Cell-to-cell transmission of α-synuclein is thought to contribute to the progression of Parkinson’s disease. Guo et al. show that microglial exosomes mediate α-synuclein transmission from microglia to neurons in vitro, in vivo and in human studies, and could thus be a promising therapeutic target.
Keywords: microglia, exosome, α-synuclein, Parkinson’s disease, transmission
Abstract
Accumulation of neuronal α-synuclein is a prominent feature in Parkinson’s disease. More recently, such abnormal protein aggregation has been reported to spread from cell to cell and exosomes are considered as important mediators. The focus of such research, however, has been primarily in neurons. Given the increasing recognition of the importance of non-cell autonomous-mediated neurotoxicity, it is critical to investigate the contribution of glia to α-synuclein aggregation and spread. Microglia are the primary phagocytes in the brain and have been well-documented as inducers of neuroinflammation. How and to what extent microglia and their exosomes impact α-synuclein pathology has not been well delineated. We report here that when treated with human α-synuclein preformed fibrils, exosomes containing α-synuclein released by microglia are fully capable of inducing protein aggregation in the recipient neurons. Additionally, when combined with microglial proinflammatory cytokines, these exosomes further increased protein aggregation in neurons. Inhibition of exosome synthesis in microglia reduced α-synuclein transmission. The in vivo significance of these exosomes was demonstrated by stereotaxic injection of exosomes isolated from α-synuclein preformed fibrils treated microglia into the mouse striatum. Phosphorylated α-synuclein was observed in multiple brain regions consistent with their neuronal connectivity. These animals also exhibited neurodegeneration in the nigrostriatal pathway in a time-dependent manner. Depleting microglia in vivo dramatically suppressed the transmission of α-synuclein after stereotaxic injection of preformed fibrils. Mechanistically, we report here that α-synuclein preformed fibrils impaired autophagy flux by upregulating PELI1, which in turn, resulted in degradation of LAMP2 in activated microglia. More importantly, by purifying microglia/macrophage derived exosomes in the CSF of Parkinson’s disease patients, we confirmed the presence of α-synuclein oligomer in CD11b+ exosomes, which were able to induce α-synuclein aggregation in neurons, further supporting the translational aspect of this study. Taken together, our study supports the view that microglial exosomes contribute to the progression of α-synuclein pathology and therefore, they may serve as a promising therapeutic target for Parkinson’s disease.
Introduction
Pathological hallmarks of Parkinson’s disease include the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the presence of cytoplasmic protein aggregates known as Lewy bodies in the remaining dopaminergic neurons. A major component of Lewy bodies is α-synuclein (α-syn). This small presynaptic protein is central to both familial and sporadic Parkinson’s disease. In the brain, α-syn can exist in several forms including soluble unfolded monomeric and polymeric forms, as well as β-sheet-containing fibrils (Mor et al., 2016). Since Braak proposed a step-by-step scheme of pathological propagation of Parkinson’s disease based on the examination of the distribution of α-syn in the brain autopsy of patients with Parkinson’s disease (Kadam and Chuan, 2016), the proposal that α-syn can spread from one cell to another in a prion-like manner has gained attention. The fibrillar form has been demonstrated to serve as seeding material for α-syn aggregation (Volpicelli-Daley et al., 2011). Indeed, a single intrastriatal injection of α-syn fibrils results in spreading of α-syn with Lewy body-like pathology to anatomically interconnected regions (Luk et al., 2012a). Consequently, cell-to-cell spread of α-syn could be a major mode of disease propagation. This process could be mediated by several mechanisms of cellular release and uptake, including exocytosis, exosomes, tunnelling nanotubes, glymphatic flow and endocytosis (Valdinocci et al., 2017). Among these different modes of intercellular transmission, exosomes facilitate transmission of α-syn over a long distance.
Exosomes are small extracellular vesicles with a typical size of 40–100 nm. Different cell types within the brain have been shown to release exosomes, including neurons, microglia, and astrocytes. Exosomes have been shown to be involved in many disorders, including Parkinson’s disease. Because exosomes can carry cargos such as mRNA and proteins that can influence gene expression and protein activity in recipient cells (Chistiakov and Chistiakov, 2017), they may contribute to the spread of misfolded proteins such as α-syn. It has been demonstrated that α-syn over-expressing neuronal cells can release exosomes capable of transferring α-syn protein to other normal neuronal cells (Alvarez-Erviti et al., 2011) where they can form aggregates and induce death in the receiving cell (Desplats et al., 2009; Hansen et al., 2011). In fact, exosome-associated α-syn oligomers are more likely to be taken up by recipient cells and are more neurotoxic compared to free α-syn oligomers (Danzer et al., 2012). Despite the evidence that exosomes are involved in the transmission of α-syn between neurons, the role of microglial exosomes in α-syn transmission requires additional research.
Microglia, the main resident immune cells in the brain, phagocytose dead cells and help clear misfolded α-syn aggregates in Parkinson’s disease (Bruck et al., 2016). However, α-syn also activates microglia, which then release pro-inflammatory cytokines. Neuroinflammation mediated by microglia has been linked to the pathogenesis of Parkinson’s disease (Hirsch et al., 2012). Recently, microglia have been observed to efficiently secrete exosomes as part of their antigen presentation and cargo release mechanisms (EL Andaloussi et al., 2013). Currently it is not clear whether microglia accelerate the spread of α-syn through promoting inflammation, exosome release, or a combination of these mechanisms. Some evidence suggests that exosomes from microglia have an active role in α-syn transmission. For example, α-syn is found in the exosomes from the microglia BV-2 cells, which have been shown to cause apoptosis in neurons (Chang et al., 2013). Another study shows that misfolded tau protein, an important pathology in Alzheimer’s disease, can spread via microglial exosomes, whereas depletion of microglia and inhibition of their exosome synthesis halt tau propagation (Asai et al., 2015). Based on these observations, we hypothesized that misfolded α-syn involved in Parkinson’s disease may also spread in a similar manner via microglia. We report in this study that microglia indeed play an active role in the process of α-syn transmission to neurons via exosomes. Additionally, proinflammatory cytokines released from activated microglia enhanced protein aggregation and spreading induced by microglial exosomes. Stereotaxic injection of exosomes derived from α-synuclein preformed fibril (PFF)-treated microglia induced motor impairment and neurodegeneration in the nigrostriatal pathway. To our knowledge, this is the first report showing that PELI1 (pellino 1), an E3 ubiquitin ligase highly expressed in activated microglia after PFF treatment, induced lysosome breakdown and autophagy inhibition, which promoted α-syn transfer via exosomes.
Materials and methods
Preparation of human α-synuclein preformed fibrils
Purified PFF were obtained using PFF monomer (provided by The Michael J. Fox Foundation) according to the accompanied protocol (The Michael J. Fox Foundation, 2017). PFF were generated by incubating α-syn monomer (5 mg/ml) with an Eppendorf orbital mixer (1000 rpm at 37°C) and shaking for 7 days, followed by aliquoting and storage at −80°C. Thioflavin T assay was performed to confirm the presence of amyloid structure and the morphology of PFF were identified by transmission electron microscopy (TEM).
Cell cultures
Primary cortical neurons
As a transmission model of cell-to-cell spreading of α-syn, cortical neurons are well-established and widely used (Volpicelli-Daley et al., 2011, 2014; Freundt et al., 2012; Mao et al., 2016). As compared to primary dopaminergic neurons, it is technically more feasibe to obtain pure cortical neurons in sufficient quantity for exosome isolation and western blot studies. The transmission patterns in these two cells are very similar because seeded α-syn aggregates also developed in dopaminergic neurons (Dryanovski et al., 2013). From the perspective of Parkinson’s disease pathology, α-syn aggregates are present in the cortex of Parkinson’s disease patients in advanced stage. More importantly, it was reported that α-syn exosome transmission also exists in cortical neurons (Danzer et al., 2012; Stuendl et al., 2016). Taken together, we believe cortical neurons represent a valid model to study α-syn transmission.
Primary cortical neuron cultures were prepared from embryonic Day 16–18 (E16–18) C57BL/6 mouse brains as reported previously (Zhao et al., 2013). Briefly, meninges-free cortices were isolated, digested in trypsin-EDTA (0.1%) for 20 min, and dispersed in serum-free Neurobasal™ Medium with 2% B27, 2 mM l-glutamine and penicillin/streptomycin for 7 days (#21103049, 17504044, 35050061, Life Technologies). The cells were plated onto poly-l-lysine-coated glass bottom cell culture dishes (#801001, NEST) or 100 mm dishes at a density of 3–10 × 105 cells/ml. Cultured neurons were used for studies on in vitro Day 7. Cell purity (>95%) was confirmed by immunofluorescence using Map-2 as a marker for neurons (Supplementary Fig. 11A).
Primary microglia cultures
Primary cultured mouse microglia were prepared from post-natal Day 0 (P0) newborn C57BL/6 pups as described previously (Floden et al., 2005). Briefly, meninges-free cortices were isolated and trypsinized. Cells were cultured in complete DMEM-F12 with 10% foetal bovine serum (FBS) and penicillin/streptomycin (#11320033, 16000044, 15140163, Life Technologies). Murine monocyte colony stimulating factor (M-CSF, 10 ng/ml, #315-02, Peprotech) was added to the medium 6 days after plating. After 15 days, the cultures were shaken vigorously (4 h; 260 rpm on a rotary shaker) to remove microglia. Cell purity (>90%) was confirmed by immunofluorescence using Iba1 as a marker for microglia (Supplementary Fig. 11B).
Drug treatment
Preformed fibril and lipopolysaccharide treatment
PFF were diluted in sterile Dulbecco’s phosphate-buffered saline (D-PBS) at 0.1 mg/ml and sonicated using a QSonica XL-2000 at (settings: power level 2, 0.5 s for each pulse for a total of 60 pulses). Sonicated PFF (2 μg/ml) was added to culture media and incubated for 24 h. Then culture media was aspirated and cells were washed three times with fresh culture media to completely remove PFF. For neurons, appropriate media was added and cells were incubated for a further 24 h. Specifically, microglia were cultured for a further 36 h after PFF removal and received lipopolysaccharide (LPS; 1 μg/ml, #L4516, Sigma Aldrich) treatment for 3 h, followed by ATP (5 mM, #A3377, Sigma Aldrich) for 15 min. Culture supernatants were then collected for exosome isolation or cytokine measurement.
GW4869 treatment of microglia
GW4869 (#D1692, Sigma-Aldrich) was initially dissolved in DMSO to make a stock solution of 0.2 mg/ml. For inhibition of exosome generation, primary cultured microglia were pretreated with 3 μM GW4869 for 24 h before PFF and LPS treatment. Culture supernatants were collected for exosome isolation and neuronal treatment.
Inflammatory factor treatment of neurons
Exosomes from PFF (2 μg/ml) treated microglia (total 20 ml culture media from two 100-mm dishes) were added to primary neurons after purification with or without TNF-α (20 ng/ml), IL-1β (20 ng/ml) or IL-6 (20 ng/ml) for 4 days. Triton™ X-100 (TX-100) insoluble α-syn aggregation was quantified using immunofluorescence and western blot.
Animal treatments
C57BL/6 mice were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. All procedures were performed according to the experimental standards of Fudan University, as well as international guidelines on the ethical treatment of experimental animals. The study was approved by the Ethics Committee of Fudan University, Shanghai, China; IRB approval number: 20150572A259. This manuscript was written in accordance with the Animal Research: Reporting In vivo Experiments (ARRIVE) guidelines.
Preformed fibril injection
C57BL/6 mice between 2 and 3 months of age were anaesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and stereotaxically injected in the right hemisphere with 5 µg PFF (2 µg/µl). Sham animals received sterile D-PBS. Dorsal neostriatum (relative to bregma: −0.2 mm caudal, ±2 mm lateral, −2.6 mm ventral from dura) injections were performed using a 10 μl syringe (Hamilton) at a rate of 0.1 μl per min (2.5 μl total per site) with the needle in place for >5 min after the end of the injection at each site.
PLX3397 treatment
PLX3397 (#S7818, Selleck Chemicals) was formulated in standard chow (Research Diets Inc.) at 290 mg/kg. Mice were fed PLX3397 or control chow for 1 month before and after the PFF injection and then sacrificed (n =4–6 per group). No obvious behavioural or health problems were observed during the PLX3397 diet. Animals were sacrificed 30 days after PFF treatment.
Exosome injection
Exosomes were isolated from D-PBS treated microglial culture medium or PFF, LPS and ATP treated medium. Total protein level was measured using Pierce BCA Protein Assay Kit. Exosomes (2.5 μl; ∼46 µg total protein at each site) were stereotaxically injected into the right dorsal neostriatum of 8–12-week-old C57BL/6 mice each site (site 1: relative to bregma: −0.2 mm caudal, +2 mm right lateral, −2.6 mm ventral from dura; site 2: relative to bregma: +1 mm caudal, +2 mm right lateral, −2.6 mm ventral from dura). Equivalent amounts of D-PBS were also injected into the same brain region of the sham groups. Animals were sacrificed 30 or 180 days after injection and the fixed brain tissues were subjected to immunohistochemistry.
Exosome purification and cell treatment
For the collection and functional assays of exosomes, exosome-free FBS (#EXOFBS50A-1, ExoPerfect) was used in cell culture. Twenty millilitres (from two 100 mm plates) of cell culture media from primary microglia or primary neurons was collected after PFF treatment as described above. Cells and cell debris were removed by centrifuging at 3000g at room temperature for 15 min. The resultant supernatant was mixed with 4 ml of ExoQuick-TC PLUS™ Exosome Purification Kit (#WQPL10TC-1, SBI System Biosciences) according to the manufacturer’s instructions. Exosome-free conditioned medium was prepared by centrifuging conditioned medium at 3000g followed by 100 000g for 1 h. The supernatant was then collected as exosome-free conditioned medium. Exosome pellets were resuspended in culture medium and added to normal neurons (70% confluence, 35 mm plate) for 4 days.
For the exosomes from patients, CD11b+ exosomes from 3 ml CSF were purified by EasySep™ Release PE Positive Kit (Stemcell, #17654) with a PE-conjugated CD11b antibody (BioLegend, #101207). Then CD11b+ exosomes were resuspended in 200 μl culture medium and added to primary neurons in 24-well plates and incubated for 4 days. To demonstrate that the exosomes pulled were of microglial origin, we performed the following two approaches: first, we used an alternative PE-conjugated CD11b antibody, which has no reactivity to human (anti-rat, BioLegend, #201807). Second, we incubated the CD11b antibody with its blocking peptide (MyBioSource, #MBS425396) at a ratio of 10:1 (blocking peptide: CD11b antibody) for 1 h at room temperature to block the antigen binding sites of CD11b antibody before the exosomes were isolated. CD11b+ exosomes from different protocols were added to primary neurons in 24-well plates and incubated for 4 days.
Transmission electron microscopy
Exosome pellets were resuspended in sterile water after purification. Exosomes were then transferred to carbon-coated 200-mesh copper electron microscopy grids and incubated for 10 min at room temperature. The exosomes were then incubated with 2% phosphotungstic acid for 3 min at room temperature. Micrographs were observed under a transmission electron microscope (Phillip CM120). To visualize PFF, this protein was diluted to 1 mg/ml in D-PBS and sonicated at power level 2 for a total of 60 pulses (0.5 s each, QSonica XL-2000). PFF and sonicated PFF were transferred to carbon-coated 200-mesh copper electron microscopy grids separately. The PFF protein were negatively stained with 1% uranium acetate for 10 s at room temperature, and the morphology of PFF were examined by TEM.
Immunofluorescence
Neurons treated with PFF or exosomes were fixed with 4% paraformaldehyde (PFA)/4% sucrose/1% TX-100 and stained with the following antibodies: anti-α-syn filament (1:1000, ab209538, Abcam), anti-total-α-syn (1:100, BD 610787), anti-p-α-syn (1:1000; Abcam, ab51253), anti-Map-2 (ab11267; ab5392, Abcam). PFA (4%) fixed brain sections were subjected to immunofluorescence using anti-Iba1 (1:1000, ab107159, Abcam), anti-PELI-1 (1:50, PA534465, Thermo Fisher), and anti-TH (1:1000, ab76442, Abcam). The PFF-treated microglia were fixed with 4% PFA and immunostained with an antibody to detect total α-syn (1:100, BD 610787) and Iba1 (1:1000, ab107159, Abcam). The PFF-treated BV-2 cells were fixed with 4% PFA and stained with the following antibodies: anti-CD63 (1:200, #143903, BioLegend), anti-LC3B (1:1000, ab48394, Abcam), and anti-p-α-syn (1:200, ab59264, Abcam). Corresponding secondary antibodies Alexa Fluor® 488 and 594 (1:1000, Life Technologies) were used. Nuclei were visualized with DAPI (Life Technologies). Images were captured using confocal microscopy (FV1200, Olympus). Photoshop (Adobe) was used to prepare figures. Microglia cell numbers were quantified with ImageJ by counting the number of Iba1+ cells in the striatum and substantia nigra from at least 15 200× objective original magnification images per group. Co-localization analysis was performed using ImageJ software from a randomly chosen field of cells (20 cells each group from three independent experiments). α-Syn aggregations in neurons were determined by using a standardized custom histogram-based coloured thresholding technique and then subjected to ‘particle analysis’ using ImageJ.
Immunohistochemistry
Vectastain Elite ABC HRP Kit (#PK6101, PK6102, Vector Laboratories, Inc.) was used and all procedures were performed according to the instructions. Briefly, 4% PFA-fixed brain sections (30 μm) from mice receiving PFF, microglial exosomes or D-PBS were incubated in 0.3% hydrogen peroxide for 30 min and then incubated in blocking serum. Samples were incubated with anti-p-α-syn (S129, 1:500, ab59264, Abcam) and anti-TH (1:500, ab112, Abcam) at 4°C overnight, followed by secondary antibody and ABC solution, each for 1 h at room temperature. DAB tablets (D5905, Sigma) were used to develop colour. The numbers of p-α-syn positive cells in different brain areas were counted and normalized to the size of corresponding area (mm2) using 400× original magnification images (20 randomly captured sections at each target region, four to six animals per group).
Quantification of dopamine nigral neurons and striatal terminals
Every fourth section (for midbrain) and every eighth section (for striatum) spanning from the caudal to rostral boundaries of these brain regions were subjected to TH immunostaining. Total numbers of TH-positive neurons in the SNpc were quantified while blinded to the treatment as previously described (Holcik, 1982). An Olympus BX-51 microscope coupled with the Optical Fractionator probe of the Stereo Investigator software was used. TH-stained neurons were counted in the ipsilateral SNpc of every fourth section through the entire extent of the SNpc. Each section was viewed at lower power and outlined. The numbers of TH-stained cells were counted at high power (400×) using a 60 × 80 mm counting frame.
TH levels in the striatum were quantified by densitometry. Sections were scanned in a high-resolution scanner and images measured using ImageJ software. The striatum boundaries were traced and optical density measured in terms of grey levels for 8-bit images as previously described (Bourdenx et al., 2015).
ELISA, HPLC and LDH assays
The contents of TNF-α, interleukin (IL)-6 and interleukin (IL)-1β in the microglia culture supernatants were determined with RayBio Mouse ELISA kits (#ELM-IL6, ELM-TNFα, ELM-IL1b, RayBiotech) according to the manufacturer’s instructions. Total exosomal α-syn was quantified by human-specific ELISA kit (Invitrogen, KHB0061), according to the manufacturer’s instructions. To quantify mouse striatal dopamine, HPLC was performed as we previously reported (Cui et al., 2009). To evaluate the cellular damage after different treatments, Lactate Dehydrogenase (LDH) Assay Kit (Colorimetric, ab102526) was used to measure the LDH levels of microglia according to the manufacturer’s instructions.
Short interfering RNA-mediated PELI1 knockdown
For PELI1 knockdown, BV-2 cells were transfected with short interfering (si)RNA (50 nM) against mouse Peli1 (5′-GUUGAAUACUCAUGACA-3′) or a scrambled control siRNA using Lipofectamine™ 2000 (Invitrogen), according to the manufacturer’s instructions (Genomeditech). After 5 h incubation, the medium was replaced with regular culture medium. After 24 h, PFF were added and the cells were cultured for an additional 24 h. The efficiency of Peli1 knockdown was confirmed by real time RT-PCR and western blot.
Ubiquitination assays
HEK293 cells were grown in 100 mm dishes and transiently transfected with 1 μg of each plasmid containing HA-ubiquitin, FLAG-LAMP2 in the presence or absence of MYC-PELI1 using Lipofectamine™ 2000 (Invitrogen). Six hours later, medium was replaced with DMEM containing 10% FBS. Transfected cells were detected by immunoblotting with antibodies against MYC, FLAG or HA (haemagglutinin).
The immunoprecipitation (IP) was performed using a standard method. Briefly, cells were collected and lysed in IP buffer and supernatant was collected for IP after centrifugation. Primary monoclonal anti-HA antibody (2 μg) or 2 μg mouse IgG (Santa Cruz) was bound to Dynabeads™ Protein G (Invitrogen) and then washed in PBS containing 0.01% Tween 20 prior to being incubated with the soluble cell lysates. The beads-Ab-Ag complexes were then washed with citrate-phosphate buffer. After washes to remove non-specific binding to beads, the bound protein was solubilized in sample buffer, and analysed by SDS-PAGE.
Autophagy flux analysis
BV-2 cells were transfected with Peli1 siRNA or a scrambled control siRNA. Five hours later medium was replaced with regular culture medium plus Ad-mCherry-GFP-LC3B adenovirus (multiplicity of infection: 40, Beyotime Biotechnology, C3011). After 24 h, PFF (2 μg/ml) were added and the cells were cultured for an additional 24 h. In these analyses, mCherry+GFP+ puncta are yellow, and mCherry+GFP− puncta are red; images were collected using a fluorescent confocal microscope. Quantification of LC3 puncta was performed using ImageJ, and the average numbers of LC3 puncta per cell were counted from more than 20 cells randomly selected from three independent experiments.
Western blot assay
Neurons were lysed with radioimmunoprecipitation assay buffer (RIPA) containing protease inhibitors (Sigma). Soluble and insoluble cell fractions for α-syn protein analysis were prepared as previously described (Volpicelli-Daley et al, 2011) with minor change: cells were incubated with TX-100 lysis buffer [150 mM NaCl, 50 mM Tris, 1% Triton™ X-100/Tris-buffered saline (TBS) and protease and phosphatase inhibitor cocktails] on ice for 25 min. The lysate was then sonicated briefly and centrifuged at 14 000g for 15 min. The supernatant was collected and is referred to as the ‘TX-100 soluble fraction’. The remaining pellet was extracted in 2% SDS in TBS and is referred to as the ‘TX-100 insoluble fraction’. Exosomes from different treated microglia were purified from culture medium and lysed with RIPA buffer containing protease inhibitors and sonicated before loading.
Proteins were separated by SDS-PAGE and then transferred onto nitrocellulose membranes. The membranes were incubated overnight at 4°C with the following primary antibodies: anti-Alix (1:1000; Abcam, ab117600), anti-Tsg101 (1:1000; Abcam, ab125011), antibody for total α-syn (1:1000; BD 610787), antibody for p-α-syn (1:1000; Abcam, ab51253), antibody for mouse α-syn (1:1000; Cell Signaling, #4179), anti-PELI-1 (abEPR19302), anti-LAMP2 (1:1000; Abcam, ab13524), anti-β-actin (1:1000; Abcam, ab1801), anti-HA (1:1000; Sigma H3663), anti-MYC (1:1000; ab32072), anti-FLAG (1:1000; ab49763), anti-LC3 (1:1000; Novus, NB100-2220), and anti-p62 (1:1000; ab227207). Secondary antibodies conjugated with horseradish peroxidase (HRP) were used, and immunoreactivity was visualized with chemiluminescence (Bio-Rad, ChemiDoc™ XRS+ System). Protein bands were analysed and quantified using Image Lab software.
For dot blot, exosomes from microglia were collected as described above. Then exosomes were diluted in 100 μl PBS/0.2% TX-100, and applied to nitrocellulose membrane (pore size 0.22 μm) placed in a dot blot apparatus (the 96-well Bio-Dot® and 48-well Bio-Dot® SF microfiltration units). Anti-α-syn filament antibody (1:1000; Abcam, ab209538), anti-total α-syn (1:1000; BD 610787) and anti-p-α-syn (1:1000; Abcam, ab51253) were used and developed with ECL chemiluminescence kit (Millipore, WBKLS0100).
Flow cytometry
CSF (150 μl) was incubated with 2 μl CD11b antibody or isotype control antibody, respectively (Abcam GR3173662-1, GR3173857-2) for 12 h. Exosomes were then isolated using exoRNeasy Serum/Plasma Maxi Kit (Qiagen, 77064) according to the manufacturer’s instructions. Exosomes were collected in clean polypropylene tubes and analysed using Apogee micro-flow cytometry. Data analysis was performed using Apogee Histogram Software.
Human studies
Nine patients with sporadic Parkinson’s disease, 10 patients with multiple system atrophy (MSA) and nine age-matched healthy controls (Supplementary Table 1) were recruited from the Department of Neurology, Huashan Hospital, Fudan University. All subjects received a PET scan using DAT to separate control subjects from patients. FDG-PET was performed to distinguish between Parkinson’s disease and MSA in the study. Before entering the study, all subjects were screened and clinically examined by two senior investigators of movement disorders. Based on the UK Brain Bank criteria (Daniel and Lees, 1993), a diagnosis of Parkinson’s disease was made in all subjects if the patients had ‘pure’ parkinsonism without a history of known causative factors such as encephalitis or neuroleptic treatment, supranuclear gaze abnormalities, or ataxia. The Unified Parkinson’s Disease Rating Scale (UPDRS) motor examination and Hoehn and Yahr scale were assessed at least 12 h after the cessation of oral antiparkinsonian medications (i.e. a practically defined ‘OFF’ condition) and not more than 2 h before the PET scan. The following exclusion criteria were used: (i) a history of neurological or psychiatric illness; (ii) prior exposure to neuroleptic agents or drug use; and (iii) an abnormal neurological examination. Diagnosis of MSA was made according to our previous study (Bu et al., 2018). Ethical approval was granted by the Huashan Hospitals Ethics Committee and written consent was obtained from each subject after detailed explanation of the procedures.
Behavioural analyses
Gait analysis
Six months after microglial exosome injection, locomotor activity was tested. The GaitLab System apparatus (Bureau Veritas) was used. While the animal was walking along the GaitLab corridor, its gait was recorded by a camera at a distance of 80 cm on the walkway. Records and results are stored and analysed by the GaitLab software. A report of the experiment was generated, thus allowing the analysis of targeted parameters.
Y-maze
Six months after microglial exosome injection, spontaneous alternation behaviour (SAB) was tested in a San Diego Y maze Instrument (model 7001-0307) using a standard protocol to evaluate hippocampal-dependent working memory deficits. SAB score during an 8-min test period was calculated as the proportion of alternations (an arm choice differing from the previous two choices) to the total number of alternation opportunities (total arm entries: 2). For example, if the mouse made the choices: C, B, A, B, C, B, A, C, its alternation opportunity is: 8 − 2 = 6 and SAB therefore is: 4/6 = 67%.
Rotarod
Sixty days after injection of microglial exosomes, mouse motor coordination and balance were assessed using a rotarod. Each mouse was given training, the training schedule consisted of three trials each day for 3 days. The rotarod accelerated from 4 rpm up to a maximum of 40 rpm over the course of 300 s. The average latency to fall over three trials was recorded.
Open field
The open-field consisted of a square area (50 cm × 50 cm) with a shelter. Mice were placed in the centre and allowed to freely explore for 5 min while the trial was videotaped. After each test, the apparatus was thoroughly cleaned with 70% ethanol. The total distance and the average speed were measured. Open field tests were carried out 60 days after beginning the PLX3397 diet and 6 months after injection of microglial exosomes. Subsequent video scoring was completed by an observer blind to treatment groups.
Nanoparticle tracking analysis
Nanoparticle tracking analysis was performed using a NanoSight NS300 (NanoSight Ltd.). The purified exosomes were diluted 1:100–1:500 in PBS and subjected to nanoparticle tracking analysis. Samples were measured in triplicates. Particle numbers were analysed with the Nanoparticle Tracking Analysis (NTA) 3.0 software (for Nanosight NS300).
Real-time PCR
Total RNA was isolated from cultured cells using TRIzol® (Takara). RNA (0.5 μg) was used for cDNA synthesis, using random primers and SuperScript™ RT-II (Invitrogen). For Peli1, we used SYBR® Green (Quantitect SYBR Green kit, Qiagen) together with mouse Actb as a control. The following sequences were used: Peli1 forward 5′-TGCCGAAATCAATCAATCAA-3′, reverse 5′-CAATGGAGTGTCACTGGGTG-3′. PCRs were performed in triplicate using a QuantStudio 5 (Thermo Fisher Scientific), and the relative amount of cDNA was calculated by the comparative CT method.
PKH67-labelled exosomes
The purified exosomes were labelled with a PKH67 green fluorescent labelling kit (Sigma-Aldrich) according to the manufacturer’s instructions. Briefly, the exosome pellets from 20 ml culture medium were resuspended in 500 µl of Dilution C, PKH67 (2 µl) was diluted in 500 µl Dilution C, then they were mixed and incubated for 3 min at room temperature. Then an equivalent volume of 1% bovine serum albumin (BSA) was added to bind the excess PKH67. Exosomes labelled with PKH67 were collected as mentioned above, resuspended in Neurobasal™ medium and added to neurons (cultured in a confocal dish).
Thioflavin T assay
Thioflavin T assay was performed as described previously (Harischandra et al., 2019; Yun et al., 2019) with modifications. For primary microglia, cells were washed three times with fresh medium after PFF were removed. Conditioned medium was collected and exosomes were purified using ExoQuick-TC PLUS Exosome Purification Kit (Cat#WQPL10TC-1, SBI system biosciences) according to manufacturer’s instructions. Exosomes from one T25 flasks were resuspended in 100 µl fresh medium. Conditional medium (95 µl) or exosomes sample (95 µl) were mixed with 95 µl of the 25 μM Thioflavin T (Sigma T3516) in a 96-well plate and incubated at room temperate for 30 min. CD11b+ exosomes from patients were isolated from 1 ml CSF sample using EasySep Release PE Positive Kit (Stemcell, #17654) with a PE-conjugated CD11b antibody (BioLegend, #101207). CD11b+ exosomes were then resuspended in 100 μl D-PBS and mixed with 2.5 μl Thioflavin T (1 mM) in a 96-well plate and incubated at room temperate for 10 min. Each sample was run in three replicates. The fluorescence was measured on a Multi-Mode Microplate Reader with an excitation of 445 nm and an emission at 480 nm.
Statistical analysis
Differences between groups were analysed using independent-sample t-tests, one-way or two-way ANOVA followed by Newman-Keuls post hoc testing for pairwise comparison using SPSS 20.0. The null hypothesis was rejected when P-value was <0.05. Data are shown as mean ± standard error of the mean (SEM) or mean ± standard deviation (SD).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
Results
Exosomes mediate transmission of α-synuclein between neurons
Small seeds of PFF generated from recombinant α-syn can be endocytosed by neurons where it recruits endogenous α-syn to form phosphorylated and insoluble aggregates (Volpicelli-Daley et al., 2014). PFF treatment has also been shown both in vitro and in vivo to induce the spread of α-syn from one cell to another (Freundt et al., 2012; Luk et al., 2012b). We obtained PFF from The Michael J. Fox Foundation and prepared them as instructed by the accompanying protocol. Using TEM, we confirmed the morphology and size of PFF and their sonicated form (Supplementary Fig. 1A). After sonication, PFF were added directly to primary neurons for 24 h to induce the formation of α-syn aggregates. As previously reported (Volpicelli-Daley et al., 2011, 2014), PFF induced TX-100 insoluble α-syn and phosphorylated-α-syn (p-α-syn) aggregation, as well as high molecular weight α-syn (Supplementary Fig. 1). To investigate whether the transmission of α-syn between neurons can be mediated by exosomes, primary neurons were first incubated with PFF for 24 h, washed and replaced with fresh medium. On the following day, conditioned medium exosome fractions from PFF-treated donor neurons (EF) or exosomes from untreated neurons (control exosomes, EF-c) were added to the recipient neurons for 4 days. As shown in Supplementary Fig. 1B, exosomes were taken up by the recipient neurons. Conditioned medium, and more importantly, EFs could serve as seeds to recruit endogenous α-syn and to induce TX-100 insoluble α-syn and p-α-syn aggregation (Supplementary Fig. 1C–E), which were found within the soma and axon of the recipient neurons (Supplementary Fig. 1C).
Exosomes prepared from conditioned medium were enriched in microvesicles 50–100 nm in diameter as demonstrated using TEM and nanoparticle tracking analysis (Supplementary Fig. 2A and B), consistent with the size of exosomes. Immunoblotting confirmed that these vesicles were immunoreactive to the exosomal markers Tsg101 and Alix (Supplementary Fig. 2C).
Microglia actively phagocytose extracellular preformed fibrils and release exosomes containing α-synuclein
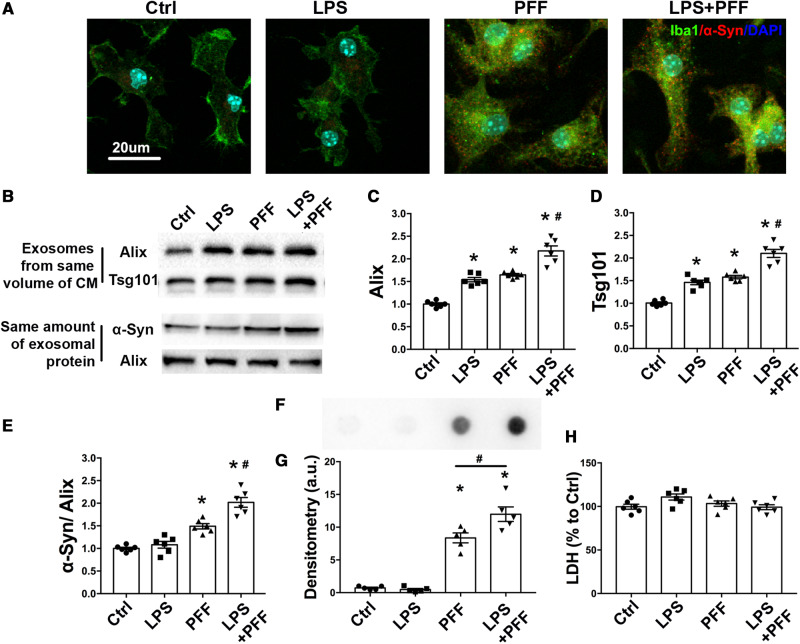
In addition to their ability to transmit α-syn aggregation between neurons, we asked whether exosomes could also transmit α-syn via another type of brain cell. Microglia, the main resident immune cells in the brain, have received significant attention largely because of their role in producing neuroinflammation. Various neurotoxins, including α-syn and LPS, have been shown to activate microglia (Aguzzi et al., 2013; Heneka et al., 2014), which release neuroinflammatory cytokines such as TNF-α, IL-1β and IL-6 (Qu et al., 2007) as shown in Supplementary Fig. 3A–C. Neuroinflammation exacerbates neurotoxicity and promotes α-syn spreading (Neumann et al., 2009). When treated with PFF, or a combination of PFF and LPS, intracellular aggregates of α-syn were detected in microglia (Fig. 1A), indicating that microglia could uptake PFF, but lack the ability to effectively degrade fibrillary α-syn. Activated microglia have been demonstrated to impair autophagy (Du et al., 2017). In addition to reducing the ability of cells to clear protein aggregation, blockade of autophagic flux also enhances the release of exosomes (Alvarez-Erviti et al., 2011; Danzer et al., 2012; Baixauli et al., 2014). To investigate the role of activated microglia in releasing exosomes, we performed immunoblotting to quantify the amount of exosomes released and the levels of α-syn within the exosomes found in conditioned medium from primary microglia that had been treated with LPS, PFF, and LPS plus PFF together. When normalized to the same amount of exosomes, using Tsg101 and Alix as markers, it was evident that these treatments increased the release of exosomes from microglia (Fig. 1B–D). Furthermore, PFF increased α-syn levels in exosomes (Fig. 1B and E), indicating that exosomes may exert an important role in eliminating intracellular abnormal α-syn. When the combination of PFF and LPS was administered, the release of exosomes and their content of α-syn were enhanced (Fig. 1B–E). Using dot blot with an antibody that detects conformation-specific α-syn filaments, we confirmed the presence of oligomeric α-syn, which is the neurotoxic species (Fig. 1F and G), in microglia-derived exosomes after PFF treatment. The levels of oligomeric α-syn were also enhanced when treated with PFF plus LPS (Fig. 1F and G). Next, a thioflavin T assay was performed to analyse the fibrillary structures of exosomal α-syn and dot blot was performed to detect p-α-syn; however, no difference was detectable (Supplementary Fig. 3D and E). Furthermore, to eliminate the possibility that the observed higher levels of exosomes in the conditioned medium from cells treated with LPS and PFF were due to broken cell plasma membrane caused by these treatments, we performed an LDH assay in the media. Figure 1H indicates that at these sublethal concentrations, no cell breakage was detectable. Together, these results indicate that microglia are capable of releasing α-syn-containing exosomes with PFF treatment. When additionally activated by LPS, this release was further enhanced. Thus, for the subsequent experiments, microglia were treated with PFF plus LPS.
Figure 1.
PFF treatment promotes the release of α-syn containing exosomes from microglia. Primary cultured microglia were treated with PFF (2 μg/ml) for 24 h, followed by treatment with or without LPS as described. (A) Primary cultured microglia were fixed and stained with an antibody to detect total α-syn. Scale bar = 20 μm. (B–E) The exosomes were extracted from the same volume of culture medium. Immunoblotting was used to detect exosomes as evidenced by the levels of the exosomal markers Alix and TSG 101 proteins (B–D). Exosomes were lysed with RIPA buffer and sonicated briefly. The same amount of exosomal protein from isolated exosomes were loaded and total α-syn levels were detected by western blotting (E). (F and G) Representative image of dot blot and scatterplots of densitometry analysis assessing misfolded α-syn in exosomes obtained from same volume of microglia culture media with different treatments. A conformation-specific antibody against α-syn oligomers was used. (H) LDH levels in culture media were measured after different treatment by ELISA. Ctrl = treatment with ATP before exosome collecting. All data represent mean ± SEM, n = 5–6 independent experiments, using one-way ANOVA followed by Newman-Keuls post hoc test, *P < 0.05 versus Ctrl, #P < 0.05 versus PFF. CM = conditioned medium.
Exosomes mediate extracellular transmission of α-synuclein between microglia and neurons
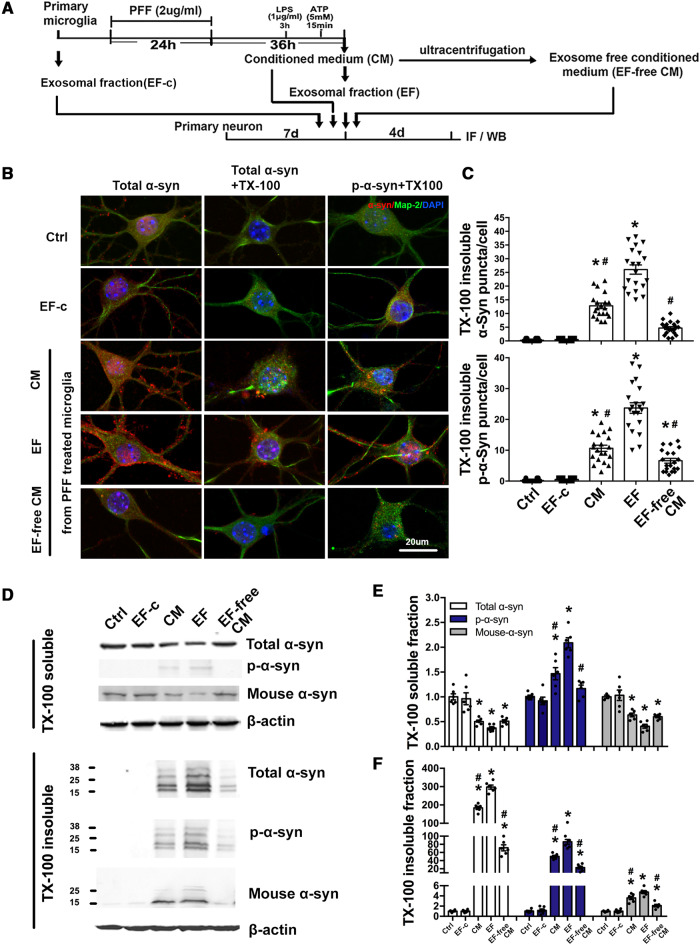
As shown in Fig. 1, exosomes obtained from the PFF-treated microglia can carry α-syn oligomers. To determine the impact of these exosomes on neurons, we used an alternative approach as illustrated in Fig. 2A. Cell culture medium from primary microglia was centrifuged at 3000g to remove cellular debris. This cleared conditioned medium—which still contained exosomes and cytokines—was then subjected to ultra-centrifugation, and the supernatant was collected as exosome-free conditioned medium. Subsequently, conditioned medium, purified EF (from the PFF-treated microglia), EF-c (exosomes from untreated microglia) or exosome-free conditioned medium were added to cultured primary cortical neurons separately and incubated for 4 days to allow internalization of exosomes to occur. TX-100 insoluble α-syn aggregation in the recipient neurons, as visualized using antibody against total α-syn or antibody against p-Ser129 α-syn, was most dramatically increased in the EF group in more than 85% of neurons, whereas this effect was largely absent in the exosome-free conditioned medium and EF-c group (Fig. 2B, C, Supplementary Fig. 3F and G). This was consistent with the immunoblot results (Fig. 2D–F). EF treatment increased TX-100 insoluble α-syn, including pathological p-α-syn, endogenous α-syn and total α-syn, while soluble endogenous α-syn was reduced, suggesting that α-syn containing exosomes recruited endogenous α-syn to form insoluble aggregation. These results indicate that α-syn containing exosomes was able to infect heathy neurons and trigger α-syn aggregation in recipient neurons. We also performed additional control experiments to demonstrate that aggregation of α-syn in the secondary neurons was induced by α-syn-containing exosomes rather than non-specific binding of residual PFF from the conditioned medium (Supplementary Fig. 4).
Figure 2.
Exosomes from PFF-treated microglia induces α-syn aggregation in recipient neurons. (A) Schematic diagram illustrating the timeline of experimental approach. Briefly, primary cultured microglia were treated with PFF, then PFF were removed and cells were washed with fresh culture medium to avoid the contamination of PFF, followed by the treatment of LPS and ATP. Exosomes from microglia culture medium were isolated. Primary cortical neurons were incubated for 4 days with conditioned medium (CM), extracted exosomes or exosomes-free medium (EF-free CM) from microglia. Ctrl = no-treatment control; EF = exosomes from PFF-treated microglia; EF-c = exosomes from untreated microglia. (B) Neurons were then fixed with PFA alone or PFA with 1% TX-100 to extract soluble proteins, followed by immunostaining for total α-syn and p-α-syn. CM and EF induced α-syn aggregation in neurons. Scale bar = 20 μm. (C) The quantitative data of TX-100 insoluble α-syn aggregation in recipient neurons. *P < 0.05 versus Ctrl, #P < 0.05 versus EF. (D–F) Representative immunoblots and quantitative data of TX-100 soluble and insoluble α-syn in recipient neurons. TX-100 soluble and insoluble fractions of neurons were isolated and analysed using indicated antibodies. *P < 0.05 versus Ctrl, #P < 0.05 versus EF. Data represent mean ± SEM, one-way ANOVA followed by Newman-Keuls post hoc test, n = 4–6 independent experiments. IF = immunofluorescence; WB = western blot.
Inhibition of exosome synthesis reduces α-synuclein transmission from microglia to neurons
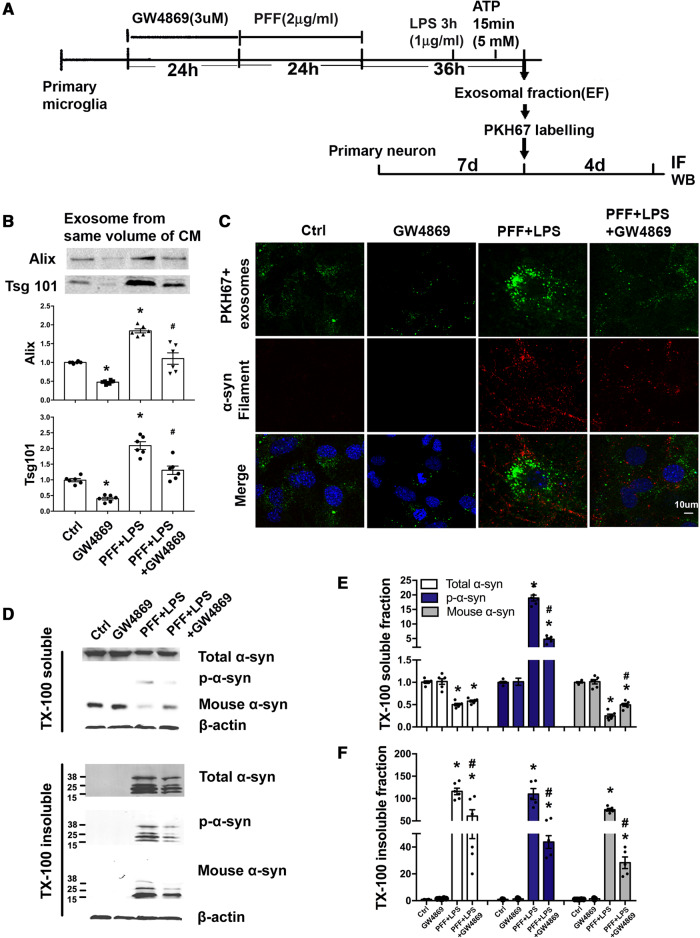
Considering that PFF treatment increased the transmission of α-syn from microglia to neurons through exosomes, we asked whether pharmacological inhibition of exosome synthesis could reduce α-syn transmission from microglia to neurons. GW4869 is a pharmacological inhibitor of nSMase2, which regulates the biogenesis of exosome (Trajkovic et al., 2008). For this study, microglia were treated with GW4869 for 24 h, followed by PFF and LPS (Fig. 3A). Exosomes were collected from microglial conditioned medium and subjected to immunoblotting. Results showed that GW4869 strikingly reduced secretion of exosomes in microglia (Fig. 3B) without inducing cell death (Supplementary Fig. 5A). To investigate whether this reduced secretion of microglial exosomes would result in lower internalization of exosomes into neurons, we labelled purified exosomes with a membrane dye PKH67 and then added these PKH67+ exosomes to primary neurons. Results showed that with GW4869 treatment, the internalization of exosomes into neurons was significantly reduced (Fig. 3C). Consistent with this, there was also less α-syn aggregation in these neurons (Fig. 3C). Neurons were then collected and separated into TX-100 soluble and insoluble fractions. Addition of GW4869 reduced TX-100 insoluble α-syn after PFF plus LPS treatment (Fig. 3D–F), suggesting that reducing the release of α-syn containing exosomes from microglia could reduce α-syn spreading to neurons. These data indicate that microglial exosomes may be a potential intervention target for inhibiting the spread of α-syn.
Figure 3.
GW4869 suppresses secretion of microglial exosomes and transmission of α-syn to neurons. (A) Schematic diagram illustrating the timeline and experimental procedures. Primary microglia were treated with either GW4869, PFF, or GW4869 first followed by PFF before LPS and ATP. Ctrl = treatment with ATP before collecting exosome; IF = immunofluorescence. (B) The same volume of microglial culture medium from different experimental conditions was extracted for exosomes, whose levels were determined by western blotting using Alix and Tsg101. (C) GW4869 treatment reduced exosome internalization into neurons resulting in less α-syn aggregation. Exosomes isolated from the same volume of microglia culture medium from different experimental conditions were labelled with the dye PKH67. These exosomes were then added to recipient neurons and incubated for 4 days. Neurons were fixed with 4% PFA and α-syn filament staining was performed. (D–F) Quantitative data and representative immunoblots of TX-100 soluble and insoluble α-syn in recipient neurons. *P < 0.05 versus Ctrl, #P < 0.05 versus PFF+LPS. Data represent mean ± SEM, one-way ANOVA followed by Newman-Keuls post hoc test, n = 3–6 independent experiments.
Depletion of microglia suppresses PFF-induced α-synuclein transmission in vivo
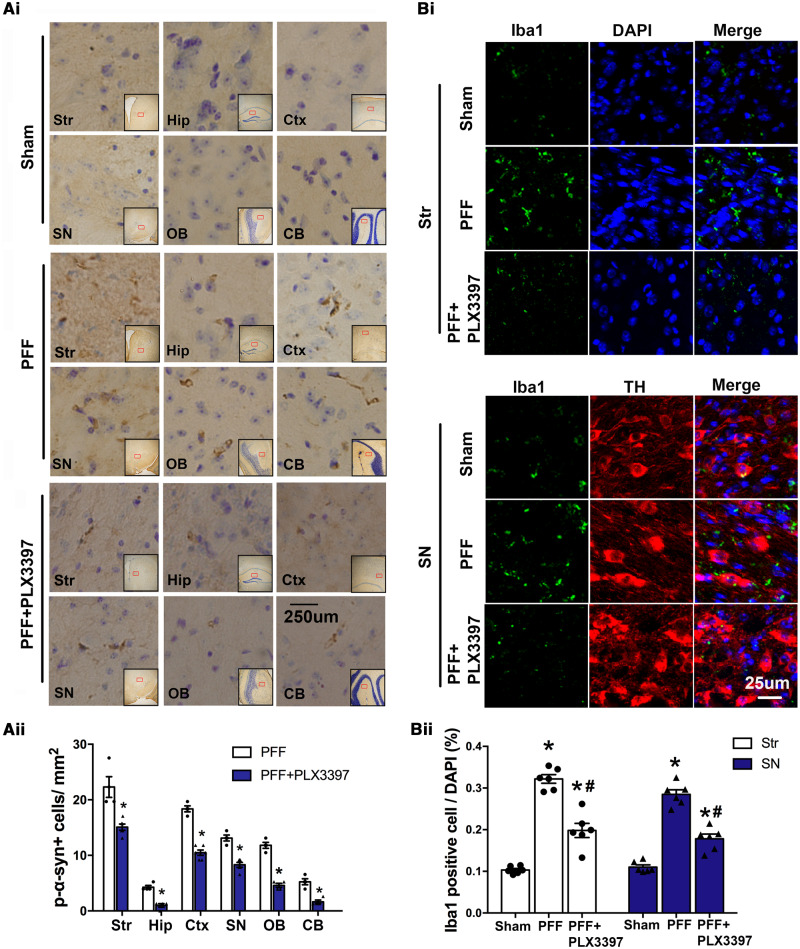
Based on the in vitro data, we next turned to in vivo studies to confirm the role of microglia and microglial exosomes in α-syn transmission. First, we performed stereotaxic injections of PFF, targeting the dorsal striatum in mice, an approach that had been established to produce transmission of pathological α-syn and other cardinal features of Parkinson’s disease (Luk et al., 2012a). After 30 days post-injection, p-α-syn accumulations were present in several brain regions ipsilateral to injection, including the striatum, and other anatomically interconnected regions such as substantia nigra (SN), cortical layers, olfactory bulb, and hippocampus [Fig. 4A(i and ii)]. Of note, the presence of abnormal p-α-syn aggregation in these areas, which have trajectory connections to the striatum, suggest α-syn pathology can spread from one brain region to another. In contrast, D-PBS injections in the sham group did not produce such an α-syn pathology. Also, we observed that Iba1 positive cells in the SN and striatum, two important brain areas in Parkinson’s disease, were increased 30 days after PFF injection [Fig. 4B(i and ii)]. Together, these results indicate that microglia are involved in the spread of α-syn.
Figure 4.
Microglial depletion suppresses α-syn propagation in PFF injected mice. Mice were injected with PFF in the striatum. One month before and after this injection, mice were fed with standard or chow containing PLX3397, a chemical that depletes microglia. Mice were then sacrificed 30 days after PFF injection and brain samples were analysed using immunostaining. [A(i)] In addition to the striatum, p-α-syn was also detected in the ipsilateral hippocampus, cortex, substantia nigra, olfactory bulb, and cerebellum 30 days after PFF injection. [A(ii)] Quantification of the p-α-syn cells in different brain regions (n = 4–6 mice/group, 20 images per region). Data represent mean ± SEM, independent t-test, *P < 0.05 versus PFF. [B(i and ii)] Using immunofluorescence for Iba1, the number of microglia in the striatum and SN 30 days after PFF injection was quantified (n = 6 mice/group, 24 images per group). One-way ANOVA followed by Newman-Keuls post hoc test, data represent mean ± SEM, *P < 0.05 versus Sham, #P < 0.05 versus PFF. Sham = mice were injected with same volume of D-PBS. CB = cerebellum; Ctx = cortex; Hip = hippocampus; OB = olfactory bulb; SN = substantia nigra; Str = striatum.
We next investigated whether depletion of microglia would affect this transmission. PLX3397 is an inhibitor of colony stimulating factor 1 receptor (CSF1R), which has previously been demonstrated to specifically deplete microglia (Elmore et al., 2014). We depleted microglia in C57BL6 mice by feeding them with a chow containing PLX3397. This treatment significantly reduced the number of Iba1-positive cells in the brain after 30 days (Supplementary Fig. 5B), but did not affect mouse body weight (Supplementary Fig. 5C). After 1 month of being on this diet, we injected PFF into the dorsal striatum and mice were maintained on the same chow for one additional month. Control mice received regular chow without PLX3397. Motor ability and anxiety levels of mice were not affected by PLX3397 after 60 days (Supplementary Fig. 5D and E) and PLX3397 did not induce TH cell death (Supplementary Fig. 6A and B). Using confocal microscopy, we observed that the number of Iba1-positive microglia in the SN and striatum was reduced in the PFF+PLX3397-treated mice compared with those of the PFF group. Moreover, the number of abnormal p-α-syn aggregations was significantly reduced in striatum and its anatomically interconnected regions in the PFF+PLX3397 group [Fig. 4A(i and ii)]. PLX3397 treatment alone had no effect on p-α-syn in the brain (Supplementary Fig. 6C). In accordance with the immunostaining data, p-α-syn protein levels in different brain regions and the whole brain were also detected by western blot (Supplementary Fig. 6D and E), which showed reduced p-α-syn by PLX3397 after PFF injection. These data demonstrate that microglial depletion partially suppresses α-syn aggregation and transmission in brain.
Microglial exosomes mediate α-synuclein transmission in brain
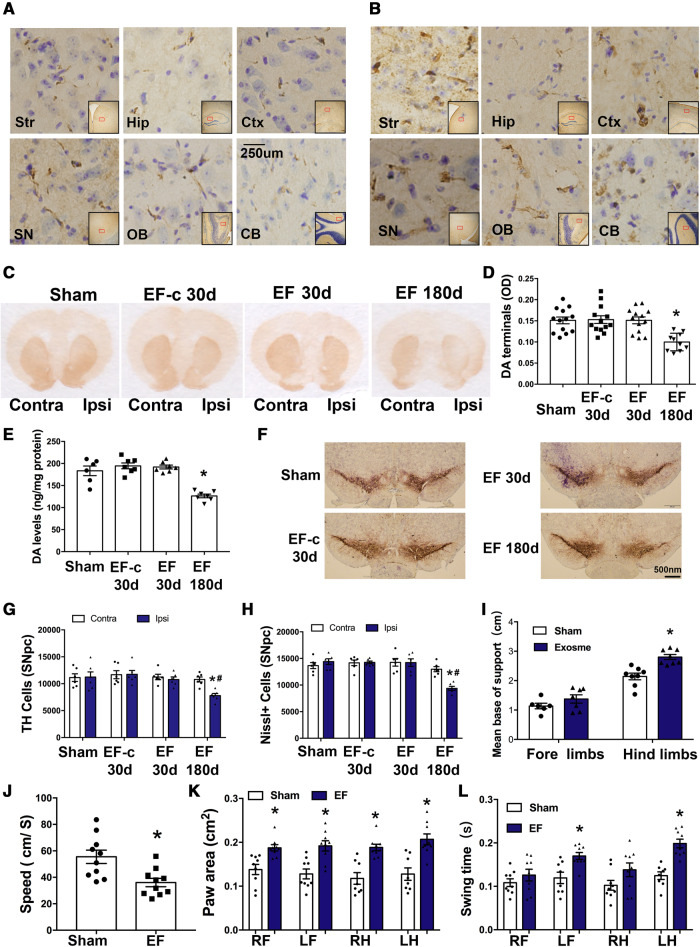
To investigate the possibility that microglial exosomes can facilitate α-syn transmission in vivo, we isolated exosomes from different treated microglia and stereotaxically injected these exosomes into the right dorsal striatum of mice. Using p-α-syn antibodies, we performed immunohistochemical analysis in multiple brain regions after injection to map out α-syn pathology. After 30 days, no p-α-syn was detected in sham mice and mice injected with exosomes from D-PBS treated microglia (EF-c) (Supplementary Fig. 7A and B). However, accumulations of p-α-syn were detectable in EF-injected mice in multiple brain regions on the ipsilateral side after 30 days (Fig. 5A), with an increase after 180 days (Fig. 5B). These results confirm that exosomal α-syn from microglia is readily transmissible in vivo, although the underlying mechanism of this transmission between brain regions is still unclear.
Figure 5.
Exosomes derived from PFF-treated microglia induces α-syn transmission, dopaminergic neuron degeneration and behavioural changes. (A and B) Mice were stereotaxically injected with EF into the right dorsal striatum. Representative images show accumulation of p-α-syn (brown) in different ipsilateral regions at 30 days (A) or 180 days (B) after the injection. (C–E) Striatal dopamine terminals and total dopamine levels were measured at either 30 days or 180 days after injection. The OD level of dopamine terminals (D) showed a 40% decrease and total dopamine levels (E) showed ∼30–35% decrease in hemisphere ipsilateral to injection. Data represent mean ± SEM, one-way ANOVA followed by Newman-Keuls post hoc test, *P < 0.05 versus Sham. (F–H) TH-immunostaining and stereological cell counting of TH-immunoreactive neurons and Nissl-positive neurons in the SNpc. Approximately 33% of TH cells were lost at 180 days in hemisphere ipsilateral to injection. Data represent mean ± SEM, two-way ANOVA followed by Newman-Keuls post hoc test *P < 0.05 versus Sham, #P < 0.05 versus contralateral side. (I–L) Gait analysis was performed 180 days after injection to evaluate locomotor activities. Results were analysed with independent t-test, *P < 0.05 versus Sham. n = 6–8 mice/group. Sham = mice were injected with same volume of D-PBS; EF-c = exosomes from D-PBS treated microglia (control exosome); and EF = exosomes from PFF treated microglia. CB = cerebellum; Contra = contralateral side; Ctx = cortex; Hip = hippocampus; Ipsi = ipsilateral side; LF = left forelimb; LH = left hindlimb; OB = olfactory bulb; RF = right forelimb; RH = right hindlimb; SN = substantia nigra; Str = striatum.
To evaluate whether striatally injected exosomes would induce cellular damage to the dopaminergic system, we quantified the density of striatal dopaminergic terminals by analysing optical density of TH-positive structures. As demonstrated in Fig. 5C and D, there was a loss of dopaminergic terminal density 6 months after injection of EF. Consistent with the damage in the nigrostriatal pathway, HPLC analysis showed a reduction in total striatal dopamine content 6 months after exosome injection (Fig. 5E). Further analyses using stereological cell counting of dopaminergic neurons in SNpc confirmed corresponding loss of TH-positive cells in the ipsilateral SNpc, whereas the number of TH neurons in the contralateral SNpc were indistinguishable from sham group (Fig. 5F and G). Nissl cell count indicated that the loss of TH is not simply due to downregulation of phenotypic markers but rather due to cell loss (Fig. 5H). However, neither the TH cell number in SNpc nor the TH staining in striatum showed any decrease 30 days after injection of EF in the ipsilateral hemisphere, indicating that there is a gradual and unilateral loss of cell function. Together these results indicated that microglial exosomes triggered retrograde cell death in the nigrostriatal pathway in a time-dependent manner.
We next evaluated if damage in the nigrostriatal system impairs locomotor function. Using a gait analysis system, we detected the behavioural changes in mice 6 months after injection of EF. The base of support refers to the distance of the average width between the front paws or the hind paws. We found that the base of support of the hind limbs after injection of exosomes was increased compared to the control group (Fig. 5I). The average walking speed (Fig. 5J) was decreased. The paw areas (Fig. 5K) were increased on both sides and in both the front limbs and hind limbs. Swing time refers to the duration of time that the animal does not place a paw on the glass plate. We observed prolonged swing time in the left limbs, contralateral to exosome injection side (Fig. 5L). These changes above may contribute to the decrease in walking speed. However, no changes in memory function were observed as assessed using the Y-maze (Supplementary Fig. 7C). In addition, open field activity remained unchanged, indicating overall motor activity was not significantly altered (Supplementary Fig. 7D and E).
Taken together, these results demonstrate that microglia exosomal α-syn was related to Parkinson’s disease progression, involved in the spreading of α-syn and the loss of dopaminergic neurons. In addition, the neuropathology and motor deficits became progressively more severe over time.
Pro-inflammatory cytokines enhance α-synuclein aggregation induced by microglial exosomes
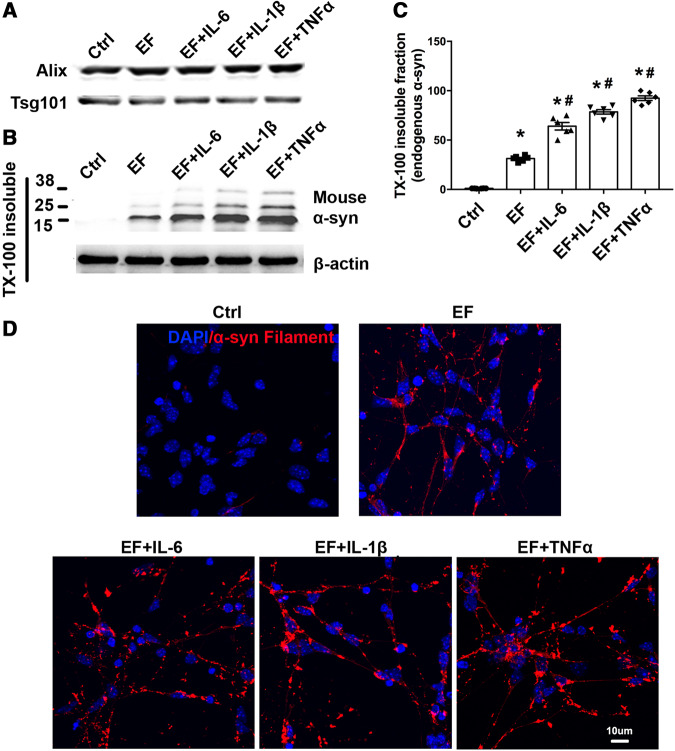
Microglial activation in areas corresponding to α-syn deposition and neurodegeneration has been reported to be an important characteristic in patients with Parkinson’s disease (Fellner and Stefanova, 2013). In our study, microglia activation was observed both in vitro and in vivo after PFF treatment. Microglia activation was accompanied by the release of pro-inflammatory factors, as shown in Supplementary Fig. 3. However, whether the inflammatory factors would have an impact on exosome-mediated α-syn spreading was unclear. To address this issue, we treated neurons with EF (Fig. 6A) in the presence or absence of various proinflammatory cytokines. Immunoblot demonstrated that TX-100 insoluble endogenous α-syn were increased when EFs were combined with pro-inflammatory cytokines (Fig. 6B and C). This finding was further supported by immunostaining to detect TX-100 insoluble α-syn aggregation, which showed that TNF-α, IL-1b, IL-6 increased intracellular α-syn deposition (Fig. 6D). These results suggest that EF plays an active role in mediating α-syn transmission and microglial inflammatory reaction accelerated this process.
Figure 6.
Inflammatory cytokines enhanced α-syn aggregation after microglial exosome treatment. (A) Exosome from PFF treated microglia were purified. (B and C) Primary neurons were treated with EF, EF+TNF-α, EF+IL-1b or EF+ IL-6 for 4 days. TX-100 insoluble fractions of neurons with different treatments were isolated and analysed by targeting endogenous α-syn. Forty micrograms of total protein was loaded. Ctrl = no treatment control. (D) TX-100 insoluble total α-syn aggregation staining was performed. Scale bar = 10 μm. Data represent mean ± SEM, n = 3–6 independent experiments. One-way ANOVA followed by Newman-Keuls post hoc testing, *P < 0.05 versus Ctrl, #P < 0.05 versus EF.
PELI1-mediated lysosomal breakdown contributes to autophagy flux dysregulation and exosome secretion in microglia
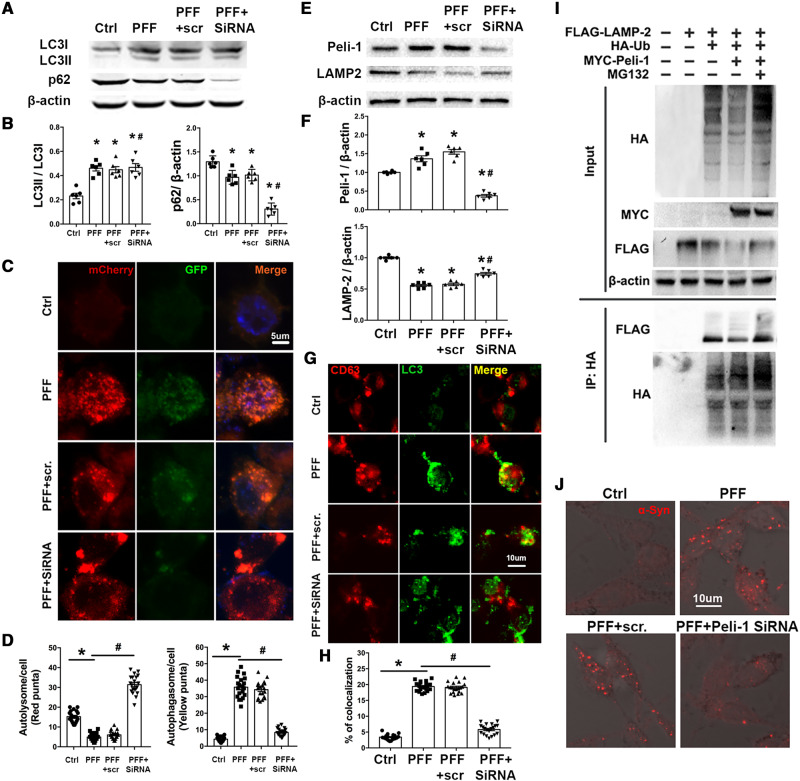
Based on our observations that PFF increased exosome secretion from microglia, we next addressed the question of why microglia activated by PFF would secrete more α-syn carrying exosomes. To this end, we reviewed the process of α-syn degradation and exosome formation. Autophagy is a vital pathway by which α-syn is degraded (Webb et al., 2003; Cuervo et al., 2004; Lee et al., 2004). The disruption of autophagy flux has been observed in affected neurons, for example, accumulation of autophagosomes and loss of lysosomal markers have been reported in cultured cells of Parkinson’s disease patients, rodent models of Parkinson’s disease and in post-mortem Parkinson’s disease brain samples (Anglade et al., 1997; Zhu et al., 2007; Chu et al., 2009; Dehay et al., 2010). In agreement with findings in neurons, we observed an induction of autophagy in PFF treated BV-2 cells (Fig. 7A and B), with autophagosome accumulation (Fig. 7C and D). Because autophagosomes are normally degraded through fusion with lysosomes to form autophagolysosomes, the accumulation of autophagosomes indicates that the lysosome-mediated clearance of autophagosomes is impaired, suggesting a blockade of autophagy flux. Consistent with this hypothesis, the levels of the lysosomal structural protein LAMP2 were markedly decreased in PFF treated BV-2 cells (Fig. 7E, F and Supplementary Fig. 8A–C).
Figure 7.
PFF treatment increases the degradation of LAMP2 through ubiquitin-proteasome system after ubiquitination by PELI1 in BV-2 cells, thus impairing the autophagy flux and enhances the fusion of autophagysomes and MVBs. (A and B) Western blotting of LC3 and p62 levels in BV-2 cells following PFF treatment (2 μg/ml; 24 h) with or without Peli1 siRNA. n = 6 independent experiments. (C) Representative images of BV-2 cells transfected with Ad-mCherry-GFP-LC3B adenovirus following PFF (2 μg/ml, 24 h) with/without Peli1 siRNA. Scale bar = 5 μm. (D) The number of red (autolysome) and yellow puncta (autophagasome) per cell were determined using ImageJ. Twenty cells from three independent experiments were analysed in each group. (E and F) Western blot analysis of LAMP2 and PELI1 levels in BV-2 cells following PFF treatment (2 μg/ml; 24 h) with or without Peli1 siRNA. n = 6 independent experiments. Data were analysed using Image Lab (version 5.2.1). (G) Confocal microscopy analysis of the MVB marker CD63 (red) and LC3 autophagysomes (green). BV-2 cells were treated with PFF (2 μg/ml, 24 h) with/without Peli1 siRNA. Scale bar = 10 μm. (H) Co-localization of CD63 (MVB) with LC3 (autophagysome) was measured in BV-2 cells. Twenty cells from three independent experiments were analysed in each group. (I) Western blot analysis of poly-ubiquitination of LAMP2 by PELI1 in vivo. FLAG-LAMP2 and HA-ubiquitin were transiently expressed in HEK293 cells with MYC-PELI1. The cells were treated with MG132 (20 μM) for 7 h before analysis. Antibodies to HA and FLAG were used to visualized ubiquitinated LAMP2 following immunoprecipitation with HA beads. (J) Confocal microscopy analysis of p-syn in BV-2 cells treated with PFF (2 μg/ml, 24 h) ± Peli1 siRNA. Scale bar = 10 μm. n = 6 independent experiments. Data represent mean ± SEM. One-way ANOVA followed by Newman-Keuls post hoc testing. *P < 0.05 versus Ctrl, #P < 0.05 versus PFF.
Exosomes are released from maturing late endocytic/multivesicular bodies (MVBs) upon fusion of MVBs with the plasma membrane. α-Syn exits MVBs through the endosomal sorting complex required for transport (ESCRT) complex and the expression levels of the MVB resident proteins such as PARK9 regulate the number of exosomes that releases α-syn into the extracellular space (Tsunemi et al., 2014). Furthermore, autophagosomes can fuse with MVBs forming amphisomes, leading to the convergence of autophagic-endosomal pathway (Berg et al., 1998; Klionsky, 2005; Fader et al., 2008; Szatmari et al., 2014). To assess whether the accumulated autophagosomes are indeed fused with MVBs, we assessed the co-localization of CD63 (a MVB marker) with LC3 (an autophagosome marker) in BV-2 cells. As shown in Fig. 7G and H, there was a marked increase in CD63+ LC3+ puncta after PFF treatment. These findings indicated that PFF caused lysosomal dysfunction, while also raising the question of why LAMP2 was decreased in PFF-treated BV-2 cells.
The ubiquitin proteasome system (UPS) plays a vital role in clearing intracellular proteins from eukaryocytes. Proteins are polyubiquitinated by ubiquitin ligase and then transferred to the proteasome for degradation. PELI1 (pellino 1), an E3 ubiquitin ligase, is reported to be abundantly expressed in activated microglia and plays an important role in their inflammatory reaction (Xiao et al., 2013; Medvedev et al., 2015). Our data show that PELI1 levels were dramatically increased in PFF treated BV-2 cells (Supplementary Fig. 8A–D) as well as in activated microglia in the brains of PFF injected mice (Supplementary Fig. 8E). To obtain direct evidence, we performed in vitro polyubiquitination assays. In the presence of HA-ubiquitin, LAMP2 was efficiently polyubiquitinated; while overexpression of PELI1 enhanced the degradation of LAMP2, evidenced by decreased levels of FLAG-tagged LAMP2 detection (Fig. 7I). In the presence of MG132 (proteasome inhibitor), the polyubiquitination of LAMP2 was significantly increased (Fig. 7I). Together, these data indicate that PELI1 targets LAMP2 for ubiquitin-mediated degradation in BV-2 cells after PFF treatment.
To examine the function of PELI1, we knocked down endogenous PELI1 expression by siRNA. PELI1 siRNA significantly reduced PELI1 mRNA and protein levels in BV-2 cells (Supplementary Fig. 9A–C). Silencing of PELI1 significantly reversed PFF induced reduction of LAMP2, attenuated autophagosome accumulation, restored autophagy flux and reduced the fusion of autophagosomes with MVBs (Fig. 7A–H). Moreover, silencing of PELI1 attenuated abnormal α-syn aggregation in PFF treated BV-2 cells (Fig. 7J). Taken together, these results indicate that PELI1 is vital in regulating lysosomal breakdown and that the blockage of autophagy flux increased the proportion of MVB-autophagosomes, leading to increased α-syn containing exosomes from microglia.
Microglia/macrophage-derived exosomes from CSF of Parkinson’s disease patients induce α-synuclein aggregation in neurons
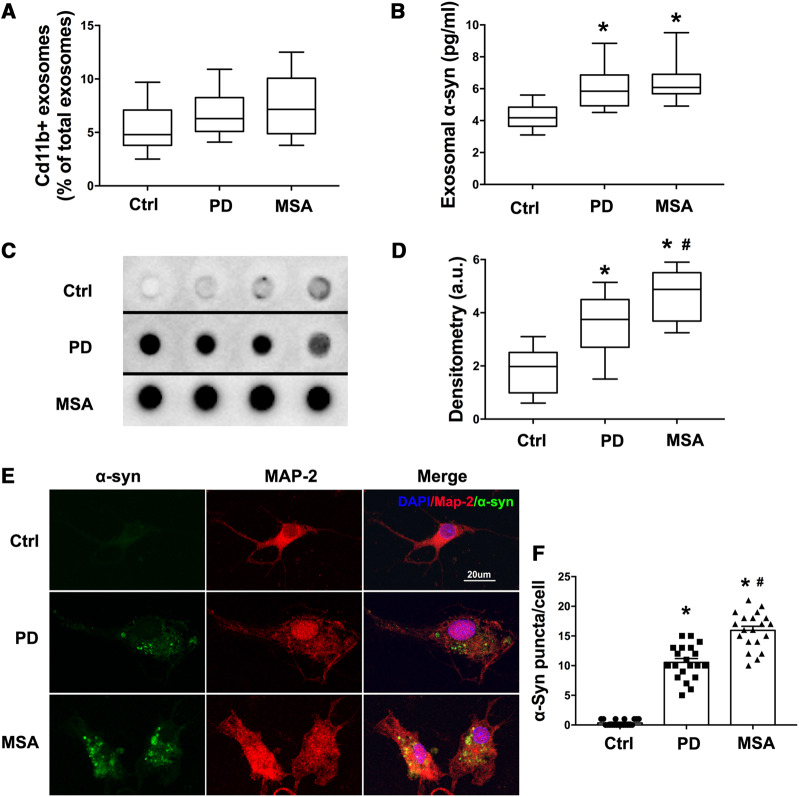
Both our in vivo and in vitro data supported the hypothesis that microglial exosomes participate in α-syn transmission to neurons. To confirm this in patients, CSF from sporadic Parkinson’s disease, control (healthy age-matched) and MSA patients was collected (Supplementary Table 1, Supplementary Fig. 10A and B). It has been reported that α-syn exists in exosomes of CSF, including in the CSF of healthy people and patients affected by neurodegenerative disorders such as Parkinson’s disease and dementia with Lewy bodies (Stuendl et al., 2016). Proteomic analysis of exosome derived from microglial cells illustrated that CD11b is a membrane-associated protein that is enriched in exosomes and thus could be used as a microglial-origin marker (Brites and Fernandes, 2015). First, to evaluate the percentage of microglia/macrophage-derived exosomes in CSF, CD11b antibody was used to label microglia/macrophage-derived exosomes. After incubation with CD11b antibodies, flow cytometry was performed to detect the ratio of CD11b+ exosomes over total exosomes in CSF. The levels of CD11b+ exosomes were not statistically different between the groups, although MSA and Parkinson’s disease patients showed a higher trend compared with the control group (Fig. 8A and Supplementary Fig. 10C). Next, we compared the content of total α-syn in microglia/macrophage-derived exosomes. CD11b+ exosomes from CSF were purified by CD11b coated beads. Using ELISA we found that total α-syn level in CD11b+ exosomes was higher in Parkinson’s disease and MSA patients than healthy controls, while no difference between Parkinson’s disease and MSA patients was identified (Fig. 8B). When comparing the oligomer forms of α-syn using a confirmation-specific antibody, dot blot showed microglia/macrophage-derived exosomes from Parkinson’s disease and MSA patients had higher concentrations of oligomeric α-syn than healthy controls (Fig. 8C and D). Importantly, CD11b+ exosomes from MSA patients contained more oligomeric α-syn than Parkinson’s disease patients (Fig. 8C and D). However, Thioflavin T assay showed no difference of fibrillary α-syn between the three groups (Supplementary Fig. 10D). To test the effect of these microglia/macrophage-derived exosomes on α-syn spreading, CD11b+ exosomes were isolated from CSF and incubated with cortical neurons. After 48 h, we observed that CD11b+ exosomes from Parkinson’s disease and MSA patients induced TX-100 insoluble α-syn in the treated neurons, with a much more severe aggregation in the MSA group (Fig. 8E and F). However, when pretreated with a CD11b blocking peptide or incubated with a CD11b antibody that had no immunoreactivity to human, no aggregation was detected (Supplementary Fig. 10E). These data demonstrate that the binding between CD11b antibody and exosome was specific and the CD11b+ exosomes isolated were indeed of microglial origin. Together these data indicate that microglia/macrophage-derived exosomes are a major subgroup of α-syn containing extracellular vesicles in the brain, playing an important role in cell-to-cell spreading of α-syn.
Figure 8.
CD11+ exosomes derived from CSF of Parkinson’s disease patients contain α-syn oligomer and are able to induce α-syn aggregation in neurons. (A) Exosomes from 150 μl CSF were isolated and then detected using flow cytometer with a CD11b-FITC antibody to label microglia/macrophage derived exosomes [minimum (Ctrl: 2.5; Parkinson’s disease: 4.1; MSA: 3.8); 25%ile (Ctrl: 3.8; Parkinson’s disease: 5.1; MSA: 4.875); median (Ctrl: 4.8; Parkinson’s disease: 6.3; MSA: 7.15); 75%ile (Ctrl: 7.1; Parkinson’s disease: 8.25; MSA: 10.06); maximum (Ctrl: 9.7; Parkinson’s disease: 10.9; MSA : 12.5)]. (B) CD11b+ exosomes were isolated from 5 ml CSF and total α-syn were measured by ELISA [minimum (Ctrl: 3.103; Parkinson’s disease: 4.509; MSA: 4.909); 25%ile (Ctrl: 3.642; Parkinson’s disease: 4.926; MSA: 5.688); median (Ctrl: 4.175; Parkinson’s disease: 5.849; MSA: 6.076); 75%ile (Ctrl: 4.842; Parkinson’s disease: 6.862; MSA: 6.904); maximum (Ctrl: 5.6; Parkinson’s disease: 8.849; MSA: 9.509)]. (C) Representative image of dot blot on CD11b+ exosomes isolated from 200 μl CSF using a conformation specific α-syn antibody, which recognizes amino acid sequence-independent oligomers of α-syn. Images of four patients in every group are shown. (D) Densitometry analysis of the dot blot assessing the misfolded α-syn in CD11b+ exosomes from patients [minimum (Ctrl: 0.6; Parkinson’s disease: 1.509; MSA: 3.249); 25%ile (Ctrl: 0.9877; Parkinson’s disease: 2.703; MSA: 3.688); median (Ctrl: 1.976; Parkinson’s disease: 3.747; MSA: 4.876); 75%ile (Ctrl: 2.509; Parkinson’s disease: 4.496; MSA: 5.507); maximum (Ctrl: 3.103; Parkinson’s disease: 5.143; MSA: 5.903)]. (E) CD11b+ exosomes isolated from the 3 ml CSF of patients were added to neurons. Proteinase K-resistant α-syn aggregation was detected after 4 days. (F) The quantitative data of α-syn puncta in neurons. Data are shown as mean ± SEM. One-way ANOVA followed by Newman-Keuls post hoc testing, *P < 0.05 versus Ctrl, #P < 0.05 versus Parkinson’s disease. PD = Parkinson’s disease.
Discussion
Based on the Braak staging hypothesis, the gut and the olfactory bulb are the initial spreading sites of misfolded α-syn (Klingelhoefer and Reichmann, 2015). Monomeric or oligomeric α-syn released from the ‘donor’ neurons can be taken up by the ‘recipient’ cells, where they act as templates—or ‘seeds’—to induce misfolding of endogenous α-syn (Valdinocci et al., 2017). Emerging evidence indicates that α-syn can spread between neurons through exosome release (Visanji et al., 2013). However, how and to what extent microglia and their exosomes impact α-syn pathology has not been well delineated. We show here that microglial exosomes mediated α-syn transmission from microglia to neuron, including in vitro, in vivo, and in a human study.
Neuroinflammation produced by activated microglia has been demonstrated to play an important role in neuropathology as seen in Parkinson’s disease (Tansey and Goldberg, 2010; Xu et al., 2016). Microglia can be activated by a wide range of neurotoxic molecules. Exogenously introduced α-syn such as PFF and those endogenously released by injured neurons are both capable of eliciting strong neuroinflammatory responses from microglia (Zhang et al., 2005; Lee, 2008). As shown in our study and by other laboratories (Zhang et al., 2005), sonicated human PFF can be taken up by microglia and activate microglia both in vivo and in vitro (Zhang et al., 2005; Lee et al., 2010; Cao et al., 2012; Harms et al., 2013). We also observed that the treatment of PFF induced the release of α-syn containing exosomes from microglia. Because microglia can be activated by any non-self substance, using LPS as a positive control, we demonstrated PFF treatment increased both total α-syn levels and α-syn oligomers in microglial exosomes, which was not observed in the presence of LPS alone.
Although understanding the mechanism of such release requires additional work, one possible explanation is that inhibition of autophagy flux is induced by α-syn in microglia. Blocking autophagic activity and lysosome dysfunction has been documented to increase the release of exosomes in neuronal cells (Alvarez-Erviti et al., 2011; Danzer et al., 2012; Minakaki et al., 2018). Previous studies have also reported that α-syn levels in exosomes increase when autophagy flux is inhibited in α-syn overexpressing neurons through promoted MVB-autophagosome fusion (Alvarez-Erviti et al., 2011; Poehler et al., 2014; Minakaki et al., 2018).
However, it is not clear whether the same mechanism exists in microglia and what the underlying mechanism is. In this study, we demonstrated that PFF induced the level of PELI1 (an E3 ubiquitin ligase highly expressed specifically in activated microglia) and mediated the degradation of LAMP2 through UPS, leading to inhibition of the autophagy-lysosome pathway in microglia. Under this condition, an increased fusion of autophagosome-MVB intracellular compartments was observed in microglia, accompanied by increased exosome release and an increase of α-syn in exosomes. Because of the specific and high expression of PELI1 in activated microglia, we have demonstrated a pathway that specifically affects the lysosomal function of microglia and their secretion of exosomes.
The present study also supports the view that when delivered through exosomes, α-syn is effective in generating protein aggregation in neurons (Danzer et al., 2012). When neurons were treated with exosomes, conditioned medium or exosome-free conditioned medium from PFF-treated microglia, the presence of exosomes dramatically increased protein aggregation in neurons. It was reported that ∼75% α-syn exist in exosomes in conditioned medium from cells over-expressing α-syn (Alvarez-Erviti et al., 2011). A recent study demonstrated that exosome associated α-syn oligomers are internalized into recipient cells whereas free α-syn oligomers are not internalized and remain bound to cell surface (Delenclos et al., 2017). Coupled with this observation, it has been found that the amount of free α-syn was sharply decreased after exosomes were purified from conditioned medium, and that exosome-associated α-syn oligomers, which present on both the outside and inside of exosomes, are more likely to be taken up by recipient neurons (Danzer et al., 2012). The uptake of intact exosomes involves multiple mechanisms, involving the caveolin-mediated endocytotic process (Harischandra et al., 2019), and cell membrane fusion (EL Andaloussi et al., 2013). It is therefore unsurprising that α-syn delivered by exosomes can induce more aggregation than free α-syn oligomers, further implicating the importance of exosome-associated α-syn.
In addition to exosomes, activated microglia also release cytokines, such as TNF-α, IL-1β and IL-6. In the CNS this pro-inflammatory role of microglia affects the surrounding neurons as cytokines diffuse to the extracellular microenvironment. To address whether neuroinflammation affects microglial exosome mediated α-syn transmission, we incubated neurons with microglial exosomes in the presence or absence of TNF-α, IL-1β and IL-6. As shown in this study, α-syn aggregation was more evident when exosomes were combined with cytokines. These results indicate that the uptake of microglial exosomes in neurons was accompanied by an immunologically synergic effect, resulting in enhanced α-syn toxicity.
We used a mouse model to investigate the effects of microglia in α-syn spreading in the brain, and showed that after receiving a single injection of synthetic α-syn fibrils in dorsal striatum, cell-to-cell transmission of phosphorylated α-syn occurred and Lewy body-like pathology was detected in anatomically interconnected regions (Luk et al., 2012a). In this study, we confirmed that this abnormal p-α-syn aggregates in the striatum and several other areas directly interconnected to the striatum such as the cortex and substantia nigra. In addition, we observed an increase in Iba1+ cells in striatum and substantia nigra, showing an active microglia morphology. A recent study focused on microglia activation and accumulation of p-α-syn inclusions in brain after PFF injection demonstrated that PFF triggered reactive microglia, with increased MHC-II expression, even months prior to nigral degeneration (Duffy et al., 2018). Thus, this mouse model enables us to study the interaction of α-syn transmission and microglia. With this animal model, we then fed mice with a chow containing PLX3397, an inhibitor of CSF1R, which depletes microglia (Elmore et al., 2014). Strikingly, PLX3397 treatment reduced the presence of p-α-syn in addition to the number of Iba1+ cells in the PFF injected mice, compared to the PFF-treated mice fed with regular chow. These data indicate that microglia act as efficient participants in the pathological spreading of α-syn after PFF injection.
Similar to direct injection of PFF to the brain, stereotaxic delivery of microglial exosomes to the striatum also induced α-syn aggregation and propagation in mice—although with a slower onset of 6 months. Strikingly, these exosomes also induced nigral dopaminergic neurodegeneration and impaired motor coordination. One limitation of our behaviour studies is the lack of measurement of behavioural asymmetry using amphetamine-induced rotational test as reported (Harischandra et al., 2019). Thus, our data suggest that oligomer carrying exosomes from microglia are sufficient to induce major behavioural and pathological features of sporadic Parkinson’s disease, leading to a vicious neurotoxic cycle.
It has been reported that injection of CSF-derived exosomes from patients with dementia with Lewy bodies and Parkinson’s disease is able to induce oligomerization of soluble α-syn in neuronal cells and at the injected site of mouse brain (Stuendl et al., 2016; Ngolab et al., 2017; Minakaki et al., 2018). These studies support the concept of exosomes from patients having infectious abilities. However, exosomes in CSF including those from neuron, astrocyte, microglia, and endothelium and exosome from different cell types have different functions and properties (Budnik et al., 2016). Mixed exosomes were used in these studies because the original sources of exosomes in the CSF were not identified. In our study, we found that microglia/macrophage-derived exosomes (CD11b+) were present in CSF of patients with Parkinson’s disease, comprising ∼4–12% of the total exosomes. Based on a previous study, only 2.17% of CSF α-syn is present in exosomes in CSF from both Parkinson’s disease and healthy controls (Stuendl et al., 2016). Thus, the amount of α-syn in microglia/macrophage-derived exosomes was limited, yet sufficient to be capable of infecting neurons and serving as a seed to induce oligomerization of soluble α-syn in neurons as we observed, which showed more strong evidence to support the function of microglial exosome in α-syn transmission.
In summary, we report that microglia, in addition to their well-established role in neuroinflammation, are also actively involved in the process of cell-to-cell transmission of α-syn through the release of exosomes(Supplementary Fig. 12). Our observations, that CD11+ exosomes isolated from CSF of Parkinson’s disease patients contained α-syn and that microglial exosomes were capable of inducing nigrostriatal degeneration, further illustrate the critical role of these exosomes in the pathogenesis of Parkinson’s disease. Because of previous reports that astrocyte-derived exosomes are also able to induce protein aggregation in mouse brain (Dinkins et al., 2014), it is most likely that glial cells in general participate in the process of protein transmission. Therefore, targeting exosome release from all cell types should be taken into consideration as a potential therapeutic strategy for Parkinson’s disease.
Supplementary Material
Acknowledgements
We are grateful to The Michael J.Fox Foundation for the gift of preformed fibrils monomer. We thank Jue Zhao for assistance in preparing PET and clinical data of patients.
Funding
This study was supported by the National Natural Science Foundation of China (81971013 to M.C.; 81870915 and 81571109 to Q.D.; 81571232 to J.W.), Grant from the Science and Technology Commission of Shanghai Municipality (16411970200). K.T. is supported by the National Institute of Environmental Health Sciences (R01-ES022274 and R35-ES030523).
Competing interests
The authors report no competing interests.
Glossary
- EF =
exosomal fractions from PFF-treated donor neurons
- LPS =
lipopolysaccharide
- MSA =
multiple system atrophy
- MVBs =
multivesicular bodies
- PFF =
preformed fibrils
- SNpc =
substantia nigra pars compacta
- α-syn =
α-synuclein
References
- Aguzzi A, Barres BA, Bennett ML.. Microglia: scapegoat, saboteur, or something else? Science 2013; 339: 156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 2011; 42: 360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol 1997; 12: 25–31. [PubMed] [Google Scholar]
- Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 2015; 18: 1584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baixauli F, Lopez-Otin C, Mittelbrunn M.. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol 2014; 5: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO.. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem 1998; 273: 21883–92. [DOI] [PubMed] [Google Scholar]
- Bourdenx M, Dovero S, Engeln M, Bido S, Bastide MF, Dutheil N, et al. Lack of additive role of ageing in nigrostriatal neurodegeneration triggered by alpha-synuclein overexpression. Acta Neuropathol Commun 2015; 3: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brites D, Fernandes A.. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci 2015; 9: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck D, Wenning GK, Stefanova N, Fellner L.. Glia and alpha-synuclein in neurodegeneration: a complex interaction. Neurobiol Dis 2016; 85: 262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu LL, Liu FT, Jiang CF, Guo SS, Yu H, Zuo CT, et al. Patterns of dopamine transporter imaging in subtypes of multiple system atrophy. Acta Neurol Scand 2018; 138: 170–6. [DOI] [PubMed] [Google Scholar]
- Budnik V, Ruiz-Canada C, Wendler F.. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 2016; 17: 160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Standaert DG, Harms AS.. The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J Neuroinflamm 2012; 9: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Lang H, Geng N, Wang J, Li N, Wang X.. Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett 2013; 548: 190–5. [DOI] [PubMed] [Google Scholar]
- Chistiakov DA, Chistiakov AA.. Alpha-Synuclein-carrying extracellular vesicles in Parkinson’s disease: deadly transmitters. Acta Neurol 2017; 117: 43–51. [DOI] [PubMed] [Google Scholar]
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH.. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis 2009; 35: 385–98. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D.. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 2004; 305: 1292–5. [DOI] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, et al. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A 2009; 106: 8043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SE, Lees AJ.. Parkinson’s Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl 1993; 39: 165–72. [PubMed] [Google Scholar]
- Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 2012; 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, et al. Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci 2010; 30: 12535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delenclos M, Trendafilova T, Mahesh D, Baine AM, Moussaud S, Yan IK, et al. Investigation of endocytic pathways for the internalization of exosome-associated oligomeric alpha-synuclein. Front Neurosci 2017; 11: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A 2009; 106: 13010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E.. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging 2014; 35: 1792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryanovski DI, Guzman JN, Xie Z, Galteri DJ, Volpicelli-Daley LA, Lee VM, et al. Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J Neurosci 2013; 33: 10154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du D, Hu L, Wu J, Wu Q, Cheng W, Guo Y, et al. Neuroinflammation contributes to autophagy flux blockage in the neurons of rostral ventrolateral medulla in stress-induced hypertension rats. J Neuroinflamm 2017; 14: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MF, Collier TJ, Patterson JR, Kemp CJ, Luk KC, Tansey MG, et al. Lewy body-like alpha-synuclein inclusions trigger reactive microgliosis prior to nigral degeneration. J Neuroinflamm 2018; 15: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ.. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013; 12: 347–57. [DOI] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014; 82: 380–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader CM, Sanchez D, Furlan M, Colombo MI.. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic 2008; 9: 230–50. [DOI] [PubMed] [Google Scholar]
- Fellner L, Stefanova N.. The role of glia in alpha-synucleinopathies. Mol Neurobiol 2013; 47: 575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Li S, Combs CK.. Beta-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor alpha and NMDA receptors. J Neurosci 2005; 25: 2566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundt EC, Maynard N, Clancy EK, Roy S, Bousset L, Sourigues Y, et al. Neuron-to-neuron transmission of alpha-synuclein fibrils through axonal transport. Ann Neurol 2012; 72: 517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 2011; 121: 715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra DS, Rokad D, Neal ML, Ghaisas S, Manne S, Sarkar S, et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Sci Signal 2019; 12. doi: 10.1126/scisignal.aau4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, et al. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci 2013; 33: 9592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Latz E.. Innate immune activation in neurodegenerative disease. Nat Rev Immunol 2014; 14: 463–77. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Vyas S, Hunot S.. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord 2012; 18 Suppl 1: S210–2. [DOI] [PubMed] [Google Scholar]
- Holcik J. A contribution to the method of mortality tables and potential demography for measurement of general health status. Cesk Zdrav 1982; 30: 491–9. [PubMed] [Google Scholar]
- Kadam PD, Chuan HH.. Erratum to: rectocutaneous fistula with transmigration of the suture: a rare delayed complication of vault fixation with the sacrospinous ligament. Int Urogynecol J 2016; 27: 505. [DOI] [PubMed] [Google Scholar]
- Klingelhoefer L, Reichmann H.. Pathogenesis of Parkinson disease–the gut-brain axis and environmental factors. Nat Rev Neurol 2015; 11: 625–36. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci 2005; 118: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson’s disease. J Mol Neurosci 2008; 34: 17–22. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Khoshaghideh F, Patel S, Lee SJ.. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci 2004; 24: 1888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, Kim WK, et al. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol 2010; 185: 615–23. [DOI] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012. a; 338: 949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM.. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 2012. b; 209: 975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016; 353: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AE, Murphy M, Zhou H, Li X.. E3 ubiquitin ligases Pellinos as regulators of pattern recognition receptor signaling and immune responses. Immunol Rev 2015; 266: 109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakaki G, Menges S, Kittel A, Emmanouilidou E, Schaeffner I, Barkovits K, et al. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy 2018; 14: 98–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor DE, Ugras SE, Daniels MJ, Ischiropoulos H.. Dynamic structural flexibility of alpha-synuclein. Neurobiol Dis 2016; 88: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ.. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 2009; 132: 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngolab J, Trinh I, Rockenstein E, Mante M, Florio J, Trejo M, et al. Brain-derived exosomes from dementia with Lewy bodies propagate alpha-synuclein pathology. Acta Neuropathol Commun 2017; 5: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehler AM, Xiang W, Spitzer P, May VE, Meixner H, Rockenstein E, et al. Autophagy modulates SNCA/alpha-synuclein release, thereby generating a hostile microenvironment. Autophagy 2014; 10: 2171–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Franchi L, Nunez G, Dubyak GR.. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 2007; 179: 1913–25. [DOI] [PubMed] [Google Scholar]
- Stuendl A, Kunadt M, Kruse N, Bartels C, Moebius W, Danzer KM, et al. Induction of alpha-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain 2016; 139: 481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari Z, Kis V, Lippai M, Hegedus K, Farago T, Lorincz P, et al. Rab11 facilitates cross-talk between autophagy and endosomal pathway through regulation of Hook localization. MBoC 2014; 25: 522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS.. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 2010; 37: 510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Michael J. Fox Foundation. Protocol for generation of Pre-Formed Fibrils from Alpha-Synuclein Monomer. Protocol [Internet]. 2017. Available from https://www.michaeljfox.org/files/accelerate/models/PFF%20Protocol%202017b.pdf (1 January 1900, date last accessed).
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319: 1244–7. [DOI] [PubMed] [Google Scholar]
- Tsunemi T, Hamada K, Krainc D.. ATP13A2/PARK9 regulates secretion of exosomes and alpha-synuclein. J Neurosci 2014; 34: 15281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdinocci D, Radford RA, Siow SM, Chung RS, Pountney DL.. potential modes of intercellular alpha-synuclein transmission. Int J Mol Sci 2017; 18:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visanji NP, Brooks PL, Hazrati LN, Lang AE.. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol Commun 2013; 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Lee VM.. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc 2014; 9: 2135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011; 72: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC.. Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 2003; 278: 25009–13. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zhang G, Han C, Ma K, Guo X, Wan F, et al. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis 2019; 10: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Jin J, Chang M, Chang JH, Hu H, Zhou X, et al. Peli1 promotes microglia-mediated CNS inflammation by regulating Traf3 degradation. Nat Med 2013; 19: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, He D, Bai Y.. Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol 2016; 53: 6709–15. [DOI] [PubMed] [Google Scholar]
- Yun X, Guoxin Z, Chao H, Kai M, Xingfang G, Fang W, et al. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis 2019; 10: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 2005; 19: 533–42. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chen F, Chen S, Liu X, Cui M, Dong Q.. The Parkinson’s disease-associated gene PINK1 protects neurons from ischemic damage by decreasing mitochondrial translocation of the fission promoter Drp1. J Neurochem 2013; 127: 711–22. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT.. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol 2007; 170: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.