See Piguet (doi:10.1093/brain/awaa119) for a scientific commentary on this article.
Murley et al. report the results of a transdiagnostic study of clinical phenotype and brain atrophy in frontotemporal lobar degeneration syndromes, showing that these syndromes exist on a multidimensional spectrum, rather than as discrete entities.
Keywords: frontotemporal dementia, progressive supranuclear palsy, corticobasal syndrome, primary progressive aphasia, semantic dementia
Abstract
The syndromes caused by frontotemporal lobar degeneration have highly heterogeneous and overlapping clinical features. There has been great progress in the refinement of clinical diagnostic criteria in the past decade, but we propose that a better understanding of aetiology, pathophysiology and symptomatic treatments can arise from a transdiagnostic approach to clinical phenotype and brain morphometry. In a cross-sectional epidemiological study, we examined 310 patients with a syndrome likely to be caused by frontotemporal lobar degeneration, including behavioural variant frontotemporal dementia, non-fluent, and semantic variants of primary progressive aphasia (PPA), progressive supranuclear palsy and corticobasal syndrome. We included patients with logopenic PPA and those who met criteria for PPA but not a specific subtype. To date, 49 patients have a neuropathological diagnosis. A principal component analysis identified symptom dimensions that broadly recapitulated the core features of the main clinical syndromes. However, the subject-specific scores on these dimensions showed considerable overlap across the diagnostic groups. Sixty-two per cent of participants had phenotypic features that met the diagnostic criteria for more than one syndrome. Behavioural disturbance was prevalent in all groups. Forty-four per cent of patients with corticobasal syndrome had progressive supranuclear palsy-like features and 30% of patients with progressive supranuclear palsy had corticobasal syndrome-like features. Many patients with progressive supranuclear palsy and corticobasal syndrome had language impairments consistent with non-fluent variant PPA while patients with behavioural variant frontotemporal dementia often had semantic impairments. Using multivariate source-based morphometry on a subset of patients (n = 133), we identified patterns of covarying brain atrophy that were represented across the diagnostic groups. Canonical correlation analysis of clinical and imaging components found three key brain-behaviour relationships, with a continuous spectrum across the cohort rather than discrete diagnostic entities. In the 46 patients with follow-up (mean 3.6 years) syndromic overlap increased with time. Together, these results show that syndromes associated with frontotemporal lobar degeneration do not form discrete mutually exclusive categories from their clinical features or structural brain changes, but instead exist in a multidimensional spectrum. Patients often manifest diagnostic features of multiple disorders while deficits in behaviour, movement and language domains are not confined to specific diagnostic groups. It is important to recognize individual differences in clinical phenotype, both for clinical management and to understand pathogenic mechanisms. We suggest that a transdiagnostic approach to the spectrum of frontotemporal lobar degeneration syndromes provides a useful framework with which to understand disease aetiology, progression, and heterogeneity and to target future treatments to a higher proportion of patients.
See Piguet (doi:10.1093/brain/awaa119) for a scientific commentary on this article.
Introduction
The clinical disorders caused by frontotemporal lobar degeneration pathologies (FTLD) are highly heterogeneous in their pathology and phenotypes (Kertesz et al., 2005; MacKenzie et al., 2010; Rohrer et al., 2011). Patients are typically diagnosed as having one of several principal syndromes, including behavioural variant frontotemporal dementia (bvFTD) (Rascovsky et al., 2011), primary progressive aphasia [with the non-fluent (nfvPPA) and semantic (svPPA) subtypes] (Gorno-Tempini et al., 2011), progressive supranuclear palsy (PSP) (Höglinger et al., 2017) or corticobasal syndrome (CBS) (Armstrong et al., 2013). The clinicopathological correlations of these syndromes are imprecise (Irwin et al., 2015). For example, bvFTD can be associated with tau, TDP-43, FUS protein inclusions or mixed neuropathology (Perry et al., 2017). Some clinical syndromes, such as PSP-Richardson’s syndrome, have good correlation with the associated pathology (Gazzina et al., 2019); however, the corresponding pathology may have diverse phenotypic expressions (Respondek et al., 2014). Recent revisions of diagnostic criteria recognize this heterogeneity (Armstrong et al., 2013; Höglinger et al., 2017), and there may be future improvements in clinicopathological correlations by imaging or fluid-based biomarkers, aiming to optimize patient selection for disease-modifying therapies (Irwin et al., 2015; Meeter et al., 2017).
Here we propose that the effort to refine diagnostic segregation of the disorders has fundamental limitations. These are not merely due to the limits of a given test or biomarker but are biologically real constraints that can in turn be informative about the nature of the disorders. We suggest that a better understanding of aetiology and pathophysiology, and more effective therapies, can be gained by examining the phenotypic patterns across the broad spectrum of all FTLD-associated disease. Symptomatic therapies may especially benefit from such a transdiagnostic approach, selecting patients based on the presence of relevant clinical features, whichever their diagnostic label or proteinopathy.
A transdiagnostic approach is increasingly used in psychiatry, epitomized by the Research Domain Criteria methodology (Kozak and Cuthbert, 2016; Grisanzio et al., 2018). A similar approach is applicable to neurodegenerative diseases with overlapping phenotypes (Lambon Ralph et al., 2003; Husain, 2017) and cognitive deficits after stroke (Butler et al., 2014; Mirman et al., 2015; Halai et al., 2017). There are many overlapping symptoms and indistinct phenotypic boundaries between FTLD syndromes (Kertesz et al., 1999, 2005). For example, executive dysfunction is a common cognitive impairment across FTLD-associated syndromes (Burrell et al., 2014; Ranasinghe et al., 2016a) and changes in behaviour, social cognition and personality, while characteristic of bvFTD, are also seen in PSP (Cordato et al., 2005; Ghosh et al., 2012; Gerstenecker et al., 2013), CBS (Huey et al., 2009) and the primary progressive aphasias (Rosen et al., 2006; Rohrer and Warren, 2010). Neuropsychiatric symptoms, including apathy and impulsivity, occur in multiple FTLD syndromes (Rohrer et al., 2010a; Lansdall et al., 2017). The movement disorders typical of PSP and CBS can also develop in patients diagnosed with bvFTD (Park et al., 2017) and nfvPPA (Santos-Santos et al., 2016). Language impairments are seen across all FTLD syndromes, including bvFTD (Hardy et al., 2015), PSP and CBS (Peterson et al., 2019).
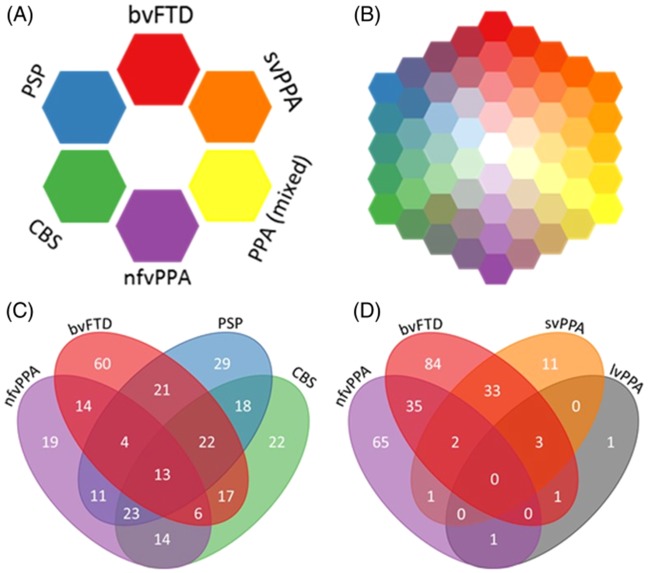
We therefore used a transdiagnostic approach to assess the phenotype of FTLD syndromes. We tested the hypothesis that syndromes associated with FTLD are multidimensional clinical spectra, rather than discrete clinical entities. The colour map in Fig. 1A symbolizes the current most widely used approach, in which patients have a distinct clinical phenotype of a singular syndrome, represented by a discrete colour patch (‘red bvFTD’ is distinct from ‘blue PSP’) (Butler et al., 2014). Our alternate hypothesis is that patients lie in a continuous colour-space, as shown in Fig. 1B. Intermediate or mixed phenotypes, such as PSP-frontal (PSP-F), CBS-NAV or svPPA with prominent behavioural disturbance, are readily placed within the continuous phenotypic space. A corollary hypothesis is that the multivariate clinical spectrum of the disorders can be mapped to multivariate regional structural brain change. Note that this is not an argument for ‘lumping’ patients into super-ordinate diagnostic groups, or for ‘splitting’ diagnoses into ever finer subtypes. This type of transdiagnostic approach recognizes the clear individual differences across patients and does not propose an unstructured pool; instead, the key hypothesis is that the underlying variations in FLTD reflect a statistical structure in the form of multiple graded dimensions rather than mutually exclusive categories. Thus, the concept of phenotypic spectra allows for both the recognition of broad similarities and unique combinations of features.
Figure 1.
The FTLD syndrome spectrum. (A) Schematic of current diagnostic criteria. (B) Schematic to highlight our hypothesis that FTLD syndromes occur on a spectrum. (C and D) Four-way Venn diagrams of overlap between FTLD syndromes in the study. The numbers in each oval refer to the number of patients who met the diagnostic criteria for those syndromes. Many patients met the diagnostic criteria for two or more syndromes. (C) Overlap between bvFTD, nfvPPA, PSP and CBS. (D) Overlap between bvFTD, nfvPPA, svPPA and lvPPA.
To test our hypotheses, we exploited the epidemiologically-based Pick’s disease and Progressive Supranuclear Palsy Prevalence and Incidence (PiPPIN) study dataset (Coyle-Gilchrist et al., 2016) and a replication dataset acquired 4 years later. We undertook a systematic behavioural, cognitive and imaging assessment of patients with syndromes associated with FTLD, in a region of 1.75 million people in the UK. We predicted that while classical syndromes of bvFTD, PPA, PSP and CBS exist, a data-driven approach would reveal phenotypic continuity without clear separation between phenotypes. With longitudinal follow-up of a subset of participants, we tested the hypothesis that clinical phenotypes merge by addition of features, with increasing overlap—analogous to the move towards the centre of the colour-space. Moreover, we predicted that clusters of symptoms would be associated with a specific pattern of brain atrophy, while the extent to which a patient has this atrophy pattern determines the severity of the associated symptoms.
Materials and methods
The rapidly evolving field of FTLD/FTD/PSP research can result in confusion in definitions and diagnostic labels. In this paper we use the current consensus nosology for clinical and pathological diagnoses. We use FTLD to refer to the pathology, subtyping to tau or TDP43 pathologies where applicable. The phrase ‘FTLD syndromes’ refers collectively to the clinical diagnoses of bvFTD (with or without motor neuron disease), PPA, nfvPPA, svPPA, PSP or CBS and their intermediate phenotypes. The term ‘corticobasal degeneration’ is limited to the pathology, while CBS refers to the clinical syndrome. Note that not all patients will have FTLD pathology (especially lvPPA and mixed PPA patients) and not all those with FTLD pathology will have had one of the corresponding syndromes.
Participant recruitment
The PiPPIN study sought to recruit all patients with a clinical diagnosis of a FTLD syndrome living in the counties of Cambridgeshire and Norfolk in the UK. Cross-sectional assessments were performed during two 24-month periods, from 1 January 2013 to 31 December 2014 and again from 1 January 2017 to 31 December 2018. Participants were recruited via multiple routes, including specialist cognitive and movement disorder clinics at tertiary and secondary healthcare services (using paper and electronic health records), patient support groups (FTD support group, PSP Association), advertisements in local newspapers and through local research databases and the National Institute for Health Research ‘Join Dementia Research’ registry. Patients were recruited at all stages of symptomatic disease. We sought to assess all participants, either at our research centre or at their home or care home. Patients alive during both study periods were invited to assessment in both periods, but only their first visit was used for the cross-sectional analysis. Three hundred and sixty-five patients were identified in the catchment area, 310 of whom were met in person by the study team for phenotypic assessment. Death or end-stage disease were the main reasons for our not assessing the remaining 55 cases. All participants provided written informed consent or, if they lacked capacity to consent, their next of kin was consulted using the ‘personal consultee’ process established by UK law. The study had ethical approval from the Cambridge Central Research Ethics Committee (REC 12/EE/0475).
Clinical assessment
We used a structured clinical assessment to record the presence or absence of symptoms and signs typically seen in FTLD syndromes, including all clinical features in the current consensus diagnostic criteria (Supplementary material) (Rascovsky et al., 2007; Bensimon et al., 2009; Gorno-Tempini et al., 2011; Armstrong et al., 2013; Höglinger et al., 2017). Each patient’s primary diagnosis was made according to these criteria, with reference to the dominant features at the time of presentation and assessment. Patients with a mixed PPA, who met the diagnostic criteria for PPA but not one of the three subtypes (Gorno-Tempini et al., 2011) were grouped with lvPPA for this study, in view of the low numbers and the association of both phenotypes with Alzheimer’s pathology (Sajjadi et al., 2012). For patients who met several sub-diagnostic criteria we grouped ‘probable’ and ‘possible’ diagnoses together, and classified by the dominant phenotype or formal MAX rules where available (Grimm et al., 2019). We reapplied the other diagnostic criteria to each patient to assess if he or she met the diagnostic criteria for any of the other FTLD syndromes (excepting the ‘mutual exclusivity’ clause included in several criteria). Patients completed the Addenbrooke’s Cognitive Examination-Revised (ACE-R) wherever possible (Mioshi et al., 2006) and a carer’s assessment was obtained using the Cambridge Behavioural Inventory (CBI-R) (Wear et al., 2008). At the time of writing, 49 participants have undergone post-mortem examination, via the Cambridge Brain Bank.
Imaging analysis
One hundred and thirty-three patients (bvFTD n = 28, nfvPPA n = 15, svPPA n = 5 PPA n = 10, PSP n = 53, CBS n = 22) from the phenotyped cohort were scanned at the Wolfson Brain Imaging Centre, University of Cambridge on a Siemens 3T system. Structural MRI was performed using a T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence. Images were preprocessed using SPM12 with default settings. Grey and white matter segments were combined to whole brain images for further analysis. The DARTEL pipeline was used to create a study-specific template using all images. Age and total intracranial volume were included in a multiple regression and regressed out of the data. Source-based morphometry was used on the residual images to identify covarying networks of grey and white matter atrophy, further details of this step are given in the next section.
Statistical analysis
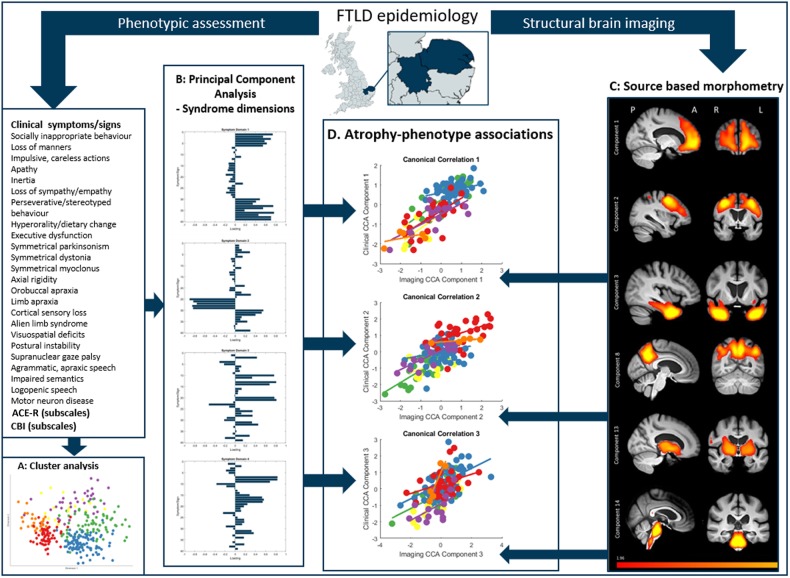
Figure 2 summarizes the analysis pipeline. First, we examined the relationships between individual clinical features using distance measures and multidimensional scaling (Shepard, 1980) (Fig. 3). The pairwise Jaccard’s distances between clinical features were calculated, resulting in a dissimilarity matrix. Non-classical two-dimensional scaling was performed on this dissimilarity matrix (Shepard, 1980).
Figure 2.
Schematic of data processing. First, patients were recruited from the study catchment area for phenotypic assessment and structural brain imaging. Second, a cluster analysis was performed on clinical features. Third, we performed PCA on all clinical features to find latent syndrome dimensions across FTLD. Fourth, we used source-based morphometry (independent component analysis on grey and white matter) to create atrophy components. Finally, we explored the relationship between phenotype (syndrome dimensions from the PCA) and brain structure (source-based morphometry imaging components) using canonical correlation analysis. A = anterior; L = left; P = posterior; R = right.
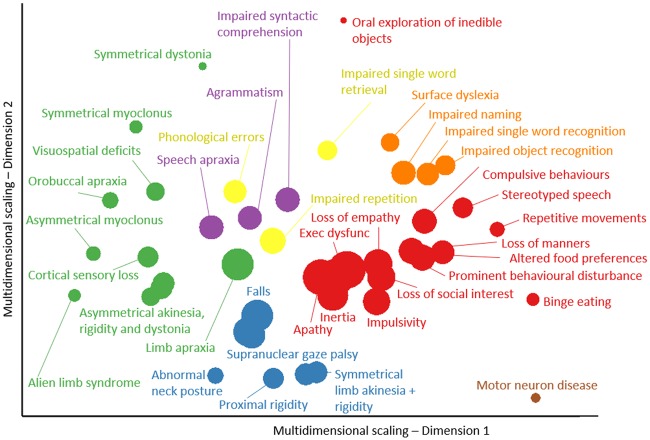
Figure 3.
Cluster analysis and multidimensional scaling of behavioural, language and motor impairments in FTLD. Each feature is colour-coded by FTLD subtype (same colour codes as Fig. 1) based on the primary diagnostic criteria to which the symptom contributes. The size of each point is scaled based on its prevalence in the cohort (larger icons have a higher prevalence). Symptoms from each FTLD syndrome cluster together, but many features are also closely located to those from other syndromes.
Second, we examined patterns of covariation in clinical features (Perry et al., 2017; Grisanzio et al., 2018; Schumacher et al., 2019). To reduce the dimensionality of the dataset, we grouped the presence of clinical symptoms and signs into 25 groups by summing the number of features present in each group. Clinical feature groups were defined a priori as those that were very closely related or were grouped together in the diagnostic criteria. For example, we grouped apathy and inertia into an ‘apathy’ feature group. A full list of clinical symptoms and signs and their groupings are provided in the Supplementary material. The clinical feature group scores, ACE-R and CBI-R results were standardized into z-scores then entered into a principal component analysis (PCA). A Kaiser-Meyer-Olkin test determined the suitability of our dataset for PCA. We selected six components using Cattell’s criteria then performed varimax rotation.
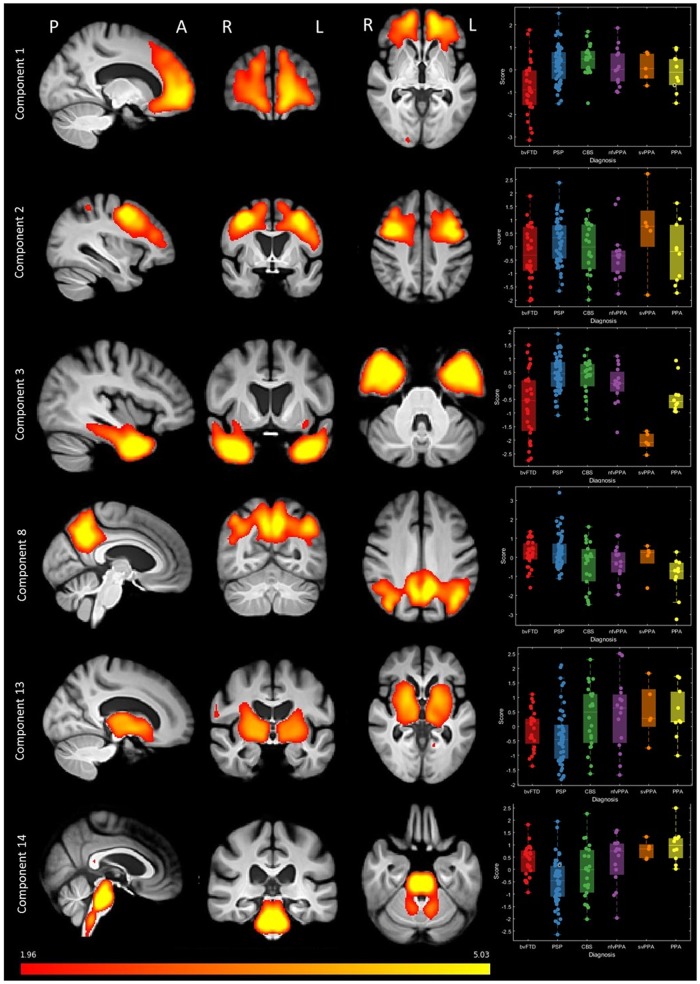
Third, we characterized patterns of covariation in grey and white matter atrophy across all participants. We used GIFT software to perform source-based morphometry, a multivariate alternative to voxel-based morphometry, which uses independent component analysis (Xu et al., 2009). Source-based morphometry was performed on the preprocessed images (see ‘Imaging analysis’ section for details). We extracted 15 independent components of covarying brain atrophy (Fig. 5), and confirmed their reliability using ICASSO with 100 repetitions (Himberg et al., 2004).
Figure 5.
Source-based morphometry (based on independent component analysis) of combined grey and white matter. A subset of components is shown (all components are provided in the Supplementary material). Fifteen components were selected, each representing a region of independently covarying grey and white matter atrophy. Images are standardized group spatial maps for each component, superimposed on an average of all brain images. The scatter-box plots show the standardized subject loading coefficients, grouped by FTLD syndrome subtype.
Fourth, we examined the relationship between clinical phenotype and brain atrophy (Fig. 6). We used canonical correlation analysis (CCA) to relate the six principal components of clinical features (Fig. 4) and the 15 imaging components (Fig. 5) (Tsvetanov et al., 2018). All inputs were standardized into z-scores before CCA. Pearson’s correlations were corrected for multiple comparisons using the false discovery rate (mafdr function in MATLAB 2018b).
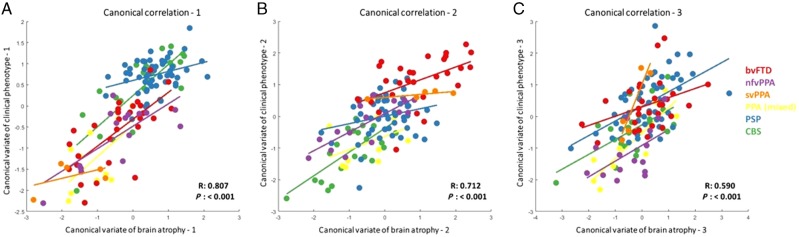
Figure 6.
Structure-phenotype associations using canonical correlation analysis with phenotypic (syndrome dimensions from PCA) and structural (atrophy components from source-based morphometry) information. Three canonical correlation components were selected, each composed of multiple imaging and clinical phenotype components. (A) First canonical correlation. Atrophy in the motor cortex and brainstem had the greatest loading onto the imaging component. Syndrome dimensions 3 (PSP-like motor features) and 4 (CBS-like motor features) had positive loadings and syndrome dimension 2 (global cognitive impairment) had negative loading on the clinical component. (B) Second canonical correlation. Atrophy in the frontal and temporal lobes had the greatest loading on the imaging component. On the clinical component, syndrome dimension one (behavioural impairment) had positive loadings. (C) Third canonical correlation. A spread of cortical and subcortical atrophy components loaded on the imaging component and syndrome dimensions 1–3 contributed to the clinical component. Plots of loadings onto all imaging and clinical components are provided in the Supplementary material.
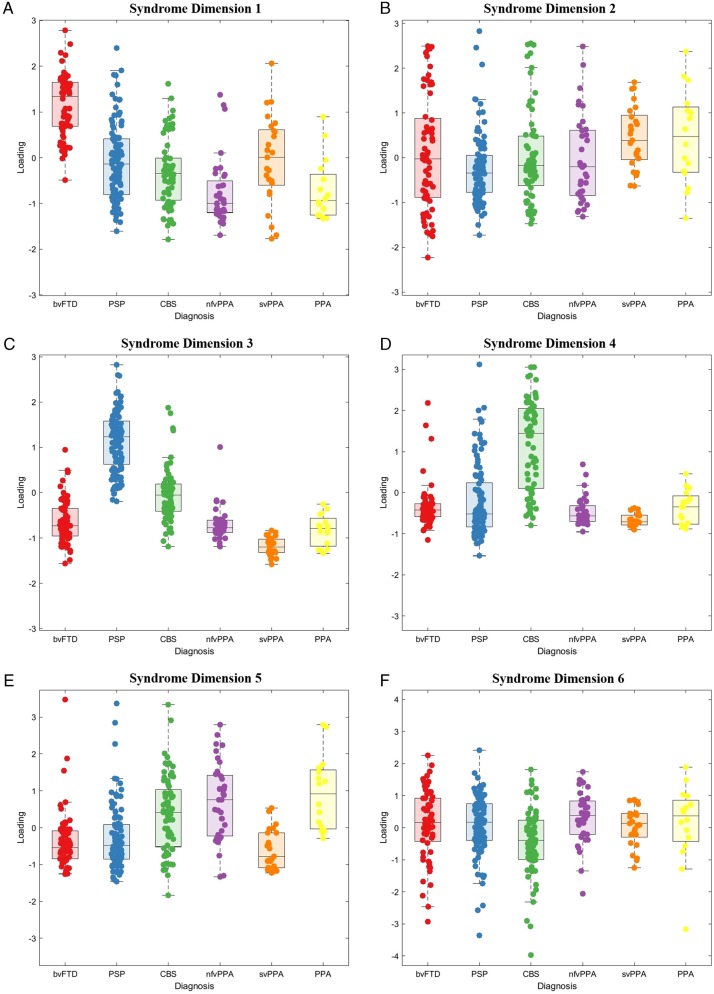
Figure 4.
Principal component analysis scores of clinical features in FTLD syndromes. Six principal components (A–F) were selected. (A) Syndrome dimension 1: clinician and carer ratings of behavioural impairment. (B) Syndrome dimension 2: global cognitive impairment, composed of all ACE-R subscores. (C) Syndrome dimension 3: supranuclear gaze palsy, postural stability and symmetrical rigidity (positive loading) and semantic language impairment (negative loading). (D) Syndrome dimension 4: asymmetrical parkinsonism, dystonia, myoclonus with limb apraxia, cortical sensory loss and alien limb syndrome. (E) Syndrome dimension 5: agrammatic, apraxic and logopenic language impairments. (F) Syndrome dimension 6: carer ratings of low mood and abnormal beliefs.
Finally, we examined longitudinal change in clinical feature component scores in 46 patients who were reviewed twice. We converted follow-up scores into z-scores based on the baseline data, by matching each score to the respective baseline z-score. This ensured that follow-up values were comparable to the baseline (cross-sectional) dataset. We multiplied these standardized follow-up z-scores by the baseline principal component coefficients to estimate follow-up principal component scores.
All patients had a clinical phenotypic assessment but other measures (including ACE-R and CBI-R) were subject to missing data. Missing data (6.32% of the total dataset) were imputed using trimmed scored regression (Folch-Fortuny et al., 2016) using the partial dataset of that participant as predictors. All statistical and imaging analysis was performed in MATLAB 2018b (MathWorks, USA) apart from ANOVA and chi-squared tests, which were performed in JASP (version 0.9.2).
Data availability
Anonymized data are available on reasonable request for academic (non-commercial) purposes, although restrictions may apply to adhere to participant consent and anonymity.
Results
A detailed epidemiological assessment of FTLD syndromes in the study area has previously been reported (Coyle-Gilchrist et al., 2016). Further demographic details of the study cohort, including the later recruitment period, are shown in Table 1.
Table 1.
Demographics of the study cohort
| All FTLD | bvFTD | nfvPPA | svPPA | PPA (lv or mixed) | PSP (all) | CBS | P-value | |
|---|---|---|---|---|---|---|---|---|
| Total in catchment area, n | 365 | 81 | 40 | 28 | 16a | 123 | 77 | – |
| Clinical phenotyping n (% of total population) | 310 (85) | 64 (79) | 36 (93) | 25 (89) | 16 (100) | 101 (82) | 68 (88) | ns* |
| Age, years, mean (SD) | 70.26 (8.57) | 64.59 (9.56) | 72.09 (8.81) | 67.55 (6.43) | 70.80 (7.05) | 72.56 (7.14) | 72.08 (7.69) | <0.001 |
| Male/female | 152/158 | 33/31 | 15/21 | 14/11 | 7/9 | 56/45 | 27/41 | ns |
| Duration of symptoms, years, mean (SD) | 4.75 (3.18) | 5.70 (4.45) | 2.83 (1.93) | 4.96 (2.69) | 2.76 (1.97) | 4.50 (2.94) | 4.71 (2.77) | ns |
| Time from diagnosis to study review, mean (SD) | 1.44 (2.77) | 1.88 (3.88) | 1.09 (1.27) | 1.65 (2.01) | 1.58 (1.67) | 1.02 (1.17) | 1.73 (2.02) | ns |
| MRI scan (% of phenotyped patients) | 133 (43) | 28 (44) | 15 (41) | 5 (20) | 10 (62) | 53 (52) | 22 (32) | ns** |
lvPPA n = 7, mixed PPA n = 9.
P-values are the result of ANOVA or χ2 test for each row on FTLD subgroups: ns = not significant (P > 0.05); *ANOVA of percentage of total population in each group; **ANOVA of percentage of phenotyped patients in each group.
We assessed in person 85% (310/365) of the patients identified as living in the study catchment area with a FTLD syndrome. Fifty-eight patients had a diagnosis of definite FTLD, either by subsequent post-mortem pathological diagnosis (n = 49) or a causative genetic mutation on clinical genetics tests. Neuropathology details of the cohort are given in the Supplementary material.
Sixty-two per cent (n = 194) met core diagnostic criteria for more than one syndrome, with patients meeting the inclusion criteria for two (n = 112), three (n = 69) or four (n = 13) diagnoses (Fig. 1C and D). The most commonly overlapping syndromes were PSP and CBS (n = 76), bvFTD and either PSP (n = 60) or svPPA (n = 38), and nfvPPA with either CBS (n = 56) or PSP (n = 51).
We used cluster analysis to investigate how closely clinical features related to each other. Multidimensional scaling of clinical features (across all patients) broadly recapitulated the phenotypic clustering as represented by the classical phenotypes of each syndrome (Fig. 3). However, there were also many close links between signs conventionally associated with distinct diagnoses. For example, progressive behavioural change, apathy, inertia and impulsivity (typical of bvFTD), were close to symmetrical parkinsonism, falls, axial rigidity and a supranuclear gaze palsy (typical of PSP). Other features suggestive of bvFTD (socially inappropriate and compulsive behaviour and stereotypy of speech), were close to features typical of svPPA features (impaired naming, single word comprehension and object recognition). PSP and CBS features were closely linked, while speech apraxia, agrammatism and impaired syntactic comprehension (indicative of nfvPPA) overlapped with limb apraxia (indicative of CBS).
First, we sought latent syndromic dimensions using PCA of the phenotypic data. Six principal components were identified using Cattell’s criteria, each representing a group of covarying features encompassing symptoms, signs, ACE-R and CBI-R scores (varimax-rotated component matrix in the Supplementary material). These six components explained 58.52% of the variance in the dataset (Kaiser-Meyer-Olkin = 0.86). Syndrome dimension 1 (Fig. 4A) reflected clinician and carer ratings of behaviour and personality change, with executive dysfunction, impulsivity and disinhibition, loss of empathy, stereotyped behaviours, hyperorality and dietary change, apathy, endorsements of abnormal behaviour, altered eating habits and stereotypic and motor behaviour subscales. This ‘behaviour’ dimension was expressed strongly by patients with bvFTD, but also a high proportion of PSP, CBS and svPPA patients. Some patients in these latter groups had weightings similar to bvFTD. Syndrome dimension 2 (Fig. 4B) reflected global cognitive function, with negative loadings from ACE-R subscores. Carer ratings of everyday function and memory also had positive loading onto this dimension (higher CBI-R score, reflecting greater impairment). There was wide variation in this dimension’s weighting across all groups, with higher scores reflecting worse cognitive impairment.
Dimension 3 (Fig. 4C) reflected axial rigidity, postural instability and a supranuclear gaze palsy (positive loading) in the absence semantic language impairments (negative loading). Thus, patients with typical PSP and typical svPPA lie at opposite ends of this dimension, with high and low scores, respectively. However other groups had a spread of scores, many patients with CBS had very high scores (PSP-like). Some bvFTD had high scores indicating a PSP-overlap, while others had low scores, implying presence of semantic impairment.
Positive scores on syndrome dimension 4 (Fig. 4D) represented asymmetrical parkinsonism, dystonia and myoclonus with cortical features of apraxia, cortical sensory loss and alien limb syndrome. Patients with CBS and a subset of patients with PSP had high scores in this dimension. Dimension 5 (Fig. 4E) represented language impairments typified by agrammatic, apraxic and logopenic speech with motor features (myoclonus and limb apraxia). Patients with CBS, nfvPPA, logopenic variant and mixed PPA had high weighting on this dimension, as did a small subset of those with clinical diagnoses of PSP and bvFTD. Finally, dimension 6 explained less variance than the other components and represented primarily carer ratings of mood and abnormal beliefs (Fig. 4F). The distribution of neuropathologically-confirmed cases is shown in the Supplementary material, section 5.
Second, we investigated the structural changes associated with FTLD, and their associations with the clinically orientated syndromic dimensions. The scanned subset of participants was similar to the population without a scan, with no statistically significant differences in age (t = 0.65, P = 0.52), sex (χ2 = 2.8, P = 0.1), disease duration (t = 0.69, P = 0.49) or scores on syndrome dimensions 1–3, 5 and 6 (all P > 0.05 uncorrected). A difference in syndrome dimension 4 (t = 2.41, P = 0.02) indicated less severe global cognitive impairment in those who were scanned. Source-based morphometry revealed 15 significant structural components, each representing a pattern of covarying atrophy (Fig. 5 and Supplementary material). The components had high stability across 100 ICASSO runs (mean = 0.981, standard deviation = 0.004). The loadings on these imaging components were not confined to single diagnostic groups.
Imaging components 1 and 2 related to the frontal and prefrontal cortex; patients with bvFTD tended to have low scores on these components (i.e. atrophy), but many patients with nfvPPA, PSP and CBS also had low scores indicating a frontal cortical atrophy (Fig. 5). Component 3, with bitemporal atrophy, had very strong negative scores in all svPPA patients, but also many bvFTD patients. Some participants with CBS, nfvPPA and PPA had negative scores on imaging component 8, which reflected biparietal atrophy. Imaging component 13 represented the volumes of corticospinal tracts and basal ganglia. Many patients with PSP, but also some patients with bvFTD, CBS and nfvPPA had low scores on this component. Component 14 represented brainstem atrophy, with large negative scores in PSP and CBS but also some nfvPPA patients. The distribution of neuropathologically-confirmed cases is shown in the Supplementary material, section 6.
Third, we looked for structure-function correlations between the clinical and imaging components, in the subset of participants with MRI. As both cognition and atrophy are intrinsically multivariate, we used canonical correlation analysis between the six cognitive dimension and 15 atrophy components. Three canonical correlations were selected for further analysis (each P < 0.05, rejecting the null hypothesis that the canonical correlation is zero). The first canonical correlation (R = 0.81, P < 0.001) represented the association between motor impairments (syndrome dimensions 3 and 4) and relatively preserved cognition (syndrome dimension 2) with motor cortex and brainstem atrophy (atrophy components 6 and 14). Patients with PSP, CBS and some patients with bvFTD had positive loadings, while patients with PPA (notably the svPPA subtype) and some with bvFTD had negative loadings (Fig. 6A). Four of six FTLD subgroups had significant correlations in this canonical correlation: PSP (Pearson’s R = 0.33, P = 0.03), CBS (R = 0.81 P < 0.001), bvFTD (R = 0.70 P < 0.001) and nfvPPA (R = 0.75 P = 0.03) (all results are provided in Supplementary material, section 4).
The second canonical correlation (R = 0.71, P < 0.001) represented another spectrum of cognitive and motor phenotypes correlating with a different pattern of atrophy (Fig. 6B). Positive loadings (most common in bvFTD, svPPA and some PSP) linked behavioural impairment (syndrome dimension 1) with atrophy in the frontal and temporal lobes (atrophy components 1 and 3). Negative loadings (most common in CBS, nfvPPA and mixed PPA) linked global cognitive impairment, apraxia, cortical sensory loss and language impairments with atrophy in the parietal cortex (atrophy components 7 and 8); bvFTD (R = 0.49, P = 0.02), nfvPPA (R = 0.79 P = 0.001) and CBS (R = 0.7, P = 0.001) most contributed to this canonical variate.
The third canonical correlation (R = 0.58 P < 0.001) represented a combination of behavioural, cognitive and motor symptoms in association with atrophy in motor and parietal cortices, basal ganglia and brainstem (Fig. 6C). This canonical correlation had positive loadings across a wide range of diagnoses. This canonical correlation was driven by CBS (R = 0.62 P = 0.005), PSP (R = 0.54 P < 0.001) and PPA (R = 0.87 P = 0.003) subgroups with a weaker contribution from svPPA (R = 0.91, P = 0.048), nfvPPA (R = 0.54, P = 0.06) and bvFTD (R = 0.37, P = 0.07). The three residual, unselected canonical covariates did not correlate in any FTLD subgroup. The distribution of neuropathologically confirmed cases is shown in the Supplementary material, section 7.
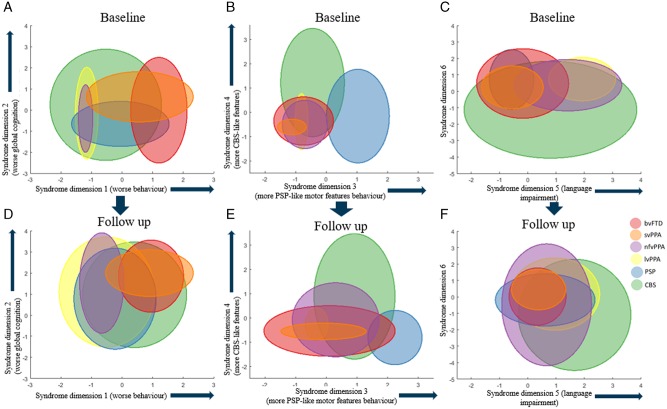
The final analysis considered the longitudinal change in the 46 patients who were alive and assessed in both 2013–14 and 2017–18. The mean time between assessments was 3.6 years (standard deviation 0.87 years). At baseline, patients with follow-up were younger (mean 67.0 versus 70.9, t = 2.8, P = 0.005) but had similar sex ratio and disease duration to those without follow-up. Patients with follow-up had lower scores on syndrome dimension 3 (t = 3.55, P < 0.001), with fewer PSP cases (χ2 = 3.94, P < 0.05). The other five dimension scores at baseline were not different between patients with and without follow-up. Between first and second assessments there was progression in all syndrome dimensions across all groups. At the second assessment there was greater overlap between diagnostic groups, across all syndrome dimensions (Fig. 7). More patients met two or more sets of diagnostic criteria (after removing mutual exclusivity criteria) at follow-up (n = 42) compared to baseline (n = 33) (χ2 with Yates correction = 4.618, P = 0.031).
Figure 7.
Longitudinal phenotype information. A subset of patients was assessed at two time points. Three arbitrary pairs of syndrome dimensions are given to illustrate the convergence of clinical phenotype in syndrome dimensions at follow-up. Each ellipse shows the 95% confidence intervals of the syndrome dimension scores for each FTLD subgroup at baseline and follow-up. At follow-up there was greater overlap across all FTLD syndromes in all syndrome dimensions.
Discussion
Using a data-driven analysis of cross-sectional phenotypes, this epidemiologically-based study revealed that the common syndromes associated with FTLD are not discrete in their clinical features or structural brain changes (Fig. 1A), but instead exist as a multidimensional spectrum (Fig. 1B). Many patients displayed the diagnostic features for multiple diagnoses (Fig. 1C and D). The dimensions of behaviour, movement and language features occurred to varying degrees across all the major diagnostic groups. Differences between groups were expressed by different weightings along these spectra, rather than by categorical clinical or imaging features.
Despite the continuity among patient phenotypes, the clinical syndromes are not random associations. There were close associations between sets of cognitive, behavioural, language and motor features, which are reminiscent of the classical phenotypes (Fig. 2). For example, syndrome dimension 3 represents supranuclear gaze palsy, falls, akinesia and preserved semantics, typical of PSP-Richardson’s syndrome. However, 44% of CBS patients expressed this pattern to the same degree as PSP patients. The recognition of such overlap has contributed to the development of intermediate diagnoses like PSP-CBS (Höglinger et al., 2017) and CBS-PSP (Armstrong et al., 2013) but our results indicate that such overlap is common rather than exceptional. However, not all potential intermediate phenotypes occur. For example, a supranuclear gaze palsy, axial and symmetrical limb rigidity rarely coexist with semantic impairment, a combination that has been reported only in exceptional cases of mixed tau and TDP43 pathology (Snowden et al., 2019).
We propose that a spectral approach is critical to understand the biological basis of the complex clinical syndromes, and to target future therapies appropriately. Rather than focus on the determinants of disease or treatment by diagnosis, one can focus on the determinants and treatment of the syndromic dimensions, in whichever diagnostic ‘group’ these dimensions are expressed. To do otherwise risks the misdirection of a treatment or the dilution of the effects of aetiological factors, whether genetic, environmental, or aggregate of pathogenic proteins. In other words, one could understand and potentially treat the ‘PSP-like’ features whether they occurred in the context of clinically diagnosed PSP-Richardson’s syndrome, CBS or bvFTD.
We do not suggest that the current diagnostic criteria are invalid. Instead, our results highlight the limitations of a categorical approach to diagnosis when the disorders are inherently multivariate spectra in their clinical and imaging features. Nor do our data suggest incorrect diagnosis: although only 49 of the patients have had post-mortem examination, the results confirmed clinicopathological correlations in keeping with the literature [very high for PSP (Gazzina et al., 2019) and svPPA (Spinelli et al., 2017), predominantly corticobasal degeneration or Alzheimer’s disease pathologies for CBS (Alexander et al., 2014), and either tau or TDP43 pathologies for bvFTD (Perry et al., 2017)]. Indeed, the symptom-based data-driven cluster analysis broadly reproduced the diagnostic criteria. But, the relative weightings on such clusters were graded, which highlights the difficulties when applying diagnostic criteria to patients with intermediate or mixed phenotypes (Spinelli et al., 2017).
Our analysis did not differentiate features that are more salient to a clinician (e.g. supranuclear gaze palsy) from those that are more salient to a relative or carer (e.g. behavioural disturbance, non-fluent aphasia or falls). This difference in perspective is relevant to diagnostic labelling. For example, a patient with apraxia, akinesia, dystonia and non-fluent agrammatic speech might be diagnosed as CBS or nfvPPA according to the dominant clinical features: but whose opinion on dominance matters most, the patient, carer or clinician? This is complicated further by the change in insight associated with many FTLD syndromes (O’Keeffe et al., 2007). A further complication for the categorical approach to diagnosis is the evolution of behavioural, motor or language features over time, which raises the question of whether the diagnosis label should be changed or complemented by a secondary, parallel diagnosis. Our approach largely resolves this issue by taking a transdiagnostic approach based on clinical and/or imaging domains, which we consider below.
The data-driven approach identified close clustering of the clinical features and six latent syndrome dimensions that demonstrated the high degree of overlap across FTLD syndromes. Behavioural features were closely clustered and loaded onto one syndrome dimension. However, they also clustered near cognitive and motor symptoms/signs. Apathy and impulsivity had a close link, reflecting the fact that they often coexist, rather than representing opposite ends of a hyper-hypo-kinetic spectrum (Lansdall et al., 2017). Many patients had apathy, which lay near the centre of the multidimensional scaling plot (Fig. 3), suggesting that it is related similarly to other features across FTLD syndromes. The behavioural syndrome dimension was expressed across multiple groups and was not restricted to the subset of the cohort with bvFTD (Fig. 4A). Furthermore, not all patients with bvFTD had very high scores on this behavioural syndrome dimension. Those with lower behaviour scores, but a clinical diagnosis of bvFTD, may represent bvFTD with prominent apathetic/dysexecutive symptoms (O’Connor et al., 2017), or reflect more advanced disease when some of the more florid behavioural changes are less pronounced (O’Connor et al., 2016). Many patients with PSP and CBS had high scores on this syndrome dimension. Behavioural changes in PSP and CBS are well recognized (Burrell et al., 2014), but are often thought to be mild. Our findings suggest that behavioural impairments in PSP and CBS can be prominent: some patients with PSP and CBS had higher scores on this syndrome dimension than patients with bvFTD. Behavioural features coexisted with all other FTLD-related features. Global cognitive impairment was represented by syndrome dimension 2. The ACE-R subscores and carer ratings of everyday skills and memory loaded onto this dimension. However, the reasons for low ACE-R scores may vary depending on which other symptom profiles are expressed: a low score on the ACE-R could be due to progressive dementia or caused by severe behavioural (syndrome dimension 1) or language (dimension 5) or motor (dimensions 3 and 4) impairment, all of which would interfere with the test session.
Our results are also relevant to the current nosology of primary progressive aphasias. Semantic impairments loaded onto a different syndrome dimension and clustered separately from the language impairments associated with nfvPPA and lvPPA. This provides partial support for the current distinction between svPPA and other forms of PPA. However, nfvPPA and lvPPA were not readily distinguished by the data-driven analysis—as has been noted in a previous independent cohort (Sajjadi et al., 2012). In contrast, patients with svPPA were similar to bvFTD in many respects (Fig. 4). Compulsive behaviours, stereotyped speech and simple repetitive habits were closely linked to semantic language impairments, including object recognition and single word comprehension (Harris et al., 2016). Other language features, including impaired syntactic comprehension, agrammatism and speech apraxia, were closely related to CBS-like motor features (syndrome dimension 3), in CBS, PSP, and nfvPPA groups—in keeping with the well characterized overlap of non-fluent (Rohrer et al., 2010a, b) and apraxic (Josephs et al., 2006, 2012) speech with PSP and CBS (Armstrong et al., 2013; Respondek and Höglinger, 2016; Peterson et al., 2019). The PPA diagnostic criteria require that language impairments are the most prominent clinical feature and the principal cause of difficulty with activities of daily living. This may not be the case in some patients with svPPA; although clinicians may note prominent semantic impairments, coexistent behavioural impairment may be more conspicuous to relatives or carers and have a greater impact on independence and daily living. In addition, we report the practical difficulties applying the current PPA diagnostic criteria. In our epidemiological-based cohort, 19 patients met criteria for primary progressive aphasia (Gorno-Tempini et al., 2011) but not one of the PPA subtypes. The current diagnostic criteria are stringent and require the presence and absence of multiple language features. Patients with language symptoms may have very isolated deficits (Josephs et al., 2012) or at the other extreme multiple impairments which span more than one PPA subtype, even at diagnosis (Utianski et al., 2019).
Many studies have correlated clinical syndromes with structural change, using computational morphometry on volume, thickness, curvature or cortical diffusivity. Typically, these compare patient groups to each other or to controls, to reveal group-based patterns of atrophy in bvFTD (Schroeter et al., 2007; Whitwell et al., 2012; Ranasinghe et al., 2016b; Meeter et al., 2017; Perry et al., 2017; Chen et al., 2018b; Illán-Gala et al., 2019), svPPA (Gorno-Tempini et al., 2004; Schroeter et al., 2007; Kumfor et al., 2016), nfvPPA (Gorno-Tempini et al., 2004; Schroeter et al., 2007; Santos-Santos et al., 2016), PSP (Brenneis et al., 2004; Lagarde et al., 2013; Piattella et al., 2015; Dutt et al., 2016; Whitwell et al., 2017a, 2019) and CBS (Josephs et al., 2010; Whitwell et al., 2010; Dutt et al., 2016). However, these previous methods are limited by the categorical approach to diagnosis. To reveal the associations between phenotypic features and structural change, across diagnostic groups, we used source-based morphometry to identify regions of covarying atrophy patterns (Xu et al., 2009). We confirmed our hypothesis that individual atrophy patterns are not confined to specific diagnostic groups. Our imaging cohort was generally representative of the whole FTLD population, with similar weightings across five of six dimensions and demographics. Participants who underwent MRI were less affected in the global cognitive impairment syndrome dimension, likely due to the practical difficulties of scanning participants with advanced dementia. Frontal lobe atrophy patterns were seen in participants from all groups, especially bvFTD and PSP. Subcortical atrophy was more prevalent in PSP and CBS but was also seen in bvFTD and PPA, and a majority of bvFTD patients had negative scores on the basal ganglia imaging component. This has been noted previously in symptomatic bvFTD and PPA (Schroeter et al., 2007; Bocchetta et al., 2018), and those at genetic risk of FTD (Rohrer et al., 2015). Brainstem atrophy, while characteristic of PSP (Whitwell et al., 2017a), was also seen in some patients with CBS and nfvPPA, but this has previously been shown not to predict PSP pathology (Whitwell et al., 2013). The source-based morphometry approach also revealed a group of patients who are not well accommodated in the current diagnostic criteria. Five patients with a nominal diagnosis of bvFTD had very low scores on the right temporal lobe imaging component, and we suggest that these might better be called the right variant of semantic dementia, which causes a combination of behavioural and semantic impairments with prosopagnosia (Chan et al., 2009; Kumfor et al., 2016). A subset of patients with CBS and mixed PPA had negative scores on component 8, indicating posterior cortical atrophy. These patients may be more likely to have Alzheimer’s disease pathology (Lee et al., 2011).
We identified three significant canonical ‘structure-function’ correlations in the cohort (Fig. 6). These represent the spectrums of anatomical change underlying behavioural, motor and language impairments. These structure-function correlations did not replicate classical nosological distinctions. Instead they provide an alternative data-driven approach with which to understand and target treatments for syndromes associated with FTLD. The first canonical correlation found an association between motor cortex and brainstem atrophy with PSP or CBS-like motor impairments. Unsurprisingly, PSP and CBS had significant correlations between these canonical covariates but so did bvFTD and nfvPPA, reflecting the motor impairments that are seen in a subgroup of these patients. The second canonical correlation represented the spectrum between frontotemporal (positive scores) and posterior cortical atrophy (negative scores). This canonical covariate may differentiate FTLD from Alzheimer’s disease pathology, as negative scores on this imaging covariate resemble an Alzheimer’s disease-like atrophy pattern (Supplementary material, section 7). The third canonical covariate was associated with significant correlations in all FTLD subgroups apart from bvFTD, and encompassed a range of cognitive, behaviour and motor clinical features associated with cortical and subcortical atrophy.
Longitudinal analysis in a subset of patients confirmed that, with disease progression, overlap between FTLD phenotypes increases (Kertesz et al., 2005). A greater number of patients met criteria for several FTLD subtypes compared to first assessment and there was greater overlap between all syndrome dimensions (Fig. 7). Our transdiagnostic approach allows disease progression to be more accurately represented, in terms of worsening clinical features rather than conflicting diagnoses. Assessing FTLD syndromes in isolation, without reference to the whole FTLD syndrome spectrum, risks missing evolving signs of other FTLD syndromes and therefore underestimating disease severity. The time between the two phenotypic assessments was relatively long (mean 3.6 years) given the mean survival in FTLD syndromes (Coyle-Gilchrist et al., 2016). We acknowledge that the longitudinal analysis may be biased towards patients with slowly progressive disease (i.e. survivors over the assessment interval). Indeed, patients with follow-up were younger than those without and included fewer with PSP, which on average has a worse prognosis than other FTLD syndromes (Lansdall et al., 2019).
A strength of our analysis is that it is embedded within an epidemiological cohort study with multi-source identification and recruitment. Previous structure-function studies of these disorders may have been influenced by low sample sizes and selection bias, by focusing only on patients at earlier disease stages who are well enough to attend subspecialist research centres for detailed phenotypic assessment. The representativeness in our study may partly explain why many of our patients overlapped diagnostic criteria.
Our study also has several limitations. Applying multiple diagnostic criteria across all patients raises challenges. For example, the criteria often incorporate an exclusion clause, that the illness is ‘not better explained by another diagnosis’. We lifted this criterion and applied the clinical features to the other positive and negative criteria. Patients may have symptoms or signs that do not quite reach the threshold needed to meet a diagnostic criterion. Our approach was to try to apply the same threshold in all groups, in asserting the presence of a symptom or sign. We included continuous measures of cognitive (ACE-R) and behavioural (CBI-R) but not motor symptoms’ severity. Severity scales for parkinsonism may be weighted towards specific illnesses (e.g. the PSP-rating scale for PSP-Richardson’s syndrome), which might bias the weighting of the motor syndrome dimensions across FTLD subtypes. We therefore focused on the presence, not severity, of individual symptoms, noting that the diagnostic criteria also do not operationalize severity. We also grouped together language features for the PCA (e.g. apraxic and agrammatic speech), which may have made it more difficult to distinguish motor-only PPA subtypes, noting that our a priori grouping was supported post hoc by the data-driven cluster analysis. Our assessment of clinical features was cross-sectional, rather than a retrospective estimate of presenting features. Some of the diagnostic criteria (e.g. for PPA, Gorno-Tempini et al., 2011) refer to the dominance of a symptom cluster (e.g. language disorder) at presentation. This sounds straightforward, but the time of presentation varies widely, is often late (Coyle-Gilchrist et al., 2016), and is partially dependent on variations in healthcare services, referral pathways and public awareness of symptoms’ significance (Bradford et al., 2009). These factors interfere with the ability of symptomatology to inform the diagnosis and likely pathology, especially in overlap syndromes such as CBS-NAV (non-fluent/agrammatic variant), or PSP-F. This transdiagnostic approach to FTLD may not be appropriate in all situations, for example trials of treatments targeting a specific proteinopathy. Robust biomarkers that can differentiate between, for example FTLD-tau and FTLD-TDP43, are thus far lacking (Bevan-Jones et al., 2017; Meeter et al., 2017). Currently, trials focus recruitment on subsets of patients with strong clinicopathological correlation such as PSP-Richardson’s syndrome (Boxer et al., 2019). However, this limits patient access to drug trials, given the poor clinicopathological correlation in many FTLD syndromes. More accurate biomarkers, whether PET, CSF or blood-based (Meeter et al., 2017; Leuzy et al., 2019), would facilitate transdiagnostic approaches and accurate drug targeting while maximizing power and generalizability of results. A further limitation is the small number to date with post-mortem confirmation of pathology. As set out in the Supplementary material, the 49 neuropathological results are in line with the literature for each syndrome, but with only 21 of the 49 also having MRI, there was insufficient power for predictive models of pathology.
Research related to disease nosology often raises the issue of whether to ‘lump’ disorders together or to ‘split’ them into subtypes (Scaravilli et al., 2005). The decision to lump or split can reveal insights into the neurobiology of disease. But, lumping and splitting can also obscure insights. We propose an alternative approach, with data-driven spectral analyses, that neither lump nor split arbitrarily, but allow phenotypic and imaging variance to elucidate pathogenesis of cognitive syndromes. We acknowledge that our brain metrics are only crude measures of atrophy. Other brain measures, of tau burden (Passamonti et al., 2017; Whitwell et al., 2017b; Bevan-Jones et al., 2020), synaptic density (Chen et al., 2018a), physiology (Hughes et al., 2018; Sami et al., 2018) and functional connectivity (Seeley et al., 2009; Rittman et al., 2019) may enrich the source-based morphometric approach, integrating PET markers of pathology (Passamonti et al., 2019) or spectroscopic measures of the neurotransmitter deficits in FTLD (Kantarci et al., 2010; Murley and Rowe, 2018). Genetic information could further inform the multivariate analysis of phenotype, mindful that while bvFTD has a strong genetic component, svPPA and PSP do not (Rohrer et al., 2009). An additional limitation is the potential for multiple pathologies, in which several pathogenic protein inclusions may coexist and be synergistic in neurodegeneration (Robinson et al., 2018).
In conclusion, we have presented evidence from a transdiagnostic, data-driven approach to the clinical and structural phenotypes in syndromes associated with FTLD. Patient categorization and selection should depend on the study or question of interest (Husain, 2017; Coulthard and Love, 2018), but for understanding the origin of symptoms, designing symptomatic treatment, and assessment of diagnostic biomarkers, we suggest that the more relevant outcomes are the data-driven axes of disease. Clinical heterogeneity and phenotypic variance are ‘noise’ in category-based analysis of disease and treatment effects, and undermine the observation of effects. However, the same variance can be informative in terms of a spectrum of structure-function abnormality, complementing data-driven approaches to characterize neurodegenerative disease using neuropathological features (Cornblath et al., 2019). The data-driven approach provides a comprehensive framework with which to understand disease progression and heterogeneity, and guide treatment.
Supplementary Material
Acknowledgements
We would like to thank the patients and their families and carers, the radiographers at the Wolfson Brain Imaging Centre, University of Cambridge and all the staff at the Cambridge Centre for Frontotemporal Dementia and Related Disorders, University of Cambridge.
Funding
This work was funded by the Holt Fellowship (A.G.M.), British Academy (K.A.T., PF160048), Wellcome Trust (J.B.R., 103838), the PSP Association, the Medical Research Council, the National Institute for Health Research Cambridge Biomedical Research Centre and Cambridge Brain Bank; and the Cambridge Centre for Parkinson Plus.
Competing interests
J.B.R. serves as an associate editor to Brain, and is a non-remunerated trustee of the Guarantors of Brain and the PSP Association (UK). He provides consultancy to Asceneuron, Biogen, UCB and has research grants from AZ-Medimmune, Janssen, Lilly as industry partners in the Dementias Platform UK.
Glossary
- ACE-R =
Addenbrooke’s Cognitive Examination-Revised
- bvFTD =
behavioural variant frontotemporal dementia
- CBS =
corticobasal syndrome
- FTLD =
frontotemporal lobar degeneration
- lv/nfv/svPPA =
logopenic/non-fluent/semantic variant primary progressive aphasia
- PCA =
principal component analysis
- PSP =
progressive supranuclear palsy
References
- Alexander SK, Rittman T, Xuereb JH, Bak TH, Hodges JR, Rowe JB.. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J Neurol Neurosurg Psychiatry 2014; 85: 925–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN.. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS Study. Brain 2009; 132: 156–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan-Jones WR, Cope TE, Jones PS, Passamonti L, Hong YT, Fryer TD, et al. [18F]AV-1451 binding in vivo mirrors the expected distribution of TDP-43 pathology in the semantic variant of primary progressive aphasia. J Neurol Neurosurg Psychiatry 2017; 89: 1032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan-Jones WR, Cope TE, Jones PS, Passamonti L, Hong YT, Fryer TD, et al. Neuroinflammation and protein aggregation co-localize across the frontotemporal dementia spectrum. Brain 2020; 143: 1010–26. [DOI] [PMC free article] [PubMed]
- Bocchetta M, Gordon E, Cardoso MJ, Modat M, Ourselin S, Warren JD, et al. Thalamic atrophy in frontotemporal dementia—not just a C9orf72 problem. NeuroImage Clin 2018; 18: 675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer AL, Qureshi I, Ahlijanian M, Grundman M, Golbe LI, Litvan I, et al. Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol 2019; 18: 549–58. [DOI] [PubMed] [Google Scholar]
- Bradford A, Kunik ME, Schulz P, Williams SP, Singh H.. Missed and delayed diagnosis of dementia in primary care. Alzheimer Dis Assoc Disord 2009; 23: 306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneis C, Seppi K, Schocke M, Benke T, Wenning GK, Poewe W.. Voxel based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 2004; 75: 246–9. [PMC free article] [PubMed] [Google Scholar]
- Burrell JR, Hodges JR, Rowe JB.. Cognition in corticobasal syndrome and progressive supranuclear palsy: a review. Mov Disord 2014; 29: 684–93. [DOI] [PubMed] [Google Scholar]
- Butler RA, Ralph MAL, Woollams AM.. Capturing multidimensionality in stroke aphasia: mapping principal behavioural components to neural structures. Brain 2014; 137: 3248–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, et al. The clinical profile of right temporal lobe atrophy. Brain 2009; 132: 1287–98. [DOI] [PubMed] [Google Scholar]
- Chen MK, Mecca AP, Naganawa M, Finnema SJ, Toyonaga T, Lin SF, et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle Glycoprotein 2A Positron Emission Tomographic Imaging. JAMA Neurol 2018. a; 75: 1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kumfor F, Landin-Romero R, Irish M, Hodges JR, Piguet O.. Cerebellar atrophy and its contribution to cognition in frontotemporal dementias. Ann Neurol 2018. b; 84: 98–109. [DOI] [PubMed] [Google Scholar]
- Cordato NJ, Duggins AJ, Halliday GM, Morris JGL, Pantelis C, Melbourne R, et al. Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain 2005; 128: 1259–66. [DOI] [PubMed] [Google Scholar]
- Cornblath EJ, Robinson JL, Lee V-Y, Trojanowski JQ, Bassett DS, Defining and predicting transdiagnostic categories of neurodegenerative disease. bioRxiv 2019; 25: 664250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulthard EJ, Love S.. A broader view of dementia: multiple co-pathologies are the norm. Brain 2018; 141: 1894–7. [DOI] [PubMed] [Google Scholar]
- Coyle-Gilchrist ITS, Dick KM, Patterson K, Vázquez Rodríquez P, Wehmann E, Wilcox A, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 2016; 86: 1736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt S, Binney RJ, Heuer HW, Luong P, Attygalle S, Bhatt P, et al. Progression of brain atrophy in PSP and CBS over 6 months and 1 year. Neurology 2016; 87: 2016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch-Fortuny A, Arteaga F, Ferrer A.. Missing data imputation toolbox for MATLAB. Chemom Intell Lab Syst 2016; 154: 93–100. [Google Scholar]
- Gazzina S, Respondek G, Compta Y, Allinson KS, Spillantini MG, Molina-Porcel L, et al. Neuropathological validation of the MDS-PSP criteria with PSP and other frontotemporal lobar degeneration. bioRxiv 2019: 520510.
- Gerstenecker A, Duff K, Mast B, Litvan I.. Behavioral abnormalities in progressive supranuclear palsy. Psychiatry Res 2013; 210: 1205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh BCP, Calder AJ, Peers PV, Lawrence AD, Acosta-Cabronero J, Pereira JM, et al. Social cognitive deficits and their neural correlates in progressive supranuclear palsy. Brain 2012; 135: 2089–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbe LI, Ohman-Strickland PA.. A clinical rating scale for progressive supranuclear palsy. Brain 2007; 130: 1552–65. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004; 55: 335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm M, Respondek G, Stamelou M, Arzberger T, Ferguson L, Gelpi E, et al. How to apply the movement disorder society criteria for diagnosis of progressive supranuclear palsy. Mov Disord 2019; 34: 1228–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanzio KA, Goldstein-Piekarski AN, Wang MY, Ahmed APR, Samara Z, Williams LM.. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry 2018; 75: 201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halai AD, Woollams AM, Lambon Ralph MA.. Using principal component analysis to capture individual differences within a unified neuropsychological model of chronic post-stroke aphasia: revealing the unique neural correlates of speech fluency, phonology and semantics. Cortex 2017; 86: 275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CJD, Buckley AH, Downey LE, Lehmann M, Zimmerer VC, Varley RA, et al. The language profile of behavioral variant frontotemporal dementia. J Alzheimers Dis 2015; 50: 359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Jones M, Gall C, Richardson AMT, Neary D, et al. Co-occurrence of language and behavioural change in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord Extra 2016; 6: 205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time-series via clustering and visualization. NeuroImage 2004; 22: 1214–22. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive Supranuclear Palsy - The Movement Disorder Society Criteria. Mov Disord 2017; 32: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey ED, Goveia EN, Paviol S, Pardini M, Krueger F, Zamboni G, et al. Executive dysfunction in frontotemporal dementia and corticobasal syndrome. Neurology 2009; 72: 453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes LE, Rittman T, Robbins TW, Rowe JB.. Reorganization of cortical oscillatory dynamics underlying disinhibition in frontotemporal dementia. Brain 2018; 141: 2486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M. Transdiagnostic neurology: neuropsychiatric symptoms in neurodegenerative diseases. Brain 2017; 140: 1535–6. [DOI] [PubMed] [Google Scholar]
- Illán-Gala I, Montal V, Borrego-Écija S, Vilaplana E, Pegueroles J, Alcolea D, et al. Cortical microstructure in the behavioural variant of frontotemporal dementia: looking beyond atrophy. Brain 2019; 142: 1121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, et al. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol 2015; 129: 469–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Jennifer L, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006; 129: 1385–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, MacHulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 2012; 135: 1522–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Boeve BF, Knopman DS, Petersen RC, Hu WT, et al. Anatomical differences between CBS-corticobasal degeneration and CBS-Alzheimer’s disease. Mov Disord 2010; 25: 1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Boeve BF, Wszolek ZK, Rademakers R, Whitwell JL, Baker MC, et al. MRS in presymptomatic MAPT mutation carriers: a potential biomarker for tau-mediated pathology. Neurology 2010; 75: 771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Munoz DG.. Clinical and pathological overlap between frontotemporal dementia, primary progressive aphasia and corticobasal degeneration: the Pick complex. Dement Geriatr Cogn Disord 1999; 10 (Suppl 1): 46–9. [DOI] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG.. The evolution and pathology of frontotemporal dementia. Brain 2005; 128: 1996–2005. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN.. The NIMH research domain criteria initiative: background, issues and pragmatics. Psychophysiology 2016; 53: 286–97. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Landin-Romero R, Devenney E, Hutchings R, Grasso R, Hodges JR, et al. On the right side? A longitudinal study of left-versus right-lateralized semantic dementia. Brain 2016; 139: 986–98. [DOI] [PubMed] [Google Scholar]
- Lagarde J, Valabrègue R, Corvol J-C, Pineau F, Le Ber I, Vidailhet M, et al. Are frontal cognitive and atrophy patterns different in PSP and bvFTD? A comparative neuropsychological and VBM study. PLoS One 2013; 8: e80353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K, Graham N, Dawson K, Hodges JR.. Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer’s disease: a cross-sectional and longitudinal study of 55 cases. Brain 2003; 126: 2350–62. [DOI] [PubMed] [Google Scholar]
- Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, Vázquez Rodríguez P, Wilcox A, Wehmann E, et al. Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain 2017; 140: 1792–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdall CJ, Coyle-Gilchrist ITS, Vázquez Rodríguez P, Wilcox A, Wehmann E, Robbins TW, et al. Prognostic importance of apathy in syndromes associated with frontotemporal lobar degeneration. Neurology 2019; 92: e1547–e1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, Dearmond SJ, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol 2011; 70: 327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzy A, Chiotis K, Lemoine L, Gillberg P-G, Almkvist O, Rodriguez-Vieitez E, et al. Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol Psychiatry 2019; 24: 1112–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010; 119: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeter LH, Kaat LD, Rohrer JD, Van Swieten JC.. Imaging and fluid biomarkers in frontotemporal dementia. Nat Rev Neurol 2017; 13: 406–19. [DOI] [PubMed] [Google Scholar]
- Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR.. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriat Psychiatry 2006; 21: 1078–85. [DOI] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan OK, Coslett HB, et al. Neural organization of spoken language revealed by lesion-symptom mapping. Nat Commun 2015; 6: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murley AG, Rowe JB, Neurotransmitter deficits from frontotemporal lobar degeneration. Brain 2018; 141: 1263–85. [DOI] [PMC free article] [PubMed]
- O’Connor C, Landin-Romero R, Clemson L, Kaizik C, Daveson N, Hodges J, et al. Behavioral-variant frontotemporal dementia: distinct phenotypes with unique functional profiles. Neurology 2017; 89: 570–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CM, Clemson L, Hornberger M, Leyton CE, Hodges JR, Piguet O, et al. Longitudinal change in everyday function and behavioral symptoms in frontotemporal dementia. Neurol Clin Pract 2016; 6: 419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe FM, Murray B, Coen RF, Dockree PM, Bellgrove MA, Garavan H, et al. Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain 2007; 130: 753–64. [DOI] [PubMed] [Google Scholar]
- Park HK, Park K, Yoon B, Lee JH, Choi SH, Joung JH, et al. Clinical characteristics of parkinsonism in frontotemporal dementia according to subtypes. J Neurol Sci 2017; 372: 51–6. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Tsvetanov KA, Jones PS, Bevan-Jones WR, Arnold R, Borchert RJ, et al. Neuroinflammation and functional connectivity in Alzheimer’s disease: interactive influences on cognitive performance. J Neurosci 2019; 39: 7218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Vázquez Rodríguez P, Hong YT, Allinson KSJ, Williamson D, Borchert RJ, et al. 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain 2017; 140: 781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Brown JA, Possin KL, Datta S, Trujillo A, Radke A, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain 2017; 140: 3329–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson KA, Patterson K, Rowe JB.. Language impairment in progressive supranuclear palsy and corticobasal syndrome. J Neurol 2019; 1–14. doi: 10.1007/s00415-019-09463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piattella MC, Upadhyay N, Bologna M, Sbardella E, Tona F, Formica A, et al. Neuroimaging evidence of gray and white matter damage and clinical correlates in progressive supranuclear palsy. J Neurol 2015; 262: 1850–8. [DOI] [PubMed] [Google Scholar]
- Ranasinghe KG, Rankin KP, Lobach IV, Kramer JH, Sturm VE, Bettcher BM, et al. Cognition and neuropsychiatry in behavioral variant frontotemporal dementia by disease stage. Neurology 2016. a; 86: 600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe KG, Rankin KP, Pressman PS, Perry DC, Lobach IV, Seeley WW, et al. Distinct subtypes of behavioral-variant frontotemporal dementia based on patterns of network degeneration. JAMA Neurol 2016. b; 73: 1078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord 2007; 21: S14–8. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Respondek G, Höglinger GU.. The phenotypic spectrum of progressive supranuclear palsy. Park Relat Disord 2016; 22: S34–S36. [DOI] [PubMed] [Google Scholar]
- Respondek G, Stamelou M, Kurz C, Ferguson LW, Rajput A, Chiu WZ, et al. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord 2014; 29: 1758–66. [DOI] [PubMed] [Google Scholar]
- Rittman T, Borchert R, Jones S, van Swieten J, Borroni B, Galimberti D, et al. Functional network resilience to pathology in presymptomatic genetic frontotemporal dementia. Neurobiol Aging 2019; 77: 169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018; 141: 2181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J, Rossor M, Warren J.. Apraxia in progressive nonfluent aphasia. J Neurol 2010. a; 257: 569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Guerreiro R, Vandrovcova J, Uphill J, Reiman D, Beck J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology 2009; 73: 1451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 2011; 134: 2565–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol 2015; 14: 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD.. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J Neurol Sci 2010; 293: 35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JDJ, Paviour D, Bronstein AAM, O’Sullivan SS, Lees A, Warren JD, et al. Progressive supranuclear palsy syndrome presenting as progressive nonfluent aphasia: a neuropsychological and neuroimaging analysis. Mov Disord 2010. b; 25: 179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Ogar JM, Amici S, Rose K, Dronkers N, et al. Behavioral features in semantic dementia versus other forms of progressive aphasias. Neurology 2006; 67: 1752–6. [DOI] [PubMed] [Google Scholar]
- Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ.. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology 2012; 78: 1670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami S, Williams N, Hughes LE, Cope TE, Rittman T, Coyle-Gilchrist ITS, et al. Neurophysiological signatures of Alzheimer’s disease and frontotemporal lobar degeneration: pathology versus phenotype. Brain 2018; 141: 2500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Santos MA, Mandelli ML, Binney RJ, Ogar J, Wilson SM, Henry ML, et al. Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurol 2016; 73: 733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaravilli T, Tolosa E, Ferrer I.. Progressive supranuclear palsy and corticobasal degeneration: lumping versus splitting. Mov Disord 2005; 20: 21–28. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Raczka K, Neumann J, Yves von Cramon D.. Towards a nosology for frontotemporal lobar degenerations-A meta-analysis involving 267 subjects. Neuroimage 2007; 36: 497–510. [DOI] [PubMed] [Google Scholar]
- Schumacher R, Halai AD, Lambon Ralph MA.. Assessing and mapping language, attention and executive multidimensional deficits in stroke aphasia. Brain 2019; 142: 3202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD.. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009; 62: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard RN. Multidimensional scaling, tree-fitting, and clustering. Science 1980; 210: 390–8. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Kobylecki C, Jones M, Thompson JC, Richardson AM, Mann D.. Association between semantic dementia and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 2019; 90: 115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli EG, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 2017; 81: 430–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov KA, Ye Z, Hughes L, Samu D, Treder MS, Wolpe N, et al. Activity and connectivity differences underlying inhibitory control across the adult life span. J Neurosci 2018; 38: 7887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Botha H, Martin PR, Schwarz CG, Duffy JR, Clark HM, et al. Clinical and neuroimaging characteristics of clinically unclassifiable primary progressive aphasia. Brain Lang 2019; 197: 104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear HJ, Wedderburn CJ, Mioshi E, Williams-Gray CH, Mason SL, Barker R. A, et al. The Cambridge Behavioural Inventory revised. Dement Neuropsychol 2008; 2: 102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Höglinger GU, Antonini A, Bordelon Y, Boxer AL, Colosimo C, et al. Radiological biomarkers for diagnosis in PSP: where are we and where do we need to be? Mov Disord 2017. a; 32: 955–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology 2010; 75: 1879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Parisi JE, Gunter JL, Weigand SD, Boeve BF, et al. Midbrain atrophy is not a biomarker of progressive supranuclear palsy pathology. Eur J Neurol 2013; 20: 1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Lowe VJ, Tosakulwong N, Weigand SD, Senjem ML, Schwarz CG, et al. [18F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov Disord 2017. b; 32: 124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Tosakulwong N, Schwarz CG, Botha H, Senjem ML, Spychalla AJ, et al. MRI outperforms [18F]AV-1451 PET as a longitudinal biomarker in progressive supranuclear palsy. Mov Disord 2019; 34: 105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, Dejesus-Hernandez M, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain 2012; 135: 794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD.. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum Brain Mapp 2009; 30: 711–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data are available on reasonable request for academic (non-commercial) purposes, although restrictions may apply to adhere to participant consent and anonymity.