Abstract
Docosahexaenoic acid (DHA), the most abundant n-3 polyunsaturated fatty acid (n-3PUFA) in the brain, has attracted great importance for a variety of neuronal functions such as signal transduction through plasma membranes, neuronal plasticity, and neuroprotection. Astrocytes that provide structural, functional, and metabolic support for neurons, express ∆6- desaturase encoded by FADS2 gene that can be, next to the plasma DHA pool, additional source of DHA in the brain. Furthermore, the genetic variations of FADS gene cluster has been found in children with developmental disorders, and are associated with cognitive functions. Since, the regulation of DHA biosynthesis in astrocytes remains poorly studied the aim of this study was to determine the effect of palmitic acid (PA), α-linolenic acid (ALA) or docosahexaenoic acid (DHA), on the transcription of FADS2 gene in astrocytes and survival of neurons challenged with oxidative compounds after co-culture with astrocytes exposed to DHA. The lipid profile in cell membranes after incubation with fatty acids was determined by gas chromatography, and FADS2 expression was analyzed using real-time PCR. The viability of neurons cocultured with PUFA-enriched astrocytes was investigated by flow cytometry after staining cells with annexin V-FITC and PI. The results showed that DHA suppressed (P <0.01), PA stimulated (P <0.01), while ALA did not change the FADS2 gene expression after 24 h incubation of astrocytes with fatty acids. Although FADS2 mRNA was down-regulated by DHA, its level in astrocytic membranes significantly increased (P <0.01). Astrocytes with DHA-enriched membrane phospholipids markedly enhanced neuronal resistance to cytotoxic compounds and neuronal survival. These results suggest that beneficial effects of supplementation with n-3 PUFA in Alzheimer disease and in psychiatric disorders is caused, in part, by increased efficacy of DHA-enriched astrocytes to protect neurons under adverse conditions in the brain.
Key Words: Docosahexaenoic acid, FADS2, astrocytes, neuroprotection
The brain is highly enriched in docosahexaenoic acid (DHA 22:6n-3) which is of particular significance for the brain development and function (1). DHA esterified at sn-2 position of phospholipids in plasma membranes forms one third of the total fatty acids in the CNS (2). It has been shown that DHA presence in membranes affects the organization of lipid rafts and plays an important role in modulation of intracellular signaling and synaptic plasticity. Hence, a reduced DHA level in brain phospholipids is associated with a decline of cognitive functions in elderly, as well in patients with Alzheimer’s disease (3) and with psychiatric disorders (4, 5). The main source of DHA for the brain is uptake from esterified blood pool of DHA after dietary fat digestion (6). Moreover, DHA can be synthesized from essential -linolenic acid (ALA, 18:3n-3) in the liver through elongation and introducing cis double bonds into the hydrocarbon chain by desaturases (7). Delta 6-desaturase encoded by FADS2 gene is considered as the rate-limiting enzyme in the biosynthesis of long chain polyunsaturated fatty acids(LC-PUFAs). Astrocytic Δ6-desaturase expression has likewise been demonstrated (8) and association between FADS2 single nucleotide polymorphisms and low LC-PUFA levels in erythrocyte membranes in patients with mild cognitive impairment was noted (9).
Astrocytes, the most abundant cells in the brain provide structural, functional and metabolic support for neurons. They are involved in maintaining ionic (10) and neurotransmitter homeostasis (11), as well participate in neuronal plasticity and interneuronal communication, as they express numerous receptors, metabolize amino acid neurotransmitters and release numerous gliotransmitters (12,13,14). Since biosynthesis of DHA in astrocytes remains poorly understood, the aim of this study was to determine DHA incorporation into plasma membranes and its effect on transcription of FADS2 gene in astrocytes in comparison with other fatty acids, such as saturated palmitic acid (PA; C16:0) and the DHA precursor, ALA. Since cooperation between astrocytes and neurons, among others relies on supply of energy and metabolic substrates and protection against oxidative stress (15), we determined survival of neurons co-cultured with DHA-enriched astrocytes in response to cytotoxic agents.
Materials and methods
Primary astrocyte culture
Astrocytes were prepared from the cerebral cortex of 1-2 days old rat pups using modified method of McCarthy and deVellis (16) and Albuqerque et al. (17). Briefly, cortices were dissected and dispersed into single cells by triturating in DMEM (Life Technologies, USA). The debris were removed by double filtration through Nitex 180 m, and then Nitex 30 m (Merck, Germany). The clear suspension was centrifuged (5 min, 800 rpm at room temperature), pellets were suspended in DMEM-F12/10% FBS medium, and cells seeded into 75 cm2 culture flasks (2 x105 cells/cm2). Astrocytes were cultured in a 5% CO2 atmosphere at 37 °C, the medium was changed every two days until the cells were 70–80% confluent, then flasks were shaken to detach the oligodendrocytes. The monolayer cells were trypsinized, counted, plated into new 75 cm2 flasks and cultured to 80% confluence. The purity of astrocyte culture was 99%, as determined by glial fibrillary acidic protein (GFAP) (Life Technologies, USA) fluorescent immunocytochemistry. The study protocol was approved by the Ethical Committee for Animal Care.
RNA preparation and real-time PCR
To examine the effect of various types of fatty acids on FADS2 gene expression astrocytes were seeded in 6-well culture plates at 5x105 cells/well and incubated with palmitic acid (PA), -linolenic acid (ALA) and docosahexaenoic acid (DHA) (Sigma Aldrich, USA) at concentration of 50 M for 24 h. Control cells were cultured in DMEM. Total RNA was isolated using the TRIZOL RNA reagent (Invitrogen, USA) according to the manufacturer's instructions. For the reverse transcription reaction pure RNA with a ratio A 260/280 of 1.9- 2.1 was used. First-strand cDNA was synthesized from 1 g of total RNA in a total 20 μl using Superscript III reverse/ImProm-IITM reverse transcriptase (Promega, USA), dNTPs and random hexamer primers (Invitrogen, USA) for 1 h at 50 °C. Then, RT products were analyzed in triplicate by real-time RT-PCR using an ABI Prism 7000 sequence detection system (Applied Biosystems, USA) with a Brilliant III SYBR QPCR kit (Agilent Technologies, USA) following the manufacturer’s protocol. PCR efficiency was validated with a standard curve. Primers used to detect the mRNA of FADS2 and housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are presented in Table 1. The results were analyzed according to the 2-ΔΔCt method. The mRNA level for each sample was normalized against GAPDH mRNA, and data are presented as relative quantification (RQ), i.e. fold change compared to the untreated control with RQ value of 1.
Table1.
Primers used in the real-time PCR analysis
| Gene | Sequence ID | Primer sequences | Amplicon (bp) |
|---|---|---|---|
| FADS2 | HQ 909027.1 | Forward:5’-GTTCTTCTTTCTCCTCCTGTCCC-3’ | 77 |
| Reverse:5’-CATTGCCGAAGTACGAGAGGAT-3’ | |||
| GAPDH | NM 017008.4 | Forward: 5’-CATGGCCTTCCGTGTTCCTA-3’ | 105 |
| Reverse: 5’-CCTGCTTCACCACCTTCTTGA-3’ |
Membrane preparation and fatty acid determin-ation
Astrocytes were plated at density of 8 x 106 in Petri dishes and incubated with 30 M DHA for 24 h. Control cells were cultured in DMEM. Then cells were harvested, re-suspended in buffer (0.25 M sucrose, 1 mM EDTA, and 10 mM Tris-HCl, pH 7.4), and homogenized in a glass homogenizer with a Teflon pestle. Crude plasma membrane and mitochondria fractions were separated by differential centrifugation and then in sucrose density gradient. Lipids were extracted by standard Folch method, subsequently separated with the mobile phase: heptane-diisopropyl ether–acetic acid (60:40:3, v/v/v) and trans-methylated to fatty acid methyl esters (FAMEs) with boron trifluoride in methanol reagent under nitrogen atmosphere at 100 °C for 30 min. The FAMEs were analyzed by gas chromatography with a flame ionization detector (FID) on a Clarus 500 Gas Chromatograph (Perkin Elmer, USA). FAMEs were then separated on a capillary column coated with Varian CP-Sil88 stationary phase (50 m ×0.25 mm, ID 0.2 μm, Varian). Operating conditions were as follows: the split-splitless injector was used in split mode with a split ratio of 1:20. The injection volume of the sample was 2 μl. The injector and FID detector temperature was kept at 260 °C. The column temperature was programmed from 150 °C (2 min) to 230 °C (10 min) at 4 °C/min. The carrier gas was helium at a flow rate of 1 ml/min. FAMEs were identified by comparing their retention time with standards and quantitated using an internal standard method.
Astrocyte-neuron co-culture
Neurons for the astrocyte-neuron co-culture were obtained from the brain of rat pups at postnatal day 0–1. After decapitation cerebral cortices were dissected and collected in cold DMEM with 10% FBS then purified from the meninges, chopped and digested at 37 C with papain (2 mg/ml) (Sigma Aldrich, USA) for 30 min and with DNase (2.5mg/ml) (Sigma Aldrich, USA) for 30 s. Following trituration through a fire-polished glass Pasteur pipette cell suspension was passed through a 70 m cell strainer and centrifuged (800 rpm, 5 min, room temperature). Obtained neurons were plated at a density of 5 x 104 cells/well in poly-L-lysine coated 12-well culture plates and incubated in DMEM for 4 h. Then medium was changed to Neurobasal A enriched with 10% B27 supplement (Life Technologies, USA) and cells were allowed to settle for 5-10 days. ThinCert™ cell culture inserts (Greiner Bio-One, Austria) were utilized to obtain co-culture. Primary astrocytes were seeded in cell culture inserts with 1,0 m pores at density 1x105 cell/insert for 24 h. Following 24 h incubation with 30 M DHA inserts with astrocytes were transferred to the wells containing neurons. Astrocytes and neurons were co-cultivated in DMEM supplemented with N2 and FBS for 24 h. Subsequently, inserts with astrocytes were removed and neurons were exposed to 40 M tBOOH and 0.45 mM H2O2 for 2 h.
Flow cytometry
The sensitivity of neurons co-cultured with DHA-enriched astrocytes to cytotoxic agents was evaluated by the quantification of viable, apoptotic and necrotic cells by Annexin V/propidium iodide (PI) (Life Technologies, USA) double staining and detection by flow cytometry. Neurons were harvested with trypsin-EDTA, washed with PBS and stained with Annexin V-FITC and PI for 15 min, at room temperature in the dark. The fluorescence intensity of 10,000 cells was measured in a FACSCanto II flow cytometer (Becton Dickinson, USA). The proportions of viable (Annexin−/PI−), apoptotic (Annexin V+/PI−), necrotic (Annexin V−/PI+) cells were analyzed using BD FACSDiva software.
Statistical analyzes
The results are presented as means ± SEM. Statistical significance using GraphPad Prism 6.0 was determined by one-way ANOVA followed by the Newman-Keuls multiple comparison test. For analysis of neuron viability student’s t-test was applied. Statistical significance was considered at a P <0.05.
Results
Expression of FADS2 gene in astrocytes
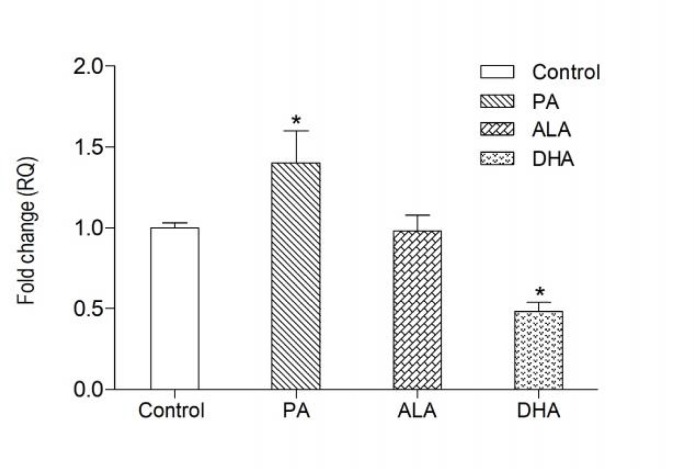
The transcription of FADS2 gene was significantly up-regulated in astrocytes supplemented with PA (P<0.01) and down-regulated by at least 50% in astrocytes incubated with DHA (P < 0.01) as compared to cells growing in the medium not supplemented with fatty acids. The level of FADS2 mRNA in astrocytes incubated with ALA was not different from that in control cells (figure 1).
Fig. 1.
FADS2 mRNA levels in astrocytes after incubation with palmitic acid (PA), -linolenic acid (ALA) and docosahexaenoic acid (DHA). All fatty acids were used at 50 M concentration. Data are mean of fold change SEM. * P < 0.01 compared to untreated astrocytes (control)
Incorporation of DHA into astrocyte membranes
The fatty acid composition in mitochondrial and plasma membrane of astrocytes incubated with DHA is shown in Table 2. Incubation of astrocytes with DHA resulted in about 2.5-fold increase in the content of this fatty acid in mitochondrial as well as about 2-fold rise in plasma membranes compared to control cells.
Table 2.
Fatty acids composition of mitochondrial and plasma membranes of astrocytes
| Fatty acids | % of total fatty acids |
||||
|---|---|---|---|---|---|
| Mitochondrial membranes | Plasma membranes | ||||
| Control | DHA-supplemented | Control | DHA-supplemented | ||
| Saturated | 59.67 0.79 | 56.06 0.77 | 48.13 0,57 | 47.63 1.1 | |
| MUFA | 25.15 0.19 | 21.10 0.14 | 29.44 0.32 | 23.92 0.42 | |
| PUFA n-6 | 8.02 0.29 | 6.28 0.06 | 10.39 0.42 | 7.18 0.37 | |
| C20:5n-3 (EPA) | 0.65 0.03 | 0.45 0.05* | 1.4 0.06 | 0.96 0.07* | |
| C22:6n-3 (DHA) | 6.51 0.22 | 16.12 0.8** | 10.65 0.11 | 20.33 0.41** | |
Values are mean SEM. *P < 0.05; **P < 0.01 compared to control.
Survival of neurons co-cultured with DHA-enriched astrocytes
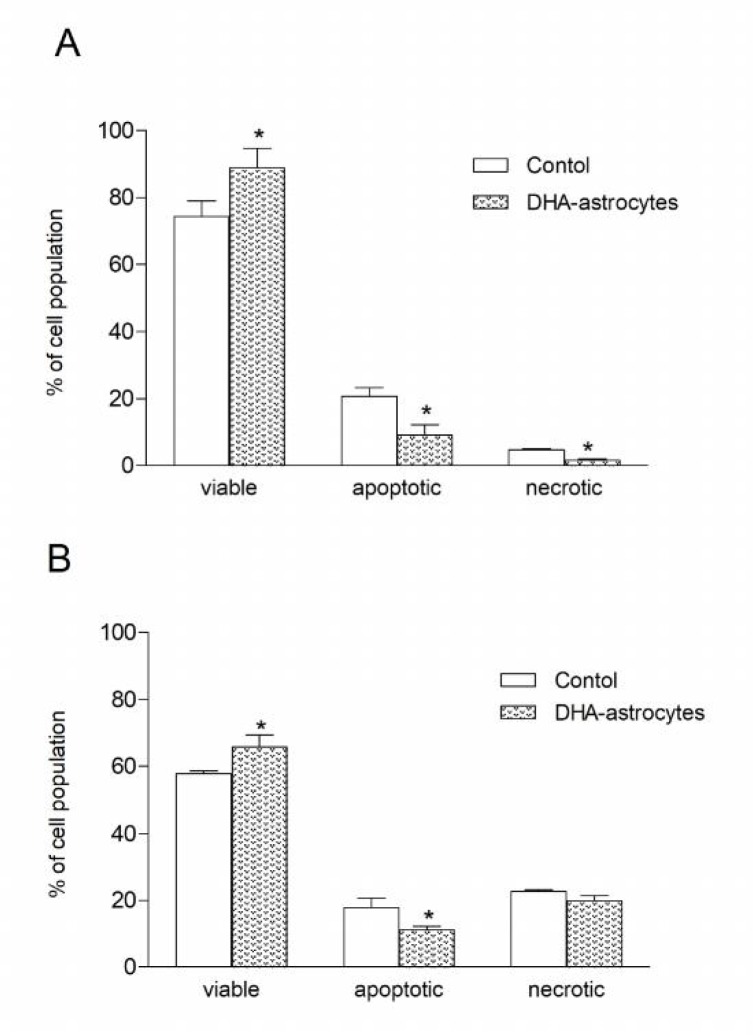
Figure 2 shows the quantification of viable, apoptotic and necrotic populations of neurons co-cultured for 24 h with DHA-enriched astrocytes compared to neurons co-cultured with astrocytes growing in standard medium without fatty acid (in figure depicted as control). Neurons co-cultured with astrocytes growing in DMEM after exposure to t-BOOH and H2O2 survived in 74% and 58%, respectively. Neurons co-culture with DHA-enriched astrocytes manifested a significant increase in percentage of viable cells after the treatment with t-BOOH and H2O2 to 89% and 66%, respectively.
Fig. 2.
Survival of neurons co-cultured with astrocytes preincubated with DHA. Cells were challenged with tBOOH (A) or H2O2 (B); cells growing in DMEM were used as control. Data are mean SEM, * P < 0.05 compared to control
Discussion
Our results showed that DHA suppressed, PA stimulated, while ALA did not change the FADS2 gene expression in astrocytes. Dietary study in animals demonstrated that a diet rich in fish oil decreased the activity of 6-desaturase in the liver microsomes (18). However, changes in the enzyme activity may not result from changes in the gene and protein expressions. In our previous study, we showed that the effect of fatty acids on FADS2 mRNA expression did not reflect the levels of FADS2 protein in astrocytes (19). It should be considered that there is a competition between specific fatty acids at the subsequent stages of their biosynthetic pathway. The experiments on cells transfected with FADS2 gene demonstrated LC-PUFA synthesis inhibition in excess of PA by competing ALA and linoleic acids for 6-desaturase which introduces double bonds at the first and the last stage of LC-PUFA biosynthesis (20).
The mechanism involved in the regulation of 6-desaturase by fatty acids is studied mostly in the liver (6) and adipose tissue (21), but rarely in astrocytes. In mammals, transcription of FADS2 is regulated by sterol regulatory element-binding protein 1 (SREBP-1) and peroxisome proliferator-activated receptor (PPAR) that act reciprocally on regulation of fatty acid metabolism (22,23) and as the sensors of PUFA levels in cells. PUFAs suppress the transcription of the hepatic lipogenic genes by reducing SREBP-1c mRNA and SREBP-1 protein levels, as well the posttranslational proteolytic activation of SREBP-1c (24), while saturated and monounsaturated fatty acids do not have such effect (25). It has been demonstrated that dietary fish oil decreased the 6-desaturase mRNA and protein expressions, both in the liver and the brain (26), and the 6-desaturase promoter has the functional sequence that binds SREBP 1 during suppression by PUFA (27). Furthermore, the 6-desaturase expression was increased in mice overexpressing the nuclear form of SREBP (23). These findings indicate that DHA-reduced FADS2 transcription in our study may result from SREBP-1c inhibition. Alternatively, DHA as an endogenous ligand of retinoid receptor (RXR) (28, 29), the obligatory member of the heterodimer RXR/ PPARα (30) may regulate the expression of FADS2 gene (31).
In a previous study (15), we showed that incubation of astrocytes with DHA enhanced the expression of the anti-oxidative GSH-dependent enzymes via the Nrf2 pathway, that is in accordance with other results (32, 33). In the present work we demonstrated that astrocytes with DHA-enriched membrane phospholipids protect neurons against death caused by cytotoxic agents. Since astrocytes supply glutathione (GSH) to neurons (our own unpublished results, 34), the neuroprotective effect of DHA-enriched astrocytes likely results from increased GSH delivery and enhanced neurons resistance to cytotoxic compounds. This is in line with the study on postmitotic human dopaminergic neurons cocultured with human or murine astrocytes before exposure to proteotoxic and oxidative stress. Astrocytes prevented neuronal apoptosis, enhanced degradation of aggregated poly-ubiquitinated proteins and increased resilience of neurons to cellular stress via continuously released GSH (35). This study demonstrates that FADS2 expression was down-regulated after incubation of astrocytes with DHA, however, the DHA levels increased in the astrocytic membranes. The astrocytes with DHA-enriched membrane phospholipids enhanced neuronal resistance to cytotoxic compounds although the synthesis of LC-PUFAs could be reduced in response to elevated DHA levels. These results suggest that beneficial effects of supplementation with n-3 PUFA in early stages of Alzheimer disease and in psychiatric disorders is caused, in part, by increased efficacy of DHA-enriched astrocytes to protect neurons under adverse conditions in the brain, such as neuroinflammation or oxidative stress during ischemia.
Acknowledgments
This work was support by The National Center for Research and Development for bilateral co-operation with Taiwan (grant DKO/PL-TW1/4/2013) and grants from the Medical University of Lodz 503/0-079-04/503-01-001 and 502-03/0-079-04/502-04-009.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Gharami K, Das M, Das S. Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem Int. 2015;89:51–62. doi: 10.1016/j.neuint.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Wysoczanski T, Sokola-Wysoczanska E, Pekala J, et al. Omega-3 Fatty Acids and their Role in Central Nervous System - A Review. Curr Med Chem. 2016;23:816–31. doi: 10.2174/0929867323666160122114439. [DOI] [PubMed] [Google Scholar]
- 3.Cunnane SC, Schneider JA, Tangney C, et al. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2012;29:691–7. doi: 10.3233/JAD-2012-110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNamara RK, Jandacek R, Tso P, et al. Lower docosahexaenoic acid concentrations in the postmortem prefrontal cortex of adult depressed suicide victims compared with controls without cardiovascular disease. J Psychiatr Res. 2013;47:1187–91. doi: 10.1016/j.jpsychires.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNamara RK, Jandacek R, Rider T, et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–99. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Igarashi M, DeMar JC Jr, Ma K, et al. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–8. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486:219–31. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 8.Moore SA, Yoder E, Murphy S. Astrocytes, not neurons, produce docosahexaenoic acid (22:6-3) and arachidonic acid (20:4-6) J Neurochem. 1991;56:518–24. doi: 10.1111/j.1471-4159.1991.tb08180.x. [DOI] [PubMed] [Google Scholar]
- 9.McNamara RK, Jandacek R, Rider T, et al. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophr Res. 2007;91:37–50. doi: 10.1016/j.schres.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidel JL, Escartin C, Ayata C, et al. Multifaceted roles for astrocytes in spreading depolarization: A target for limiting spreading depolarization in acute brain injury? Glia. 2016;64:5–20. doi: 10.1002/glia.22824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmoud S, Gharagozloo M, Simard C, et al. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells. 2019:8. doi: 10.3390/cells8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santello M, Calì C, Bezzi P. Gliotransmission and the tripartite synapse. Adv Exp Med Biol. 2012;970:307–31. doi: 10.1007/978-3-7091-0932-8_14. [DOI] [PubMed] [Google Scholar]
- 13.Devaraju P, Sun MY, Myers TL, et al. Astrocytic group I mGluR-dependent potentiation of astrocytic glutamate and potassium uptake. J Neurophysiol. 2013;109:2404–14. doi: 10.1152/jn.00517.2012. [DOI] [PubMed] [Google Scholar]
- 14.Skowronska K, Obara-Michlewska M, Zielinska M, et al. NMDA Receptors in Astrocytes: In Search for Roles in Neurotransmission and Astrocytic Homeostasis. Int J Mol Sci. 2019:20. doi: 10.3390/ijms20020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zgorzynska E, Dziedzic B, Gorzkiewicz A, et al. Omega-3 polyunsaturated fatty acids improve the antioxidative defense in rat astrocytes via an Nrf2-dependent mechanism. Pharmacol Rep. 2017;69:935–42. doi: 10.1016/j.pharep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albuquerque C, Joseph DJ, Choudhury P, et al. Dissection, plating, and maintenance of cortical astrocyte cultures. Cold Spring Harb Protoc. 2009;2009:pdb prot5273. doi: 10.1101/pdb.prot5273. [DOI] [PubMed] [Google Scholar]
- 18.Stawarska A, Bialek A, Tokarz A. The type of dietary fat and dietary energy restriction affects the activity of the desaturases in the liver microsomes. Prostaglandins Leukot Essent Fatty Acids. 2018;128:62–6. doi: 10.1016/j.plefa.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Dziedzic B, Bewicz-Binkowska D, Zgorzynska E. DHA upregulates FADS2 expression in primary cortical astrocytes exposed to vitamin A. Physiol Res. 2018;67:663–8. doi: 10.33549/physiolres.933708. [DOI] [PubMed] [Google Scholar]
- 20.Park HG, Kothapalli KSD, Park WJ, et al. Palmitic acid (16:0) competes with omega-6 linoleic and omega-3 a-linolenic acids for FADS2 mediated Delta6-desaturation. Biochim Biophys Acta. 2016;1861:91–7. doi: 10.1016/j.bbalip.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ralston JC, Matravadia S, Gaudio N, et al. Polyunsaturated fatty acid regulation of adipocyte FADS1 and FADS2 expression and function. Obesity (Silver Spring) 2015;23:725–8. doi: 10.1002/oby.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura MT, Nara TY. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot Essent Fatty Acids. 2003;68:145–50. doi: 10.1016/s0952-3278(02)00264-8. [DOI] [PubMed] [Google Scholar]
- 23.Matsuzaka T, Shimano H, Yahagi N. Dual regulation of mouse Δ5-and Δ6-desaturase gene expression by SREBP-1 and PPARα. J Lipid Res. 2002;43:107–14. [PubMed] [Google Scholar]
- 24.Xu J, Cho H, O'Malley S. Dietary polyunsaturated fats regulate rat liver sterol regulatory element binding proteins-1 and-2 in three distinct stages and by different mechanisms. J Nutr. 2002;132:3333–9. doi: 10.1093/jn/132.11.3333. [DOI] [PubMed] [Google Scholar]
- 25.Vallim T, Salter AM. Regulation of hepatic gene expression by saturated fatty acids. Prostaglandins Leukot Essent Fatty Acids (PLEFA) 2010;82:211–8. doi: 10.1016/j.plefa.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu H, Fan C, Xu F. Dietary fish oil n-3 polyunsaturated fatty acids and alpha-linolenic acid differently affect brain accretion of docosahexaenoic acid and expression of desaturases and sterol regulatory element-binding protein 1 in mice. J Nutr Biochem. 2010;21:954–60. doi: 10.1016/j.jnutbio.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Nara TY, He WS, Tang C, et al. The E-box like sterol regulatory element mediates the suppression of human Delta-6 desaturase gene by highly unsaturated fatty acids. Biochem Biophys Res Commun. 2002;296:111–7. doi: 10.1016/s0006-291x(02)00851-3. [DOI] [PubMed] [Google Scholar]
- 28.Bordoni A, Di Nunzio M, Danesi F, et al. Polyunsaturated fatty acids: From diet to binding to ppars and other nuclear receptors. Genes Nutr. 2006;1:95–106. doi: 10.1007/BF02829951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyall SC, Michael GJ, Michael-Titus AT. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res. 2010;88:2091–102. doi: 10.1002/jnr.22390. [DOI] [PubMed] [Google Scholar]
- 30.Rastinejad F. Retinoid X receptor and its partners in the nuclear receptor family. Curr Opin Struct Biol. 2001;11:33–8. doi: 10.1016/s0959-440x(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 31.Tang C, Cho HP, Nakamura MT. Regulation of human Δ-6 desaturase gene transcription identification of a functional direct repeat-1 element. J Lipid Res. 2003;44:686–95. doi: 10.1194/jlr.M200195-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Bell KF, Al-Mubarak B, Fowler JH, et al. Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci U S A. 2011;108:E1. doi: 10.1073/pnas.1015229108. 2; author reply E3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta K, Chandran S, Hardingham GE. Human stem cell-derived astrocytes and their application to studying Nrf2-mediated neuroprotective pathways and therapeutics in neurodegeneration. Br J Clin Pharmacol. 2013;75:907–18. doi: 10.1111/bcp.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chowdhury T, Allen MF, Thorn TL, et al. Interleukin-1beta Protects Neurons against Oxidant-Induced Injury via the Promotion of Astrocyte Glutathione Production. Antioxidants (Basel) 2018:7. doi: 10.3390/antiox7080100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutbier S, Spreng AS, Delp J, et al. Prevention of neuronal apoptosis by astrocytes through thiol-mediated stress response modulation and accelerated recovery from proteotoxic stress. Cell Death Differ. 2018;25:2101–17. doi: 10.1038/s41418-018-0229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]