Abstract
Localized hypertrophic neuropathy is a rare Schwann cell proliferation that usually affects single nerves from the extremities, and it is of unclear etiology in its pure form. RASopathies are a defined group of genetic diseases with overlapping clinical features, usually secondary to germline mutations in genes encoding either components or regulators of the RAS/MAPK pathway. Herein, we report an 11-year-old boy presenting with café au lait spots and right leg length discrepancy. A fascicular nerve biopsy of the tibial nerve demonstrated a Schwann cell proliferation with prominent onion-bulb formation, satisfying criteria for localized hypertrophic neuropathy. Molecular genetic analysis demonstrated identical KRAS mutations (c38_40dupGCG) in the peripheral nerve lesion and melanocytes from café au lait spots, but not in blood, supporting a diagnosis of a KRAS-mediated rasopathy with mosaicism. Immunohistochemical staining in the peripheral nerve lesion demonstrated strong pERK staining consistent with downstream MAPK pathway activation. This report suggests that at least a subset of localized hypertrophic neuropathies are bonafide, well-differentiated Schwann cell neoplasms developing through oncogenic RAS signaling, which provides new insights into the controversial entity historically known as localized hypertrophic neuropathy.
Keywords: KRAS, Localized hypertrophic neuropathy, Peripheral nerve, RASopathy, Schwann cell
INTRODUCTION
Localized hypertrophic neuropathy (LHN) is a rare Schwann cell proliferation that usually affects single nerves from the extremities. Histologically, it is characterized by “onion bulb” formation, composed of layers of Schwann cell processes and historically interpreted as a reactive entity (1, 2). However, it was understood over time that a subset, if not most of these cases, represented a cellular proliferation with a perineurial phenotype (i.e. intraneural perineurioma) (3, 4). The latter is now recognized as a true neoplasm with clonal mutations in TRAF7 or NF2 (5). Therefore, true LHN can be currently defined as a mass-like Schwann cell lesion in the absence of a generalized neuropathy, and remains very rare after excluding cases that represent intraneural perineuriomas. Morphologic entities in the differential diagnosis include inherited neuropathies associated with onion bulb formation, such as Charcot-Marie-Tooth. Schwannosis are rare localized nonneoplastic Schwann cell proliferations in the spinal cord, which may affect older patients or develop in the context of neurofibromatosis type 2. However, clinical presentation, morphology, immunophenotype, and distribution of these lesions are different from LHN.
RASopathies are a defined group of genetic diseases with overlapping clinical features, usually secondary to germline mutations in genes encoding either components or regulators of the RAS/MAPK pathway. Abnormalities of this pathway comprise one of the largest known groups of developmental syndromes, affecting ∼1 in 1000 individuals (6). The best-known syndromes related to this pathway and their main alterations include neurofibromatosis type 1 (NF1), capillary malformation-arteriovenous malformation, Noonan and Noonan with multiple lentigines, Costello, Legius, and cardio-facio-cutaneous syndromes. However, the list of genetic alterations and phenotypes under this common biologic umbrella continues to grow. Herein, we present a case of extensive LHN developing in a patient satisfying clinical and genetic criteria of mosaic RASopathy.
MATERIALS AND METHODS
Pathologic Analysis
Clinical information was abstracted from electronic chart records. All available imaging, including magnetic resonance (MR) imaging, was reviewed. All available histologic slides were reviewed. Immunohistochemistry was performed using antibodies targeting EMA (Cellmarque, clone E29), GLUT1 (Cellmarque, polyclonal), S100 (Ventana, clone 4C4.9), neurofilament protein (SM31, Sternberger), phospho-ERK (Cell Signaling, D13.14.4E, XP Rabbit mAb; 1:250), and Ki-67 (Ventana, clone 30-9). One piece of peripheral nerve tissue was glutaraldehyde-fixed and epon-embedded. Semithin sections were examined and blocks with onion bulbs subjected to electron microscopy analysis.
Molecular Analysis
Peripheral nerve sheath tumor targeted next generation sequencing (NGS) and copy number analysis protocol was performed at the University of Alabama, Birmingham for the following genes: KRAS (exon 2), PTPN11, NF1, NF2, SMARCB1, and LZTR1. DNA was extracted from fresh lesional tissue from peripheral nerve, as well as cultured Schwann cells from the peripheral nerve lesion and melanocytes from one pigmented skin spot. The average coverage for NGS testing is ∼1600× with >98% of the coding region ≥350× and >99% ≥200×. This allows for the detection of low-level mosaicism, down to 3%–5% variant allele fraction with 95% confidence. DNA extracted from blood lymphocytes was tested by Sanger sequencing. Additional details on the testing methods may be gathered from the UAB laboratory website (https://www.uab.edu/medicine/genetics/medical-genomics-laboratory/testing-services/nf1-legius-syndrome-and-rasopathies/ras-ng).
RESULTS
Clinical History
The patient was an 11-year-old boy with no significant personal or family medical history. He presented with right leg length discrepancy for one year, as well as 4 subtle café au lait spots observed under Wood’s lamp involving the face, trunk and extremities bilaterally. Radiographs showed bilateral, multiple stress fractures of the lower limbs. MR imaging showed diffuse, smooth enlargement of the lumbosacral plexus and right sciatic nerve extending to the tibial, common peroneal, and deep and superficial peroneal nerves throughout their visualized course without associated enhancement or bulbous enlargement (Fig. 1). The patient underwent corrective surgery and a concurrent fascicular biopsy of the tibial nerve was performed.
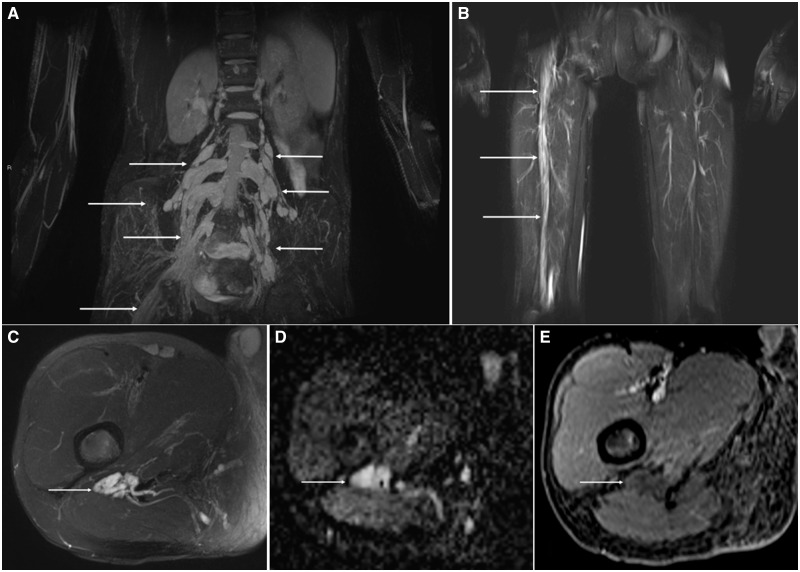
FIGURE 1.
Imaging features of localized hypertrophic neuropathy in RASopathy. Coronal Short tau inversion recovery (STIR) Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE) maximum intensity projection (MIPs) through the pelvis (A) and thighs (B) shows multifocal, bilateral lumbar nerve root thickening (arrows) extending into the right lumbosacral plexus and sciatic nerve root (arrow). Axial T2 fat suppressed (FS) image through the proximal thigh (C) shows marked thickening of the sciatic nerve (arrow) without a discrete mass. Note, the absence of imaging features of a peripheral nerve tumor such as a target sign. Apparent diffusion coefficient map through the right thigh (D) also reveals marked thickening of the sciatic nerve (arrow) without a discrete mass or restricted diffusion to suggest a hypercellular neoplasm. Axial T1-FS post contrast image through right thigh (E) also reveals marked thickening of the sciatic nerve (arrow) without internal enhancement.
Pathology
The 0.6-cm specimen consisted of a nerve fascicle showing sclerosis of the epineurium and slight irregularity of the perineurium. There were concentric whorls in the form of “onion bulbs” (Fig. 2). Longitudinal sections demonstrated areas of increased cellularity, but mitotic activity was inconspicuous. Immunohistochemical stain for S100 was strongly positive and outlined the onion bulbs (Fig. 2). EMA was negative and neurofilament protein highlighted sparse, residual axons, some in the center of the onion bulbs (Fig. 2), suggesting that the Schwann cell process did not involve every axon. GLUT1 showed weak, nonspecific immunoreactivity, with strong labeling limited to erythrocytes which served as internal positive control. Strong immunoreactivity for phospho-ERK was detected (Fig. 2). This antibody recognizes levels of p44 and p42 MAP Kinase (Erk1 and Erk2) when phosphorylated at 2 relevant sites (Thr202 and Tyr204) of Erk1 and (Thr185 and Tyr187) of Erk2, as well as singly phosphorylated at Thr202. This provides evidence of MAPK pathway activation, downstream of RAS. We have previously detected similar immunoreactivity in neoplasms associated with other RASopathies (Noonan) (7) or activating somatic BRAF alterations (8). Ki67 labeling index was very low (Fig. 2). Electron microscopy examination was limited by a predominant longitudinal orientation of the sections examined. However, numerous Schwann cell processes were concentrically arranged in areas, and axonal loss was also detected (Fig. 2), which correlated with nerve conduction loss identified intraoperatively. This suggests a degree of chronicity with associated partial axonal loss in the lesion and provided further support for the Schwann cell nature of the process.
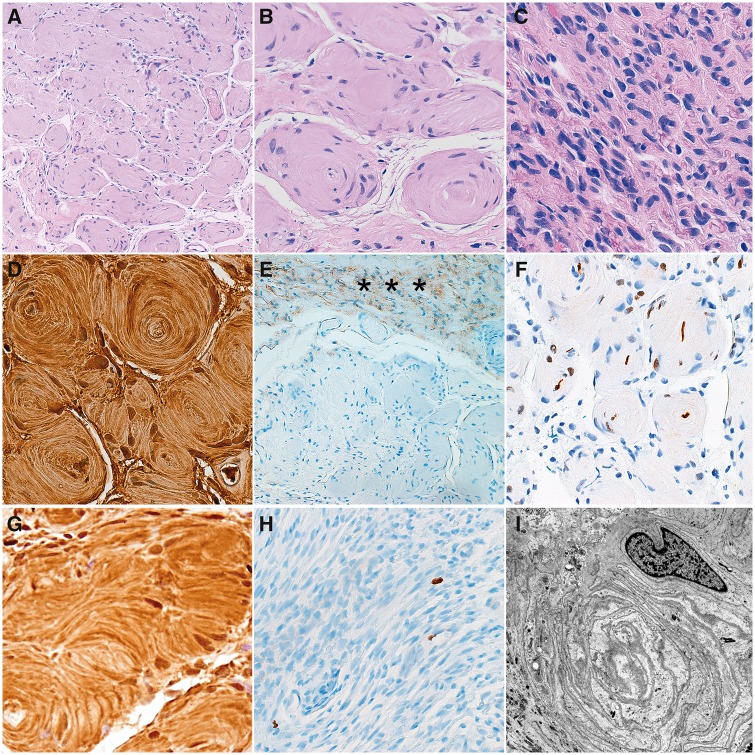
FIGURE 2.
Pathologic features of localized hypertrophic neuropathy in RASopathy. Cross sections of the peripheral nerve fascicle demonstrated circular arrangements of Schwann cell processes around individual axons (“onion bulbs”) (A, B). Longitudinal sections showed an increase in endoneurial cellularity, but no mitotic activity (C). Immunohistochemical stains demonstrated strong reactivity for S100 in onion bulbs (D), while EMA immunoreactivity was limited to perineurium (asterisks) (E). Neurofilament protein highlighted individual axons (F). Strong pERK staining was detected in Schwann cells (G). Ki67 labeling index was very low (<1%) (H). Electron microscopy demonstrated concentric arrangements of Schwann cell processes (I).
Molecular Genetics
NGS molecular analysis performed in fresh peripheral nerve lesional tissue identified a KRAS duplication (c38_40dupGCG, mutant allele fraction of ∼16%, 192 mutation reads/∼1150 wild type reads). The same alteration was found in cultured Schwann cells from the nerve lesion and at a low level in cultured melanocytes from the café au lait spot. No genetic alterations were detected in blood by Sanger sequencing (1040 reads over ∼5 amplicons). The same KRAS alteration has been reported in other neoplasms (COSV55499554), including tumors of the large and small bowel (9). The potential functional impact of the in-frame insertion variant was ascertained by Ensembl’s Variant Effect Predictor (VEP) (10) and Sorting Intolerant From Tolerant (SIFT) programs (11). VEP predicted a moderate effect whereas SIFT predicted a damaging effect on gene function (Supplementary Data Table S1). As described above, strong immunohistochemical staining for phosphor-ERK, a known downstream mediator of the MAPK pathway, was detected in lesional tissue consistent with an activating mutation. Taken together, these findings supported a diagnosis of LHN developing in the context of a KRAS-driven RASopathy with possible mosaicism.
DISCUSSION
LHN is a rare, unique lesion of the peripheral nerve that has undergone nosologic change through the past several decades. The histological and imaging based differential diagnosis of LHN includes primarily intraneural perineurioma, but also neurofibroma with onion bulb-like formation, and other hereditary neuropathies like Charcot-Marie-Tooth. Additionally, most of the lesions that were diagnosed in the past as LHN are now believed to represent intraneural perineuriomas (3, 4). In contrast to true LHN using a strict definition of a Schwann cell lesion, perineuriomas are EMA-positive and S100-negative neoplasms. Even though neurofibromas with onion bulb-like formation are also positive for S100, they display their classic diffuse or plexiform morphology, with onion bulb-like formation present only focally. Schwannosis, although having some histologic similarities, is typically limited to the spinal cord in older patients or those with NF2, and therefore the distribution is different from LHN.
Molecular analysis can be also helpful in characterizing these lesions. No alterations were identified in the NF1 gene in the current case, but rather a pathogenic mutation in KRAS was found. Neurofibromas uniformly carry inactivating mutations in NF1 (12), and therefore, we interpreted this Schwann cell proliferation as most compatible with LHN. Molecular analyses of this lesion are lacking in the literature given its rarity, which is now accentuated by the exclusion of intraneural perineurioma, that was likely responsible for most cases previously described as LHN in the literature.
Of interest, the patient had a KRAS alteration not only in the peripheral nerve lesion, but also in samples from cutaneous melanocytes while being negative in blood, which supported a mosaic presentation of a RASopathy, a heterogeneous and expanding group of genetic disorders with variable genetic drivers that have in common alterations in the MAPK/RAS pathway (6, 13). The patient’s activating KRAS gene mutation could lead to hyperactivation of RAS signaling cascades resulting in an increased risk for the development of other tumors in the future, and may thus require regular clinical and radiographic surveillance, as is done routinely for other syndromes in this category. The best characterized ones are NF1, caused by NF1 mutations, and Noonan syndrome, caused by several mutations, including PTPN11. More recently, Legius syndrome was recognized as a member of this group, a disorder resulting from mutations in the Sprouty protein family and RAS regulator, SPRED1 (14). Interestingly, most of the RASopathies other than NF1 are not usually associated with the development of peripheral nerve sheath tumors, with very few exceptions. Soft tissue (15) and intraneural perineuriomas (16) may rarely develop in NF2 patients. There is a report of multiple spinal nerve or plexi enlargements in Noonan patients with PTPN11 (17, 18) or SOS1 mutations (19). One Noonan patient with a PTPN11 mutation presented with a neuropathy and enlargement of the sacral plexus but no biopsy was performed (20). Another Noonan patient with a PTPN11 mutation, identified in a study of 3 families, developed enlargements of multiple nerves and fascicular biopsy of the tibial nerve demonstrated onion bulb formation as well as a separate neurofibroma (17), changes similar to the case presented herein. Of interest to the present case, one patient with a germline KRAS mutation presented with enlargement of multiple nerve roots and plexi, and biopsy of a superficial nodule revealed a nerve sheath tumor interpreted as schwannoma (21).
Several features in our opinion help characterize this current lesion as neoplastic: It resembles intraneural perineurioma in all respects, a recognized clonal neoplasm of nerve, except that the lesional cells are Schwann cells; it is a mass-like lesion (on imaging favored to represent tumor) with an increase in cellularity attributable to Schwann cells, despite low level proliferation; and, finally, an oncogenic mutation involving a known oncogenic driver was identified in the lesional cells (but not in the germline). This was associated with strong phospho-ERK immunoreactivity of the peripheral nerve lesion, consistent with downstream MAPK pathway activation. The oncogenic mutation identified in the skin lesion raises an analogy to other neoplastic disorders of neural crest origin, such as neurocutaneous melanocytosis, where the skin and intracranial lesions, considered bonafide neoplasms, share the same oncogenic mutation leading to MAPK pathway activation, usually in the form of a NRAS mutation (in contrast to KRAS mutation in our case). The fascinating embryologic origin of these lesions appears to involve a postzygotic mutation in a melanocytic/Schwann cell precursor, with the timing of the mutation reflecting the precise phenotype observed (22). Migration of the precursor cell may be wide, since the lesions were bilateral in our case.
In conclusion, true LHN is a rare entity that may be associated with activating KRAS gene mutations, and therefore a subset may represent true neoplasms of Schwann cells developing in the context of a RASopathy. RASopathies are among the largest groups of genetic syndromes, and it is important to recognize them since targeted therapies are developing or undergoing clinical trials to potentially treat patients affected by this group of diseases.
Supplementary Material
This study was supported in part by NIH grant P30 CA006973 to the Sidney Kimmel Comprehensive Cancer Center (PI: W. Nelson).
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Norris B, Gonzales M, Drummond KJ.. Solitary localised hypertrophic neuropathy of the cauda equina. J Clin Neurosci 2011;18:712–4 [DOI] [PubMed] [Google Scholar]
- 2. Suarez GA, Giannini C, Smith BE, et al. Localized hypertrophic neuropathy. Mayo Clin Proc 1994;69:747–8 [DOI] [PubMed] [Google Scholar]
- 3. Stanton C, Perentes E, Phillips L, et al. The immunohistochemical demonstration of early perineurial change in the development of localized hypertrophic neuropathy. Hum Pathol 1988;19:1455–7 [DOI] [PubMed] [Google Scholar]
- 4. Tsang WY, Chan JK, Chow LT, et al. Perineurioma: An uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol 1992;16:756–63 [DOI] [PubMed] [Google Scholar]
- 5. Klein CJ, Wu Y, Jentoft ME, et al. Genomic analysis reveals frequent TRAF7 mutations in intraneural perineuriomas. Ann Neurol 2017;81:316–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tidyman WE, Rauen KA.. Expansion of the RASopathies. Curr Genet Med Rep 2016;4:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. n MK, Jallo GI, Ayars M, et al. Rosette forming glioneuronal tumor in association with Noonan syndrome: Pathobiological implications. NP 2011;30:297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodriguez FJ, Ligon AH, Horkayne-Szakaly I, et al. BRAF duplications and MAPK pathway activation are frequent in gliomas of the optic nerve proper. J Neuropathol Exp Neurol 2012;71:789–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tate JG, Bamford S, Jubb HC, et al. COSMIC: The catalogue of somatic mutations in cancer. Nucleic Acids Res 2019;47:D941–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McLaren W, Gil L, Hunt SE, et al. The ensembl variant effect predictor. Genome Biol 2016;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaser R, Adusumalli S, Leng SN, et al. SIFT missense predictions for genomes. Nat Protoc 2016;11:1–9 [DOI] [PubMed] [Google Scholar]
- 12. Pemov A, Li H, Patidar R, et al. The primacy of NF1 loss as the driver of tumorigenesis in neurofibromatosis type 1-associated plexiform neurofibromas. Oncogene 2017;36:3168–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lissewski C, Kant SG, Stark Z, et al. Copy number variants including RAS pathway genes—How much RASopathy is in the phenotype? Am J Med Genet A 2015;167:2685–90 [DOI] [PubMed] [Google Scholar]
- 14. Brems H, Chmara M, Sahbatou M, et al. Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nat Genet 2007;39:1120–6 [DOI] [PubMed] [Google Scholar]
- 15. Pitchford CW, Schwartz HS, Atkinson JB, et al. Soft tissue perineurioma in a patient with neurofibromatosis type 2: A tumor not previously associated with the NF2 syndrome. Am J Surg Pathol 2006;30:1624–9 [DOI] [PubMed] [Google Scholar]
- 16. White B, Belzberg A, Ahlawat S, et al. Intraneural perineurioma in neurofibromatosis type 2 with molecular analysis. Clin Neuropathol 2020; in press. [DOI] [PubMed] [Google Scholar]
- 17. Conboy E, Dhamija R, Wang M, et al. Paraspinal neurofibromas and hypertrophic neuropathy in Noonan syndrome with multiple lentigines. J Med Genet 2016;53:123–6 [DOI] [PubMed] [Google Scholar]
- 18. Spatola M, Wider C, Kuntzer T, et al. PTPN11 mutation manifesting as LEOPARD syndrome associated with hypertrophic plexi and neuropathic pain. BMC Neurol 2015;15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santoro C, Giugliano T, Melone MAB, et al. Multiple spinal nerve enlargement and SOS1 mutation: Further evidence of overlap between neurofibromatosis type 1 and Noonan phenotype. Clin Genet 2018;93:138–43 [DOI] [PubMed] [Google Scholar]
- 20. Maridet C, Sole G, Morice-Picard F, et al. Hypertrophic neuropathy in Noonan syndrome with multiple lentigines. Am J Med Genet A 2016;170:1570–2 [DOI] [PubMed] [Google Scholar]
- 21. Bertola DR, Pereira AC, Brasil AC, et al. Multiple, diffuse schwannomas in a RASopathy phenotype patient with germline KRAS mutation: A causal relationship? Clin Genet 2012;81:595–7 [DOI] [PubMed] [Google Scholar]
- 22. Kusters-Vandevelde HV, Kusters B, van Engen-van Grunsven AC, et al. Primary melanocytic tumors of the central nervous system: A review with focus on molecular aspects. Brain Pathol 2015;25:209–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.