Abstract
Twenty-five years ago the first small molecule inhibitors of Hsp90 were identified. In the intervening years there has been dramatic progress in basic scientific understanding of the Hsp90 chaperone machinery and in the role of Hsp90 in malignancy. The first-in-class Hsp90 inhibitor 17-AAG entered into Phase I clinical trials in 1999. There are now 13 Hsp90 inhibitors in clinical trial, representing multiple drug classes, and hundreds of patients have been treated in adult oncology and pediatric oncology trials. This review will provide an overview of the clinical trial results thus far. In addition, pivotal issues in further development of Hsp90 inhibitors as anticancer drugs will be discussed.
Keywords: Keywords, Hsp90 inhibitors, targeted therapy, clinical trial
INTRODUCTION
The benzoquinone ansamycins, including geldanamycin, herbimycin and macbecin, are natural products originally isolated for their antibiotic activity [1]. In the mid 1980s a series of experiments demonstrated that the benzoquinone ansamycins reverse the malignant phenotype of v-src transformed cells. At this time it was thought that the anticancer activity of these small molecules was due to their acting as inhibitors of the tyrosine kinase activity of src family proteins [2]. In experiments published in 1994, it was demonstrated that these small molecules bind specifically to heat shock protein (Hsp)90. Furthermore, it was demonstrated that binding results in src degradation, and that these events occur at drug concentrations considerably lower than concentrations required for inhibition of src enzymatic activity [3]. Thus, small molecules that were thought to be classical tyrosine kinase inhibitors (TKIs) could result in loss of tyrosine kinase activity because they caused degradation of the kinase. This discovery had several ramifications. One was that it provided a class of bioprobes that were enormously useful in discovery of myriad Hsp90 client proteins and in elucidation of how the Hsp90 machinery functions and the role of Hsp90 in the cell. Secondly, these experiments led directly to the first clinical Hsp90 inhibitor, 17-AAG (17-allylamino-17-demethoxygeldanamycin, tanespimycin). For many years 17-AAG was the only Hsp90 inhibitor in clinical trial. After 5 years a more water-soluble geldanamycin, 17-dimethylamino-ethylamino-17-demethoxygeldanamycin (17-DMAG, alvespimycin), was introduced in the clinic. Strikingly, one year later, a clinical trial opened with IPI-504 (retaspimycin), a hydroquinone form of 17-AAG, and within the next four years 11 additional Hsp90 inhibitors, from nine big pharma and small biotech companies, entered clinical trial (see Table 1). Clearly, there is unabated and widespread interest in Hsp90 as a molecular target. Here we provide an update on the status of Hsp90 inhibitor clinical trials. Additional recent reviews of this topic are suggested for the interested reader [4–7].
Table 1.
Hsp90 Inhibitors in Clinical Development
| Inhibitor | Structure | Route | Phase | Company |
|---|---|---|---|---|
| Tanespimycin (17-AAG) | 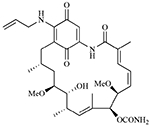 |
i.v. | III | BMS Kosan |
| Retaspimycin hydrochloride (IPI-504) | 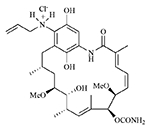 |
i.v. | II | Infinity |
| IPI-493 | 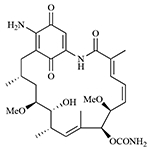 |
oral | I | Infinity |
| SNX-5422 mesylate | 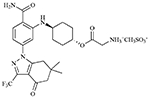 |
oral | I | Pfizer Serenex, Inc. |
| AUY922 | 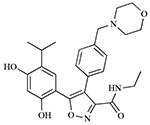 |
i.v. | I/II | Novartis |
| BIIB021 CNF-2024 | 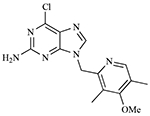 |
oral | II | Biogen Idec |
| BIIB028 | Small molecule* | i.v. | I | Biogen Idec |
| STA-9090 | Resorcinol derivative* | i.v. | I/II | Synta |
| KW-2478 | Small molecule* | i.v. | I | Kyowa Hakko Kirin |
| AT13387 | Small molecule* | oral, i.v. | I | Astex Therapeutics |
| XL888 | Small molecule* | oral | I | Exelixis |
| HSP990 | Small molecule* | oral | I | Novartis |
| MPC-3100 | Small molecule* | oral | I | Myriad Pharmaceuticals |
| ABI-010 | Nanoparticle albumin-bound 17-AAG | i.v. | I | Abraxis Bioscience |
The structures are not reported.
HSP90 INHIBITORS IN CLINICAL DEVELOPMENT
The first-in-class Hsp90 inhibitor, 17-allylamino-17-demethoxygeldanamycin (17-AAG, tanespimycin), entered clinical trial in 1999. There are now 40 completed and 23 active Hsp90 inhibitor oncology trials (see Tables 2 & 3). The majority of completed trials studied 17-AAG in various schedules, formulations and cancer indications. Currently, 17-AAG is being tested in disease-specific trials in monotherapy and combination therapy protocols.
Table 2.
Closed/Suspended Hsp90 Inhibitor Clinical Trials
| Inhibitor | Combined Drug | Phase | Sponsor | Indication* |
|---|---|---|---|---|
| Tanespimycin (KOS-953) | Bortezomib | II/III | BMS | Relapsed/refractory MM |
| Tanespimycin (KOS-953) | Bortezomib | III | BMS | MM in first relapse |
| 17-AAG | - | II | NCI | Stage III or IV melanoma |
| 17-AAG | - | II | NCI | Von Hippel-Lindau disease and renal tumors |
| 17-AAG | - | II | NCI | Metastatic papillary or clear cell RCC |
| 17-AAG | - | II | NCI | Refractory locally advanced or metastatic breast cancer |
| 17-AAG | - | II | NCI | Metastatic malignant melanoma |
| 17-AAG | - | II | NCI | Inoperable locoregionally advanced or metastatic medullary or differentiated thyroid carcinoma |
| 17-AAG | - | II | NCI | Hormone-refractory metastatic prostate cancer |
| 17-AAG | - | II | NCI | Relapsed or refractory anaplastic large cell lymphoma, MCL, or classical Hodgkin’s lymphoma |
| 17-AAG | - | II | NCI | Systemic mastocytosis |
| 17-AAG | - | I | Other** | Advanced malignancies |
| 17-AAG | - | I | NCI | Refractory or advanced solid tumors or hematologic malignancies |
| 17-AAG | - | I | NCI | Unresectable solid tumors or relapsed lymphoma |
| 17-AAG | - | I | NCI | Advanced epithelial cancer, malignant lymphoma, or sarcoma |
| 17-AAG | - | I | NCI | Advanced solid tumors |
| 17-AAG | - | I | NCI | Recurrent or refractory leukemia or selected solid tumors |
| 17-AAG | - | I | NCI | Imatinib mesylate-resistant chronic phase CML |
| 17-AAG | - | I | NCI | Metastatic or unresectable solid tumors or lymphoma |
| 17-AAG | Docetaxel | I | NCI | Progressive metastatic prostate cancer or other progressive metastatic or unresectable solid tumors |
| 17-AAG | Imatinib | I | NCI | CML |
| 17-AAG | - | I | NCI | Pediatric patients with relapsed or refractory solid tumors or leukemia |
| 17-AAG | Paclitaxel | I | NCI | Metastatic or unresectable solid malignancy |
| 17-AAG | Cytarabine | I | NCI | Relapsed or refractory AML, ALL, CML, CMML, or high-grade MDS |
| 17-AAG | w/wo Rituximab | I | NCI | Relapsed B-cell chronic lymphocytic leukemia or prolymphocytic leukemia |
| 17-AAG | Bortezomib | I | NCI | Relapsed or refractory AML, ALL, CLL, or NHL |
| 17-AAG | Irinotecan | I | NCI | Locally advanced or metastatic solid tumors |
| 17-AAG | Sorafenib | I | NCI | Metastatic or unresectable solid tumors |
| 17-DMAG | - | I | NCI | Advanced solid tumor or lymphoma |
| 17-DMAG | - | I | NCI | Metastatic or unresectable solid tumors or lymphomas |
| 17-DMAG | - | I | NCI | Metastatic or unresectable solid tumors |
| 17-DMAG | I | NCI | Unresectable or metastatic solid tumors | |
| IPI-504 | - | III | Infinity | Metastatic and/or unresectable GIST following failure of at least imatinib and sunitinib |
| IPI-504 | - | I/II | Infinity | Relapsed/refractory stage IIIb, or stage IV NSCLC |
| IPI-504 | - | II | Infinity | Hormone-resistant prostate cancer |
| IPI-504 | - | I | Infinity | Relapsed and relapsed refractory MM |
| IPI-504 | - | I | Infinity | GIST or STS |
| IPI-504 | Trastuzumab | II | Infinity | Pretreated, locally advanced or metastatic HER2 positive breast cancer |
| IPI-504 | Docetaxel | I | Infinity | Advanced solid tumors |
| BIIB021 | - | I | Biogen Idec | Advanced solid tumors |
multiple myeloma (MM), acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia (CML), acute myelogenous leukemia (AML), chronic lymphocytic leukemia (CLL), chronic myelomonocytic leukemia (CMML), myelodysplastic syndrome (MDS), mantle cell lymphoma (MCL), metastatic breast cancer (MBC), non-small cell lung cancer (NSCLC), gastrointestinal stromal tumor (GIST), non-Hodgkin’s lymphoma (NHL), soft tissue sarcoma (STS), renal cell carcinoma (RCC)
Cancer Research UK Clinical Groups at Guy’s, King’s & St. Thomas’ Hospitals.
Table 3.
Active Hsp90 Inhibitor Clinical Trials
| Inhibitor | Combined drug | Phase | Sponsor | Indication |
|---|---|---|---|---|
| 17-AAG | Gemcitabine | II | NCI | Stage IV pancreatic adenocarcinoma |
| 17-AAG | Gemcitabine | II | NCI | Advanced ovarian epithelial or peritoneal cavity cancer |
| 17-AAG | - | I | NCI | Advanced solid tumors |
| 17-AAG | Bortezomib | I | NCI | Advanced solid tumors or lymphomas |
| IPI-504 | Docetaxel | I | Infinity | Advanced solid tumors/NSCLC |
| IPI-504 | Trastuzumab | II | Infinity | HER2-positive MBC |
| IPI-504 | - | II | Infinity | NSCLC |
| IPI-493 | - | I | Infinity | Advanced solid tumors |
| SNX-5422 | - | I | Pfizer | Refractory solid tumor malignancies |
| SNX-5422 | - | I | Pfizer | Refractory hematological malignancies |
| SNX-5422 | - | I | NCI | Refractory solid tumors or lymphomas |
| AUY922 | - | I/II | Novartis | Advanced solid malignancies, HER2+ or ER+ locally advanced or metastatic breast cancer |
| AUY922 | w/wo Bortezomib | I/II | Novartis | Relapsed or refractory MM |
| STA-9090 | - | I/II | Synta | AML, CML, MDS and myeloproliferative disorders |
| STA-9090 | - | I | Synta | Solid tumors |
| BIIB021 | w/wo Trastuzumab | I | Biogen Idec | HER2− (single dose) or HER2+ (combination with Trastuzumab) advanced breast cancer |
| BIIB021 | - | I | Biogen Idec | Advanced solid tumors |
| BIIB021 | - | II | Biogen Idec | GIST |
| BIIB028 | - | I | Biogen Idec | Solid tumors |
| KW-2478 | - | I | Kyowa Hakko Kirin | MM, CLL or B-cell NHL |
| AT13387 | - | I | Astex Therapeutics | Metastatic solid tumors |
| XL888 | - | I | Exelixis | Solid tumors |
| HSP990 | - | I | Novartis | Advanced solid tumors |
| MPC-3100 | - | I | Myriad Pharmaceuticals | Cancer patients who have failed other treatments |
| ABI-010 | w/wo ABI-007 | I | Abraxis Bioscience | Advanced non-hematologic malignancies |
A major limitation of 17-AAG has been its poor solubility in water. To improve solubility and bioavailability of 17-AAG, several formulations have been developed. Initially, a 4% DMSO in egg phospholipid formulation, delivered at 1 mg/ml was developed by the National Cancer Institute (NCI). Kosan Biosciences developed a Cremophor-based formulation delivered at 1.4 mg/ml. Disadvantages included a requirement for steroid and antihistamine premedications and requirements for specialized administration sets. Kosan then developed an isotonic injectable suspension product, delivered at 5 mg/ml, with greater peripheral vein tolerability and venous access device compatibility, which did not require steroid premedications or specialized tubing/bags. The suspension product and Cremophor formulation were compared in multiple myeloma in a study of tanespimycin plus bortezomib at the recommended Phase II dose, and it was concluded that future trials would use the suspension formulation [8]. In June of 2008 Bristol Myers Squibb (BMS) acquired Kosan and is continuing tanespimycin development.
Conforma Therapeutics developed an oil-in-water nanoemulsion of 17-AAG (CNF 1010) that also avoided DMSO or Cremaphor. CNF1010 was acquired by Biogen Idec with its acquisition of Conforma. 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin, KOS-1022, alvespimycin) is a water-soluble geldanamycin analog developed by Kosan that can be administered both intravenously and orally. An albumin-bound form of 17-AAG (ABI-010) was developed by Abraxis Bioscience, Inc. Their data suggest that the binding of albumin to 17-AAG and other anticancer agents creates a nanometer-sized particle that is readily taken up by tumor cells, enabling higher concentrations of drug at the target site, as well as improved drug solubility. ABI-010 is scheduled to enter Phase I testing as a single agent or in combination with ABI-007 (Abraxane, a Cremophor-free nanoparticle paclitaxel) in patients with advanced non-hematologic malignancies.
The water-soluble Hsp90 inhibitor, IPI-504 (retaspimycin hydrochloride), was developed by Infinity Pharmaceuticals, Inc. It is the hydroquinone hydrochloride derivative of 17-AAG, prepared by the reduction of the quinone moiety of 17-AAG to the hydroquinone using sodium hydrosulfite followed by the formation of the hydrochloride salt. It is administered intravenously and is currently in Phase II evaluation. Infinity Pharmaceuticals, Inc. also developed an oral formulation of 17-AG (17-amino-17-demethoxygeldanamycin, IPI-493), a metabolite of 17-AAG and IPI-504 that retains Hsp90 inhibitory activity. IPI-493 is currently in a Phase I dose escalation study in patients with advanced solid tumors.
CLINICAL TRIALS
Table 4 lists the Hsp90 inhibitor clinical trials that have been reported since 2004. Some of these trials are described below in more detail.
Table 4.
Abstracts of Hsp90 Clinical Trials, 2004 - 2009
| Inhibitor | Combined drug(s) | N1 | Phase | Route | Indication | Refs |
|---|---|---|---|---|---|---|
| Tanespimycin | - | 14 | II | i.v. | Metastatic melanoma | [57] |
| Tanespimycin | Bortezomib | 49 | * | i.v. | MM | [58] |
| Tanespimycin | Bortezomib | 72 | I/II | i.v. | Relapsed and refractory MM | [31] |
| Tanespimycin | Trastuzumab | 29 | II | i.v. | HER2-positive metastatic breast cancer | [30] |
| 17-AAG | - | 17 | I | i.v. | Relapsed/refractory pediatric patients with solid tumors | [59] |
| 17-AAG | - | 12 | I | i.v. | Children with solid tumors | [60] |
| 17-AAG | - | 13 | I | i.v. | Relapsed refractory MM | [61] |
| 17-AAG | - | 12 | I | i.v. | Advanced cancer | [62] |
| 17-AAG | - | 45 | I | i.v. | Advanced tumors | [63] |
| 17-AAG | Sorafenib | 16 | I | i.v. | Pretreated advanced malignancy | [64] |
| 17-AAG | Irinotecan | 22 | I | i.v. | Solid tumors | [65] |
| 17-AAG | Bortezomib | 20 | I | i.v. | Relapsed refractory MM | [66] |
| 17-AAG | Trastuzumab | 25 | I | i.v. | Advanced solid tumors | [67] |
| 17-AAG | Docetaxel | 40 | I | i.v. | Solid tumors | [68] |
| 17-AAG | Docetaxel | 16 | I | i.v. | Advanced solid tumors | [69] |
| 17-AAG | Gemcitabine, Cisplatin | 12 | I | i.v. | Solid tumors | [70] |
| CNF1010 | - | 30 | I | i.v. | Advanced solid tumors | [71] |
| IPI-504 | - | 60 | I | i.v. | Advanced cancer | [72] |
| IPI-504 | - | 54 | I | i.v. | Metastatic, TKI-resistant GIST or advanced/metastatic solid tumors | [36] |
| IPI-504 | - | 21 | I | i.v. | Metastatic TKI-resistant GIST | [44] |
| IPI-504 | - | 19 | II | i.v. | Relapsed and/or refractory stage IIIb or stage IV NSCLC | [33] |
| IPI-504 | - | 19 | II | i.v. | Castration-resistant prostate cancer | [34] |
| IPI-504 | - | 12 | I/II | i.v. | Relapsed and/or refractory stage IIIb or stage IV NSCLC | [73] |
| IPI-504 | Docetaxel | 16 | lb | i.v. | Advanced solid tumors | [32] |
| Alvespimycin | - | 25 | I | i.v. | Advanced solid tumors | [74] |
| Alvespimycin | - | 47 | * | oral | Solid tumors | [75] |
| Alvespimycin | - | 10 | I | i.v. | Advanced, solid tumours | [76] |
| Alvespimycin | - | 28 | I | oral | Advanced malignancies | [77] |
| Alvespimycin | - | 23 | I | i.v. | Solid tumors | [78] |
| Alvespimycin | - | 13 | I | i.v. | Refractory hematological malignancies | [79] |
| Alvespimycin | - | 37 | I | i.v. | Advanced solid tumors | [80] |
| Alvespimycin | Trastuzumab | 21 | I | i.v. | H-refractory HER2+ metastatic breast cancer and refractory ovarian cancer | [81] |
| BIIB021 | - | 23 | I | oral | Advanced solid tumors or CLL | [82] |
| SNX-5422 | - | 11 | I | oral | Refractory solid tumors or lymphomas | [83] |
| KW-2478 | - | 15 | I | i.v. | Refractory MM, CLL, NHL | [84] |
| AUY922 | - | 44 | I | i.v. | Advanced solid malignancies | [85] |
| AUY922 | - | 40 | I | i.v. | Solid tumors | [86] |
number of patients;
not specified
1. 17-AAG/tanespimycin
In Phase I studies, 17-AAG was evaluated using schedules of daily x5, daily x3, 2x weekly, and weekly. The maximum tolerated dose (MTD) was clearly schedule-dependent with intermittent dosing schedules being less toxic [9]. For example, after administration of 17-AAG daily for 5 days every 3 weeks, the maximum tolerated dose (MTD) was 40-56 mg/m2 [10]. In contrast, with 2x weekly dosing the MTD has varied between 175-220 mg/m2, and with once weekly dosing (3 weeks on, 1 week off treatment) it varied between 295 mg/m2 and 450 mg/m2 [11–15]. The improved tolerability of intermittent dosing must be balanced against the impact on biologic activity of less frequent 17-AAG administration. Since 17-AAG and other Hsp90 inhibitors persist in tumor significantly longer than in normal tissues and blood [16], the majority of follow-on adult trials have adopted either twice weekly or once weekly dosing schedules.
17-AAG also was evaluated in two pediatric Phase I trials using a 2x weekly (2 weeks out of 3) schedule [17, 18]. Although no objective responses were observed, biologic activity was detected in peripheral blood mononuclear cells without dose-limiting toxicity. The recommended Phase II dose was either 270 mg/m2 [17] or 360 mg/m2 [18]. Both trials used DMSO as the diluent and one of the groups concluded that use of non-DMSO-containing formulations might improve acceptance of this drug by the children and their families [18].
CNF1010 was under evaluation in Phase I trial in patients with advanced solid tumors and in Phase I trial in chronic lymphocytic leukemia patients expressing ZAP-70. However, the trials were terminated due to discontinuation of the program prior to its acquisition by Bristol Myers Squibb (BMS) [19].
17-AAG has been evaluated in several Phase II trials (see Table 2), including in patients with papillary and clear cell renal cell carcinoma [20], in patients with metastatic melanoma [21], and in patients with hormone refractory metastatic prostate cancer [22]. In the renal cell carcinoma study, patients were administered 17-AAG intravenously at a dose of 220 mg/m2 twice weekly for two weeks followed by a week of rest. No objective responses were seen. In the metastatic melanoma study, patients received 17-AAG intravenously at a dose of 450 mg/m2 once per week for six weeks. Tumor biopsies were obtained pre-treatment and 18 - 50 hours following the first dose. While no objective responses were seen, pharmacodynamic evaluation of the tumor biopsies before and after drug treatment revealed that anti-Hsp90 biologic activity was short-lived. These data suggest that a formulation and schedule that provide a more prolonged suppression of Hsp90 may be necessary to obtain significant activity in these settings. A similar conclusion may be drawn from the prostate cancer trial, in which patients with castration-resistant disease, at least one prior systemic therapy, and rising prostate specific antigen (PSA) received 17-AAG at a dose of 300 mg/m2 once weekly for three out of every four weeks. With this schedule, no activity was observed.
Preclinical studies have reported synergistic interactions between 17-AAG and paclitaxel [23, 24]. A Phase I study evaluating this drug combination in patients with solid tumors refractory to standard therapy was reported recently [25]. 17-AAG was administered on a twice weekly schedule for three out of every four weeks and paclitaxel was administered once weekly for three out of every four weeks. Disease stabilization was noted in six of twenty-five patients, and the recommended Phase II doses of 17-AAG and paclitaxel following this schedule were 175 mg/m2 and 80 mg/m2, respectively.
Two Hsp90 client proteins, Chk1 and Wee1, are kinases that regulate cell cycle progression by modulating the G2 checkpoint. Preclinical data show that both kinases are destabilized by Hsp90 inhibition, and that Hsp90 inhibitors sensitize tumor cells to DNA damaging agents (the tumor cells cannot arrest in G2 to repair the DNA damage) [26, 27]. Following up on these observations, a Phase I study recently evaluated the combination of 17-AAG and irinotecan, a topoisomerase I poison, in patients with a variety of solid tumors that were refractory to standard therapy [28]. Patients received intravenous irinotecan followed by 17-AAG once weekly for two weeks in a three-week cycle. Tumor shrinkage was observed in six of twenty-seven patients, although none qualified as a partial response by RECIST criteria. Five of ten patients whose tumors contained mutant p53 had stable disease. Tumor biopsies obtained at the MTD showed biologic evidence of Hsp90 inhibition, including loss of phospho-Chk1, abrogation of the G2 checkpoint, and cell death. The recommended Phase II doses using this schedule were 100 mg/m2 irinotecan and 300 mg/m2 17-AAG. The DNA damaging agent gemcitabine is also being evaluated in combination with 17-AAG in two Phase II trials - one in patients with stage IV pancreatic cancer and the other in patients with recurrent advanced ovarian epithelial or peritoneal cavity cancer [http://www.clinicaltrials.gov].
One of the most promising indications for Hsp90 inhibitor treatment is in HER-2-overexpressing breast cancer patients who have progressed on trastuzumab. A Phase I dose-escalation trial of trastuzumab and tanespimycin (17-AAG) conducted by Hudis and colleagues at Memorial Sloan-Kettering Cancer Center in patients with nonhematologic malignancy with evidence of progression on standard therapy demonstrated that the combination of trastuzumab and tanespimycin was safe and active in patients with trastuzumab-refractory HER-2-overexpressing breast cancer [29]. This group has gone on to undertake a Phase II trial of patients with HER2-positive metastatic breast cancer and progressive disease following first line trastuzumab or progressive disease within three months following adjuvant trastuzumab. Patients received standard weekly doses of trastuzumab followed by tanespimycin (450 mg/m2). The primary endpoint was the overall response rate by RECIST criteria. Early patients received Cremophor-based tanespimycin with antihistamine/steroid premedication and later patients received the suspension product without premedications. The preliminary conclusion from the Phase II trial was that the combination of trastuzumab and tanespimycin is active in patients with HER2-positive metastatic breast cancer with progression on trastuzumab therapy (response rate of 24% and clinical benefit rate of 57%) [30]. This trial was completed in May 2009.
Another promising indication is in relapsed and refractory multiple myeloma in combination with bortezomib. Richardson, Anderson and colleagues presented the final results of a Phase I/II study at ASCO 2009 [31], in which 58 patients with measurable disease treated with the bortezomib/tanespimycin combination, including a Phase I tanespimycin dose-escalation component and an expanded Phase II component, had response rates of 41%, 20% and 14% in bortezomib-naïve, -pretreated, and -refractory patients respectively. The median duration of response for the combination compared favorably to bortezomib monotherapy. In addition, no severe peripheral neuropathy was observed, consistent with the neuroprotective effect of tanespimycin in pre-clinical models. These investigators are initiating a Phase III study of tanespimycin and bortezomib versus bortezomib monotherapy.
2. IPI-504/Retaspimyrin
IPI-504 is being studied in combination with docetaxel on multiple schedules in a Phase 1b dose-escalation study. The study initially enrolled patients with advanced solid tumors and in late 2008 expanded to focus on patients with advanced non-small cell lung cancer (NSCLC) that, in addition to HER2-positive breast cancer and multiple myeloma, is a third promising indication for Hsp90 inhibitor therapy. Preliminary data on 22 patients were presented at ASCO 2009 [32]. An MTD of 450 mg/m2 was reached when administered every 3 weeks. The trial will continue with IPI-504 at a dose of 300 mg/m2 in combination with docetaxel.
Lynch and colleagues reported at ASCO 2009 on a trial of IPI-504 given as monotherapy twice weekly for 2 out of 3 weeks to patients with stage IIIb (with malignant effusion) or stage IV NSCLC that had progressed on tyrosine kinase inhibitor (TKI) treatment [33]. Two cohorts were studied on the basis of EGFR mutation status. IPI-504 monotherapy was well tolerated with evidence of antitumor activity, which was seen particularly, and unexpectedly, in NSCLC patients with wild-type EGFR. Further evaluation is continuing in the expansion phase of this trial and future trials in NSCLC will depend on a final analysis of the Phase II data.
At the ASCO 2009 Genitourinary Cancers Symposium, researchers from the Dana-Farber Cancer Institute reported that IPI-504 did not demonstrate efficacy as monotherapy in castration-resistant prostate cancer in a single-arm Phase II trial. Of 19 patients enrolled there were grade 3/4 toxicities in 8 and two deaths on study, one from hepatic failure and one from hyperglycemic ketoacidosis, which were determined to be related to intravenous IPI-504. One of four patients without bony metastases had a PSA decline of 48% from baseline after 9 cycles of treatment [34]. Fifteen patients on this study had bony metastatic disease and no PSA or RECIST responses were observed in this cohort. Taken together with a recent preclinical report that experimental prostate cancer growth in bone may be adversely affected by 17-AAG [35], these data suggest that more detailed evaluation of Hsp90 inhibitor efficacy in this setting may be warranted.
A Phase II study of IPI-504 in patients with metastatic gastrointestinal stromal tumors (GIST) refractory to tyrosine kinase inhibitors reported early evidence of activity (defined responses were seen in 78% of evaluable patients, with the best response being stable disease) [36]. Based on these data, in May of 2008 Infinity opened the RING trial, a Phase III randomized, double-blind, placebo-controlled registration trial of IPI-504 in patients with metastatic and/or unresectable GIST following failure of at least imatinib and sunitinib. They were planning to enroll approximately 195 patients and to follow the patients with imaging at weeks 2, 5, 8, 14 and every 6 weeks thereafter (http://clinicaltrials.gov/ct2/show/NCT00688766). However, on April 15, 2009, Infinity announced that it had elected to terminate the trial upon the recommendation of its independent data monitoring committee based on an early review of safety data from the first 46 patients enrolled, which showed a higher than anticipated mortality rate among patients enrolled in the treatment arm (http://www.liferaftgroup.org/docs/newsletters/May2009nwsltr.pdf). Julian Adams, Ph.D., president of R&D and CSO expressed the commitment of the company to the continued development of IPI-504 and IPI-493 (http://investor.ipi.com/releasedetail.cfm?ReleaseID=377328).
A Phase II trial of IPI-504 combined with trastuzumab has been initiated to establish the safety of IPI-504 with trastuzumab, as well as to demonstrate the anti-tumor activity of this combination in patients with HER2-positive metastatic breast cancer. Infinity is also evaluating a companion registration path in earlier line HER2-positive metastatic breast cancer, with IPI-504 plus trastuzumab plus chemotherapy (Julian Adams, personal communication).
3. 17-DMAG/Alvespimycin
17-DMAG was studied in four Phase I monotherapy trials at the NCI (days 1 and 4 or 2 and 5 weekly for 4 weeks), Memorial Sloan-Kettering Cancer Center (days 1, 8, 15 q 28 days), University of Pittsburgh (days 1-3 or 1-5 q 21 days), and Institute of Cancer Research (day 1, 8, 15, 22 q 28 days). At the 2007 ASCO Breast Cancer Symposium Kosan reported clinical benefit in 42% of patients (8 out of 19 evaluable) with refractory HER2-positive metastatic breast cancer and in patients with refractory ovarian cancer who were progressing on standard chemotherapy (http://www.medicalnewstoday.com/articles/94089.php). At the 2006 Annual Meeting of the American Society of Hematology it was reported that 17-DMAG in combination with chemo therapy resulted in complete responses in 3 of 17 patients with refractory acute myelogenous leukemia [37]. However, despite some evidence of promising anticancer activity 17-DMAG was found to be associated with unacceptable toxicity, which was target-unrelated [38], and, in 2008, 17-DMAG was dropped from further clinical development by Kosan. After its acquisition of Kosan, BMS continued an ongoing clinical trial of intravenous 17-DMAG plus trastuzumab with or without paclitaxel in patients with advanced solid tumor malignancies or HER2-positive metastatic breast cancer that had previously failed trastuzumab therapy. The purpose of the study was to determine the MTD of 17-DMAG when administered weekly in combination with trastuzumab or in combination with trastuzumab and paclitaxel in this patient population. The primary outcome measures were assessment of response by RECIST criteria after two cycles (8 weeks) of therapy and every two cycles thereafter in patients with measurable disease. The study enrolled 50 patients and was completed September 2009 (http://ctr.bms.com/OneBmsCtd/InitTrialDetailAction.do?pnum=CA201-001).
4. BIIB021
Synthetic Hsp90 inhibitors based on diverse non-ansamycin chemical scaffolds have been developed and are now undergoing clinical evaluation. Data suggest these compounds have several desirable properties compared to 17-AAG, including improved water solubility, ease of synthesis, and lowered susceptibility to MDR-mediated efflux [39, 40]. The first synthetic Hsp90 inhibitor to enter clinical trial was BIIB021 (formerly CNF2024, 6-chloro-9-[(4-methoxy-3,5-dimethylpyridin-2-yl-)methyl]-9H-purin-2-amine), developed initially by Conforma Therapeutics, which was acquired by Biogen Idee in May 2006. BIIB021 is currently in three clinical trials, including a Phase I single-agent trial in patients with HER2-negative advanced breast cancer or in combination with trastuzumab in patients with HER2-positive advanced breast cancer. A Phase I study of BIIB021 administered twice weekly in patients with advanced solid tumors defined the MTD at 600mg/m2. It is now being evaluated in a Phase I study in advanced solid tumors at a schedule of once or twice daily administration, with a goal of achieving more sustained and complete inhibition of Hsp90 (http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=588718&version=HealthProfessional&protocolsearchid=6735733). BIIB021 is also being studied in a Phase II trial in GIST in which the primary outcome measure is change in FDG-PET imaging.
5. SNX-5422
SNX-5422 mesylate (amino-acetic acid 4-[2-carbamoyl-5-(6,6-dimethyl-4-oxo-3-trifluoromethyl-4,5,6,7-tetrahydroindazol-l-yl)-phenylamino]-cyclohexyl ester methanesulfonate), developed by Serenex and acquired by Pfizer, is a prodrug of active SNX-2122 (4-[6,6-dimethyl-4-oxo-3-(trifluoromethyl)-4,5,6,7-tetrahydro-lH-indazol-l-yl]-2-[(trans-4-hydroxycyclohexyl)amino]benzamide), an indazolone 2-aminobenzamide analog. It is orally bioavailable and is currently undergoing three Phase I trials: an industry-sponsored dose-escalation safety and pharmacokinetics study of SNX-5422 tablets twice daily in patients with refractory solid tumors or non-Hodgkin’s lymphoma, an industry-sponsored dose-escalation safety and pharmacokinetics study of SNX-5422 tablets twice daily in patients with refractory hematologic malignancies, and an NCI-sponsored trial in patients with refractory solid tumors and lymphomas that includes pharmacodynamic endpoints. Primary outcomes are determination of MTD, safety and toxicity, and secondary outcome is determination of the PK profile of SNX-5422 and its active metabolite SNX-2112.
6. AUY-922 & HSP990
AUY-922, a 4,5-diarylisoxazole derivative developed by Novartis, is in Phase I/II trial in patients with advanced solid malignancies, especially HER2-positive or ER-positive locally advanced or metastatic breast cancer. Also, it is undergoing Phase I/II evaluation with or without Bortezomib in patients with relapsed or refractory multiple myeloma.
Vemalis and Novartis have also developed Hsp990, an orally bioavailable synthetic small molecule Hsp90 inhibitor. A Phase I dose escalation trial in patients with advanced solid tumors has been approved but is not yet active. A second dose escalation study in Japan and Korea is planned to start December 2009/January 2010.
7. STA-9090
Synta Pharmaceuticals developed a synthetic, small molecule Hsp90 inhibitor, STA-9090. Two dose-escalation Phase I trials in solid tumor patients evaluating STA-9090 administered once weekly or twice weekly for three weeks followed by a dose-free one-week interval opened in October 2007. A Phase I/II safety and efficacy study of STA-9090 administered twice weekly for the treatment of AML, CML, myelodysplastic syndromes and myeloproliferative disorders opened in March 2009. An open-label Phase I/II study to assess safety and efficacy of once-weekly STA-9090 in subjects with AML, ALL and blast-phase CML has been approved but is not yet open. In March 2009, Synta announced an additional Phase I/II trial for hematologic cancers, with plans for opening an additional trial for solid tumors later in 2009.
8. KW-2478
Kyowa Hakko Kirin has developed a non-ansamycin, non-purine Hsp90 inhibitor, KW-2478, which is administered intravenously. A Phase I trial is underway in patients with multiple myeloma, chronic lymphocytic leukemia (CLL), or B-cell non-Hodgkin’s lymphoma.
9. MPC-3100, XL888, AT13387
Myriad Pharmaceuticals has developed a fully synthetic, small molecule, orally bioavailable Hsp90 inhibitor, MPC-3100, that has entered Phase I study. Exelixis has developed a synthetic, orally bioavailable Hsp90 inhibitor, XL888, which is currently in Phase I trial in patients with solid tumors. Astex Therapeutics has developed the Hsp90 inhibitor AT13387 using a high-throughput x-ray crystallography fragment-based drug discovery platform. The company expects that their preclinical xenograft studies demonstrating tumor-specific drug retention combined with long duration of biomarker knockdown will allow for once weekly dosing. An IND was approved in January 2008 and an “oligo-specific” Phase I study has been initiated to assess safety and tolerability of AT 13387 in patients with advanced refractory solid tumors, including HER2-positive metastatic breast cancer, hormone-refractory prostate cancer, metastatic melanoma, stage IIIb or IV NSCLC, small cell lung carcinoma, high grade gliomas, and GIST (http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=641045&version=HealthProfessional&protocolsearchid=6524666).
PHARMACODYNAMIC ASSAY OF HSP90 INHIBITION
The majority of pharmacodynamic (PD) assays in the Phase I trials were performed on peripheral blood mononuclear cells. Among the Phase I 17-AAG trials, only Banerji et al. examined tumor biopsies [13]. Two Hsp90 client proteins, CDK4 and RAF-1 were depleted in 8 of 9 and 4 of 6 informative patients respectively, 24 hours posttreatment compared to pretreatment samples. Solit et al. explored the weekly 450 mg/m2 dose of 17-AAG and correlated outcome with pretreatment and posttreatment tumor sample PD markers in a melanoma Phase II study. There were no clinical responses in this Phase II trial, nor was robust anti-Hsp90 biologic activity observed in posttreatment tumor biopsies. The investigators concluded that, rather than rejecting Hsp90 inhibition as a therapeutic approach to melanoma, the data emphasized the necessity for a more potent Hsp90 inhibitor or for an improved formulation/pharmacokinetic properties that would permit prolonged engagement of the target in tumor [21].
Because all Hsp90 inhibitors in clinical trial bind to the Hsp90 amino-terminal ATP binding pocket and are predicted to cause client protein degradation, initial PD assays focused on evaluation of client protein levels. In the majority of trials, tumor has not been available, and client protein degradation was assessed in peripheral blood mononuclear cells (PBMC). Perhaps not surprisingly, this PD endpoint has had limited usefulness. In particular, client protein degradation has not been shown to correlate with dose or clinical response, and is not seen in all patients. Hsp90 inhibitors have been shown to accumulate preferentially in tumor versus normal cells, and the avidity of Hsp90 for inhibitors appears to be fundamentally different in tumor versus normal cells. Furthermore, the client proteins most sensitive to Hsp90 inhibition are preferentially expressed in tumor cells. Thus it is not unexpected that a need exists for alternate PD approaches.
Although client protein degradation in PBMC has been problematic, greater success with PBMC has been achieved measuring the induction of Hsp70 in response to therapy. The mechanism of Hsp70 induction is distinct from client protein degradation, but nonetheless is Hsp90-dependent. In the absence of Hsp90 inhibitor, the transcription factor HSF1 is held in the cytoplasm in an inactive monomeric state bound to Hsp90 [41]. In cells exposed to an Hsp90 inhibitor, the HSF1 monomer is released from Hsp90, resulting in its trimerization, nuclear translocation and activation as a transcription factor whereupon it activates expression of heat shock genes including Hsp70 [41]. However, although modulation of Hsp70 in PBMC has been more reliable across trials than has been client protein degradation, this marker has not correlated with drug dose or clinical response.
With the goal of developing a more correlative PD test of tumor-specific Hsp90 inhibitor biologic activity, several noninvasive functional imaging techniques are being explored. Positron emission tomography (PET) with [18F] fluorodeoxyglucose (FDG-PET) evaluates tumor glucose uptake, and is increasingly used to evaluate efficacy of anticancer drugs [42, 43]. This modality has been incorporated into a Phase I trial of IPI-504 in patients with metastatic GIST [44]. A reduced FDG-PET signal in tumors correlated with the dose of IPI-504 and reactivation of tumor FDG uptake correlated with planned breaks in drug administration. Decreased FDG uptake re-appeared upon retreatment with IPI-504. These findings suggest that, at least in highly glycolytic tumors, FDG-PET may provide a useful PD correlate of anti-tumor activity.
Smith-Jones and colleagues at Memorial Sloan-Kettering Cancer Center have reported preclinical data evaluating HER-2 PET [45]. These investigators utilized a F(ab’)2 fragment of Herceptin linked to 68Ga-DOTA as a PET probe. This proof of principle study demonstrated that HER-2 PET is a sensitive and robust PD assay able to monitor tumor HER-2 expression in real time following systemic Hsp90 inhibitor administration in vivo.
An additional noninvasive approach to PD that has been employed in Hsp90 inhibitor clinical trials is the analysis of plasma levels pre- and post-therapy of insulin-like growth factor binding protein 2 (IGFBP-2) and HER-2 extracellular domain (HER-2 ECD). Eiseman et al. analyzed both biomarkers [46] in plasma samples from patients treated with 17-AAG alone or in combination with docetaxel, and compared levels with plasma from healthy volunteers. IGFBP-2 levels were significantly lower in healthy donors than in cancer patients. However, 17-AAG treatment was not consistently associated with decreases in IGFBP-2 or HER-2 ECD concentrations in patient plasma. In studying a different Hsp90 inhibitor, BIIB021, Elfiky et al. reported a dose-related decrease in serum HER-2 ECD [47].
CONCLUSION
The first Hsp90 inhibitor entered clinical trial 10 years ago. Although currently there are no drugs approved in this class, there have been significant advances on several fronts and potential routes to approval are becoming apparent. One important advance has been in the drugs themselves. The first-in-class Hsp90 inhibitor, 17-AAG (tanespimycin) is moving into Phase III development with an improved formulation that overcomes the dose-limiting toxicities of earlier trials, which had been ascribed to target-independent formulation-associated toxicity. Drugs based on a variety of non-ansamycin scaffolds have entered the clinical arena in the past 2 years, and new agents will soon move forward into the clinic.
A second area of advance is in choosing the appropriate indication. Recent disease-specific clinical studies have illuminated some key points for further development of Hsp90 inhibitors. The promising results in HER-2-positive breast cancer highlight the value of choosing a cancer dependent on a client that is exceptionally sensitive to Hsp90 inhibition. Importantly, Hsp90 inhibitors can induce responses when used in combination with trastuzumab in women who have progressed on trastuzumab therapy.
A second indication is in NSCLC, where Hsp90 inhibitors have activity in combination with TKIs, even in patients who have progressed on TKI therapy. In this setting, the relevant biology may relate to the ability of an Hsp90 inhibitor to block oncogenic switching to signaling via other receptor tyrosine kinases [48–50]. The oncogenic switch has been shown to be induced as a resistance mechanism to TKIs, but most of the alternative kinases in cancer cells are Hsp90 clients that are sensitive to Hsp90 inhibition. Even alternative EGFR mutations that provide resistance to TKIs remain sensitive to Hsp90 inhibitors [51, 52].
A third scenario is seen in multiple myeloma, where Hsp90 inhibitors have activity in combination with bortezomib in patients progressing on bortezomib. In response to Hsp90 inhibition, many cellular proteins are targeted for degradation in the proteasome. When combined with a proteasome inhibitor Hsp90 inhibition can overload the protein degradation system, triggering the unfolded protein response (UPR) and potentially additional apoptotic responses [53, 54]. Here it is likely that the biology of multiple myeloma, a cell type that is highly secretory and dependent on the proteasome and UPR machinery for cell viability, may cause the tumor cell to be more sensitive to Hsp90 inhibition, and contribute to the enhanced activity of the Hsp90 inhibitor-proteasome inhibitor combination [55]. Furthermore, there is some evidence that inhibition of Hsp90 may ameliorate bortezomib-induced neuropathy, potentially through induction of Hsp70, which has been shown to have pro-survival function in neuronal cells [56].
Together these studies highlight the importance of understanding the molecular mechanisms of disease and disease resistance in developing clinical applications of Hsp90 inhibitors. The rapid progress being made in personalized molecular characterization of primary and recurrent tumor will be instrumental in exposing the unique vulnerabilities of tumor cells to Hsp90 monotherapy and combination therapy, and in fulfilling the promise of Hsp90 inhibitors in the treatment of cancer.
REFERENCES
- [1].Uehara Y Natural product origins of Hsp90 inhibitors. Curr. Cancer Drug Targets, 2003, 3, 325–330. [DOI] [PubMed] [Google Scholar]
- [2].Uehara Y; Hori M; Takeuchi T; Umezawa H Phenotypic change from transformed to normal induced by benzoquinonoid ansamycins accompanies inactivation of p60src in rat kidney cells infected with Rous sarcoma virus. Mol. Cell. Biol, 1986, 6, 2198–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Whitesell L; Mimnaugh EG; De Costa B; Myers CE; Neckers LM Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA, 1994, 91, 8324–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Banerji U Heat shock protein 90 as a drug target: some like it hot. Clin. Cancer Res, 2009, 15, 9–14. [DOI] [PubMed] [Google Scholar]
- [5].Erlichman C Tanespimycin: the opportunities and challenges of targeting heat shock protein 90. Expert Opin. Investig. Drugs, 2009, 18, 861–868. [DOI] [PubMed] [Google Scholar]
- [6].Mitsiades CS; Hideshima T; Chauhan D; McMillin DW; Klippel S; Laubach JP; Munshi NC; Anderson KC; Richardson PG Emerging treatments for multiple myeloma: beyond immunomodulatory drugs and bortezomib. Semin. Hematol, 2009, 46, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Taldone T; Gozman A; Maharaj R; Chiosis G Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr. Opin. Pharmacol, 2008, 8, 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Richardson PG; Chanan-Khan A; Lonial S; Krishman A;Carroll M; Cropp GF; Kersey K; Abitar M; Johnson RG; Hannah AL; Anderson KC Tanespimycin (T) + Bortezomib (BZ) in Multiple Myeloma (MM): Confirmation of the recommended dose using a novel formulation [abstract]. ASH Annu. Meet. Abstr, 2007, 110, 1165. [Google Scholar]
- [9].Solit DB; Ivy SP; Kopil C; Sikorski R; Morris MJ; Slovin SF; Kelly WK; DeLaCruz A; Curley T; Heller G; Larson S; Schwartz L; Egorin MJ; Rosen N; Scher HI Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin. Cancer Res, 2007, 13, 1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grem JL; Morrison G; Guo XD; Agnew E; Takimoto CH; Thomas R; Szabo E; Grochow L; Grollman F; Hamilton JM; Neckers L; Wilson RH Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J. Clin. Oncol, 2005, 23, 1885–1893. [DOI] [PubMed] [Google Scholar]
- [11].Ramanathan RK; Egorin MJ; Eiseman JL; Ramalingam S; Friedland D; Agarwala SS; Ivy SP; Potter DM; Chatta G; Zuhowski EG; Stoller RG; Naret C; Guo J; Belani CP Phase I and pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with refractory advanced cancers. Clin. Cancer Res, 2007, 13, 1769–1774. [DOI] [PubMed] [Google Scholar]
- [12].Nowakowski GS; McCollum AK; Ames MM; Mandrekar SJ; Reid JM; Adjei AA; Toft DO; Safgren SL; Erlichman C A phase I trial of twice-weekly 17-allylamino-demethoxygeldanamycin in patients with advanced cancer. Clin. Cancer Res, 2006, 12, 6087–6093. [DOI] [PubMed] [Google Scholar]
- [13].Banerji U; O’Donnell A; Scurr M; Pacey S; Stapleton S; Asad Y; Simmons L; Maloney A; Raynaud F; Campbell M; Walton M; Lakhani S; Kaye S; Workman P; Judson I Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J. Clin. Oncol, 2005, 23, 4152–4161. [DOI] [PubMed] [Google Scholar]
- [14].Goetz MP; Toft D; Reid J; Ames M; Stensgard B; Safgren S; Adjei AA; Sloan J; Atherton P; Vasile V; Salazaar S; Adjei A; Croghan G; Erlichman C Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J. Clin. Oncol, 2005, 23, 1078–1087. [DOI] [PubMed] [Google Scholar]
- [15].Ramanathan RK; Trump DL; Eiseman JL; Belani CP; Agarwala SS; Zuhowski EG; Lan J; Potter DM; Ivy SP; Ramalingam S; Brufsky AM; Wong MK; Tutchko S; Egorin MJ Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein. 90,. in patients with refractory advanced cancers. Clin. Cancer Res, 2005, 11, 3385–3391. [DOI] [PubMed] [Google Scholar]
- [16].Chiosis G; Neckers L Tumor selectivity of Hsp90 inhibitors: the explanation remains elusive. ACS Chem. Biol, 2006, 1, 279–284. [DOI] [PubMed] [Google Scholar]
- [17].Bagatell R; Gore L; Egorin MJ; Ho R; Heller G; Boucher N; Zuhowski EG; Whitlock JA; Hunger SP; Narendran A; Katzenstein HM; Arceci RJ; Boklan J; Herzog CE; Whitesell L; Ivy SP; Trippett TM Phase I pharmacokinetic and pharmacodynamic study of 17-N-allylamino-17-demethoxygeldanamycin in pediatric patients with recurrent or refractory solid tumors: a pediatric oncology experimental therapeutics investigators consortium study. Clin. Cancer Res, 2007, 13, 1783–1788. [DOI] [PubMed] [Google Scholar]
- [18].Weigel BJ; Blaney SM; Reid JM; Safgren SL; Bagatell R; Kersey J; Neglia JP; Ivy SP; Ingle AM; Whitesell L; Gilbertson RJ; Krailo M; Ames M; Adamson PC A phase I study of 17-allylaminogeldanamycin in relapsed/refractory pediatric patients with solid tumors: a Children’s Oncology Group study. Clin. Cancer Res, 2007, 13, 1789–1793. [DOI] [PubMed] [Google Scholar]
- [19].Williams R Discontinued drugs in 2008: oncology drugs. Expert Opin. Investig. Drugs, 2009. [DOI] [PubMed] [Google Scholar]
- [20].Ronnen EA; Kondagunta GV; Ishill N; Sweeney SM; Deluca JK; Schwartz L; Bacik J; Motzer RJ A phase II trial of 17-(Allylamino)-17-demethoxygeldanamycin in patients with papillary and clear cell renal cell carcinoma. In-vest. New Drugs, 2006, 24, 543–546. [DOI] [PubMed] [Google Scholar]
- [21].Solit DB; Osman I; Polsky D; Panageas KS; Daud A; Goydos JS; Teitcher J; Wolchok JD; Germino FJ; Krown SE; Coit D; Rosen N; Chapman PB Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin. Cancer Res, 2008, 14, 8302–8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Heath EI; Hillman DW; Vaishampayan U; Sheng S; Sarkar F; Harper F; Gaskins M; Pitot HC; Tan W; Ivy SP; Pili R; Carducci MA; Erlichman C; Liu G A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin. Cancer Res, 2008, 14, 7940–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sausville EA Combining cytotoxics and 17-allylamino, 17-demethoxygeldanamycin: sequence and tumor biology matters. Commentary re: P. Munster et al., Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner. Clin. Cancer Res, 7: 2228–2236, 2001. [PubMed] [Google Scholar]; Clin. Cancer Res, 2001, 7, 2155–2158. [PubMed] [Google Scholar]
- [24].Nguyen DM; Lorang D; Chen GA; Stewart J.H.t.; Tabibi E; Schrump DS Enhancement of paclitaxel-mediated cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: in vitro and in vivo analysis. Ann. Thorac. Surg, 2001, 72, 371–378; discussion 378–379. [DOI] [PubMed] [Google Scholar]
- [25].Ramalingam SS; Egorin MJ; Ramanathan RK; Remick SC; Sikorski RP; Lagattuta TF; Chatta GS; Friedland DM; Stoller RG; Potter DM; Ivy SP; Belani CP A phase I study of 17-allylamino-17-demethoxy geldanamycin combined with paclitaxel in patients with advanced solid malignancies. Clin. Cancer Res, 2008, 14, 3456–3461. [DOI] [PubMed] [Google Scholar]
- [26].Arlander SJ; Eapen AK; Vroman BT; McDonald RJ; Toft DO; Karnitz LM Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J. Biol. Chem, 2003, 278, 52572–52577. [DOI] [PubMed] [Google Scholar]
- [27].Tse AN; Sheikh TN; Alan H; Chou TC; Schwartz GK 90-kDa heat shock protein inhibition abrogates the topoisomerase I poison-induced G2/M checkpoint in p53-null tumor cells by depleting Chk1 and Wee1. Mol. Pharmacol, 2009, 75, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tse AN; Klimstra DS; Gonen M; Shah M; Sheikh T; Sikorski R; Carvajal R; Mui J; Tipian C; O’Reilly E; Chung K; Maki R; Lefkowitz R; Brown K; Manova-Todorova K; Wu N; Egorin MJ; Kelsen D; Schwartz GK A phase 1 dose-escalation study of irinotecan in combination with 17-allylamino-17-demethoxygeldanamycin in patients with solid tumors. Clin. Cancer Res, 2008, 14, 6704–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Modi S; Stopeck AT; Gordon MS; Mendelson D; Solit DB; Bagatell R; Ma W; Wheler J; Rosen N; Norton L; Cropp GF; Johnson RG; Hannah AL; Hudis CA Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J. Clin. Oncol, 2007, 25, 5410–5417. [DOI] [PubMed] [Google Scholar]
- [30].Modi S; Sugarman S; Stopeck A; Linden H; Ma W; Kersey K; Johnson RG; Rosen; Hannah AL; Hudis CA Phase II trial of the Hsp90 inhibitor tanespimycin (Tan) + trastuzumab (T) in patients (pts) with HER2-positive metastatic breast cancer (MBC) [abstract]. J. Clin. Oncol, 2008, 26, 1027.18309938 [Google Scholar]
- [31].Richardson PG; Chanan-Khan A; Lonial S; Krishnan A; Carroll M; Alsina M; Albitar M; Berman D; Kaplita S; Anderson K Tanespimycin plus bortezomib in patients with relapsed and refractory multiple myeloma: Final results of a phase I/II study [abstract]. J. Clin. Oncol, 2009, 27, 8503. [Google Scholar]
- [32].Riely GJ; Stoller R; Egorin M; Solit D; Dunbar J; Savage A; Walker J; Grayzel D; Ross R; Weiss GJ A phase Ib trial of IPI-504 (retaspimycin hydrochloride), a novel Hsp90 inhibitor, in combination with docetaxel [abstract]. J. Clin. Oncol, 2009, 27, 3547.19546404 [Google Scholar]
- [33].Sequist LV; Gettinger S; Natale R; Martins R; Lilenbaum R; Janne P; Gray J; Samuel TA; Grayzel D; Lynch TJ A phase II trial of IPI-504 (retaspimycin hydrochloride), a novel Hsp90 inhibitor, in patients with relapsed and/or refractory stage IIIb or stage IV non-small cell lung cancer (NSCLC) stratified by EGFR mutation status [abstract]. J. Clin. Oncol, 2009, 27, 8073. [Google Scholar]
- [34].Oh WK; Stadler WM; Srinivas S; Chu F; Bubley G; Quigley M; Goddard J; Dunbar J; Grayzel D; Ross RW A single arm phase II trial of IPI-504 in patients with castration resistant prostate cancer (CRPC) [abstract]. Genitourinary Cancers Symp. Abstr, 2009, 219. [Google Scholar]
- [35].Yano A; Tsutsumi S; Soga S; Lee MJ; Trepel J; Osada H; Neckers L Inhibition of Hsp90 activates osteoclast c-Src signaling and promotes growth of prostate carcinoma cells in bone. Proc. Natl. Acad. Sci. USA, 2008, 105, 15541–15546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wagner AJ; Morgan JA; Chugh R; Rosen LS; George S; Gordon MS; Devine CM; Van den Abbeele AD; Grayzel D; Demetri GD Inhibition of heat shock protein 90 (Hsp90) with the novel agent IPI-504 in metastatic GIST following failure of tyrosine kinase inhibitors (TKIs) or other sarcomas: Clinical results from phase I trial [abstract]. J. Clin. Oncol, 2008, 26, 10503. [Google Scholar]
- [37].Lancet J; Baer MR; Gojo I; Burton M; Quinn M; Tighe SM; Bhalla K; Kersey K; Wells S; Zhong Z; Sadler B; Albitar M; Johnson R; Hannah A Phase I, pharmacokinetic (PK) and pharmacodynamic (PD) study of intravenous alvespimycin (KOS-1022) in patients with refractory hematological malignancies [abstract]. ASHAnnu. Meet. Abstr, 2006, 108, 1961. [Google Scholar]
- [38].Chiosis G; Caldas Lopes E; Solit D Heat shock protein-90 inhibitors: a chronicle from geldanamycin to today’s agents. Curr. Opin. Investig. Drugs, 2006, 7, 534–541. [PubMed] [Google Scholar]
- [39].Chiosis G; Kang Y; Sun W Discovery and development of purine-scaffold Hsp90 inhibitors. Expert Opin. Drug Discov, 2008, 3, 99–114. [DOI] [PubMed] [Google Scholar]
- [40].McDonald E; Jones K; Brough PA; Drysdale MJ; Workman P Discovery and development of pyrazole-scaffold Hsp90 inhibitors. Curr. Top. Med. Chem, 2006, 6, 1193–1203. [DOI] [PubMed] [Google Scholar]
- [41].Zou J; Guo Y; Guettouche T; Smith DF; Voellmy R Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell, 1998, 94, 471–480. [DOI] [PubMed] [Google Scholar]
- [42].Jimeno A; Rudek MA; Kulesza P; Ma WW; Wheelhouse J; Howard A; Khan Y; Zhao M; Jacene H; Messersmith WA; Laheru D; Donehower RC; Garrett-Mayer E; Baker SD; Hidalgo M Pharmacodynamic-guided modified continuous reassessment method-based, dose-finding study of rapamycin in adult patients with solid tumors. J. Clin. Oncol, 2008, 26, 4172–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ma WW; Jacene H; Song D; Vilardell F; Messersmith WA; Laheru D; Wahl R; Endres C; Jimeno A; Pomper MG; Hidalgo M [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J. Clin. Oncol, 2009, 27, 2697–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Demetri GD; George S; Morgan JA; Wagner AJ; Quigley MT; Polson K; Pokela J; Van den Abbeele AD; Adams J; Grayzel D Inhibition of the Heat Shock Protein 90 (Hsp90) chaperone with the novel agent IPI-504 to overcome resistance to tyrosine kinase inhibitors (TKIs) in metastatic GIST: Updated results of a phase I trial [abstract]. J. Clin. Oncol, 2007, 25, 10024. [Google Scholar]
- [45].Smith-Jones PM; Solit D; Afroze F; Rosen N; Larson SM Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J. Nucl. Med, 2006, 47, 793–796. [PMC free article] [PubMed] [Google Scholar]
- [46].Eiseman JL; Guo J; Ramanathan RK; Belani CP; Solit DB; Scher HI; Ivy SP; Zuhowski EG; Egorin MJ Evaluation of plasma insulin-like growth factor binding protein 2 and Her-2 extracellular domain as biomarkers for 17-allylamino-17-demethoxygeldanamycin treatment of adult patients with advanced solid tumors. Clin. Cancer Res, 2007, 13, 2121–2127. [DOI] [PubMed] [Google Scholar]
- [47].Elfiky A; Saif MW; Beeram M; O’ Brien S; Lammanna N; Castro JE; Woodworth J; Perea R; Storgard C; Von Hoff DD BIIB021, an oral, synthetic non-ansamycin Hsp90 inhibitor: Phase I experience. J. Clin. Oncol, 2008, 26, 2503. [Google Scholar]
- [48].Montagut C; Sharma SV; Shioda T; McDermott U; Ulman M; Ulkus LE; Dias-Santagata D; Stubbs H; Lee DY; Singh A; Drew L; Haber DA; Settleman J Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res, 2008, 68, 4853–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pashtan I; Tsutsumi S; Wang S; Xu W; Neckers L Targeting Hsp90 prevents escape of breast cancer cells from tyrosine kinase inhibition. Cell Cycle, 2008, 7, 2936–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang S; Pashtan I; Tsutsumi S; Xu W; Neckers L Cancer cells harboring MET gene amplification activate alternative signaling pathways to escape MET inhibition but remain sensitive to Hsp90 inhibitors. Cell Cycle, 2009, 8, 2050–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sawai A; Chandarlapaty S; Greulich H; Gonen M; Ye Q; Arteaga CL; Sellers W; Rosen N; Solit DB Inhibition of Hsp90 down-regulates mutant epidermal growth factor receptor (EGFR) expression and sensitizes EGFR mutant tumors to paclitaxel. Cancer Res, 2008, 68, 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Xu W; Soga S; Beebe K; Lee MJ; Kim YS; Trepel J; N L Sensitivity of epidermal growth factor receptor and ErbB2 exon 20 insertion mutants to Hsp90 inhibition. Br. J. Cancer, 2007, 97, 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mimnaugh EG; Xu W; Vos M; Yuan X; Isaacs JS; Bisht KS; Gius D; Neckers L Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol. Cancer Ther, 2004, 3, 551–566. [PubMed] [Google Scholar]
- [54].Mimnaugh EG; Xu W; Vos M; Yuan X; Neckers L Endoplasmic reticulum vacuolization and valosin-containing protein relocalization result from simultaneous hsp90 inhibition by geldanamycin and proteasome inhibition by velcade. Mol. Cancer Res, 2006, 4, 667–681. [DOI] [PubMed] [Google Scholar]
- [55].Mitsiades CS; Mitsiades NS; McMullan CJ; Poulaki V; Kung AL; Davies FE; Morgan G; Akiyama M; Shringarpure R; Munshi NC; Richardson PG; Hideshima T; Chauhan D; Gu X; Bailey C; Joseph M; Libermann TA; Rosen NS; Anderson KC Antimyeloma activity of heat shock protein-90 inhibition. Blood, 2006, 107, 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Frebel K; Wiese S Signalling molecules essential for neuronal survival and differentiation. Biochem. Soc. Trans, 2006, 34, 1287–1290. [DOI] [PubMed] [Google Scholar]
- [57].Kefford R; Millward M; Hersey P; Brady B; Graham M; Johnson RG; Hannah AL Phase II trial of tanespimycin (KOS-953), a heat shock protein-90 (Hsp90) inhibitor in patients with metastatic melanoma [abstract]. J. Clin. Oncol, 2007, 25, 8558. [Google Scholar]
- [58].Richardson PG; Chanan-Khan A; Lonial S; Krishnan A; Carroll M; Cropp GF; Albitar M; Johnson RG; Hannah A; Anderson K Tanespimycin (T) + bortezomib (BZ) in multiple myeloma (MM): Pharmacology, safety and activity in relapsed/refractory (rel/ref) patients (Pts) [abstract]. J. Clin. Oncol, 2007, 25, 3532. [Google Scholar]
- [59].Weigel B; Blaney S; Kersey J; Bagatell R; Ivy SP; Whitesell L; Krailo M; Reid J; Ames M; Adamson P A phase I study of 17-AAG in relapsed/refractory pediatric patients with solid tumors: A Children’s Oncology Groups study [abstract]. J. Clin. Oncol, 2006, 24, 9018. [Google Scholar]
- [60].Bagatell R; Gore L; Egorin M; Ho R; Boucher N; Heller G; Trippett T Phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of 17-Allylamino-17-demethoxygeldanamycin (17AAG) in children with solid tumors [abstract]. J. Clin. Oncol, 2006, 24, 9022. [Google Scholar]
- [61].Mitsiades C; Chanan-Khan A; Alsina M; Doss D; Landrigan B; Kettner D; Albitar M; Cropp GF; Hannah AL; Richardson P Phase 1 trial of 17-AAG in patients with relapsed and refractory multiple myeloma (MM) [abstract]. J. Clin. Oncol, 2005, 23, 3056. [Google Scholar]
- [62].Erlichman C; Toft D; Reid J; Goetz M; Ames M; Mandrekar S; Ajei A; McCollum A; Ivy P A phase I trial of 17-allylamino-geldanamycin (17AAG) in patients with advanced cancer [abstract]. J. Clin. Oncol, 2004, 22, 3030. [Google Scholar]
- [63].Ramanathan RK; Trump DL; Eiseman JL; Belani CP; Agarwala SS; Zuhowski EG; Lan J; Ivy P; Tutchko S; Egorin ME A phase I pharmacokinetic (PK) and pharmaco dynamic (PD) trial of weekly 17-allylamino-17 demethoxygeldanamycin (17AAG, NSC-704057) in patients with advanced tumors [abstract]. J. Clin. Oncol, 2004, 22, 3031. [Google Scholar]
- [64].Vaishampayan UN; Sausville EA; Horiba MN; Quinn M; Heilbrun LK; Burger A; Ivy P; Li J; Lorusso P Phase I trial of intravenous 17-allylaminogeldanamycin (A) and oral sorafenib (B) in pretreated advanced malignancy: Plasma Hsp90{alpha} induction correlates with clinical benefit [abstract]. J. Clin. Oncol, 2007, 25, 3531. [Google Scholar]
- [65].Tse AN; Carvajal R; Shah M; Dials H; Fogel M; O’Reilly E; Chung K; Maki R; Wu N; Egorin M; Schwartz GK Phase 1 dose-escalation study of 17-allylamino-17-demethoxy-geldanamycin (17AAG) in combination with irinotecan in patients with solid tumors [abstract]. J. Clin. Oncol, 2007, 25, 3533. [Google Scholar]
- [66].Chanan-Khan A; Richardson P; Alsina M; Lonial S; Krishnan A; Carroll M; Albitar M; Hannah AL; Johnson RG; Anderson K Phase 1 clinical trial of KOS-953 + bortezomib (BZ) in relapsed refractory multiple myeloma (MM) [abstract]. J. Clin. Oncol, 2006, 24, 3066. [Google Scholar]
- [67].Modi S; Stopeck A; Gordon MS; Solit D; Ma W; Wheler J; Cropp GF; Johnson RG; Hannah A; Hudis C Phase I trial of KOS-953, a heat shock protein 90 inhibitor, and trastuzumab (T) [abstract]. J. Clin. Oncol, 2006, 24, 501. [Google Scholar]
- [68].Solit DB; Egorin M; Kopil C; Delacruz A; Shaffer D; Slovin S; Morris M; Kelly WK; Rosen N; Scher H Phase I pharmacokinetic and pharmacodynamic trial of docetaxel and 17AAG (17-allylamino-17-demethoxygeldanamycin) [abstract]. J. Clin. Oncol, 2005, 23, 3051. [Google Scholar]
- [69].Solit DB; Egorin M; Valentin G; Delacruz A; Ye Q; Schwartz L; Larson S; Rosen N; Scher HI Phase I pharmacokinetic and pharmacodynamic trial of docetaxel and 17AAG (17-allylamino-17-demethoxygeldanamycin) [abstract]. J. Clin. Oncol, 2004, 22, 3032.15210738 [Google Scholar]
- [70].Haluska P; Toft DO; Steinmetz SM; Furth A; Mandrekar S; Stensgard BA; McCollum AK; Hanson LJ; Adjei AA; Erlichman C A phase I trial of gemcitabine (Gem), 17-allylaminogeldanamycin (17-AAG) and cisplatin (CDDP) in solid tumor patients [abstract]. J. Clin. Oncol, 2004, 22, 3058. [Google Scholar]
- [71].Saif MW; Erlichman C; Dragovich T; Mendelson D; Toft D; Timony G; Burrows F; Padgett C; De Jager R; Von Hoff D Phase I study of CNF1010 (lipid formulation of 17-(allylamino)-17-demethoxygeldanamycin: 17-AAG) in patients with advanced solid tumors [abstract]. J. Clin. Oncol, 2006, 24, 10062. [Google Scholar]
- [72].MacRae C; Richardson PG; Walker J; Grayzel DS; Demetri GD Cardiovascular safety profile of IPI-504 (retaspimycin hydrochloride), a novel Hsp90 inhibitor: Results from two independent phase I trials in patients with advanced cancer [abstract]. J. Clin. Oncol, 2009, 27, e14539. [Google Scholar]
- [73].Sequist LV; Janne PA; Walker J; Sweeney J; Grayzel D; Lynch TJ Phase I/II trials of the novel Hsp90 inhibitor, IPI-504, in patients with relapsed and/or regractory stage IIIB or stage IV non-small cell lung cancer stratified by EGFR mutation status [abstract]. AACR-NCI-EORTC Int. Conf, 2007, B79. [Google Scholar]
- [74].Pacey SC; Wilson R; Walton M; Eatock M; Zetterlund A; Arkenau H; Beecham R; Raynaud F; Workman P; Judson I A phase I trial of the HSP90 inhibitor, alvespimycin (17-DMAG) administered weekly, intravenously, to patients with advanced, solid tumours. J. Clin. Oncol, 2009, 27, 3534. [Google Scholar]
- [75].Flaherty KT; Gore L; Avadhani AN; Spratlin JL; Harlacker K; Zhong Z; Johnson RG; Hannah AL; O’Dwyer PJ; Eckhardt SG First use of an oral Hsp90 inhibitor in patients (Pts) with solid tumors: Alvespimycin (A) administered QOD or Q [abstract]. J. Clin. Oncol, 2008, 26, 2502. [Google Scholar]
- [76].Pacey SC; Wilson R; Walton M; Eatock M; Moreno-Farre J; Gallerani E; Davergne V; Raynaud F; Workman P; Judson I A phase I trial of the heat shock protein 90 (HSP90) inhibitor 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG, alvespimycin) administered weekly [abstract]. J. Clin. Oncol, 2007, 25, 3568. [Google Scholar]
- [77].Flaherty KT; Gore L; Avadhani A; Leong S; Harlacker K; Zhong Z; Johnson RG; Hannah AL; O’Dwyer P; Eckhardt SG Phase. 1,. pharmacokinetic (PK) and pharmacodynamic (PD) study of oral alvespimycin (A; KOS-1022; 17-DMAG): Two different schedules in patients with advanced malignancies [abstract]. J. Clin. Oncol, 2007, 25, 14059. [Google Scholar]
- [78].Murgo AJ; Kummar S; Gardner ER; Figg W; Chen X; Yancey M; Ivy P; Conley B; Doroshow JH; Gutierrez ME Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) administered twice weekly [abstract]. J. Clin. Oncol, 2007, 25, 3566. [Google Scholar]
- [79].Lancet J; Gojo I; Baer M; Burton M; Klein M; Nowadly C; Gorre M; Zhong Z; Johnson RG; Hannah AL Phase. 1,. pharmacokinetic (PK) and pharmacodynamic (PD) study of of the Hsp-90 inhibitor, KOS-1022 (17-DMAG), in patients with refractory hematological malignancies [abstract]. J. Clin. Oncol, 2006, 24, 2081. [Google Scholar]
- [80].Egorin MJ; Belani CP; Remick SC; Erlichman C; Teneyck CJ.; Holleran JL; Ivy SP; Ramalingam S; Naret CL; Ramanathan RK Phase I, pharmacokinetic (PK), & pharmacodynamic (PD) study of 17-dimethylaminoethylamino-17-demethoxygeldanamycin, (17DMAG, NSC 707545) in patients with advanced solid tumors [abstract]. J. Clin. Oncol, 2006, 24, 3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Miller K; Rosen LS; Modi S; Schneider B; Roy J; Chap L; Paulsen M; Kersey K; Hannah A; Hudis C Phase I trial of alvespimycin (KOS-1022; 17-DMAG) and trastuzumab (T) [abstract]. J. Clin. Oncol, 2007, 25, 1115. [Google Scholar]
- [82].Rugo HS; Herbst RS; Liu G; Park JW; Kies MS; Pithavala YK; McShane TM; Steinfeldt HM; Reich SD; Wilding G Clinical and dynamic imaging results of the first phase I study of AG-013736, an oral anti-angiogenesis agent, in patients (pts) with advanced solid tumors [abstract]. J. Clin. Oncol, 2008, 26, 2503. [Google Scholar]
- [83].Bryson JC; Infante JR; Ramanathan RK; Jones SF; Von Hoff DD; Burris HA III. A Phase I dose-escalation study of the safety and pharmacokinetics (PK) of the oral Hsp90 inhibitor SNX-5422 [abstract]. J. Clin. Oncol, 2008, 26, 14613. [Google Scholar]
- [84].Cavenagh JD; Yong K; Byrne J; Cavet J; Johnson P; Morgan G; Williams C; Akinaga S; Francis G; Kilborn J The Safety, Pharmacokinetics and Pharmacodynamics of KW-2478, a Novel Hsp90 Antagonist, in Patients with B-Cell Malignancies: A First-in-Man, Phase I, Multicentre, Open-Label, Dose Escalation Study [abstract]. ASHAnnu. Meet. Abstr, 2008, 112, 2777. [Google Scholar]
- [85].Sessa C; Sharma SK; Britten CD; Vogelzang NJ; Bhalla KN; Mita MM; Pluard TJ; Stiegler P; Quadt C; Shapiro GI A phase I dose escalation study of AUY922, a novel HSP90 inhibitor, in patients with advanced solid malignancies [abstract]. J. Clin. Oncol, 2009, 27, 3532. [Google Scholar]
- [86].Ide S; Motwani M; Jensen MR; Wang J; Huseinovic N; Stiegler P; Wang X; Quadt C Pharmacodynamics and pharmacokinetics of AUY922 in a phase I study of solid tumor patients [abstract]. J. Clin. Oncol, 2009, 27, 3533.19546402 [Google Scholar]


