Abstract
Demands of modern society force many work operations into the night when the internal circadian timekeeping system is promoting sleep. The combination of disturbed daytime sleep and circadian misalignment, which is common in overnight shift work, decreases cognitive performance, yet how performance may differ across multiple consecutive nights of shift work is not fully understood. Therefore, the primary aim of this study was to use a simulated night-shift protocol to examine the cognitive performance and ratings of sleepiness and clear-headedness across the hours of a typical daytime shift, a first night shift with an afternoon nap and extended wakefulness, and 2 subsequent overnight shifts. We tested the hypothesis that cognitive performance would be worse on the first night shift as compared with the baseline and subsequent nighttime shifts and that performance during nighttime shifts would be reduced as compared with the baseline daytime shift. Fifteen healthy adults (6 men) were studied in the 6-day in-laboratory protocol. Results showed that working during the night increased subjective sleepiness and decreased clear-headedness and performance on the Psychomotor Vigilance Task (i.e., slower median, fastest and slowest reaction times, and increased attentional lapses), Stroop color word task (decreased number of correct responses and slower median reaction time), and calculation addition performance task (decreased number attempted and correct). Furthermore, we observed limited evidence of sleepiness, clear-headedness, or performance adaptation across subsequent nights of simulated night work. Our findings demonstrate that night-shift work, regardless of whether it is the first night shift with a nap and extended wakefulness or subsequent night shifts, decreases performance and clear-headedness as compared with the day shift.
Keywords: circadian misalignment, mood, sleep deprivation, sleepiness, performance
In the modern 24-h society, many occupations require work during the night and sleep during the day, times at which these actions are not promoted by the internal circadian timing system (Borbély and Achermann, 1992; Dijk and Czeisler, 1994). This circadian misalignment is commonly reported to disturb sleep and impair performance in shift workers and in laboratory studies of circadian misalignment (Torsvall et al., 1989; Axelsson et al., 2004; Akerstedt and Wright, 2009; Burke et al., 2013). Permanent, rotating, or occasional night-shift workers report “nodding off” during work shifts at a higher incidence than those working during the day (Gold et al., 1992) and have an increased risk of accidents (Barger et al., 2005; Smith et al., 1994). These increases in reported sleepiness and decreases in performance among night-shift workers are often due to a combination of disturbed daytime sleep, circadian misalignment with limited ability to adapt circadian rhythms to the night-shift schedule (Folkard, 2008; Gumenyuk et al., 2012), and extended work hours, especially on the first night of a series of overnight shifts (Folkard, 1992). The first night shift is often preceded by short daytime naps and wakefulness for several hours prior to the start of the shift (Folkard, 1992; Härmä et al., 1989), thus resulting in extended wakefulness by the end of the shift. In addition, cognitive performance decreases with time awake (Dijk et al., 1992), and the timing of the night shift typically occurs during the circadian low in cognition (Dijk et al., 1992; Frey et al., 2004; Burke et al., 2015). Extended shifts have been found to increase the risk of accidents, with a 12-h shift having more than double the average risk of an 8-h shift (Folkard and Tucker, 2003).
Whether performance decrements are most profound on the first night shift or become more severe across multiple night shifts remains unclear. Findings from field studies show that the risk of an accident is higher and medical performance worsens across successive night shifts (Dula et al., 2001; Folkard and Tucker, 2003; Folkard and Lombardi, 2006), but other findings suggest that the first night is the most vulnerable to performance impairment because of the combination of extended wakefulness and circadian misalignment (Purnell et al., 2002; Chang et al., 2017; Leff et al., 2008). Findings simulating shift work in controlled laboratory settings show that the first night of shift work has more pronounced performance decrements as compared with the subsequent night shifts (Lamond et al., 2004; Santhi et al., 2007). However, these simulated laboratory studies did not include a nap opportunity prior to the first night shift, a schedule that is common in shift work (Härmä et al., 1989; Rosa, 1993) and has been shown to improve performance during the night in the laboratory (Dinges et al., 1987; Schweitzer et al., 2006; Bonnet, 1991; Macchi et al., 2002) and improve alertness during a night shift in the field (Härmä et al., 1989). Contrary to these laboratory and field findings, on days with self-selected naps in the field, performance on some tasks was reported to be worse than on no-nap days (Rosa, 1993). These conflicting findings require additional studies to better characterize the time course of performance changes across subsequent night shifts. Therefore, the aim of this study was to investigate changes in cognitive performance and ratings of sleepiness and clear-headedness across a simulated shift-work protocol that allowed for comparisons between a daytime work shift, a transition day with an afternoon nap prior to the first night shift, and 2 subsequent night shifts with daytime sleep opportunities. Uniquely, we focused our laboratory performance assessments during the hours of the work shift rather than the entirety of scheduled wakefulness to best estimate the influence of the night shift on work performance. We hypothesized that cognitive performance during nighttime working shifts would be reduced as compared with the day shift. Further, we hypothesized that cognitive performance would be worse on the first night shift with extended hours of wakefulness as compared with the day shift and 2 subsequent nighttime working shifts.
MATERIALS AND METHODS
Participants
The study included 15 healthy participants (6 men) aged 21 to 34 years (26.1 ± 4.5 years; mean ± SD) with no reported diagnoses of any medical, psychiatric, or sleep disorders. Data from 1 additional participant who did not complete the study were not included. Participants gave written informed consent, and study procedures were approved by institutional review boards. Participants reported that they were not current smokers, not pregnant, and did not maintain habitual sleep durations of <7 h or >9.25 h. Participants were deemed healthy by a physician-administered physical examination, psychological tests, and psychological interview with a trained clinician, blood chemistries (complete blood cell count and comprehensive metabolic panel), clinical electrocardiogram, and medication-free status. Participants reported no regular night work in the preceding year or crossing more than 1 time zone in the previous 3 weeks. Health screenings were conducted at the Sleep and Chronobiology Laboratory and at the Clinical Translational Research Center (CTRC) at the University of Colorado Boulder.
Ambulatory Monitoring
For 1 week prior to study admission, participants maintained a self-selected sleep schedule of ~8 h per night. The sleep schedule was verified with sleep-wakefulness logs, call-in bed and wake times to a time-stamped voice mailbox recorder, and wrist activity with light exposure recordings (Actiwatch-L, Mini Mitter Respironics, Bend, OR). Drug use (including caffeine and nicotine) was proscribed for 1 week prior to in-laboratory testing, alcohol 3 days prior to, and all drugs throughout the remainder of the protocol. Drug use was determined by self-report and verified by urine toxicology at screening and by urine toxicology and a breath alcohol test upon laboratory admission.
In-laboratory Protocol
Participants lived in the CTRC at the University of Colorado Hospital for ~6 days under a constant posture protocol, because energy expenditure controlling for posture and activity was the primary focus of the study (McHill et al., 2014a). At admittance, participants were given cognitive performance batteries for practice to control for/remove the steep portion of the learning curve (Wertz et al., 2006) and were instructed that speed and accuracy were equally important in completing tasks. Participants were exposed to each task until achieving ≥90% on accuracy measures. Polysomnography (PSG) was used to record an 8-h baseline nighttime sleep opportunity at the participants’ habitual bedtime, as determined from the ambulatory monitoring week, and to verify that they were free from sleep disorders. Light levels upon admittance to the hospital on day 1 were <40 lux. Beginning on day 2 (daytime shift), participants were studied in a constant posture protocol with ambient dim lighting (<1 lux in the angle of gaze, <5 maximum) throughout scheduled wakefulness and 0 lux during scheduled sleep. Participants remained in a semirecumbent seated posture in a hospital bed with their head raised to ~35° during wakefulness and lowered to a supine position during scheduled sleep opportunities. Temperature was maintained at 22 °C to 24 °C. Day 2 consisted of 16 h of daytime wakefulness and an 8-h nighttime sleep opportunity at the habitual time. The transition to the first night shift consisted of wakefulness at habitual wake time and a 2-h afternoon nap opportunity 6 h prior to the first night shift, a schedule common in many shift workers (Härmä et al., 1989; Åkerstedt and Torsvall, 1985). After the first night shift, participants were provided an 8-h daytime sleep opportunity that began 1 h after their habitual baseline wake time. The next 2 study days consisted of 2 days of night-shift work and daytime sleep. Scheduled wakefulness and participant compliance during the constant posture routine were verified via continuous monitoring by research staff and electroencephalography recordings. Detailed sleep, circadian phase, appetitive hormones, and energy expenditure data from this study have been reported previously (McHill et al., 2014a).
PSG Recordings
PSG recordings were obtained with Siesta digital sleep recorders (Compumedics USA Ltd, Charlotte, NC) from C3-A2, C4-A1, O1-A2, F3-A2, right and left electro-oculogram, chin electromyogram, and electrocardiogram. Sleep was manually scored in 30-s epochs from brain region C3-A2 according to standard guidelines (Rechtschaffen and Kales, 1968). If the C3-A2 trace contained an artifact, the C4-A1 trace was used to determine the sleep stage.
Performance Battery
Participants completed a battery of performance tests and questionnaires, which lasted ~35 min, every 2 h during wakefulness. The first test battery was given at 2 h into scheduled wakefulness and the last 2 h prior to scheduled sleep (Fig. 1). The hours into the working shift from 0 to 8 h were equivalent to hours awake of 2 to 10 h for the day shift and 7 to 15 h for the night shifts, not including the wakefulness prior to the nap opportunity for night shift 1. Cognitive performance tasks were selected for their sensitivity to sleepiness and so that we could assess brain functions that are critical for work performance, decision making, and public safety. The computerized performance battery consisted of the Karolinska Sleepiness Scale (KSS), visual analog scales (VAS), Psychomotor Vigilance Task (PVT), Stroop color word test (STROOP; 200 stimuli [120 for congruent, 40 incongruent, 40 neutral]), calculation addition performance task (ADD), and a conjunction visual search task (CONJ search; Burke et al., 2015). We included multiple cognitive outcomes because factor analyses show that different tasks provide unique information on cognitive impairments during sleep loss (i.e., subjects may show impairment on some tasks but not on others; Frey et al., 2004; Drummond et al., 2006) and circadian misalignment (Chellappa et al., 2018). The KSS is a questionnaire that assesses subjective sleepiness by asking the subject to rate on a scale of 1 to 9 how alert or sleepy they feel at the moment of the test (Åkerstedt and Gillberg, 1990). The VAS assess clear-headedness by prompting the subject to identify on a 100-mm horizontal line how they feel at that moment, with each end of the line labeled with the extremes of a subjective continuum (e.g., words or phrases such as very clear-headed and very groggy; McHill et al., 2014b). The PVT is a 10-min reaction time test that assesses sustained vigilance by presenting a millisecond number counter stimulus and determining the reaction time with a button press (Dinges and Powell, 1985). Our primary outcome for performance was median reaction time and the number of lapses on the PVT, 2 highly sensitive measures to sleep loss (Lim and Dinges, 2008). The STROOP color word test assesses accuracy, cognitive speed, and the inhibitory control component of executive function. This task requires participants to respond by a button press to indicate the color of the text stimulus presented on the computer screen. The text is colored such that the word is either the same as the color (e.g., the word blue is colored blue, congruent) or not (e.g., the word blue is colored green, incongruent) or nonwords (colored XXXX, neutral; Burke et al., 2015). The ADD task assesses the accuracy and speed of mathematical calculations (Wertz et al., 2006) by presenting the subject with randomly generated pairs of 2-digit numbers (e.g., 15+26). The CONJ search task assesses selective attention by requiring subjects to identify whether a target is present (e.g., a red vertical bar) among a field of distracters (e.g., horizontal red and green bars; Treisman and Sato, 1990; Wolfe, 1994; Burke et al., 2015). The task is scored as the number of correctly identified targets (target correctly identified as present) and blanks (target correctly identified as absent; Burke et al., 2015).
Figure 1.

Study protocol. Clock hour is plotted as the relative time of day with scheduled waking arbitrarily assigned a time of 0800 h and all events referenced to this time. Open bars indicate wakefulness in room lighting (<40 lux), black bars scheduled polysomnography-recorded sleep opportunities, and gray bars scheduled wakefulness in dim lighting (<1 lux). T represents the timing of the ~35-min test battery.
Data Analysis
Performance measures were aligned such that data were compared across an 8-h “workday” during simulated daytime and nighttime shift work (Fig. 1). Thus, the first 5 out of 7 performance batteries during the day shift (relative timing 0900-1700 h for an individual with a habitual wake time of 0800 h) and the last 5 out of 7 performance batteries on night shifts 1 to 3 (relative timing 2300-0700 h) were analyzed.
The PVT was statistically analyzed for the reciprocal of the median reaction time, reciprocal of the 10% slowest reaction time, the 10% fastest reaction time, and lapses in attention (reaction time > 500 ms; Lim and Dinges, 2008; Jung et al., 2011). The STROOP color word task was analyzed as the overall number correct for congruent presentations, incongruent presentations, and neutral presentations (Burke et al., 2015). The median correct reaction time for the STROOP congruent, incongruent, neutral, and both incongruent minus congruent (Burke et al., 2015) and incongruent minus neutral (Cain et al., 2011; measures of inhibitory control) presentations, the number of problems attempted and answered correctly during the 4-min ADD task, and the number of correct responses divided by the reaction time during the CONJ search task were analyzed as the difference from the start of the work shift (performance at 2 h into wakefulness) to account for observed improvements on night shifts 1 and 3 compared with the day shift. These improvements potentially indicate evidence of continued learning of tasks during acute night-shift work.
Sleep data were analyzed for sleep opportunities after the day shift (habitual bedtime baseline) and prior to night shifts 2 and 3 (daytime sleep; McHill et al., 2014a). Sleep efficiency was binned per hour of each sleep opportunity. The average performance over the work shift and sleep efficiency during the sleep opportunities were analyzed using mixed model analysis of variance with work shift and time as fixed factors and subject as a random factor using STATISTICA, version 10.0 (StatSoft Inc, Tulsa, OK). Planned comparisons were conducted for work shift averages and for each 2-h test and sleep efficiency comparisons using dependent t tests. To correct for multiple planned comparisons and to reduce type 1 error, modified Bonferroni correction factors were used on all planned comparisons (Keppel, 1991). Based on the modified Bonferroni, p < 0.025 was required for significance for comparisons across study days, and p < 0.02 was required for significance for comparisons at each hour awake.
RESULTS
Sleep
Figure 2 shows that sleep efficiency was significantly higher at the beginning and lower at the end of the daytime sleep opportunity as compared with nighttime sleep (interaction between sleep opportunity and hours into sleep opportunity; p < 0.0001). Planned comparisons revealed significantly higher sleep efficiency for the first 3 h of the day versus nighttime sleep opportunity and lower sleep efficiency for the last 2 h of the day versus nighttime sleep opportunity (Fig. 2; p < 0.01). Participants had an average sleep efficiency of 81.2% ± 0.05% during the 2-h daytime nap opportunity on the transition to the first night shift, equating to 1.6 ± 0.1 h out of the 2-h nap.
Figure 2.

Hourly sleep efficiency during the baseline night and daytime sleep opportunities. The gray line represents nighttime sleep, the black solid line the sleep opportunity prior to night shift 2, and the dashed line the sleep opportunity prior to night shift 3. # indicates differences between nighttime sleep and sleep prior to night shift 2 (p < 0.01) and ¥ between nighttime sleep and sleep prior to night shift 3 (p < 0.01). Error bars are SEM.
Subjective Sleepiness and Clear-headedness
Participants reported higher sleepiness during simulated night-shift work (main effect for work shift; Fig. 3A, B; p < 0.0001) with significant interactions between work shift and hours into the work shift (Fig. 3A, B; p < 0.0001). Specifically, higher average sleepiness ratings were observed on all night shifts versus the day shift (Fig. 3A; p < 0.025), and there was a nonsignificant comparison for higher sleepiness ratings on night shift 1 versus night shift 3 (p = 0.04). Higher sleepiness on night shifts versus the day shift was observed for hours 4 to 8 of the work shift and on night shift 1 versus night shift 2 and night shift 3 at hour 8 of the work shift (Fig. 3B; p < 0.02).
Figure 3.

Karolinska Sleepiness Scale scores and clear-headedness. The left panels show the average values for each work shift. Solid lines represent significant differences at the end of each line (p < 0.025). The right panel shows performance every 2 h across the work shift. * indicates differences between day shift and night shift 1, # between day shift and night shift 2, ¥ between day shift and night shift 3, † between night shift 1 and night shift 2, and ‡ between night shift 1 and night shift 3 (all p < 0.02). Error bars are SEM.
Clear-headedness ratings were lower during night-shift work versus the day shift (main effect for work shift; p < 0.001), with significant interactions for work shift and hours into the work shift (Fig. 3C, D; p < 0.0001). Figure 3C shows that average clear-headedness ratings were lower on all night shifts versus the day shift (Fig. 3C; p < 0.025). In addition, there were nonsignificant comparisons for lower clear-headedness ratings on night shift 1 as compared with night shift 2 (p = 0.047) and night shift 3 (p = 0.03). Lower clear-headedness on the night shifts versus the day shift was observed for hours 6 to 8 of the work shift and on night shift 1 versus night shift 3 at hour 0 and on night shift 1 versus the day shift at hour 4 (Fig. 3D; p < 0.02).
PVT
Figure 4 shows decreased performance for all PVT outcomes during simulated night-shift work (main effect for work shift; p < 0.0001), with significant interactions for work shift and hours into the work shift (p < 0.01). Planned comparisons revealed significantly slower average median reaction time, slowest 10% reaction time, and fastest 10% reaction time (Fig. 4A, C, E; p < 0.025) and significantly increased lapses of attention (Fig. 4G; p < 0.001) on most night shifts as compared with the day shift. There were nonsignificant comparisons for increased slowest 10% reaction time between the day shift and night shift 3 (p = 0.048) and for increased lapses of attention between the day shift and night shifts 2 (p = 0.03) and 3 (p = 0.046). Significant decreases in PVT performance were observed on night shifts for hours 4 to 8 of the work shift for most PVT measures (Fig. 4B, D, F, H; p < 0.02) compared with the day shift. Furthermore, the median reaction time performance was worse at the beginning of night shift 2 as compared with the day shift (Fig. 4B; p < 0.02), and reaction time measures were worse on night shift 1 versus night shift 2 for hours 6 or 8 of the work shift (Fig. 4B, D, F; p < 0.02).
Figure 4.

Psychomotor vigilance task reciprocal median reaction time, reciprocal slowest reaction time, mean fastest reaction time, and mean lapses. The left panel shows the average performance for each work shift. Solid lines represent significant (p < 0.025) differences at the end of each line. The right panel shows performance every 2 h across the work shift. * indicates differences between day shift and night shift 1, # between day shift and night shift 2, ¥ between day shift and night shift 3, and † between night shift 1 and night shift 2 (all p < 0.02). Error bars are SEM.
STROOP Color Word Task
The average number of correct responses for congruent, neutral, and incongruent stimuli was significantly decreased during simulated night-shift work (main effect for work shift; p < 0.025). Specifically, a decreased number of average correct responses on the STROOP test was observed on night shifts 1 and 3 versus the day shift for the number of congruent and neutral correct and on night shifts 1 and 2 for incongruent correct (Fig. 5A–F; p < 0.025). In addition, the average number of neutral correct responses was decreased for night shift 1 versus night shift 3 (Fig. 5E; p < 0.025). There were nonsignificant comparisons for decreased congruent correct and neutral correct between the day shift and night shift 2 (p = 0.03) and for incongruent correct between the day shift and night shift 3 (p = 0.03). The hours of the work shift during which the numbers of correct responses were significantly decreased on the night shift versus day shift and between night shifts were inconsistent. Generally, a higher number of correct responses was observed on the day shift as compared with night shifts. In addition, a higher number of correct congruent responses was observed on night shift 3 versus night shift 1 at hour 8, a higher number of correct incongruent responses was observed on night shift 1 versus night shifts 2 and 3 at hour 0, and a lower number of correct neutral responses was observed on night shift 1 versus night shifts 2 and 3 at hour 4 (Fig. 5B, D, F; p < 0.02).
Figure 5.

STROOP color word task congruent, incongruent, and neutral correct responses. The left panel shows the average number of correct responses for each work shift. Solid lines represent significant differences and for points at the end of each line (p < 0.01). The right panel shows performance every 2 h across the work shift. * indicates differences between day shift and night shift 1, # between day shift and night shift 2, ¥ between day shift and night shift 3, † between night shift 1 and night shift 2, and ‡ between night shift 1 and night shift 3 (all p < 0.02). Error bars are SEM.
Figure 6 shows a slower median reaction time, presented as a difference from baseline reaction time, for STROOP stimuli during night-shift work (main effect for work shift; p < 0.025), with significant interactions for work shift and hours into the work shift for congruent correct median reaction time (p < 0.001). Planned comparisons revealed significantly slower average congruent, incongruent, and neutral correct median reaction time on most night shifts versus the day shift (Fig. 6A, C, E; p < 0.001), with a nonsignificant comparison for slower incongruent reaction time on night shift 2 versus the day shift (p = 0.03). A significantly slower congruent correct median reaction time was observed on all night shifts for hours 2 to 6 of the work shift and hour 8 for night shift 1 (Fig. 6B; p < 0.02) versus the day shift. The incongruent correct median reaction time was slower for hours 2 and 4 on night shift 2 and for hour 4 on night shift 1 versus the day shift (Fig. 6D; p < 0.02). A significantly slower neutral correct median reaction time was observed on night shift 2 for hour 6 versus the day shift (Fig. 6F; p < 0.02).
Figure 6.

Difference in STROOP congruent correct, incongruent correct, neutral correct, and inhibitory control median reaction times from the start of the shift. The left panel shows the average performance for each work shift. Solid lines represent significant (p < 0.025) differences at the end of each line. The right panel shows performance every 2 h across the work shift. * indicates differences between day shift and night shift 1, # between day shift and night shift 2, ¥ between day shift and night shift 3, and ‡ between night shift 1 and night shift 3 (all p < 0.02). Error bars are SEM.
The inhibitory control reaction time measured using the incongruent-congruent median reaction time was not significantly different between study days but was significantly slower on night shift 3 versus night shift 1 at hour 2 of the work shift (p < 0.02). The inhibitory control reaction time measured using the incongruent-neutral median reaction time was not significantly different between study days (Fig. 6G; p > 0.025). There was also no significant difference in inhibitory control reaction time between hours into the work shift (Fig. 6H; p > 0.02).
ADD and Visual Search Tasks
The number of calculations attempted and answered correctly on the ADD task was significantly higher on the day shift versus night shift 1 (Fig. 7A–D; significant main effects of work shift, p < 0.025). Specifically, the average number attempted and correct was higher on the day shift versus night shift 1 (Fig. 7A, C; p < 0.025), and there was a nonsignificant comparison for higher number attempted on the day shift versus night shift 2 (p = 0.04). Significant decreases in ADD task performance were observed on night shift 1 at hours 2, 4, and 8 for number attempted and correct versus the day shift (Fig. 7B, D; p < 0.02). CONJ throughput, as measured by the number of correct answers divided by reaction time, did not have a significant main effect for work shift (p = 0.31) but did have a nonsignificant comparison for an interaction effect between work shift and hours into the work shift (Fig. 7E, F; p = 0.026). In planned comparisons, there was no difference in CONJ throughput for the day shift versus any of the night shifts (Fig. 7E; all p > 0.025); however, the throughput was decreased at hour 8 of night shift 1 versus the day shift (Fig. 7F; p < 0.02).
Figure 7.
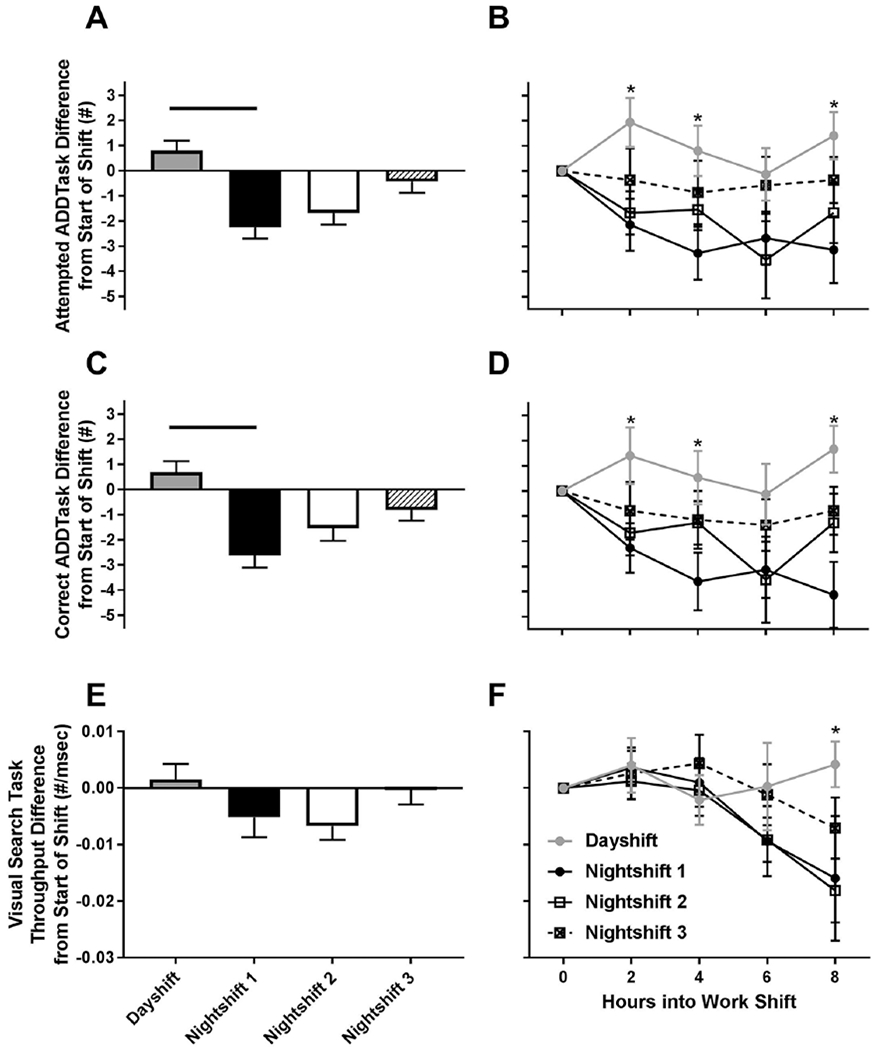
Average and hourly calculation addition performance and conjunction visual search task difference from start of shift across circadian misalignment. The left panel shows the average performance for each work shift. Solid lines represent significant (p < 0.025) differences at the end of each line. The right panel shows performance every 2 h across the work shift. * indicates differences between day shift and night shift 1 (p < 0.02). Error bars are SEM.
DISCUSSION
Findings from the current study further our knowledge about how sleepiness and cognitive performance change across the transition to and during subsequent simulated night shifts. We found that daytime sleep efficiency was increased in the first 3 h but decreased in the last 2 h of the sleep opportunity and that working at night increased sleepiness, decreased subjective clear-headedness, slowed reaction time and increased lapses of attention on the PVT, decreased the number of correct responses and correct reaction times on the STROOP task, and decreased the number of attempted and correct mathematical additions on the ADD task when compared with a day shift. In general, performance was relatively stable across the 8-h day shift, whereas performance worsened across the 8-h night shift. The decrements of performance during simulated night-shift work could contribute to impaired decision making and increased risk of accidents and injury when work operations occur at night (Gold et al., 1992; Barger et al., 2005; Smith et al., 1994).
Consistent with prior findings, we found that sleep was disturbed during daytime sleep opportunities (Wright et al., 2013; Akerstedt and Wright, 2009). Further, we found that sleep efficiency was lower near the end of the daytime sleep opportunities of the simulated shift work schedule yet was higher at the beginning of the daytime opportunity relative to baseline nighttime sleep. As the magnitude of reduced sleep efficiency later in the sleep opportunity was greater than the increased sleep efficiency at the beginning of the sleep opportunity, the net result was a reduction in total sleep time of about 1 h compared with nighttime sleep. The decreased sleep efficiency in the latter portion of the daytime sleep opportunities is likely a consequence of the decrease in sleep pressure during sleep and the circadian promotion of arousal at this circadian time (Dijk and Czeisler, 1994; Dijk et al., 1992), thereby making it difficult to maintain sleep. Reduced sleep efficiency during daytime sleep in shift workers may lead to accumulated sleep debt over successive nights of shift work and negatively affect performance concurrently with circadian misalignment (Tilley et al., 1982). The increased sleep efficiency at the beginning of the daytime sleep opportunities provides evidence for higher sleep pressure during the shift work schedule. Yet the increased sleep pressure appears to be insufficient to override the circadian arousal signal later in the daytime sleep opportunity.
Concurrent with sleep disruption, sleepiness is a common complaint among individuals working night shifts, particularly during extended wakefulness (Akerstedt and Wright, 2009). The higher subjective sleepiness scores and decreased clear-headedness observed in the present study during the night shift are in agreement with increased sleepiness as a primary consequence of shift work (Akerstedt, 1988; Axelsson et al., 2004). At the end of the first and second night shift, KSS scores were on average greater than 7, a score that is associated with physiological signs of sleepiness and increasing likelihood of unwanted transitions to sleep (Akerstedt and Wright, 2009). However, KSS scores were found to slightly decrease and clear-headedness increase on night shift 3 as compared with night shift 1, which may indicate some adaption to these subjective experiences. These ratings did not return to baseline levels, and as noted, performance on most tasks was worse/decreased on the night shift versus day shift with little evidence of adaptation across subsequent night shifts. Although subjective experiences did not return to baseline levels, any disconnect between subjective levels of arousal and objective levels of performance could have serious consequences for safety during the end of a night shift or during other attention-requiring activities (i.e., driving). However, because the participants in the current study were not blind to the study length or time cues, and because we did not have a day-shift control condition, an end-of-study effect on improving any of the observed improvements on the last day of the study cannot be ruled out. Further, we did not have a no-nap control condition to determine the effectiveness of a nap to improve performance on the transition day to the first night shift, although findings from studies that did not include naps show that performance on the first night shift is worse than subsequent night shifts (Santhi et al., 2007; Lamond et al., 2004).
As noted, our primary outcome for performance was median reaction time and the number of lapses on the PVT. The number of lapses nearly doubled on night shifts versus the day shift, and the average lapse number on the night shift were comparable with that seen during days of insufficient sleep (e.g., 3-6 h nightly sleep opportunities [Van Dongen et al., 2003; Belenky et al., 2003; McHill et al., 2018] or 1 night of total sleep deprivation [Akerstedt and Wright, 2009]). We also examined the 10% slowest and 10% fastest reaction times to examine how the subject’s performance changed in the lapse domain and optimum response time domain, respectively. Compared with the day shift, PVT reaction time measures were slower and worsened across the night shift. These findings show that attention is decreased and worsens across the work shift, not only on night shift 1 with extended wakefulness but also on subsequent night shifts. The worsening of attention performance across the night shift is consistent with the circadian rhythm of performance, with the worst performance in the early morning hours (Dijk et al., 1992; Frey et al., 2004; Burke et al., 2015). Contrary to our hypothesis, our primary outcomes of PVT performance did not differ significantly across night shift 1 versus subsequent night shifts. PVT performance improvements have been found across successive simulated night shifts after the first night shift in other laboratory studies. For example, Santhi et al. (2007) found that PVT performance, specifically lapses, improved when averaging 2 subsequent nights of shift work in laboratory settings, and Lamond et al. (2004) observed improved PVT performance across 1 week of simulated overnight shift work. Our findings may differ from previous laboratory studies for several reasons. These prior studies simulating the first night of shift work did not allow for a daytime sleep opportunity before the first night shift, whereas the current study included an afternoon nap before night shift 1, a schedule common in shift work (Härmä et al., 1989; Åkerstedt and Torsvall, 1985; Rosa, 1993). The nap opportunity may have attenuated performance decrements observed on night shift 1, and thus performance was more similar to subsequent shifts, as seen in other laboratory studies incorporating naps prior to a simulated night shift/sleep deprivation (Dinges et al., 1987; Schweitzer et al., 2006; Bonnet, 1991). This attenuation in performance, however, may be limited as reaction times became slower at the end of night shift 1 as compared with the other night shifts. Further, we compared performance only during hours of the work shift so as to capture differences that would occur when attention would be necessary while on the job. Interestingly, chronic shift workers exposed to a similar in-laboratory circadian misalignment protocol as our current study displayed comparable performance decrements, particularly 11 h after awakening (Chellappa et al., 2019). It should also be noted that we studied participants in a constant posture protocol to eliminate any potential confound of activity during the protocol. This lack of movement across the protocol may have influenced our results and not account for potential strategies used by individuals during the night to improve alertness or performance. Future work should combine a prophylactic nap strategy in combination with activity to simulate more real-world changes in performance across nights of circadian misalignment.
The STROOP color word task was used to assess performance accuracy, speed, and the inhibitory control component of executive function. We found that the number of correct responses decreased and reaction time during the STROOP increased during circadian misalignment, regardless of the type of stimulus presented (e.g., congruent, incongruent, or neutral). The slower reaction times to the 3 stimuli presented (e.g., congruent, incongruent, or neutral) are similar to previous findings of slower Stroop performance during extended wakefulness (Cain et al., 2011; Bratzke et al., 2009); however, no difference in inhibitory control during night-shift work is inconsistent with previous findings of a strong circadian modulation of inhibitory control with slowest reaction times during the circadian night (Burke et al., 2015). Our observations of decreases in correct responses on most nights of the study as compared with baseline, combined with the concurrent increases in median reaction time during the night shift, may further highlight mechanisms by which errors can increase during shift work.
Performance on the ADD mathematical calculation task was also impaired during night-shift work as compared with the day shift, with a slight but nonsignificant improvement across successive night shifts. This lack of significant improvement in ADD performance is in agreement with prior findings that indicated mathematical performance worsened or did not improve during total sleep deprivation and during weeks of chronic circadian misalignment (Johnson et al., 1992; Wright et al., 2006). As noted previously, participants were given frequent practice tests to eliminate steep portions of the learning curve. All participants achieved ≥90% on all tasks prior to data collection. It is possible that the learning of the ADD task continued across our acute circadian misalignment protocol, as previous work has shown that multiple days of circadian misalignment after fully learning the task during circadian alignment worsen ADD performance (Chellappa et al., 2018). In contrast to our current findings, Santhi et al. (2007) observed decreased CONJ visual search accuracy performance during night-shift work but found the worst performance on the first night of simulated shift work. We did not observe statistical differences in CONJ visual search throughput performance, a measure of accuracy, between the day shift and night shifts or across night shifts, potentially because of the daytime nap prior to the first shift. Further research directly comparing consecutive days of night-shift work with and without a daytime nap and other countermeasure strategies is therefore needed to better understand the influence of circadian misalignment on visual search performance.
Understanding the time course of performance decrements across subsequent days of night-shift work is vital in the development of evidence-based countermeasure strategies. Our findings contribute to the existing knowledge and further demonstrate that working at adverse circadian times, regardless of whether it is the first night shift with a nap and extended wakefulness or subsequent night shifts, results in decreased performance and mood as compared to working during the day. Contrary to other simulated laboratory night-shift work protocols (Lamond et al., 2004; Santhi et al., 2007) and some field studies (Dula et al., 2001; Folkard and Tucker, 2003), we observed little evidence of large changes in performance across subsequent nights of shift work. When differences were observed in those previous studies, the first night shift was generally worse than subsequent night shifts (Dula et al., 2001; Lamond et al., 2004; Santhi et al., 2007). Thus, our results are consistent with findings that a nap prior to the first night shift helps to attenuate performance decrements of extended wakefulness (Dinges et al., 1987; Härmä et al., 1989), but further countermeasures such as combining wakefulness and sleep-promoting strategies (e.g., caffeine and bright light [Wright et al., 1997], caffeine and naps [Schweitzer et al., 2006], or bright light and evening sleep [Chinoy et al., 2016]), naps and wakefulness-promoting medications (Batejat and Lagarde, 1999), or prophylactic naps with daytime anchor sleep may be necessary to better combat fatigue on subsequent night shifts (Mollicone et al., 2008).
ACKNOWLEDGMENTS
The authors would like to thank the participants, Clinical Translational Research Center staff, and Sleep and Chronobiology Laboratory staff as well as T. Moehlman, E. Stothard, B. Birks, B. Smith, B. Brainard, B. Griffin, T. Dear, E. Chinoy, and G. Wright for study assistance. This work was supported by the National Institutes of Health grants R21 DK092624 (K.P.W.), R01 HL109706 (K.P.W.), KL2 TR002370 (A.W.M.), F32 DK107146 (A.W.M.), and T32 HL007901 (A.W.M.), National Institutes of Health (NIH)/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award grant UL1 TR002535. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. These funding sources had no involvement in study design, collection, analysis, interpretation, writing of study results, or decision to submit for publication.
CONFLICT OF INTEREST STATEMENT
A.W.M. reports speaker honorarium or travel reimbursement fees from the Utah Sleep Research Society and the California Precast Concrete Association; K.P.W. received funding from the NIH, Office of Naval Research, Philips Inc., Torvec Inc; consulting fees from or served as a paid member of scientific advisory boards for NIH, CurAegis Inc. Circadian Therapeutics, Kellogg; and speaker honorarium or travel reimbursement fees from the American Academy of Sleep Medicine, American College of CHEST Physicians, American College of Sports Medicine, American Diabetes Association, Associated Professional Sleep Societies, Daylight Academy, Illuminating Engineering Society, and the Sleep Research Society.
REFERENCES
- Akerstedt T (1988) Sleepiness as a consequence of shift work. Sleep 11:17–34. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T and Gillberg M (1990) Subjective and objective sleepiness in the active individual. Int J Neurosci 52:29–37. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T and Torsvall L (1985) Napping in shift work. Sleep 8:105–109. [DOI] [PubMed] [Google Scholar]
- Akerstedt T and Wright KP (2009) Sleep loss and fatigue in shift work and shift work disorder. Sleep Med Clin 4:257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson J, Akerstedt T, Kecklund G, and Lowden A (2004) Tolerance to shift work—how does it relate to sleep and wakefulness? Int Arch Occup Environ Health 77:121–129. [DOI] [PubMed] [Google Scholar]
- Barger LK, Cade BE, Ayas NT, Cronin JW, Rosner B, Speizer FE, and Czeisler CA; Harvard Work Hours, Health, and Safety Group (2005) Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med 352:125–134. [DOI] [PubMed] [Google Scholar]
- Batejat DM and Lagarde DP (1999) Naps and modafinil as countermeasures for the effects of sleep deprivation on cognitive performance. Aviat Space Environ Med 70:493–498. [PubMed] [Google Scholar]
- Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, and Balkin TJ (2003) Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 12:1–12. [DOI] [PubMed] [Google Scholar]
- Bonnet MH (1991) The effect of varying prophylactic naps on performance, alertness and mood throughout a 52-hour continuous operation. Sleep 14:307–315. [DOI] [PubMed] [Google Scholar]
- Borbély AA and Achermann P (1992) Concepts and models of sleep regulation: an overview. J Sleep Res 1:63–79. [DOI] [PubMed] [Google Scholar]
- Bratzke D, Steinborn MB, Rolke B, and Ulrich R (2009) Effects of sleep loss and circadian rhythm on executive inhibitory control in the Stroop and Simon tasks. Chronobiol Int 29:55–61. [DOI] [PubMed] [Google Scholar]
- Burke TM, Markwald RR, Chinoy ED, Snider JA, Bessman SC, Jung CM, and Wright KP Jr (2013) Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep 36:1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke TM, Scheer FA, Ronda JM, Czeisler CA, and Wright KP Jr (2015) Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J Sleep Res 24:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SW, Silva EJ, Chang A-M, Ronda JM, and Duffy JF (2011) One night of sleep deprivation affects reaction time, but not interference or facilitation in a Stroop task. Brain Cogn 76:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Wu YH, Chen HL, and Hsu CY (2017) Four night shifts have a degree of performance adaptation. Hum Factors 59:925–936. [DOI] [PubMed] [Google Scholar]
- Chellappa SL, Morris CJ, and Scheer F (2018) Daily circadian misalignment impairs human cognitive performance task-dependently. Sci Rep 8:3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL, Morris CJ, and Scheer F (2019) Effects of circadian misalignment on cognition in chronic shift workers. Sci Rep 9:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinoy ED, Harris MP, Kim MJ, Wang W, and Duffy JF (2016) Scheduled evening sleep and enhanced lighting improve adaptation to night shift work in older adults. Occup Environ Med 73:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk D-J and Czeisler CA (1994) Paradoxical timing of the circadian rhythm of sleep propensity serves to console-date sleep and wakefulness in humans. Neurosci Lett 166:63–68. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, and Czeisler CA (1992) Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res 1:112–117. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Orne MT, Whitehouse WG, and Orne EC (1987) Temporal placement of a nap for alertness: contribu-tions of circadian phase and prior wakefulness. Sleep 10:313–329. [PubMed] [Google Scholar]
- Dinges DF and Powell JW (1985) Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput 17:652–655. [Google Scholar]
- Drummond SP, Paulus MP, and Tapert SF (2006) Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res 15:261–265. [DOI] [PubMed] [Google Scholar]
- Dula DJ, Dula NL, Hamrick C, and Wood GC (2001) The effect of working serial night shifts on the cognitive functioning of emergency physicians. Ann Emerg Med 38:152–155. [DOI] [PubMed] [Google Scholar]
- Folkard S (1992) Is there a ‘best compromise’ shift system? Ergonomics 35:1453–1463. [DOI] [PubMed] [Google Scholar]
- Folkard S (2008) Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int 25:215–224. [DOI] [PubMed] [Google Scholar]
- Folkard S and Lombardi DA (2006) Modeling the impact of the components of long work hours on injuries and “accidents.” Am J Ind Med 49:953–963. [DOI] [PubMed] [Google Scholar]
- Folkard S and Tucker P (2003) Shift work, safety and productivity. Occup Med 53:95–101. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Badia P, and Wright KP (2004) Inter-and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res 13:305–315. [DOI] [PubMed] [Google Scholar]
- Gold DR, Rogacz S, Bock N, Tosteson TD, Baum TM, Speizer FE, and Czeisler CA (1992) Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am J Public Health 82:1011–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumenyuk V, Roth T, and Drake CL (2012) Circadian phase, sleepiness, and light exposure assessment in night workers with and without shift work disorder. Chronobiol Int 29:928–936. [DOI] [PubMed] [Google Scholar]
- Härmä M, Knauth P, and Ilmarinen J (1989) Daytime napping and its effects on alertness and short-term memory performance in shiftworkers. Int Arch Occup Environ Health 61:341–345. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, and Czeisler CA (1992) Short-term memory, alertness and performance: a reappraisal of their relationship to body temperature. J Sleep Res 1:24–29. [DOI] [PubMed] [Google Scholar]
- Jung CM, Ronda JM, and Czeisler CA (2011) Comparison of sustained attention assessed by auditory and visual psychomotor vigilance tasks prior to and during sleep deprivation. J Sleep Res 20:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G (1991) Design and Analysis: A Researcher’s Handbook. Upper Saddle River (NJ): Prentice-Hall. [Google Scholar]
- Lamond N, Dorrian J, Burgess HJ, Holmes A, Roach G, McCulloch K, Fletcher A, and Dawson D (2004) Adaptation of performance during a week of simulated night work. Ergonomics 47:154–165. [DOI] [PubMed] [Google Scholar]
- Leff DR, Aggarwal R, Rana M, Nakhjavani B, Purkayastha S, Khullar V, and Darzi AW (2008) Laparoscopic skills suffer on the first shift of sequential night shifts: program directors beware and residents prepare. Ann Surg 247:530–539. [DOI] [PubMed] [Google Scholar]
- Lim J and Dinges DF (2008) Sleep deprivation and vigilant attention. Ann N Y Acad Sci 1129:305–322. [DOI] [PubMed] [Google Scholar]
- Macchi MM, Boulos Z, Ranney T, Simmons L, and Campbell SS (2002) Effects of an afternoon nap on nighttime alertness and performance in long-haul drivers. Accid Anal Prev 34:825–834. [DOI] [PubMed] [Google Scholar]
- McHill A, Melanson E, Higgins J, Connick E, Moehlman TM, Stothard ER, and Wright KP Jr (2014a) Impact of circadian misalignment on energy metabolism during simulated shiftwork. Proc Natl Acad Sci U S A 111:17302–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHill AW, Hull JT, Wang W, Czeisler CA, and Klerman EB (2018) Chronic sleep curtailment, even without extended (>16-h) wakefulness, degrades human vigilance performance. Proc Natl Acad Sci USA 115:6070–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHill AW, Smith BJ, and Wright KP (2014b) Effects of caffeine on skin and core temperatures, alertness, and recovery sleep during circadian misalignment. J Biol Rhythms 29:131–143. [DOI] [PubMed] [Google Scholar]
- Mollicone DJ, Van Dongen HP, Rogers NL, and Dinges DF (2008) Response surface mapping of neurobehavioral performance: testing the feasibility of split sleep schedules for space operations. Acta Astronaut 63:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell MT, Feyer AM, and Herbison GP (2002) The impact of a nap opportunity during the night shift on the performance and alertness of 12-h shift workers. J Sleep Res 11:219–227. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A and Kales AE (1968) A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Information Service, Brain Research Institute; 10. [Google Scholar]
- Rosa RR (1993) Napping at home and alertness on the job in rotating shift workers. Sleep 16:727–735. [DOI] [PubMed] [Google Scholar]
- Santhi N, Horowitz TS, Duffy JF, and Czeisler CA (2007) Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS One 2:e1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer PK, Randazzo AC, Stone K, Erman M, and Walsh JK (2006) Laboratory and field studies of naps and caffeine as practical countermeasures for sleep-wake problems associated with night work. Sleep 29:39–50. [DOI] [PubMed] [Google Scholar]
- Smith L, Folkard S, and Poole CJM (1994) Increased injuries on night shift. Lancet 344:1137–1139. [DOI] [PubMed] [Google Scholar]
- Tilley AJ, Wilkinson RT, Warren PS, Watson B, and Drud M (1982) The sleep and performance of shift workers. Hum Factors 24:629–641. [DOI] [PubMed] [Google Scholar]
- Torsvall L, Akerstedt T, Gillander K, and Knutsson A (1989) Sleep on the night shift: 24-hour EEG monitoring of spontaneous sleep/wake behavior. Psychophysiology 26:352–358. [DOI] [PubMed] [Google Scholar]
- Treisman A and Sato S (1990) Conjunction search revisited. J Exp Psychol Hum Percept Perform 16:459–478. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Maislin G, Mullington JM, and Dinges DF (2003) The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26: 117–129. [DOI] [PubMed] [Google Scholar]
- Wertz AT, Ronda JM, Czeisler CA, and Wright KP Jr (2006) Effects of sleep inertia on cognition. JAMA 295:159–164. [DOI] [PubMed] [Google Scholar]
- Wolfe JM (1994) Guided Search 2.0: a revised model of visual search. Psychon Bull Rev 1:202–238. [DOI] [PubMed] [Google Scholar]
- Wright KP, Badia P, Myers B, and Plenzler SC (1997) Combination of bright light and caffeine as a countermeasure for impaired alertness and performance during extended sleep deprivation. J Sleep Res 6:26–35. [DOI] [PubMed] [Google Scholar]
- Wright KP, Bogan RK, and Wyatt JK (2013) Shift work and the assessment and management of shift work disorder (SWD). Sleep Med Rev 17:41–54. [DOI] [PubMed] [Google Scholar]
- Wright KP, Hull JT, Hughes RJ, Ronda JM, and Czeisler CA (2006) Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci 18:508–521. [DOI] [PubMed] [Google Scholar]


