is not prevented by sHsp23 and sHsp26 RNAi (Fig 5B). Therefore, additional Pkm targets participate in synapse formation and neural activity. Taking all these data together, we conclude that sHsps and pkm expression participate in synapse formation during development and neuronal activity.
Discussion
Synapse regulation is a central event during nervous system development and adult life. Disruptions in the establishment of synapses is associated with morphological, cognitive and psychiatric disorders, but the precise mechanisms underlying these disorders remain unknown [42]. Changes in synapse structure and function are related to paralysis and muscular atrophy in amyotrophic lateral sclerosis (ALS) [43, 44], impairment of the neuromuscular junction function and therefore, motor decline [45] or social and cognitive behaviors related to autism [46]. Thus the study of relevant mechanisms for synapse formation during development is a need.
Chaperones participate in protein folding maintenance as a mechanism to regulate function and pathological conditions, but the specific contribution to synapse number during development was not addressed. Here we describe the combined contribution of two sHSPs (sHSP23 and sHSP26) to synapse formation and the modulation by a novel putative kinase protein Pkm. sHsp23 mRNA and total protein amount increases upon pkm knockdown, suggesting a transcriptional negative regulation by Pkm.
The results show that sHsp26 mRNA levels do not show significant changes after pkm expression interference. However, HSP26 in yeast is degraded via a ubiquitin/proteasome-dependent mechanisms [47], the results from western blot experiments show HSP26 protein accumulation upon pkm RNAi expression. Therefore, as Pkm is proposed to be a kinase, we cannot discard that Pkm could promote HSP26 post-transcriptional modifications (phosphorylation) to promote its degradation. According to the putative domains present in Pkm protein, there are no DNA binding domains and hence it is unlikely that Pkm acts as a transcription factor. We postulate that the transcriptional regulation of sHsp23 is determined by transcription factors sensible to posttranslational modifications as direct targets of Pkm. However, the precise mechanisms and molecular details of Pkm-sHSPs relation require be further investigated.
Genetic modifications of one single sHsp (sHsp23 or sHsp26) cause an imbalance in the equilibrium between both genes, as a consequence it causes a reduction in the number of synapses. These results suggest that single alterations in sHSPs are detrimental for the number of synapses. However, we propose that sHSP23 function in synaptogenesis requires forming a complex with sHSP26. sHsp26 upregulation and downregulation modify synapse number in the same direction (synapse number reduction). However, sHSP23 is the one modulated by Pkm but sHsp23 downregulation does not change synapse number. It is tempting to speculate that a reduction in sHSP23 does not affect to CNS development and it has a protective function more than synaptogenic. However, when sHSP23 and sHSP26 play together they modulate synapses, upregulation of both genes cause an increase in synapse number. Moreover, pkm knockdown increases synapse number but does not further increase synapse number upon sHsps overexpression, indicating that pkm effect on synapses is mediated by sHsps regulation. In addition, the combined silencing of sHsps does not alter synapse number, in line with the proposal of sHSPs equilibrium. But pkm knockdown increases significantly the number of synapses in sHsps knockdown background. These results suggest that pkm is a repressor of sHsps and pkm RNAi counteracts the reduction of sHSPs. Thus we speculate with the hypothesis of sHSP26 acting as a synapse modulator and sHSP23 as a protective partner regulated by Pkm.
A direct consequence of sHsps modulation is a reduction in neuronal intracellular calcium levels in the brain, an indicator of neuronal activity. Co-overexpression of both sHSPs results in enhanced intracellular calcium activity directly associated to neuronal activity. Therefore, small chaperones are required for the formation of the correct synapse number in NMJs and also can stimulate brain activity. These results connect neural activity and chaperones, which are proteins that sense environmental changes and in consequence, link neural activity and environment during development. In particular, these two chaperons are associated to temperature changes [48, 49], environmental-stress-induced degeneration [50, 51] and lifespan [52]. Besides, maternal loading of sHSP23 determines embryonic thermal tolerance pointing to a physiological role during development [53]. All these evidences support that sHsp disruption during embryogenesis and development can be associated to physiological defects in adulthood, therefore Pkm-sHSPs contribution during development is proposed as a central mechanism for nervous system correct formation, function and response to environmental stress.
Supporting information
(A-B) To validate if the antibody that we generated against sHsp26 is specific, we knocked down sHsp26 in the posterior compartment of wing imaginal disc (engrailed-Gal4) and visualized the specific domain with the co-expression of GFP. (C) The quantifications of pixel intensity show that anti-sHsp26 recognizes the reduction of sHsp26 expression caused by UAS-sHsp26 RNAi. Unpaired T-test Welch´s correction **** p value<0,0001. Error bars show S.D.
(TIF)
(PDF)
Acknowledgments
We thank Professor Alberto Ferrús, Dr. F.A. Martín, María Losada, Patricia Jarabo and anonymous reviewers for critiques of the manuscript and for helpful discussions. Clemencia Cuadrado for fly stocks maintenance. We are grateful to the Vienna Drosophila Resource Centre, the Bloomington Drosophila stock Centre and the Developmental Studies Hybridoma Bank for supplying fly stocks and antibodies, and FlyBase for its wealth of information. We acknowledge the support of the Confocal Microscopy unit and Molecular Biology unit at the Cajal Institute for their help with this project. We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
We would like to declare that this research has been funded by grant BFU2015-65685P from the Spanish MICINN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26(30):7870–4. Epub 2006/07/28. 26/30/7870 [pii] 10.1523/JNEUROSCI.1184-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakthisaran R, Tangirala R, Rao Ch M. Small heat shock proteins: Role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854(4):291–319. Epub 2015/01/04. S1570-9639(14)00335-5 [pii] 10.1016/j.bbapap.2014.12.019 . [DOI] [PubMed] [Google Scholar]
- 3.Beere HM. "The stress of dying": the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117(Pt 13):2641–51. Epub 2004/06/01. 10.1242/jcs.01284 [pii]. . [DOI] [PubMed] [Google Scholar]
- 4.Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, et al. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280(12):11059–66. Epub 2005/01/18. M413382200 [pii] 10.1074/jbc.M413382200 . [DOI] [PubMed] [Google Scholar]
- 5.Wettstein G, Bellaye PS, Micheau O, Bonniaud P. Small heat shock proteins and the cytoskeleton: an essential interplay for cell integrity? Int J Biochem Cell Biol. 2012;44(10):1680–6. Epub 2012/06/12. S1357-2725(12)00190-2 [pii] 10.1016/j.biocel.2012.05.024 . [DOI] [PubMed] [Google Scholar]
- 6.Bai F, Xi J, Higashikubo R, Andley UP. Cell kinetic status of mouse lens epithelial cells lacking alphaA- and alphaB-crystallin. Mol Cell Biochem. 2004;265(1–2):115–22. Epub 2004/11/17. 10.1023/b:mcbi.0000044365.48900.82 . [DOI] [PubMed] [Google Scholar]
- 7.Van Montfort R, Slingsby C, Vierling E. Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem. 2001;59:105–56. Epub 2002/03/01. 10.1016/s0065-3233(01)59004-x . [DOI] [PubMed] [Google Scholar]
- 8.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–32. Epub 2011/07/22. nature10317 [pii] 10.1038/nature10317 . [DOI] [PubMed] [Google Scholar]
- 9.Raut S, Mallik B, Parichha A, Amrutha V, Sahi C, Kumar V. RNAi-Mediated Reverse Genetic Screen Identified Drosophila Chaperones Regulating Eye and Neuromuscular Junction Morphology. G3 (Bethesda). 2017;7(7):2023–38. Epub 2017/05/14. g3.117.041632 [pii] 10.1534/g3.117.041632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagla T, Dubinska-Magiera M, Poovathumkadavil P, Daczewska M, Jagla K. Developmental Expression and Functions of the Small Heat Shock Proteins in Drosophila. Int J Mol Sci. 2018;19(11). Epub 2018/11/08. ijms19113441 [pii] 10.3390/ijms19113441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol Life Sci. 2005;62(21):2460–76. Epub 2005/09/07. 10.1007/s00018-005-5190-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahvic JL, Ji Y, Marin P, Zuflacht JP, Springel MW, Wosen JE, et al. Small heat shock proteins are necessary for heart migration and laterality determination in zebrafish. Dev Biol. 2013;384(2):166–80. Epub 2013/10/22. S0012-1606(13)00540-X [pii] 10.1016/j.ydbio.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster JM, Darling AL, Uversky VN, Blair LJ. Small Heat Shock Proteins, Big Impact on Protein Aggregation in Neurodegenerative Disease. Front Pharmacol. 2019;10:1047 Epub 2019/10/18. 10.3389/fphar.2019.01047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houck SA, Clark JI. Dynamic subunit exchange and the regulation of microtubule assembly by the stress response protein human alphaB crystallin. PLoS One. 2010;5(7):e11795 Epub 2010/07/30. 10.1371/journal.pone.0011795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kriehuber T, Rattei T, Weinmaier T, Bepperling A, Haslbeck M, Buchner J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010;24(10):3633–42. Epub 2010/05/27. fj.10-156992 [pii] 10.1096/fj.10-156992 . [DOI] [PubMed] [Google Scholar]
- 16.Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol. 2015;427(7):1537–48. Epub 2015/02/15. S0022-2836(15)00081-9 [pii] 10.1016/j.jmb.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller P, Ruckova E, Halada P, Coates PJ, Hrstka R, Lane DP, et al. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene. 2013;32(25):3101–10. Epub 2012/07/25. onc2012314 [pii] 10.1038/onc.2012.314 . [DOI] [PubMed] [Google Scholar]
- 18.Karunanithi S, Barclay JW, Robertson RM, Brown IR, Atwood HL. Neuroprotection at Drosophila synapses conferred by prior heat shock. J Neurosci. 1999;19(11):4360–9. Epub 1999/05/26. 10.1523/JNEUROSCI.19-11-04360.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JY, Kim JW, Yenari MA. Heat Shock Protein Signaling in Brain Ischemia and Injury. Neurosci Lett. 2019:134642 Epub 2019/11/24. S0304-3940(19)30745-1 [pii] 10.1016/j.neulet.2019.134642 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warrick JM, Chan HY, Gray-Board GL, Chai Y, Paulson HL, Bonini NM. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet. 1999;23(4):425–8. Epub 1999/12/02. 10.1038/70532 . [DOI] [PubMed] [Google Scholar]
- 21.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6(1):11–22. Epub 2004/12/22. nrn1587 [pii] 10.1038/nrn1587 . [DOI] [PubMed] [Google Scholar]
- 22.Klose MK, Robertson RM. Stress-induced thermoprotection of neuromuscular transmission. Integr Comp Biol. 2004;44(1):14–20. Epub 2004/02/01. 44/1/14 [pii] 10.1093/icb/44.1.14 . [DOI] [PubMed] [Google Scholar]
- 23.Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13(4):240–50. Epub 2012/03/08. nrn3200 [pii] 10.1038/nrn3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyer MM, Phillips LL, Neigh GN. Sex Differences in Synaptic Plasticity: Hormones and Beyond. Front Mol Neurosci. 2018;11:266 Epub 2018/08/16. 10.3389/fnmol.2018.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosoda R, Nakayama K, Kato-Negishi M, Kawahara M, Muramoto K, Kuroda Y. Thyroid hormone enhances the formation of synapses between cultured neurons of rat cerebral cortex. Cell Mol Neurobiol. 2003;23(6):895–906. Epub 2004/02/18. 10.1023/b:cemn.0000005318.53810.de . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettio L, Thacker JS, Hutton C, Christie BR. Modulation of synaptic plasticity by exercise. Int Rev Neurobiol. 2019;147:295–322. Epub 2019/10/15. S0074-7742(19)30048-0 [pii] 10.1016/bs.irn.2019.07.002 . [DOI] [PubMed] [Google Scholar]
- 27.Wishart TM, Parson SH, Gillingwater TH. Synaptic vulnerability in neurodegenerative disease. J Neuropathol Exp Neurol. 2006;65(8):733–9. Epub 2006/08/10. 10.1097/01.jnen.0000228202.35163.c4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 28.Gillingwater TH, Wishart TM. Mechanisms underlying synaptic vulnerability and degeneration in neurodegenerative disease. Neuropathol Appl Neurobiol. 2013;39(4):320–34. Epub 2013/01/08. 10.1111/nan.12014 . [DOI] [PubMed] [Google Scholar]
- 29.Dickman DK, Lu Z, Meinertzhagen IA, Schwarz TL. Altered synaptic development and active zone spacing in endocytosis mutants. Curr Biol. 2006;16(6):591–8. Epub 2006/03/21. S0960-9822(06)01209-7 [pii] 10.1016/j.cub.2006.02.058 . [DOI] [PubMed] [Google Scholar]
- 30.Jordan-Alvarez S, Santana E, Casas-Tinto S, Acebes A, Ferrus A. The equilibrium between antagonistic signaling pathways determines the number of synapses in Drosophila. PLoS One. 2017;12(9):e0184238 Epub 2017/09/12. 10.1371/journal.pone.0184238 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24(8):1008–24. Epub 1993/08/01. 10.1002/neu.480240803 . [DOI] [PubMed] [Google Scholar]
- 32.Keshishian H, Broadie K, Chiba A, Bate M. The drosophila neuromuscular junction: a model system for studying synaptic development and function. Annu Rev Neurosci. 1996;19:545–75. Epub 1996/01/01. 10.1146/annurev.ne.19.030196.002553 . [DOI] [PubMed] [Google Scholar]
- 33.Mason JM, Strobel E, Green MM. mu-2: mutator gene in Drosophila that potentiates the induction of terminal deficiencies. Proc Natl Acad Sci U S A. 1984;81(19):6090–4. Epub 1984/10/01. 10.1073/pnas.81.19.6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin R, Valet JP, Tanguay RM. hsp23 and hsp26 exhibit distinct spatial and temporal patterns of constitutive expression in Drosophila adults. Dev Genet. 1993;14(1):69–77. Epub 1993/01/01. 10.1002/dvg.1020140109 . [DOI] [PubMed] [Google Scholar]
- 35.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–15. Epub 1993/06/01. . [DOI] [PubMed] [Google Scholar]
- 36.Guruharsha KG, Rual JF, Zhai B, Mintseris J, Vaidya P, Vaidya N, et al. A protein complex network of Drosophila melanogaster. Cell. 2011;147(3):690–703. Epub 2011/11/01. 10.1016/j.cell.2011.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parcellier A, Schmitt E, Brunet M, Hammann A, Solary E, Garrido C. Small heat shock proteins HSP27 and alphaB-crystallin: cytoprotective and oncogenic functions. Antioxid Redox Signal. 2005;7(3–4):404–13. Epub 2005/02/12. 10.1089/ars.2005.7.404 . [DOI] [PubMed] [Google Scholar]
- 38.Weeks SD, Baranova EV, Heirbaut M, Beelen S, Shkumatov AV, Gusev NB, et al. Molecular structure and dynamics of the dimeric human small heat shock protein HSPB6. J Struct Biol. 2014;185(3):342–54. Epub 2014/01/03. S1047-8477(13)00336-5 [pii] 10.1016/j.jsb.2013.12.009 . [DOI] [PubMed] [Google Scholar]
- 39.Baldwin AJ, Lioe H, Robinson CV, Kay LE, Benesch JL. alphaB-crystallin polydispersity is a consequence of unbiased quaternary dynamics. J Mol Biol. 2011;413(2):297–309. Epub 2011/08/16. S0022-2836(11)00770-4 [pii] 10.1016/j.jmb.2011.07.016 . [DOI] [PubMed] [Google Scholar]
- 40.Masuyama K, Zhang Y, Rao Y, Wang JW. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J Neurogenet. 2012;26(1):89–102. Epub 2012/01/13. 10.3109/01677063.2011.642910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9(5):703–9. Epub 2006/04/04. nn1681 [pii] 10.1038/nn1681 . [DOI] [PubMed] [Google Scholar]
- 42.Das SC, Chen D, Callor WB, Christensen E, Coon H, Williams ME. DiI-mediated analysis of presynaptic and postsynaptic structures in human postmortem brain tissue. J Comp Neurol. 2019;527(18):3087–98. Epub 2019/06/04. 10.1002/cne.24722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shahidullah M, Le Marchand SJ, Fei H, Zhang J, Pandey UB, Dalva MB, et al. Defects in synapse structure and function precede motor neuron degeneration in Drosophila models of FUS-related ALS. J Neurosci. 2013;33(50):19590–8. Epub 2013/12/18. 33/50/19590 [pii] 10.1523/JNEUROSCI.3396-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bercier V, Hubbard JM, Fidelin K, Duroure K, Auer TO, Revenu C, et al. Dynactin1 depletion leads to neuromuscular synapse instability and functional abnormalities. Mol Neurodegener. 2019;14(1):27 Epub 2019/07/12. 10.1186/s13024-019-0327-3 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Connor E, Cairns G, Spendiff S, Burns D, Hettwer S, Mader A, et al. Modulation of Agrin and RhoA Pathways Ameliorates Movement Defects and Synapse Morphology in MYO9A-Depleted Zebrafish. Cells. 2019;8(8). Epub 2019/08/10. cells8080848 [pii] 10.3390/cells8080848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeuchi K, Gertner MJ, Zhou J, Parada LF, Bennett MV, Zukin RS. Dysregulation of synaptic plasticity precedes appearance of morphological defects in a Pten conditional knockout mouse model of autism. Proc Natl Acad Sci U S A. 2013;110(12):4738–43. Epub 2013/03/15. 1222803110 [pii] 10.1073/pnas.1222803110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh LR, Kruger WD. Functional rescue of mutant human cystathionine beta-synthase by manipulation of Hsp26 and Hsp70 levels in Saccharomyces cerevisiae. J Biol Chem. 2009;284(7):4238–45. Epub 2008/12/17. M806387200 [pii] 10.1074/jbc.M806387200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franzmann TM, Menhorn P, Walter S, Buchner J. Activation of the chaperone Hsp26 is controlled by the rearrangement of its thermosensor domain. Mol Cell. 2008;29(2):207–16. Epub 2008/02/05. S1097-2765(07)00820-9 [pii] 10.1016/j.molcel.2007.11.025 . [DOI] [PubMed] [Google Scholar]
- 49.Colinet H, Lee SF, Hoffmann A. Knocking down expression of Hsp22 and Hsp23 by RNA interference affects recovery from chill coma in Drosophila melanogaster. J Exp Biol. 2010;213(Pt 24):4146–50. Epub 2010/11/30. 213/24/4146 [pii] 10.1242/jeb.051003 . [DOI] [PubMed] [Google Scholar]
- 50.Michaud S, Tanguay RM. Expression of the Hsp23 chaperone during Drosophila embryogenesis: association to distinct neural and glial lineages. BMC Dev Biol. 2003;3:9 Epub 2003/11/18. 10.1186/1471-213X-3-9 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawasaki F, Koonce NL, Guo L, Fatima S, Qiu C, Moon MT, et al. Small heat shock proteins mediate cell-autonomous and -nonautonomous protection in a Drosophila model for environmental-stress-induced degeneration. Dis Model Mech. 2016;9(9):953–64. Epub 2016/08/03. dmm.026385 [pii] 10.1242/dmm.026385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang HD, Kazemi-Esfarjani P, Benzer S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proc Natl Acad Sci U S A. 2004;101(34):12610–5. Epub 2004/08/17. 10.1073/pnas.0404648101 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lockwood BL, Julick CR, Montooth KL. Maternal loading of a small heat shock protein increases embryo thermal tolerance in Drosophila melanogaster. J Exp Biol. 2017;220(Pt 23):4492–501. Epub 2017/11/04. jeb.164848 [pii] 10.1242/jeb.164848 [DOI] [PMC free article] [PubMed] [Google Scholar]