Abstract
Cartilage integration remains a clinical challenge for treatment of focal articular defects. Cartilage exhibits limited healing capacity that declines with tissue maturation. Many approaches have been investigated for their ability to stimulate healing of mature cartilage or integration of repair tissue or tissue-engineered constructs with native cartilage. Growth factors present in immature tissue may enhance chondrogenesis and promote integrative repair of cartilage defects. In this study, we assessed the role of one such factor, fibroblast growth factor 18 (FGF18). Studies using FGF18 have shown a variety of positive effects on cartilage, including stimulation of chondrocyte proliferation, matrix biosynthesis, and suppression of proteinase activity. To explore the role of FGF18 on cartilage defect repair, we hypothesized that treatment with recombinant human FGF18 (sprifermin) would increase matrix synthesis in a defect model, thus improving integration strength. To test this hypothesis, 6 mm cartilage cylinders were harvested from juvenile bovine knees. A central 3 mm defect was created in each explant, and this core was removed and replaced. Resulting constructs were cultured in control or sprifermin-containing medium (weekly 24-hour exposure of 100 ng/ml sprifermin) for 4 weeks. Mechanical testing, biochemical analysis, micro-CT, scanning electron microscopy, and histology were used to assess matrix production, adhesive strength, and structural properties of the cartilage-cartilage interface. Results showed greater adhesive strength, increased collagen content, and larger contact areas between core and annular cartilage in the sprifermin-treated group. These findings present a novel treatment for cartilage injuries that have potential to enhance defect healing and lateral cartilage-cartilage integration.
Keywords: FGF18, sprifermin, cartilage repair, explant model, cartilage integration
Introduction
Repair of focal chondral defects remains a major clinical challenge in orthopedics. Advanced focal defects are associated with decreased tibiofemoral cartilage volume, increased collagen type II breakdown, and osteophyte formation.1 It is commonly thought that these defects may ultimately progress to osteoarthritis, eventually requiring intervention and ultimately total knee arthroplasty.2 Non-physiologic mechanical stress on the rim surrounding the defect is likely to play a role in the progressive deterioration of adjacent cartilage.3,4 For example, a recent study by Sanders et al. found that at 16-year follow-up, patients that underwent excision of an osteochondritis dissecans lesion experienced significantly higher rates of osteoarthritis and joint arthroplasty than patients that had received lesion fixation or osteochondral grafts.5 These findings suggest a major role for altered cartilage mechanics in the pathogenesis of osteoarthritis (OA). These studies further suggest that stable implantation and integration of cartilage or a cartilage-like material can improve long term outcomes.
Arthritis is an ever-increasing clinical problem affecting the quality of life of an increasingly active and aging population. A recent report by the Centers for Disease Control and Prevention estimates that one in four adults in the United States are affected by some form of arthritis. The direct medical costs of arthritis exceed $81 billion annually in the U.S. and the number of individuals afflicted by this disease is expected to increase from 54 million to 78 million by 2040.6 This projection underscores the importance of developing reliable cartilage restoration strategies that can provide long-lasting repair and prevent progression to osteoarthritic disease.7
Commonly utilized interventional techniques for the repair of chondral defects include biologic approaches such as autologous chondrocyte implantation (ACI) and marrow-stimulating techniques such as microfracture. Large defects with osseous involvement may be treated with osteochondral grafting. While bone exhibits excellent healing at the host-graft interface, integration of reparative and healthy cartilage remains a clinical challenge for successful treatment of focal chondral defects.8 Current interventional techniques such as osteochondral autograft transfer (OATS) and mosaicplasty result in complete osseous integration with limited lateral integration of graft cartilage with host cartilage.9 Similarly, repair tissue produced by microfracture exhibits poor lateral integration with adjacent cartilage.10,11 Thus, integration of any implant material to the cartilage cut surfaces is limited, and most repair approaches show the same failure of lateral integration with the surrounding native cartilage.
Multiple studies have demonstrated that current techniques are inadequate for long-term defect repair. A systematic review of 28 clinical studies by Mithoefer et al., found that microfracture repair of chondral lesions results in functional improvement at 2 years, but noted that long-term functional results are quite variable.12 In one study by Solheim et al., long-term follow-up (10-14 years) of patients that had received microfracture for a focal chondral lesion in the patellofemoral joint revealed poor outcomes in 46% of patients (defined as a Lysholm score <64). Furthermore, 39% of the cohort required additional surgery following the original procedure, including debridement, additional microfracture, tibial osteotomy, or knee replacement.13 Long-term outcomes following first generation ACI appear slightly better than microfracture, with studies reporting high degrees of patient satisfaction at 10-20 years follow-up.14,15 The current iteration of ACI, matrix-associated autologous chondrocyte implantation (MACI), offers some improvements on the original technique, and has promising outcomes at 2-year follow-up.16 The early success of MACI is encouraging and highlights the importance of the continued development of new biomaterials and tissue engineering approaches for cartilage repair.
In vivo and in vitro models can be used to explore improvements on current therapeutics and have, for example, demonstrated the enhanced healing potential of immature cartilage over mature cartilage.17,18 Age-related changes limit the ability of mature cartilage to heal, including reduced cellularity and decreases in collagen synthesis and cross-linking.17 Work by Fisher and colleagues showed that integration strength was improved when a maturing (but not a mature) engineered cartilage tissue was implanted into a cartilage defect in vitro, highlighting the need for cellular activity in fostering lateral integration with native tissue.19 Growth factors that are present in immature cartilage may enhance chondrogeneisis and promote integrative repair. In addition to the intrinsic properties of adult cartilage that limit healing, Hunziker et al., found that surgical debridement of articular cartilage in adult rabbits and minipigs resulted in hypocellularity in adjacent healthy cartilage.20 Tew et al., showed that defect creation with a trephine drill in a cartilage explant model can induce apoptosis in superficial zone chondrocytes as far as 400 μm from the defect margin and necrosis in all zones immediately adjacent to the defect.21 Together, these results indicate that the surgical preparation of a defect site for graft implantation or marrow-stimulation may limit the ability of surrounding cartilage to integrate with repair tissue.
Given that standard surgical approaches have a common unsolved challenge, several strategies to enhance cartilage-cartilage integration have been proposed, including controlled enzymatic degradation to enhance cell migration to the defect margin, tissue glues to increase adhesion during early integrative repair, and growth factors to stimulate cell proliferation and matrix synthesis.22-26 One promising factor, fibroblast growth factor 18 (FGF18) increases mature chondrocyte proliferation, proteoglycan synthesis, and collagen type II expression.27,28 FGF18, unlike other FGFs, is predominately expressed earlier in development and may have specialized features that can be harnessed for promoting regeneration in adult tissues. FGF18 is currently under investigation as a treatment for knee OA. Moore et al., showed that intraarticular injection of FGF18 in a rat meniscal tear OA model resulted in a dose-dependent increase in cartilage formation. A recent clinical trial examined changes in tibiofemoral cartilage thickness in OA patients following intraarticular injection of recombinant human FGF18 (rhFGF18, sprifermin) and found that sprifermin prevented cartilage loss in both the lateral and medial (including central medial) femorotibial compartments.29 The chondrogenic properties and established safety record of sprifermin make it an appealing therapeutic for the treatment of acute cartilage defects. In this study, we investigated the effects of weekly rhFGF18 (sprifermin) exposure on integrative cartilage repair in an in vitro bovine articular cartilage wound explant model. We hypothesized that sprifermin would enhance chondrocyte proliferation, matrix biosynthesis, and cartilage-cartilage integration characteristics and strength.
Methods
Explant Preparation and Culture:
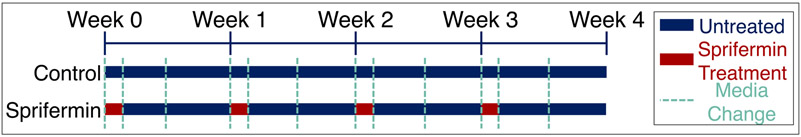
Full thickness articular cartilage explants were harvested using aseptic techniques from the trochlear groove of juvenile (3-6 month old) bovine stifle joints (Research 87, Boylston, MA) using a sterile 6 or 8 mm diameter biopsy punch unless otherwise noted. Explants were washed three times in sterile phosphate buffered saline (PBS) with 200 units/mL penicillin, 200 μg/mL streptomycin and 0.5 μg/mL fungizone (Life Technologies, Carlsbad, CA). Following the wash, explants were dissected to remove any remaining bone and cultured overnight in complete medium consisting of Dulbecco’s Modified Eagle Medium (Life Technologies, Carlsbad, CA) with 10% by volume fetal bovine serum, 100 units/mL of penicillin, 100 μg/mL of streptomycin, 2.5 μg/mL of Amphotericin B (Life Technologies, Carlsbad, CA), 1x MEM Vitamins (Corning Cellgro, Manassas, VA), 25 mM HEPES Buffer, (Life Technologies, Carlsbad, CA) and 50 μg/ml L-Ascorbic Acid 2-Phosphate (Sigma-Aldrich, St. Louis, MO). Following overnight culture, a biopsy punch half the diameter of the explant was used to create a defect in the center of each explant. This central core was removed and replaced, and the resulting construct was cultured for an additional 4 weeks. Throughout the duration of culture, the medium was supplemented once per week for 24 hours with recombinant human fibroblast growth factor 18 (rhFGF18, sprifermin, Merck KGaA, Darmstadt, Germany) at a concentration of 100 ng/ml (Fig 1). The medium was changed two additional times each week and 1 ml was collected at each change and stored at −80°C for future analysis. Control samples had medium changed at the same intervals, but without supplementation with sprifermin. After four weeks of culture, samples were either mechanically tested and frozen for biochemical analysis or fixed in 4% paraformaldehyde for micro-CT and scanning electron microscopy (SEM) analysis.
Fig 1.
Treatment schedule for cultured constructs. Experimental constructs received media containing 100 ng/ml sprifermin for a 24-hour period each week. Control constructs received media changes at the same intervals but were not supplemented with sprifermin.
Micro-CT:
Fixed explants were stained in Lugol’s solution (2.5% I2 / 5% KI in diH2O) for 24 hours prior to micro-CT scanning (SCANCO μCT50, Wayne PA). Explants were placed in a sample holder with PBS-soaked gauze to prevent drying. All samples were scanned at an isotropic voxel size of 6 μm, utilizing the following scan parameters - tube voltage: 45 kVp; current: 133 μA; exposure time: 900 ms x 5 exposures/projection; 3000 projections. Gap volume/total defect volume was calculated by defining a region of interest around the gap separating the defect from the surrounding explant cartilage.
Scanning Electron Microscopy:
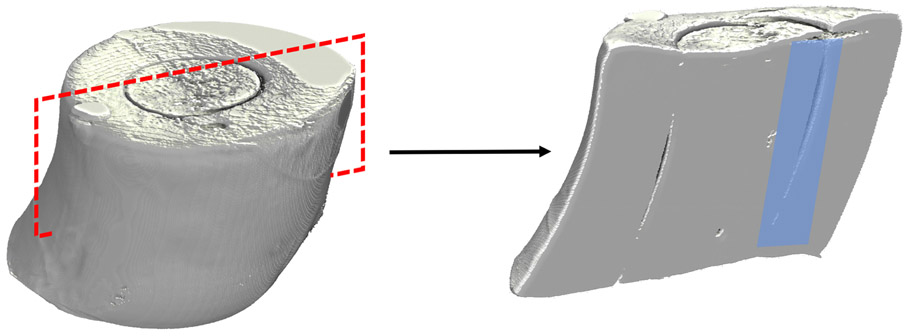
Control and sprifermin treated explants were cut in half in the sagittal plane using a microtome blade to expose the interface between the inner cartilage core and outer cartilage ring (Fig. 2). Samples were fixed with glutaraldehyde before dehydration via critical point drying and sputter coating. After processing, samples were placed on adhesive specimen mounts in the correct orientation for imaging. Images of the cartilage-cartilage interface were acquired at regular intervals at 160X magnification. Following acquisition, images were stitched together in FIJI using the MosaicJ plugin.
Fig 2.
Micro-CT volume renderings of a cartilage explant. The red dashed box demonstrates the plane in which explants were cut prior to SEM analysis. Samples were mounted such that the inner cartilage interface was exposed for imaging (blue shaded region).
Mechanical Testing:
To assay the strength of the integration between the core and outer annulus, a push-out test was performed utilizing a custom fixture used previously by our group.19,30 A constant displacement of 0.1 mm/sec was applied to the core of the construct using an Instron 5848 electromechanical testing frame (Instron, Norwood, MA). Load and displacement were recorded and the resulting data were analyzed using a custom MATLAB program to calculate peak load and integration strength, defined as the peak load divided by the contact area of the outer annulus and inner core of the construct.
Biochemical Analysis:
After mechanical testing, wet and dry weights were recorded before digesting each sample in proteinase K at 60 °C for 18 hours with frequent mixing. The core and annulus of each sample were processed separately to analyze regional differences. Glycosaminoglycan (GAG) and collagen content was quantified using the dimethylmethylene blue and orthohydroxyproline assays, respectively.31,32 Both GAG and collagen content were normalized by dividing by the wet weight to account for differences in sample size. The solid volume fraction was also determined, expressed as a percentage and determined by dividing the dry weight by the wet weight.
Histology:
Following micro-CT, samples were dehydrated in graded ethanol solutions. Samples were cleared with Citrisolv (Fisher Scientific) prior to paraffin embedding. Blocks were sectioned to 7 μm. Sections were stained with Safranin O/Fast Green (SafO/FG), and Hematoxylin and Eosin (H&E). Brightfield images were obtained with an Aperio CS2 slide scanner (Leica Biosystems). Detection of apoptotic cells was performed using a terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (In Situ Cell Death Detection Kit, Fluorescein, Roche). Paraffin sections were rehydrated before labeling with fluorescein-conjugated (FITC) nucleotides. All samples were mounted with DAPI-containing mountant (ProLong Gold, Life Technologies) for visualization of all cell nuclei. Positive controls were treated with nuclease prior to labeling and negative controls were incubated with labeled nucleotides in the absence of terminal deoxynucleotidyl transferase. All images were acquired on an Eclipse upright microscope (Nikon) using the same settings for all samples. Stitched images encompassing the entire tissue section were analyzed in ImageJ to quantify the number of apoptotic cells (FITC-labeled) and total cells (DAPI-labeled). Briefly, images were binarized, a watershed transformation was applied, and the ‘analyze particles’ tool was used to quantify cell number in each channel. All images were subjected to the same analysis process using identical parameters for each step.
Statistical Analysis:
All statistics were performed using GraphPad Prism version 6.07 for Windows (GraphPad Software, La Jolla California, USA). Differences in mechanical properties, μCT gap volume, and cell number from the TUNEL assay were compared using the Mann-Whitney Test. Statistical significance was set as p<0.05 for all analyses.
Results
Mechanical Testing:
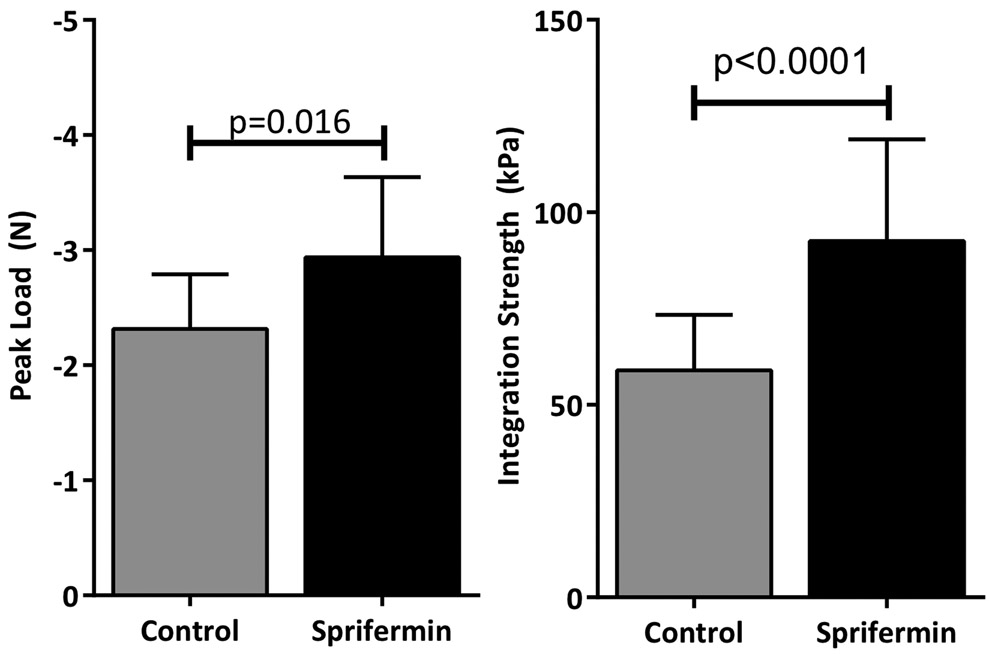
Push-out testing of the cartilage repair explants showed that constructs in the sprifermin treatment group had a higher peak load (2.9 N versus 2.3 N, p=0.016). To account for differences in construct size, the peak load was normalized to the contact area of the core-annulus interface to determine the integration strength. This showed a more pronounced difference between groups; sprifermin-treated samples had an integration strength of 93 kPa versus 59 kPa for control samples (p<0.0001) (Fig 3).
Fig 3.
Peak load and integration strength from push-out testing of 4 week constructs. Sprifermin treatment resulted in significantly higher peak load and integration strength (n=13/group).
Biochemical Analysis:
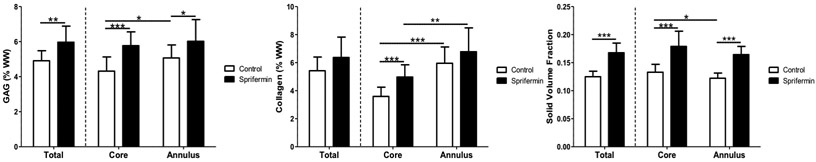
Analysis of total biochemical composition showed that sprifermin treated samples had significantly higher GAG content (6.0% (w/w) vs. 4.9% (w/w) for control samples, p=0.0033). When looking at regional differences, the GAG content was significantly higher in sprifermin treated samples in both the core (5.8% vs. 4.2%, p=0.0002) and annulus (6.0% vs 5.0%, p=0.032). Additionally, control samples had a higher GAG content in the annulus (5.0% vs 4.2%, p=0.029) whereas sprifermin treated samples did not show any differences between regions (6.0% vs 5.8%, p=0.72) (Fig 4). Sprifermin treated samples had higher collagen content in the construct core (5.0% vs 3.6% for control, p=0.0032) but not in the outer annulus (6.4% vs 5.4%, p=0.07). Comparing regional differences, the collagen content in control samples was higher in the annulus when compared to their core (6.0% vs 3.6%, p<0.0001) but this difference was not attenuated with sprifermin treatment (6.8% vs 5.0%, p=0.0032) (Fig 4). The solid volume fraction of the sprifermin treated samples was higher than that of the control samples (0.17 vs 0.12, p<0.0001). This result extended regionally, to the core (0.18 vs 0.13, p<0.0001) and annulus (0.16 vs 0.12, p<0.0001). Additionally, under control conditions, the core had a higher solid volume fraction than the annulus (0.13 vs 0.12, p=0.04) whereas there was no significant regional difference in sprifermin treated samples (0.18 vs 0.16, p=0.11) (Fig 4).
Fig 4.
Biochemical analysis. Sprifermin-treated constructs had significantly higher total and regional GAG content, as well as significantly higher collagen content in the core of the explant. The total and regional solid volume fractions (dry weight/wet weight) were significantly lower in the control group (n=15-23/group).
Micro-CT:
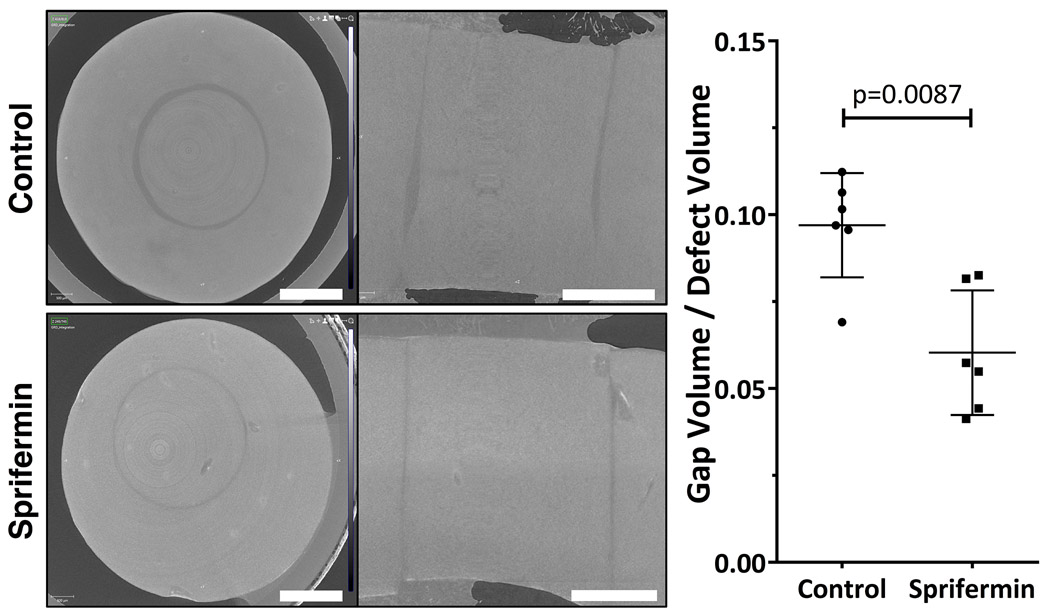
All explants had a visible margin between the defect and surrounding cartilage. Sprifermin-treated explants had a significantly lower gap volume/defect volume when compared to control explants (0.06 versus 0.10; n=6/group; p<0.05) (Fig 5). In both groups, the interface distance was variable throughout the depth of the explant.
Fig 5.
Transverse and sagittal micro-CT slices through the middle of control and sprifermin treated constructs. The gap volume/defect volume was significantly lower in the sprifermin group (p=0.0087, n=6/group). Graph shows individual data points along with bar indicating mean with SD. Scale bars = 1.5mm.
Scanning Electron Microscopy:
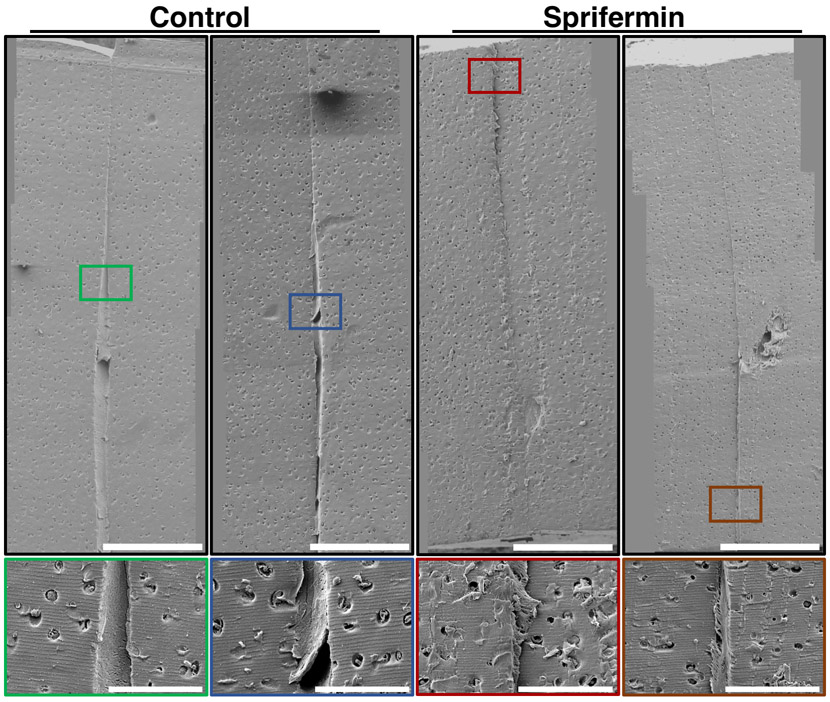
SEM analysis supported the micro-CT findings that a larger gap was present between the core and annulus of control samples (Fig 6). In the control samples, this gap was filled with a dense fibrillar material. Fibrils generally appeared to be laterally oriented, spanning across the interface. In sprifermin-treated samples, there was a smaller gap between inner and outer cartilage. This gap was either devoid of fibrillar material or contained less dense fibrillar material when compared with the control samples.
Fig 6.
SEM images of control and sprifermin-treated cartilage explants. Top row: Stitched images showing cartilage-cartilage interface in the sagittal plane. 160X magnification, scale bars = 400μm. Bottom row: Insets show interface at 600X magnification. Control samples displayed a larger gap between native cartilage borders that was filled with dense fibrous tissue. Sprifermin-treated samples had a small gap between native cartilage borders. Scale bars = 100μm.
Histology:
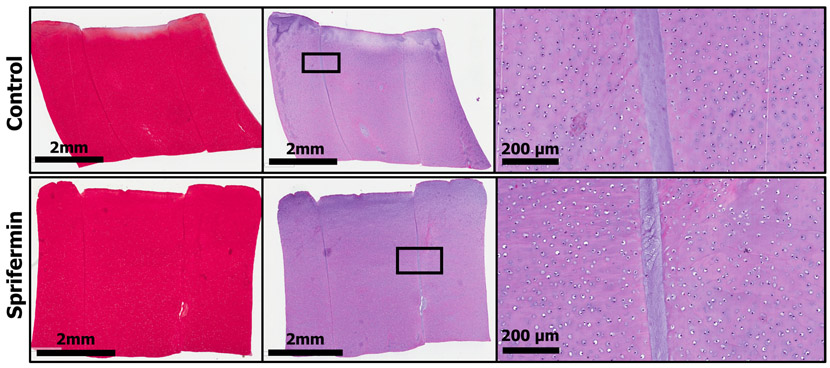
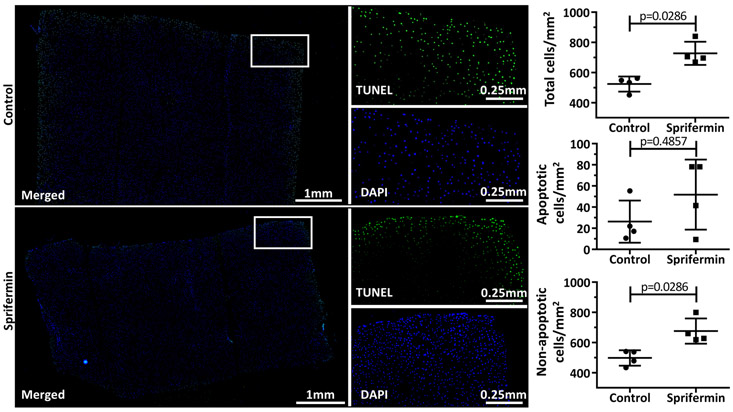
Safranin O/Fast Green (SafO/FG) staining revealed no qualitative differences between control and sprifermin groups, with some samples from both groups exhibiting reduced staining intensity adjacent to the cartilage-cartilage interface. Hematoxylin & Eosin (H&E) staining supported the observation that control samples have a larger gap between the core and annular cartilage, but as revealed through micro-CT analysis, the interface distance was variable. H&E staining also allowed for the visualization of the fibrillar material at the interface in both groups (Fig 7). The TUNEL assay demonstrated a region of apoptotic cells surrounding the margin of the tissue in samples from both groups. There was no difference in the number of apoptotic cells between groups when normalized to the cross-sectional area; however, sprifermin-treated samples had significantly more total cells/mm2, and significantly more non-apoptotic cells/mm2 than control samples (Fig 8).
Fig 7.
Histology of control and sprifermin-treated samples. From left to right: SafO/FG, H&E, and magnified view from H&E region of interest. In the magnified views, fibrillar material is visible at the interface in both groups.
Fig 8.
TUNEL-labeling of apoptotic cells. Green/FITC = TUNEL, Blue/DAPI = all nuclei. All samples displayed a rim of apoptotic cells around the margin of the tissue section. Sprifermin treated samples showed a significant increase in total cell number and non-apoptotic cell number when normalized to cross-sectional area (n=4, p<0.05).
Discussion
The current generation of cartilage repair techniques all have a common problem, that is, poor integration of repair or graft tissue with the existing native cartilage. Even when integration occurs, it is substandard, and this lack of adequate graft and host integration results in altered stress distribution during joint loading that can ultimately lead to the degradation and failure of the repair tissue.33 Studies have demonstrated that chondrocyte proliferation and matrix synthesis enhance the adhesive strength between two cartilage surfaces.34,35 Therefore, growth factors that stimulate chondrocyte anabolism may improve the integrative repair of chondral defects. In this study, we utilized a cartilage explant wound model to examine the effects of a truncated fibroblast growth factor (rhFGF18, sprifermin) and postulated that sprifermin may act to enhance growth and proliferation and matrix expression between the two surfaces. We modeled our treatment after previous in vitro and clinical trial design that used a once weekly dose for 4 weeks. In this study, our main finding was that weekly exposure to sprifermin resulted in a significant increase in cartilage-cartilage integration strength when assessed after 4 weeks of treatment. An assay of mechanical strength, a pushout test, showed that sprifermin treatment resulted in an average integration strength of 93 kPa compared to untreated control samples, which had an average integration strength of 59 kPa. Studies examining cartilage integration strength in similar explant model systems report control failure stresses comparable to ours, with integration enhancing techniques resulting in failure stresses between 70-100 kPa.17,32-34
Biochemical assessment of overall cartilage extracellular matrix demonstrated a significant increase in GAG (proteoglycan) content and an upward trend in collagen content in the sprifermin group. In addition, histological analysis demonstrated a significant increase in overall tissue cell density in the sprifermin-treated group. FGF18 can act as a trophic factor in mature articular chondrocytes, increasing proteoglycan and type II collagen matrix deposition, and stimulating chondrocyte proliferation.27,28 FGF18 primarily activates the FGF receptor IIIc (FGFR3c), which also plays an important role in development of the skeleton, such as regulation of long bone development, as well as FGF receptor IIc (FGFR2c).39-41 FGF18 expression and FGFR3 activation in growth plate chondrocytes inhibits chondrocyte proliferation and hypertrophy.42 Indeed, activating mutations of the FGFR3 receptor are responsible for multiple chondrodysplasias due to inhibitory effects at the immature growth plate.43 However, the effects of FGF18 on articular chondrocytes appear stimulatory rather than inhibitory, as shown in our study and others.27,44-46 Mori et al., demonstrated that FGF18 is expressed in mature articular chondrocytes, but not growth plate chondrocytes, in adult rats.44 Sprifermin has been shown to activate the ERK1/2 signaling pathway.46 Blockade of this pathway inhibits the effects of sprifermin, suggesting a potential mechanism for the chondrogenic effects of this factor.
Building from what is known about the effects on chondrocytes, and the clinical interest in FGF18, this study utilized sprifermin in an ex vivo healing model and used high-resolution imaging methods (micro-CT and SEM) to assess the structural properties of the cartilage-cartilage wound interface. With micro-CT, it was determined that there was greater contact area between adjacent cartilage surfaces in the sprifermin group compared to the control group. SEM imaging confirmed this finding, and provided some elucidation on the composition of this interface, showing densely packed fibrils in the control group, and closely abutted surfaces in the FGF18 group.
While studies on the effects of sprifermin in humans have primarily examined safety, several studies have reported positive effects of intraarticular sprifermin injection on cartilage volume and bone marrow lesions in osteoarthritic knees.47-49 Lohmander et al., demonstrated with quantitative MRI that weekly injections of sprifermin increased cartilage thickness in the lateral femorotibial compartment in a concentration-dependent manner following 12 months of treatment.49 In a separate paper examining the same patient cohort, Roemer et al., demonstrated that sprifermin also increased cartilage thickness in the patellofemoral junction.48 In addition, bone marrow lesions showed improvement between months 6-12 of sprifermin treatment. Hochberg et al., showed a significant increase in total tibiofemoral joint cartilage thickness after two years of sprifermin treatment (injection every 6 months). These studies demonstrate the potential of sprifermin to enhance matrix production, even in an osteoarthritic environment.
In our study, we utilized a similar once-weekly dosing regimen and saw a significant increase in both GAG content and collagen content in the core of the explant. As with any mitogenic compound, it is critical to limit exposure to the target cells and tissue and to minimize prolonged exposure to the compound. The ability of this intermittent dosing regimen to increase ECM production in vitro and increase cartilage thickness in human patients is promising for the future clinical application of sprifermin.
While sprifermin is currently under investigation as a therapeutic for the treatment of primary knee osteoarthritis, we have demonstrated in this study that it may also be useful to enhance healing of acute cartilage lesions. In our in vitro model, we demonstrated that treatment with sprifermin enhanced the integration strength between two adjacent pieces of articular cartilage. Furthermore, we showed through quantitative measurement and ultrastructural analysis that treatment with sprifermin increased GAG and collagen synthesis and resulted in ECM formation spanning the gap between cartilage pieces. As with any cartilage repair study, the choice of model system is an important consideration, as age and species-specific variation in cartilage-healing capacity are well-established. In our study, we examined the effects of sprifermin on juvenile bovine cartilage, which exhibits an enhanced healing capacity over adult bovine cartilage.50 While it is possible that the effects of sprifermin were enhanced by the innate healing capacity of juvenile bovine cartilage, our results are in agreement with studies that have examined the effects of sprifermin on osteoarthritic human chondrocytes and adult human articular cartilage.28,47 Future studies will examine the effects of sprifermin treatment on a large animal model of acute chondral defect healing and will investigate delivery mechanisms to further improve the safety and efficacy of this promising therapeutic.
Acknowledgements
This work was supported by Merck KGaA of which one of the authors (H.G.) is a paid employee. Additional support was provided by the Department of Veteran’s Affairs (I01 RX001213) and the NIH via the Penn Center for Musculoskeletal Diseases Biomechanics Core (P30-AR069619).
References
- 1.Ding C, Garnero P, Cicuttini F, et al. 2005. Knee cartilage defects: Association with early radiographic osteoarthritis, decreased cartilage volume, increased joint surface area and type II collagen breakdown. Osteoarthr Cartil 13(3):198–205. [DOI] [PubMed] [Google Scholar]
- 2.Messner K, Maletius W. 1996. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: A 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthop Scand 67(2):165–168. [DOI] [PubMed] [Google Scholar]
- 3.Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. 2004. Osteochondral defects in the human knee: Influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am J Sports Med 32(6):1451–1458. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JA, Anderson DD, Brown TD, et al. 2013. The Roles of Mechanical Stresses in the Pathogenesis of Osteoarthritis: Implications for Treatment of Joint Injuries. Cartilage 4(4):286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders TL, Pareek A, Obey MR, et al. 2017. High rate of osteoarthritis after osteochondritis dissecans fragment excision compared with surgical restoration at a mean 16-Year Follow-up. Am J Sports Med 45(8):1799–1805. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2017. Arthritis in America: Time to Take Action! CDC Vital Signs :4. [Google Scholar]
- 7.Osteoarthritis Research Society International (OARSI). 2016. OA as a Serious Disease.
- 8.Stevenson S, Emery SE, Goldberg VM. 1996. Factors affecting bone graft incorporation. Clin Orthop Relat Res (324):66–74. [DOI] [PubMed] [Google Scholar]
- 9.Horas U, Pelinkovic D, Herr G, et al. 2003. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am 85–A(2):185–92. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro F, Koide S, Glimcher MJ. 1993. Cell origin and differentiation in the repair of full thickness defects of articular cartilage. J Bone Jt Surg 75A(4):532–553. [DOI] [PubMed] [Google Scholar]
- 11.Mithoefer K, Williams RJ, Warren RF, et al. 2005. The Microfracture Technique for the Treatment of Articular Cartilage Lesions in the Knee. J Bone Jt Surg 87(9):1911–1920. [DOI] [PubMed] [Google Scholar]
- 12.Mithoefer K, Mcadams T, Williams RJ, et al. 2009. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: An evidence-based systematic analysis. Am J Sports Med 37(10):2053–2063. [DOI] [PubMed] [Google Scholar]
- 13.Solheim E, Hegna J, Inderhaug E, et al. 2016. Results at 10–14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surgery, Sport Traumatol Arthrosc 24(5):1587–1593. [DOI] [PubMed] [Google Scholar]
- 14.Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. 2010. Autologous Chondrocyte Implantation. Am J Sports Med 38(6):1117–1124. [DOI] [PubMed] [Google Scholar]
- 15.Niemeyer P, Porichis S, Steinwachs M, et al. 2014. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med 42(1):150–157. [DOI] [PubMed] [Google Scholar]
- 16.Saris D, Price A, Widuchowski W, et al. 2014. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Two-year follow-up of a prospective randomized trial. Am J Sports Med 42(6):1384–1394. [DOI] [PubMed] [Google Scholar]
- 17.Dimicco MA, Waters SN, Akeson WH, Sah RL. 2002. Integrative articular cartilage repair: Dependence on developmental stage and collagen metabolism. Osteoarthr Cartil 10(3):218–225. [DOI] [PubMed] [Google Scholar]
- 18.Tam HK, Srivastava A, Colwell CW, D’Lima DD. 2007. In vitro model of full-thickness cartilage defect healing. J Orthop Res 25(9):1136–1144. [DOI] [PubMed] [Google Scholar]
- 19.Fisher MB, Henning EA, Söegaard NB, et al. 2014. Maximizing cartilage formation and integration via a trajectory-based tissue engineering approach. Biomaterials 35(7):2140–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunziker EB, Quinn TM. 2003. Surgical removal of articular cartilage leads to loss of chondrocytes from cartilage bordering the wound edge. J Bone Joint Surg Am 85–A Suppl:85–92. [DOI] [PubMed] [Google Scholar]
- 21.Tew SR, Kwan APL, Hann A, et al. 2000. The reactions of articular cartilage to experimental wounding: Role of apoptosis. Arthritis Rheum 43(1):215–225. [DOI] [PubMed] [Google Scholar]
- 22.Janssen LM, In Der Maur CD, Koen Bos P, et al. 2006. Short-duration enzymatic treatment promotes integration of a cartilage graft in a defect. Ann Otol Rhinol Laryngol 115(6):461–468. [DOI] [PubMed] [Google Scholar]
- 23.Seol D, Yu Y, Choe H, et al. 2014. Effect of short-term enzymatic treatment on cell migration and cartilage regeneration: in vitro organ culture of bovine articular cartilage. Tissue Eng Part A 20(13–14):1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang DA, Varghese S, Sharma B, et al. 2007. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater 6(5):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jürgensen K, Aeschlimann D, Cavin V, et al. 1997. A new biological glue for cartilage cartilage interfaces: tissue transglutaminase. J Bone Jt Surg-Amer Vol 79A(2):185–193. [DOI] [PubMed] [Google Scholar]
- 26.Hunziker EB. 2001. Growth-factor-induced healing of partial-thickness defects in adult articular cartilage. Osteoarthr Cartil 9(1):22–32. [DOI] [PubMed] [Google Scholar]
- 27.Ellsworth JL, Berry J, Bukowski T, et al. 2002. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthr Cartil 10(4):308–320. [DOI] [PubMed] [Google Scholar]
- 28.Gigout A, Guehring H, Froemel D, et al. 2017. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthr Cartil 25(11):1858–1867. [DOI] [PubMed] [Google Scholar]
- 29.Hochberg M, Guermazi A, Guehring H, et al. 2017. Efficacy and Safety of Intra-Articular Sprifermin in Symptomatic Radiographic Knee Osteoarthritis: Results of the 2-Year Primary Analysis from a 5-Year Randomised, Placebo-Controlled, Phase II Study [abstract]. Arthritis Rheumatol. 69(Supplement S10). [Google Scholar]
- 30.Ionescu LC, Mauck RL. 2013. Porosity and Cell Preseeding Influence Electrospun Scaffold Maturation and Meniscus Integration In Vitro. Tissue Eng Part A 19(3–4):538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stegemann H, Stalder K. 1967. Determination of hydroxyproline. Clin Chim Acta 18(2):267–273. [DOI] [PubMed] [Google Scholar]
- 32.Farndale RW, Buttle DJ, Barrett AJ. 1986. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. BBA - Gen Subj 883(2):173–177. [DOI] [PubMed] [Google Scholar]
- 33.Ahsan T, Sah RL. 1999. Biomechanics of integrative cartilage repair. Osteoarthr Cartil 7(1):29–40. [DOI] [PubMed] [Google Scholar]
- 34.Obradovic B, Martin I, Padera RF, et al. 2001. Integration of engineered cartilage. J Orthop Res 19(6):1089–1097. [DOI] [PubMed] [Google Scholar]
- 35.DiMicco MA, Sah RL. 2001. Integrative cartilage repair: Adhesive strength is correlated with collagen deposition. J Orthop Res 19(6):1105–1112. [DOI] [PubMed] [Google Scholar]
- 36.Englert C, McGowan KB, Klein TJ, et al. 2005. Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum 52(4):1091–1099. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Xu Y, Yin Z, et al. 2013. Chondrocyte Migration Affects Tissue-Engineered Cartilage Integration by Activating the Signal Transduction Pathways Involving Src, PLCγ1, and ERK1/2. Tissue Eng Part A 19(21–22):2506–2516. [DOI] [PubMed] [Google Scholar]
- 38.Dimicco MA, Waters SN, Akeson WH, Sah RL. 2002. Integrative articular cartilage repair: Dependence on developmental stage and collagen metabolism. Osteoarthr Cartil 10(3):218–225. [DOI] [PubMed] [Google Scholar]
- 39.Davidson D, Blanc A, Filion D, et al. 2005. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem 280(21):20509–15. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Ibrahimi OA, Olsen SK, et al. 2006. Receptor specificity of the fibroblast growth factor family: The complete mammalian FGF family. J Biol Chem 281(23):15694–15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwata T, Chen L, Li C, et al. 2000. A neonatal lethal mutation in FGFR3 uncouples proliferation and differentiation of growth plate chondrocytes in embryos. Hum Mol Genet 9(11):1603–1613. [DOI] [PubMed] [Google Scholar]
- 42.Karuppaiah K, Yu K, Lim J, et al. 2016. FGF signaling in the osteoprogenitor lineage non-autonomously regulates postnatal chondrocyte proliferation and skeletal growth. Development 143(10):1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horton WA. 1997. Fibroblast growth factor receptor 3 and the human chondrodysplasias. Curr Opin Pediatr 9(4):437–42. [DOI] [PubMed] [Google Scholar]
- 44.Y. M, T. S, C.H. L, et al. 2013. Identification of fibroblast growth FACTOR-18 As a molecule to protect and regenerate articular cartilage. Osteoarthr Cartil 21:S107. [Google Scholar]
- 45.Shimoaka T, Ogasawara T, Yonamine A, et al. 2002. Regulation of osteoblast, chondrocyte, and osteoclast functions by fibroblast growth factor (FGF)-18 in comparison with FGF-2 and FGF-10. J Biol Chem 277(9):7493–7500. [DOI] [PubMed] [Google Scholar]
- 46.Gigout A, Guehring H, Froemel D, et al. 2017. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthr Cartil 25(11):1858–1867. [DOI] [PubMed] [Google Scholar]
- 47.Dahlberg LE, Aydemir A, Muurahainen N, et al. 2016. A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clin Exp Rheumatol 34(3):445–450. [PubMed] [Google Scholar]
- 48.Roemer FW, Aydemir A, Lohmander S, et al. 2016. Structural effects of sprifermin in knee osteoarthritis: a post-hoc analysis on cartilage and non-cartilaginous tissue alterations in a randomized controlled trial. BMC Musculoskelet Disord 17(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohmander LS, Hellot S, Dreher D, et al. 2014. Intraarticular Sprifermin (Recombinant Human Fibroblast Growth Factor 18) in Knee Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol 66(7):1820–1831. [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Zhao Z, Clarke RB, et al. 2013. Enhanced tissue regeneration potential of juvenile articular cartilage. Am J Sports Med 41(11):2658–2667. [DOI] [PubMed] [Google Scholar]