Abstract
BACKGROUND
Hypertension (HTN) is associated with target organ damage such as cardiac, vascular, and kidney injury. Several studies have investigated circulating microRNAs (miRNAs) as biomarkers of cardiovascular disease, but few have examined them as biomarker of target organ damage in HTN. We aimed to identify circulating miRNAs that could serve as biomarkers of HTN-induced target organ damage using an unbiased approach.
METHODS AND RESULTS
Fifteen normotensive subjects, 16 patients with HTN, 15 with HTN associated with other features of the metabolic syndrome (MetS), and 16 with HTN or chronic kidney disease (CKD) were studied. Circulating RNA extracted from platelet-poor plasma was used for small RNA sequencing. Differentially expressed (DE) genes were identified with a threshold of false discovery rate <0.1. DE miRNAs were identified uniquely associated with HTN, MetS, or CKD. However, only 2 downregulated DE miRNAs (let-7g-5p and miR-191-5p) could be validated by reverse transcription-quantitative PCR. Let-7g-5p was associated with large vessel stiffening, miR-191-5p with MetS, and both miRNAs with estimated glomerular filtration rate (eGFR) and neutrophil and lymphocyte fraction or number and neutrophil-to-lymphocyte ratio. Using the whole population, stepwise multiple linear regression generated a model showing that let-7g-5p, miR-191-5p, and urinary albumin/creatinine ratio predicted eGFR with an adjusted R2 of 0.46 (P = 8.5e−7).
CONCLUSIONS
We identified decreased circulating let-7g-5p and miR-191-5p as independent biomarkers of CKD among patients with HTN, which could have pathophysiological and therapeutic implications.
Keywords: biomarker, blood pressure, hypertension, microRNA, target organ damage
Hypertension (HTN) is associated with subclinical target organ damage such as left ventricular hypertrophy,1 arterial stiffness and remodeling,2,3 and kidney damage.4 HTN is an early and common risk factor for chronic kidney disease (CKD).5 There is an epidemiological association between HTN and CKD, and the prevalence of high blood pressure (BP) has been reported to be over 85% in stage 3 and over 90% in stage 4 and stage 5 CKD patients.5 CKD was an independent risk factor for cardiovascular events and death in a large community-based population.6 HTN can also be a component of the metabolic syndrome (MetS), a cluster of risk factors for type 2 diabetes mellitus, cardiovascular disease, and death,7 which contributes to amplify HTN-induced target organ damage.7 Accordingly, determination of asymptomatic complications of HTN is important for the design of optimal antihypertensive therapeutic therapies to reduce the risk of unfavorable cardiovascular disease outcomes.
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression post-transcriptionally by binding to the 3′ untranslated regions of their target mRNAs and lead to translational repression and mRNA degradation.8–10 Circulating miRNAs can be found in plasma packaged in microparticles (microvesicles, exosomes, and apoptotic bodies) or associated with the RNA-binding protein Argonaute2 or lipoprotein complexes (such as high density lipoprotein), which may render circulating miRNAs resistant to degradation.11 Analysis of 1,323 small RNA sequencing samples from 13 different human tissue types revealed that the repertoire of human miRNAs is far more extensive than reported in public repositories, and that there are a significant number of tissue-specific miRNAs, which indicates the relevance of miRNAs as biomarkers for diagnosis or prognosis.12
Several studies have investigated the role of circulating miRNAs as biomarkers for diagnosis or prognosis of cardiovascular disease (reviewed by refs. 11,13). Only 2 studies investigated circulating miRNAs as biomarkers of HTN-induced target organ damage.14,15 Although interesting, these studies were limited to profiling known miRNAs,12 and restricted to HTN in association with 2 forms of target organ damage. Circulating miRNA biomarkers have not been identified for other forms of HTN-associated target organ damage such as CKD.
In this study, we aimed to identify circulating miRNAs that could serve as biomarkers of HTN-induced target organ damage using an unbiased approach. This was carried out by comparing the profile of platelet-poor plasma miRNA and indexes of subclinical target organ damage in normotensive subjects (NTN), and patients with HTN associated or not with features of the MetS or with CKD.
METHODS
Experimental design
The study protocol was approved by the Human Research Ethics Review Committee of the Jewish General Hospital, where the study was carried out.
Male and female NTN, HTN, HTN with MetS, and HTN with CKD were recruited at visit 1 according to the inclusion and exclusion criteria described in the Expanded Methods in the Online Data Supplement. All subjects included in the study provided written informed consent to participate.
At visit 1, a complete physical examination was performed, body weight, height, and waist and hip circumference were determined and brachial BP was measured as recommended by Hypertension Canada guidelines as unattended automated office BP with a BpTRU BPM-300 device (VSM MedTech Devices, Coquitlam, BC, Canada) with HTN defined as BP ≥135/85 mm Hg,16 or treatment with antihypertensive medications for at least 6 months. Blood and urine samples were collected in the morning under fasting conditions. Blood was collected in BD Vacutainer EDTA for white blood cell count and differential and plasma isolation or BD Vacutainer Plus serum tubes for blood biochemistry and urine analysis determined in the Department of Diagnostic Medicine at the Jewish General Hospital according to routine methods. Estimated glomerular filtration rate (eGFR) was determined by the Modification of Diet in Renal Disease formula.17 Blood samples on EDTA were centrifuged at 1,000 g for 15 minutes at 4 °C to remove blood cells, followed by centrifugation at 10,000 g for 10 minutes at 4 °C with no brake to remove platelets. Platelet-poor plasma was transferred to new tubes by pipetting the supernatant without disturbing the platelet pellet, and stored at −80 °C until used for RNA extraction.
At visit 2, BP was measured as above. End-diastolic internal diameter, stroke change in diameter, and intima media thickness (IMT) were measured on the right common carotid artery 2 cm before the bifurcation with a high-precision echotracking device (ArtLab, Esaote, Maastricht, The Netherlands) previously described and validated.18–20 Aortic stiffness was measured with the carotid to femoral pulse wave velocity between the 2 sites by the foot-to-foot velocity method (Sphygmocor, Atcor Medical, Sydney, Australia).
Circulating RNA was isolated from platelet-poor plasma with the QIAamp Circulating Nucleic Acid kit (Qiagen, Venlo, Netherlands); 4–20 ng of circulating RNA was used for small RNA library construction that was sequenced using the HiSeq 2500 sequencing system (Illumina, San Diego, CA) at the CHU Sainte-Justine Integrated Centre for Pediatric Clinical Genomics; and a bioinformatics pipeline used to profile circulating miRNAs (Supplementary Figure S1 online).
Differentially expressed (DE) miRNAs were validated by reverse transcription-quantitative PCR (RT-qPCR). Pearson correlations were determined between validated DE miRNAs and clinical parameters/vascular/biological and a stepwise multiple linear regression was used to identify predictors of eGFR.
Statistical analysis
Results are presented as means ± SD. Comparisons between multiple groups were done by one-way analysis of variance (ANOVA) followed by a Student–Newman–Keuls post hoc test or Kruskal–Wallis one-way ANOVA on ranks with Dunn’s multiple comparison post hoc test in absence of normal distribution, as appropriate, using SigmaPlot version 13 (Systat Software, San Jose, CA). For RNA sequencing data analysis, an ANOVA-like test in EdgeR based on generalized linear models was used for differential expression analyses using a threshold of false discovery rate (or q) <0.1. The Pearson correlations and the stepwise multiple linear regression done on the whole population using log2 transformed RT-qPCR fold change data were performed using IBM SPSS Statistics version 24. P < 0.05 was considered statistically significant.
RESULTS
Clinical characteristics
We included a total of 62 individuals, 15 NTN, 16 HTN, 15 MetS, and 16 CKD subjects. Patients were older in the MetS and CKD, and more males were recruited among CKD compared with NTN (Table 1). HTN, MetS, and CKD presented elevated BP or were treated with antihypertensive drugs (Tables 1). In addition to being hypertensive, MetS presented at least two of the following criteria: central obesity, dyslipidemia (high triglycerides, low high-density lipoprotein cholesterol, or taking cholesterol lowering drugs), or increased fasting blood glucose (or treated with hypoglycemic drugs) (Table 2). Diabetes was present in 67% of MetS and 6% of CKD (Table 1). Besides being hypertensive, CKD was characterized by an eGFR <60 ml/min/1.73 m2. CKD presented increased serum creatinine compared with NTN, HTN, and MetS and elevated urinary albumin/creatinine ratio (UACR) compared with NTN and HTN (Table 2). Microalbuminuria defined by a cutoff value of UACR of 2.0 mg/mmol was observed in 63% of CKD, 27% of MetS, and <15% of HTN and NTN (Table 1). MetS parameters were also observed in 44–56% of the CKD. Dyslipidemia was also found in 81% of HTN and half of the NTN. It should be noted that ~50% of the HTN, MetS, and CKD were taking acetyl salicylic acid compared with 7% of NTN, and that ~50% of NTN and CKD and ≥75% of HTN and MetS were treated with a statin. CKD patients presented increased number and fraction of neutrophils, decreased fraction of lymphocytes and increased neutrophil-to-lymphocyte ratio (NLR) (Supplementary Table S1 online) suggesting the presence of low-grade inflammation. The number and fraction of neutrophils were increased in HTN and MetS, respectively, but the lymphocyte fraction and NLR were unaltered.
Table 1.
Demographic parameters and medications of the 4 groups of subjects
| Parameters | NTN | HTN | MetS | CKD |
|---|---|---|---|---|
| Number of subjects | 15 | 16 | 15 | 16 |
| Demographic parameters | ||||
| Age (y) | 52 ± 11 | 59 ± 10 | 62 ± 6* | 66 ± 7** |
| Male sex (%) | 53 | 56 | 60 | 81 |
| Obesity (%) | 27 | 6 | 60 | 31 |
| Central obesity (%) | 27 | 13 | 93 | 44 |
| Dyslipidemia (%) | 53 | 81 | 93 | 56 |
| Diabetes (%) | 0 | 0 | 67 | 6 |
| Microalbuminuria (%) | 14 | 13 | 27 | 63 |
| Medications | ||||
| ASA (%) | 7 | 56 | 47 | 44 |
| ARBs or ACE inhibitors (%) | 0 | 81 | 73 | 81 |
| MR blockers (%) | 0 | 0 | 7 | 0 |
| Diuretics (%) | 0 | 44 | 73 | 56 |
| CCBs (%) | 0 | 38 | 67 | 75 |
| β-blockers (%) | 0 | 25 | 27 | 31 |
| α2-agonists (%) | 0 | 6 | 13 | 0 |
| Statins (%) | 47 | 75 | 80 | 50 |
| Fibrates (%) | 0 | 6 | 7 | 0 |
| CA inhibitors (%) | 0 | 0 | 7 | 0 |
| Insulin (%) | 0 | 0 | 7 | 0 |
| Insulin secretagogues (%) | 0 | 0 | 20 | 0 |
| Insulin sensitizers (%) | 0 | 0 | 53 | 0 |
Demographic parameters and medication were assessed in normotensive subjects (NTN), and patients with hypertension (HTN) associated or not with other features of the metabolic syndrome (MetS) or with chronic kidney disease (CKD) at visit 1. Data are shown as % for categorical and as means ± SD for continuous variable. Comparisons between multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by a Student–Newman–Keuls post hoc. *P < 0.05 and **P < 0.001 vs. NTN. Abbreviations: α2-agonists, α2-adrenergic receptor agonists; ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor type 1 blockers; ASA, acetylsalicylic acid; β-blockers, β-adrenergic receptor blockers; CA inhibitors, cholesterol absorption inhibitors; CCBs, calcium channel blockers; MR, mineralocorticoid receptor.
Table 2.
Clinical parameters of the 4 groups of subjects
| Parameters | NTN | HTN | MetS | CKD |
|---|---|---|---|---|
| Number of subjects | 15 | 16 | 15 | 16 |
| Body weight (kg) | 78 ± 18 | 76 ± 9 | 89 ± 17* ,† | 83 ± 17 |
| BMI (kg/m2) | 26 ± 4 | 26 ± 3 | 32 ± 4* ,† | 29 ± 5 |
| Waist circumference (cm) | 91 ± 12 | 91 ± 9 | 106 ± 12* ,† | 101 ± 14 |
| Systolic BP (mm Hg) | 113 ± 10 | 121 ± 15** | 129 ± 14** | 126 ± 18** |
| Diastolic BP (mm Hg) | 73 ± 7 | 78 ± 10 | 79 ± 8 | 76 ± 7 |
| Mean BP (mm Hg) | 87 ± 7 | 93 ± 11** | 96 ± 8** | 93 ± 9** |
| Pulse pressure (mm Hg) | 39 ± 6 | 42 ± 11* | 50 ± 14* | 50 ± 17* |
| Heart rate (beats/min) | 64 ± 7 | 66 ± 12 | 66 ± 14 | 64 ± 12 |
| Total cholesterol (mmol/l) | 5.0 ± 0.9 | 4.5 ± 0.9 | 4.2 ± 1.1 | 4.4 ± 0.9 |
| Triglycerides (mmol/l) | 1.0 ± 0.6 | 1.1 ± 0.5 | 1.8 ± 1.4 | 1.4 ± 0.8 |
| LDL-cholesterol (mmol/l) | 2.7 ± 0.7 | 2.3 ± 0.8 | 2.1 ± 0.7 | 2.5 ± 0.7 |
| HDL-cholesterol (mmol/l) | 1.8 ± 0.6 | 1.7 ± 0.5 | 1.4 ± 0.4* | 1.4 ± 0.3* |
| FBG (mmol/l) | 4.7 ± 0.5 | 4.8 ± 0.7 | 6.1 ± 1.4* ,† | 5.0 ± 0.8‡ |
| Hemoglobin A1c (%) | 5.5 ± 0.5 | 5.5 ± 0.3 | 6.4 ± 0.9* ,† | 5.5 ± 0.5‡ |
| Creatinine (µmol/l) | 69 ± 10 | 76 ± 15 | 74 ± 12 | 144 ± 38** ,††,‡‡ |
| eGFR (ml/min/1.73 m2) | 99 ± 12 | 87 ± 11 | 92 ± 17 | 45 ± 11** ,††,‡‡ |
| UACR (mg/mmol) | 0.8 ± 0.8 | 2.7 ± 7.3 | 7.8 ± 18.4 | 21.5 ± 32.3** ,† |
Clinical parameters were evaluated in normotensive subjects (NTN), and patients with hypertension (HTN) associated or not with other features of the metabolic syndrome (MetS) or with chronic kidney disease (CKD) at visit 1. Data are shown as means ± SD for continuous variable. Comparisons between multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by a Student–Newman–Keuls post hoc test or in absence of normal distribution, a Kruskal–Wallis one-way ANOVA followed by a Dunn’s multiple comparison test, as appropriate. *P < 0.05 and **P < 0.001 vs. NTN, †P < 0.05 and ††P < 0.001 vs. HTN, ‡P < 0.05 and ‡‡P < 0.001 vs. MetS. Abbreviations: BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; UACR, urinary albumin/creatinine ratio.
The right common carotid artery IMT, wall/lumen, and wall cross-sectional area were similar in all groups (Table 3). However, the carotid circumferential wall stress was increased 1.4-fold in MetS and tended to be greater in HTN and CKD compared with NTN. The carotid young elastic modulus was increased ~2-fold and the carotid stiffness 1.4-fold in HTN and CKD and 1.9-fold in MetS, whereas carotid distensibility was decreased by ~50% in HTN and CKD and ~60% in MetS compared with NTN. Carotid to femoral pulse wave velocity, index of aortic stiffness, was increased 1.2-fold in HTN and CKD and 1.3-fold in MetS.
Table 3.
Right common carotid artery mechanical properties and aortic stiffness in the 4 groups of subjects
| Parameters | NTN | HTN | MetS | CKD |
|---|---|---|---|---|
| Number of subjects | 15 | 16 | 15 | 16 |
| Right common carotid artery | ||||
| Systolic BP (mm Hg) | 102 ± 14 | 122 ± 16** | 133 ± 18** | 121 ± 16** |
| Diastolic BP (mm Hg) | 69 ± 8 | 77 ± 9* | 78 ± 8* | 76 ± 8* |
| Mean BP (mm Hg) | 83 ± 10 | 96 ± 11** | 99 ± 10** | 94 ± 9* |
| Pulse pressure (mm Hg) | 33 ± 9 | 45 ± 12* | 55 ± 17** | 45 ± 14** |
| Internal diameter (mm) | 5.36 ± 0.59 | 5.55 ± 0.75 | 5.86 ± 1.29 | 5.94 ± 0.78 |
| IMT (µm) | 876 ± 132 | 861 ± 149 | 831 ± 142 | 928 ± 121 |
| Wall/lumen | 0.17 ± 0.03 | 0.16 ± 0.04 | 0.15 ±0.04 | 0.16 ± 0.03 |
| WCSA (mm2) | 17.2 ± 3.5 | 17.3 ± 3.2 | 17.6 ± 4.9 | 20.1 ± 4.5 |
| Circumferential wall stress (kPa) | 33.9 ± 4.8 | 41.7 ± 11.9 | 46.8 ± 16.3* | 40.4 ± 9.6 |
| Young elastic modulus (kPa) | 217 ± 477 | 477 ± 347** | 1,009 ± 832** | 448 ± 260** |
| Distensibility (kPa−1 × 10−3) | 37.1 ± 13.0 | 19.4 ± 7.5* | 13.3 ± 8.7** | 20.2 ± 8.8* |
| Stiffness (m/s) | 5.5 ± 1.1 | 7.7 ± 1.9** | 10.6 ± 4.4** ,† | 7.6 ± 2.0** ,‡ |
| Aortic stiffness | ||||
| Carotid femoral PWV (m/s) | 7.8 ± 1.3 | 9.4 ± 1.3* | 10.4 ± 2.7* | 9.7 ± 2.2* |
Large vessel study was performed in normotensive subjects (NTN), and patients with hypertension (HTN) associated or not with other features of the metabolic syndrome (MetS) or with chronic kidney disease (CKD) at visit 2. Data are shown as means ± SD. Comparisons between multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by a Student–Newman–Keuls post hoc test or in absence of normal distribution, a Kruskal–Wallis one-way ANOVA followed by a Dunn’s multiple comparison test, as appropriate. *P < 0.05 and **P < 0.001 vs. NTN, †P < 0.05 vs. HTN, ‡P < 0.05 vs. MetS. Abbreviations: BP, blood pressure; IMT, intima media thickness; PWV, pulse wave velocity; WCSA, wall cross-sectional area.
Platelet-poor plasma small RNA profiling
Circulating RNA was extracted from 15 samples per group. On average, 25.7 ng (3.8–99.0 ng) of RNA was extracted from 6 ml of platelet-poor plasma sample (Supplementary Table S2 online). Analysis of the Agilent 2100 bioanalyzer electrophoresis profiles revealed that platelet-poor RNA samples were mostly constituted of small RNA enriched in miRNAs (36 ± 9%, Supplementary Table S2 and Figure S2 online). All the samples were used to construct small RNA libraries. Twenty ng of total RNA or the maximum available were used for small RNA sequencing, amounting to 16.6 ± 4.9, 14.1 ± 6.8, 12.7 ± 5.6, and 17.6 ± 5.1 ng of RNA for NTN, HTN, MetS, and CKD, respectively. One CKD RNA sample with low base quality (Q < 20), another CKD sample with low read counts, and one MetS sample that could not be processed by miRDeep2 due to error in the sequence file were excluded from further small RNA analyses. An average of 12.7 million qualified single-end reads per sample was obtained in the small RNA sequencing data, of which 8 ± 3% mapped to a single locus and 82 ± 7% to multiple loci.
DE circulating miRNAs and their validation by RT-qPCR
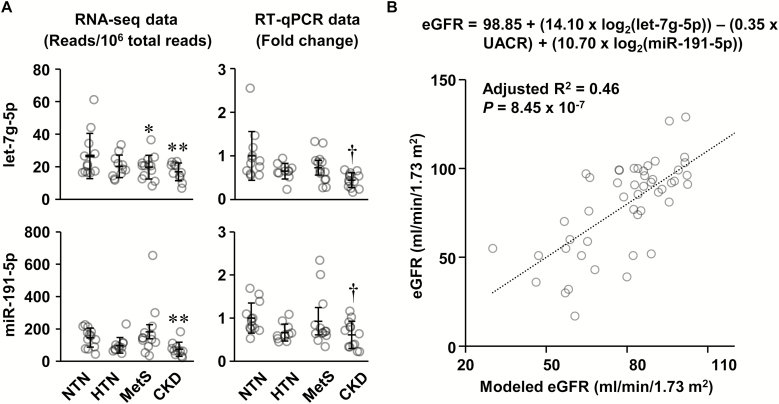
One novel and 29 known DE miRNAs were identified using small RNA sequencing in the platelet-poor plasma of HTN, MetS, and CKD compared with NTN. The most abundant DE miRNAs that had in average more than 2,000 read counts were selected for RT-qPCR validation. The geometric mean of 3 least variable miRNAs (let-7f-5p, miR-92b-3p, and miR-126-5p) was used for normalization of RT-qPCR results. However, only 2 of the top 11 DE miRNAs, let-7g-5p and miR-191-5p, could be validated by RT-qPCR (Figure 1a), which does not allow ruling out that sequencing results of other DE miRNAs that could not be validated by RT-qPCR might be unreliable. Accordingly, the relationship of the non validated microRNAs with clinical parameters and conditions was not evaluated. Sequences of miRNAs used for RT-qPCR primer design are shown in Supplementary Table S3 online. RT-qPCR confirmed indeed that let-7g-5p and miR-191-5p were downregulated in CKD, but did not validate the downregulation of let-7g-5p in MetS.
Figure 1.
Circulating let-7g-5p and miR-191-5p are decreased in chronic kidney disease (CKD) patients, and let-7g-5p, miR-191-5p, and urinary albumin/creatinine ratio (UACR) are independent predictors of the estimated glomerular filtration rate (eGFR). (a) Two of the top 11 most abundant differentially expressed microRNAs were validated by reverse transcription-quantitative PCR (RT-qPCR). RNA sequencing (RNA-seq) data are presented in the left panels and the RT-qPCR data in the right panels. Means ± SD and individual value of the normotensive subjects (NTN), and patients with hypertension (HTN) associated or not with other features of the metabolic syndrome (MetS) or with CKD are presented, n = 13–15 for RNA-seq data and 12–14 for RT-qPCR data. The geometric mean of miR-92b-3p, let-7f-5p, and miR-126-5p was used for normalization of RT-qPCR results. RNA-seq data were analyzed using an analysis of variance (ANOVA) one-way like test in EdgeR based on generalized linear models with a threshold of false discovery rate (q) <0.1. RT-qPCR log2 fold change data were analyzed using one-way ANOVA followed by a Student–Newman–Keuls post hoc test with a threshold of P < 0.05. *q < 0.1, **q < 0.05, and †P < 0.05 vs. NTN. (b) Stepwise multiple linear regression was used to determine the best model to predict the eGFR using the let-7g-5p and miR-191-5p log2 fold change determined by RT-qPCR and clinical parameters (age, UACR, carotid distensibility, neutrophil and lymphocyte fractions, neutrophil number, and neutrophil-to-lymphocyte ratio) that are correlated with eGFR. The best model of 3 is shown that included let-7g-5p (P = 0.001), UACR (P = 0.006), and miR-191-5p (P = 0.014). n = 49.
Correlation of DE let-7g-5p and miR-191-5p with clinical data
The potential of let-7g-5p and miR-191-5p as biomarkers for target organ damage in HTN was investigated by demonstrating associations between these DE miRNAs determined by RT-qPCR and parameters presented in Tables 1–3. Let-7g-5p correlated negatively with age and carotid to femoral pulse wave velocity, and positively with carotid distensibility (Table 4). MiR-191-5p correlated positively with lymphocyte number. Both miRNAs correlated positively with eGFR and lymphocyte fraction, and negatively with the neutrophil fraction and NLR.
Table 4.
Correlation between differentially expressed miRNAs and estimated glomerular filtration rate, other clinical parameters, and vascular mechanical properties
| Parameters | let-7g-5p | miR-191-5p | eGFR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R 2 | Type | P | R 2 | Type | P | R 2 | Type | P | |
| Age | 0.208 | Neg | <0.001 | 0.275 | Neg | <0.001 | |||
| eGFR | 0.297 | Pos | <0.001 | 0.259 | Pos | <0.001 | NA | NA | NA |
| UACR | 0.125 | Neg | <0.05 | ||||||
| Carotid distensibility | 0.106 | Pos | <0.05 | 0.083 | Pos | <0.05 | |||
| Carotid femoral PWV | 0.099 | Neg | <0.05 | ||||||
| Neutrophil fraction | 0.219 | Neg | <0.05 | 0.187 | Neg | <0.05 | 0.144 | Neg | <0.05 |
| Lymphocyte fraction | 0.176 | Pos | <0.05 | 0.139 | Pos | <0.05 | 0.119 | Pos | <0.05 |
| Neutrophil number | 0.268 | Neg | <0.001 | 0.102 | Neg | <0.05 | 0.102 | Neg | <0.05 |
| Lymphocyte number | 0.086 | Pos | <0.05 | ||||||
| Neutrophils/lymphocytes | 0.216 | Neg | <0.05 | 0.193 | Neg | <0.05 | 0.153 | Neg | <0.05 |
The log2 fold change of let-7g-5p and miR-191-5p expression levels determined by reverse transcription-quantitative PCR was used to determine simple linear correlations with clinical parameters and vascular mechanical properties. The square of the Pearson correlation coefficient (R2), the type of correlation (positive (Pos) or negative (Neg)), and the probability (P) are shown. n = 50 for let-7g-5p and miR-191-5p and 61–62 for estimated glomerular filtration rate (eGFR). Abbreviations: NA, non applicable; PWV, pulse wave velocity; UACR, urinary albumin/creatinine ratio.
Let-7g-5p and miR-191-5p as biomarkers for CKD
Since let-7g-5p and miR-191-5p were downregulated in CKD and presented the greatest correlation with eGFR (Table 4), we questioned using stepwise multiple linear regression whether a model including let-7g-5p and miR-191-5p with parameters that correlated with eGFR could strongly predict eGFR. First, we determined that besides let-7g-5p and miR-191-5p, eGFR correlated negatively with age, UACR, neutrophil fraction and number, and NLR, and positively with carotid distensibility and the lymphocyte fraction (Table 4). The parameters entered into the regression were tested for multicollinearity and demonstrated sufficient independence from each other (Supplementary Table S4 online). The best model of 3 included let-7g-5p, miR-191-5p, and UACR, and predicted eGFR with an adjusted R2 of 0.46 (P = 8.45e−7, Figure 1b).
DISCUSSION
This study allowed identifying 2 downregulated circulating miRNAs, let-7g-5p and miR-191-5p, in hypertensive patients with CKD. Associations were demonstrated between let-7g-5p and large vessel stiffening, miR-191-5p and MetS, and both circulating miRNAs with eGFR and inflammatory markers. Stepwise multiple linear regression modeling demonstrated that circulating let-7g-5p and miR-191-5p and UACR are independent predictors of eGFR, and thus potential biomarkers of CKD in hypertensive patients, and could have pathophysiological and therapeutic significance.
Circulating miRNAs that originate from tissues have great potential to be biomarkers of subclinical target organ damage in HTN as there are tissue-specific miRNAs.12 In this study, platelet-poor plasma was used to profile circulating miRNAs in HTN associated or not with other features of the MetS or with CKD. It was important to eliminate platelets from plasma samples as much as possible as they are an important source of miRNA,21,22 which could confound detection of changes in tissue-derived miRNAs. Although serum does not contain platelets, it was not used as miRNAs could be released on activation of platelets and other blood cells during the coagulation process.23
This study was designed to identify DE circulating miRNAs associated to asymptomatic complications of HTN by comparing the profile of platelet-poor plasma miRNAs of NTN subjects with that of patients with HTN associated or not with other features of the MetS or with CKD. This experimental design allowed the ability to identify biomarkers associated with HTN, complications of HTN, or both. Only 2 other studies have investigated circulating known miRNAs as biomarkers of target organ damage in HTN patients using a similar experimental design.14,15 Huang et al.15 examined let-7 levels in plasma by RT-PCR in untreated hypertensive patients and healthy subjects with or without thickening (>0.9 mm) of carotid IMT. These authors observed positive correlations between plasma let-7 with systolic BP, carotid IMT, and C-reactive protein, and showed that let-7 is an independent predictor of carotid IMT thickening. These findings were not found in the present study. Firstly, let-7 is a multimember miRNA family. Secondly, although not clearly stated, let-7 may have been determined in platelet-rich plasma as plasma was isolated after one centrifugation of blood samples, and could therefore originate from platelets.21 In the present study, RNA sequencing revealed that expression levels of several let-7 members were downregulated in HTN, MetS, and CKD (Supplementary Table S3 online). No correlation was observed between let-7g-5p with BP or carotid IMT. However, associations were observed between let-7g-5p and increased number and fraction of neutrophils, decreased fraction of lymphocytes, and increased NLR. Kaneto et al.14 profiled miRNAs in serum of healthy subjects and patients with HTN associated or not with left ventricular hypertrophy. These authors identified serum miR-7-5p and miR-26b-5p as biomarkers of left ventricular hypertrophy in HTN. These miRNAs were not found to be altered in the present study.
Our finding that both let-7g-5p and miR-191-5p were associated with neutrophil and lymphocyte fractions, neutrophil number, and NLR is of interest because activation of the immune system may play a role in HTN-induced target organ damage.24 In humans, stiffening of large vessels and CKD have been shown to be associated with inflammation.25 NLR is a novel inflammatory marker that has been shown to be a predictor of cardiovascular complications.26,27 NLR was associated with arterial stiffness in postmenopausal women with osteoporosis.28 Higher NLR was independently associated with arterial stiffness and coronary calcium score in a cohort of 849 Korean adults in a health examination program29 NLR was also an independent predictor of aortic stiffening in patients with type 1 diabetes.30 Some studies have also examined the role of let-7g-5p or miR-191-5p in inflammation.31,32 Using microarrays, a study in human volunteers demonstrated that let-7g was downregulated in blood leukocytes during acute inflammation triggered by Escherichia coli lipopolysaccharide infusion in vivo.31 However, this failed to be confirmed by RT-qPCR. Another study demonstrated using profiling of serum miRNAs by microarray and validation by RT-qPCR that circulating miR-191-5p was lower in patients with sepsis with or without acute kidney injury compared with the healthy volunteers.33
Associations have been reported between let-7g or miR-191 and kidney diseases not associated with HTN, or without mention of HTN. An association between platelet-rich plasma miR-191 and renal damage was shown in Chinese subjects with chronic exposure to arsenic.34,35 Associations were also reported between serum, urine, and kidneys miR-191 levels and kidney disease in Chinese nephrotic children.36,37 Urinary let-7g-5p and miR-191a-5p levels were increased at day 5 and not changed or decreased, respectively, at day 7 after induction of nephropathy with cisplatin in rats.38 However, BP was not reported in the above studies.
In this study the strongest association was found between both let-7g-5p and miR-191-5p and eGFR. Interestingly, a similar observation was made in a study investigating the miRNA signature in serum of CKD patients using nanoString technology.23 Let-7g-5p was downregulated and miR-191-5p tended to be decreased in patients with stage 5 CKD compared with healthy controls. However, BP was not reported and these DE miRNAs were not studied further. These data support the finding that circulating let-7g-5p and miR-191-5p could be biomarkers of subclinical renal injury in HTN. However, whether these 2 miRNAs are associated with CKD in general is unknown and remains to be determined. Microalbuminuria is a marker of glomerular filtration barrier dysfunction.4 Although a correlation was observed between UACR and eGFR, UACR was not correlated with let-7g-5p and miR-191-5p. However, using stepwise multiple linear regression, we generated a model that showed that let-7g-5p, miR-191-5p, and UACR are independent determinants of eGFR. Furthermore, this model demonstrated that combining these 3 parameters has the potential to predict progression of CKD as it predicted 46% of the variation in eGFR, and that age was excluded from the model, thus avoiding tautology. A meta-analysis has shown that lower eGFR and higher albuminuria are risk factors for all-cause and cardiovascular mortality, independent of each other and cardiovascular risk factors.39 It would be therefore important to determine whether the addition of circulating let-7g-5p and miR-191-5p as biomarkers could be useful for prediction and improving outcomes of patients with CKD and HTN by contributing to the design of optimal antihypertensive therapies to reduce the risk of cardiovascular disease events.
Limitations
The number of subjects studied in each group is small because of the vascular phenotyping, and the use of Next Generation Sequencing to analyze the noncoding RNAs. The age range of the recruited subjects is wide, and MetS and CKD patients were older than NTN subjects, due to difficulty in recruiting participants, in particular NTN and CKD subjects. Thus, these results need to be confirmed in larger cohorts and their pathophysiological significance further clarified in the future. Secondly, in a diverse population of middle-aged and elderly patients in North America it is not possible to recruit hypertensive subjects who may not be slightly overweight or dyslipidemic, and the same for CKD patients or even normotensive individuals in this age group. However, the absence of reduction of eGFR and of at least 3 criteria for MetS allow categorizing subjects as hypertensive or normotensive according to the definition of HTN.16 Thirdly, the validation efficiency of RNA sequencing DE miRNA data by RT-qPCR was low. This could be due to differences in normalization and gene expression determination methods. Therefore, the fact that some of the DE miRNAs could not be validated by RT-qPCR does not allow ruling out that the sequencing results of those DE miRNAs that were not validated by RT-qPCR might be unreliable, and accordingly, their relationship with clinical parameters and conditions was not evaluated.
This study showed that lower circulating let-7g-5p and miR-191-5p were associated with different indices of subclinical target organ damage in HTN. More importantly, lower circulating let-7g-5p and miR-191-5p were identified as independent markers of renal injury that when combined with UACR provided an improved prediction of progression of renal injury as determined by increased eGFR, and could have pathophysiological significance and therapeutic implications to reduce the risk of cardiovascular disease events in CKD, which needs to be further investigated and confirmed in larger cohorts.
FUNDING
This work was supported by Canadian Institutes of Health Research (CIHR) grant 123465 and CIHR First Pilot Foundation grant 143348, a Tier 1 Canada Research Chair (CRC) on HTN and Vascular Research by the CRC Government of Canada/CIHR Program, by the Canada Fund for Innovation, and by a Discovery Grant from Servier France, all to ELS; by a Terry Fox New Frontiers Program grant 217967 to DS; and by a fellowships to JCFA from Science without Borders [CsF] of the National Council for Scientific and Technological Development [CNPq] of Brazil; and to OB from Société québécoise d’hypertension artérielle.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Asia Rehman for initial contribution, Kathy Delouya R.N. and Tracy Hodge R.N. for excellent nursing support and Virginie Sailour for excellent bioinformatics support. Echotracking and tonometry were donated by INSERM for support of this (and other) research programs. This research was also enabled in part by the computing infrastructure provided by Calcul Québec (http://www.calculquebec.ca/) and Compute Canada (www.computecanada.ca). This study was part of a thesis submitted by Kugeng Huo to the Division of Experimental Medicine in the Department of Medicine, McGill University, in partial fulfillment of the requirements for the degree of Doctor of Philosophy.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, Fauad-Tarazi F, Horan MJ, Marcus M, Massie B. The heart in hypertension. N Engl J Med 1992; 327:998–1008. [DOI] [PubMed] [Google Scholar]
- 2. Boutouyrie P, Laurent S, Girerd X, Benetos A, Lacolley P, Abergel E, Safar M. Common carotid artery stiffness and patterns of left ventricular hypertrophy in hypertensive patients. Hypertension 1995; 25:651–659. [DOI] [PubMed] [Google Scholar]
- 3. Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens 2001; 19:921–930. [DOI] [PubMed] [Google Scholar]
- 4. Mulè G, Castiglia A, Cusumano C, Scaduto E, Geraci G, Altieri D, Di Natale E, Cacciatore O, Cerasola G, Cottone S. Subclinical kidney damage in hypertensive patients: a renal window opened on the cardiovascular system. focus on microalbuminuria. Adv Exp Med Biol 2017; 956:279–306. [DOI] [PubMed] [Google Scholar]
- 5. Rao MV, Qiu Y, Wang C, Bakris G. Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999–2004. Am J Kidney Dis 2008; 51(Suppl 2):S30–S37. [DOI] [PubMed] [Google Scholar]
- 6. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 7. Cuspidi C, Sala C, Zanchetti A. Metabolic syndrome and target organ damage: role of blood pressure. Expert Rev Cardiovasc Ther 2008; 6:731–743. [DOI] [PubMed] [Google Scholar]
- 8. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004; 5:522–531. [DOI] [PubMed] [Google Scholar]
- 9. Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010; 466:835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 2009; 10:126–139. [DOI] [PubMed] [Google Scholar]
- 11. Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res 2012; 110:483–495. [DOI] [PubMed] [Google Scholar]
- 12. Londin E, Loher P, Telonis AG, Quann K, Clark P, Jing Y, Hatzimichael E, Kirino Y, Honda S, Lally M, Ramratnam B, Comstock CE, Knudsen KE, Gomella L, Spaeth GL, Hark L, Katz LJ, Witkiewicz A, Rostami A, Jimenez SA, Hollingsworth MA, Yeh JJ, Shaw CA, McKenzie SE, Bray P, Nelson PT, Zupo S, Van Roosbroeck K, Keating MJ, Calin GA, Yeo C, Jimbo M, Cozzitorto J, Brody JR, Delgrosso K, Mattick JS, Fortina P, Rigoutsos I. Analysis of 13 cell types reveals evidence for the expression of numerous novel primate- and tissue-specific microRNAs. Proc Natl Acad Sci USA 2015; 112:E1106–E1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viereck J, Thum T. Circulating noncoding RNAs as biomarkers of cardiovascular disease and injury. Circ Res 2017; 120:381–399. [DOI] [PubMed] [Google Scholar]
- 14. Kaneto CM, Nascimento JS, Moreira MCR, Ludovico ND, Santana AP, Silva RAA, Silva-Jardim I, Santos JL, Sousa SMB, Lima PSP. MicroRNA profiling identifies miR-7-5p and miR-26b-5p as differentially expressed in hypertensive patients with left ventricular hypertrophy. Braz J Med Biol Res 2017; 50:e6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang YQ, Huang C, Chen JY, Li J, Feng YQ. Plasma expression level of miRNA let-7 is positively correlated with carotid intima-media thickness in patients with essential hypertension. J Hum Hypertens 2017; 31:843–847. [DOI] [PubMed] [Google Scholar]
- 16. Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, Harris KC, Nakhla M, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Padwal RS, Tran KC, Grover S, Rabkin SW, Moe GW, Howlett JG, Lindsay P, Hill MD, Sharma M, Field T, Wein TH, Shoamanesh A, Dresser GK, Hamet P, Herman RJ, Burgess E, Gryn SE, Grégoire JC, Lewanczuk R, Poirier L, Campbell TS, Feldman RD, Lavoie KL, Tsuyuki RT, Honos G, Prebtani APH, Kline G, Schiffrin EL, Don-Wauchope A, Tobe SW, Gilbert RE, Leiter LA, Jones C, Woo V, Hegele RA, Selby P, Pipe A, McFarlane PA, Oh P, Gupta M, Bacon SL, Kaczorowski J, Trudeau L, Campbell NRC, Hiremath S, Roerecke M, Arcand J, Ruzicka M, Prasad GVR, Vallée M, Edwards C, Sivapalan P, Penner SB, Fournier A, Benoit G, Feber J, Dionne J, Magee LA, Logan AG, Côté AM, Rey E, Firoz T, Kuyper LM, Gabor JY, Townsend RR, Rabi DM, Daskalopoulou SS; Hypertension Canada . Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34:506–525. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 18. Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int 2006; 69:350–357. [DOI] [PubMed] [Google Scholar]
- 19. Briet M, Maruani G, Collin C, Bozec E, Gauci C, Boutouyrie P, Houillier P, Laurent S, Froissart M. Age-independent association between arterial and bone remodeling in mild-to-moderate chronic kidney disease. Nephrol Dial Transplant 2010; 25:191–197. [DOI] [PubMed] [Google Scholar]
- 20. Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P; Nephrotest Study Group . Arterial remodeling associates with CKD progression. J Am Soc Nephrol 2011; 22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Osman A, Fälker K. Characterization of human platelet microRNA by quantitative PCR coupled with an annotation network for predicted target genes. Platelets 2011; 22:433–441. [DOI] [PubMed] [Google Scholar]
- 22. Willeit P, Zampetaki A, Dudek K, Kaudewitz D, King A, Kirkby NS, Crosby-Nwaobi R, Prokopi M, Drozdov I, Langley SR, Sivaprasad S, Markus HS, Mitchell JA, Warner TD, Kiechl S, Mayr M. Circulating microRNAs as novel biomarkers for platelet activation. Circ Res 2013; 112:595–600. [DOI] [PubMed] [Google Scholar]
- 23. Ulbing M, Kirsch AH, Leber B, Lemesch S, Münzker J, Schweighofer N, Hofer D, Trummer O, Rosenkranz AR, Müller H, Eller K, Stadlbauer V, Obermayer-Pietsch B. MicroRNAs 223-3p and 93-5p in patients with chronic kidney disease before and after renal transplantation. Bone 2017; 95:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caillon A, Paradis P, Schiffrin EL. Role of immune cells in hypertension. Br J Pharmacol 2019; 176:1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int 2012; 82:388–400. [DOI] [PubMed] [Google Scholar]
- 26. Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther 2016; 14:573–577. [DOI] [PubMed] [Google Scholar]
- 27. Mozos I, Malainer C, Horbańczuk J, Gug C, Stoian D, Luca CT, Atanasov AG. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front Immunol 2017; 8:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu XY, Li XS, Li Y, Liu T, Wang RT. Neutrophil-lymphocyte ratio is associated with arterial stiffness in postmenopausal women with osteoporosis. Arch Gerontol Geriatr 2015; 61:76–80. [DOI] [PubMed] [Google Scholar]
- 29. Park BJ, Shim JY, Lee HR, Lee JH, Jung DH, Kim HB, Na HY, Lee YJ. Relationship of neutrophil-lymphocyte ratio with arterial stiffness and coronary calcium score. Clin Chim Acta 2011; 412:925–929. [DOI] [PubMed] [Google Scholar]
- 30. Ayhan H, Kasapkara HA, Aslan AN, Durmaz T, Keleş T, Akçay M, Akar Bayram N, Baştuğ S, Bilen E, Sarı C, Bozkurt E. Relationship of neutrophil-to-lymphocyte ratio with aortic stiffness in type 1 diabetes mellitus. Can J Diabetes 2015; 39:317–321. [DOI] [PubMed] [Google Scholar]
- 31. Schmidt WM, Spiel AO, Jilma B, Wolzt M, Müller M. In vivo profile of the human leukocyte microRNA response to endotoxemia. Biochem Biophys Res Commun 2009; 380:437–441. [DOI] [PubMed] [Google Scholar]
- 32. Guo Y, Chao L, Chao J. Kallistatin attenuates endothelial senescence by modulating Let-7g-mediated miR-34a-SIRT1-eNOS pathway. J Cell Mol Med 2018; 22:4387–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ge QM, Huang CM, Zhu XY, Bian F, Pan SM. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS One 2017; 12:e0173292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu Y, Zou Z, Liu Y, Wang Q, Sun B, Zeng Q, Liu Q, Zhang A. miR-191 is involved in renal dysfunction in arsenic-exposed populations by regulating inflammatory response caused by arsenic from burning arsenic-contaminated coal. Hum Exp Toxicol 2020; 39:37–46. [DOI] [PubMed] [Google Scholar]
- 35. Zeng Q, Zou Z, Wang Q, Sun B, Liu Y, Liang B, Liu Q, Zhang A. Association and risk of five miRNAs with arsenic-induced multiorgan damage. Sci Total Environ 2019; 680:1–9. [DOI] [PubMed] [Google Scholar]
- 36. Lu M, Wang C, Yuan Y, Zhu Y, Yin Z, Xia Z, Zhang C. Differentially expressed microRNAs in kidney biopsies from various subtypes of nephrotic children. Exp Mol Pathol 2015; 99:590–595. [DOI] [PubMed] [Google Scholar]
- 37. Luo Y, Wang C, Chen X, Zhong T, Cai X, Chen S, Shi Y, Hu J, Guan X, Xia Z, Wang J, Zen K, Zhang CY, Zhang C. Increased serum and urinary microRNAs in children with idiopathic nephrotic syndrome. Clin Chem 2013; 59:658–666. [DOI] [PubMed] [Google Scholar]
- 38. Kanki M, Moriguchi A, Sasaki D, Mitori H, Yamada A, Unami A, Miyamae Y. Identification of urinary miRNA biomarkers for detecting cisplatin-induced proximal tubular injury in rats. Toxicology 2014; 324:158–168. [DOI] [PubMed] [Google Scholar]
- 39. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium . Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.