Abstract
In this contribution, we provide a comprehensive overview of C–H activation methods promoted by NHC–transition-metal-complexes, covering the literature since 2002 (the year of the first report on metal–NHC catalyzed C–H activation) through June 2019 focusing on both NHC ligands and C–H activation methods. The review covers C–H activation reactions catalyzed by group 8 to 11 NHC–metal complexes. Through discussing the role of NHC ligands in promoting challenging C–H activation methods, the Reader is provided with an overview of this important area and its crucial role in forging carbon–carbon and carbon–heteroatom bonds by directly engaging ubiquitous C–H bonds.
Graphical Abstract

1. Introduction
Since the seminal isolation of 1,3-diadamantylimidazol-2-ylidene by Arduengo in 1991, N-heterocyclic carbenes (NHCs) have played a central role as ligands in transition-metal-catalysis.1–59 Of particular importance is the ability of NHC ligands to enable difficult oxidative additions through strong σ-donation to various metal centers.41–52 Furthermore, NHC ligands represent one of the most wide-ranging ways to vary steric environment around the metal by N-wingtip substitution and backbone modifications of the ligand.35,51,53–59 Moreover, recent studies have emphasized the importance of π-backbonding from the metal to the empty p-orbitals of the NHC, which is an important component in transition-metal-catalysis employing electrophilic metals.43 Finally, NHC ligands coordinated to metal centers are surprisingly robust to oxidative decomposition, thus enabling the use of NHC-metal complexes in oxidative transformations.18
In the last twenty years, tremendous advances have been achieved in C–H functionalization reactions.60–86 At present, the synthesis of target molecules by selective activation of C–H bonds ranks amongst the most important objectives of modern synthetic chemistry and transition-metal-catalysis.60,61 New C–H activation methods for the direct conversion of ubiquitous C–H bonds have spurred the development of synthetic organic technologies through atom-, cost- and step-economic installation of functional groups and functional group interconversion.63–86 As a result, C–H functionalization methods are of broad academic and industrial importance and find widespread application in pharmaceutical, agrochemical, natural product, functional materials, polymers, and fine chemicals fields. Due to potential important economic benefits,85,86 the development of new and efficient C–H activation methods is a major driving methodology leading to a significantly more efficient construction of organic molecules. Despite the fact that transition-metal-catalyzed reactions exploiting NHC ligands have become invaluable in catalysis and organic synthesis in the last twenty years and significant advances have been reported, a comprehensive review on C–H activation reactions enabled by NHC ligands has not been published to date.
In this contribution, we provide a comprehensive overview of C–H activation methods promoted by NHC–transition-metal-complexes, covering the literature since 2002 (the year of the first report on metal–NHC catalyzed C–H activation) through June 2019 focusing on both NHC ligands and C–H activation methods. The review covers C–H activation reactions catalyzed by group 8 to 11 NHC–metal complexes, which encompasses all metals that have been used for catalytic C–H activation reactions using NHC-metal complexes to date. We believe that by discussing the role of NHC ligands in promoting challenging C–H activation methods, the Reader will be provided with an overview of this important area and its crucial role in forging carbon–carbon and carbon–heteroatom bonds by directly engaging ubiquitous C–H bonds.
The review is organized by metal and further by the type of C–H bonds undergoing functionalization. Unsurprisingly, the dominant metal used in C–H functionalization promoted by NHC ligands is palladium, and thus, the review starts with discussing C–H bond activation catalyzed by precious metal–NHC complexes, followed by first row metal–NHC complexes and coinage metal–complexes. Several comprehensive reviews and monographs on NHC ligands have been published.1–59 These publications have addressed general aspects of NHC ligands,1–40 electronic properties of NHCs,41–52 and steric properties of NHCs,35,51,53–59 among other applications.
Structures of the most common NHC ligands used in C–H activation reactions together with their respective acronyms are shown in Figure 1. An important practical aspect of using metal–NHCs involves different ways of generating active complexes that could affect the outcome of C–H activation reactions.1–5,26,34 The most common route involves the use of bench-stable NHC salts followed by an in situ deprotonation and metal complexation. Some C–H activation methods are sensitive to the presence of base, necessitating the use of free NHCs. Arguably, the most convenient way of applying metal–NHC complexes involves the use of well-defined metal–NHC pre-catalysts that are converted in situ into a catalytically-active species.
Figure 1. Structures of the Most Common NHCs Used in C–H Activation Reactions.
It is further important to point out the key unique features of NHC ligands compared to other ligands used in C–H activation reactions. First, NHC ligands are strong σ-donors, which facilitates oxidative addition in nucleophilic C–H activation reactions. For example, comparison of the relative donor ability of NHCs and phosphines using the Tolman electronic parameter (TEP) of [(L)Ir(CO)2Cl] complexes demonstrates that NHCs are significantly stronger donors than commonly used tertiary phosphines (e.g., PPh3, TEP = 2068.9 cm−1; PCy3, TEP = 2056.4 cm−1; IMes, TEP = 2050.7 cm−1; IPr, TEP = 2051.5 cm−1; ICy, TEP = 2049.6 cm−1; IAd, TEP = 2049.5 cm−1), which leads to a stronger binding to the metal, improved stability to air and moisture and more facile oxidative addition as compared with metal-phosphine counterparts.49–51 Note that the strong σ-donation rendered possible by NHCs also allows for the high stability of metals in high oxidation states in oxidative C–H activation reactions, which are problematic with tertiary phosphines due to facile oxidation to phosphine oxides. Second, in terms of steric environment, NHCs are significantly larger than common tertiary phosphines (buried volume, %Vbur, [(L)AuCl] complexes, M–L = 2.00 Å: PPh3, %Vbur = 27.3%; PCy3, %Vbur = 38.8%; PtBu3, %Vbur = 43.9%; IMes, %Vbur = 36.5%; IPr, %Vbur = 45.4%; IPr*, %Vbur = 50.4%).52,53 As an illustrative example, buried volume of common NHCs and tertiary phosphines can be correlated with bond dissociation energies (BDEs) in [Ni(CO)3(L)] complexes, wherein NHCs exhibit much higher bond dissociation energies than the corresponding tertiary phosphines with comparable steric demand (e.g., PPh3: %Vbur = 22%; BDE = 26.7 kcal mol−1; PtBu3: %Vbur = 30%; BDE = 28.0 kcal mol−1; IPr: %Vbur = 29%; BDE = 38.5 kcal mol−1; ICy: %Vbur = 23%; BDE = 39.6 kcal mol−1).52,53 Third, unlike phosphines, NHCs can be more easily modified by N-wingtip substitution, backbone modification and type of the NHC carbene, which allows for gradual tuning of the ligand for the optimum performance in catalysis.
Having pointed out the advantages of NHCs as ligands in C–H activation reactions, it is also important to note several disadvantages of this class of ligands and areas for further improvement. First, it should be considered that in general NHC ligands are more labor and cost intensive to synthesize than common tertiary phosphines. Accordingly, fewer types of NHC ligands are commercially available for rapid testing in catalysis as compared to common tertiary phosphines. Second, except for standard NHC ligands, straightforward synthetic methods that permit for expeditious synthesis of diverse NHC ligands for catalyst screening and identification of active ligands are lacking. Third, NHCs are a relatively new class of ligands and there is a lack of systematic structure-activity-relationship studies that would aid the development of catalytic methods. We hope that this review will stimulate the additional use of NHC ligands in C–H activation methods by a wide range of synthetic researchers.
2. Palladium–NHC Complexes
In 1995, Herrmann and coworkers reported the first example of a Pd–NHC complex in cross-coupling reactions.87 Although not optimum in terms of ligand to metal ratio and reaction conditions, this study constitutes one of the first examples of a transition-metal–NHC complex employed in catalysis. After this seminal report, the use of Pd–NHCs in cross-coupling reactions has rapidly increased in the following decades.1–59 In particular, the vast majority of transition-metal-catalyzed cross-couplings have been significantly improved by employing NHCs instead of phosphines as ancillary ligands by taking advantage of their strong σ-donating abilities and variable steric bulk.17,23,54 Similarly, palladium remains the most common metal used in C–H activation reactions promoted by NHC ligands.
2.1. C(sp2)–H Activation
2.1.1. Aldehyde Directed C(sp2)–H Activation
In 2005, one of the first examples of the Pd–NHC catalyzed C(sp2)–H activation was reported by Cetinkaya and co-workers (Scheme 1).88 To our knowledge, this report represents the first comprehensive study on using metal–NHC complexes for small molecule synthesis via directed C–H functionalization. The direct C(sp2)–H arylation of benzaldehydes was achieved with the assistance of bulky, electron-rich imidazolidinylidene ligands 1–5. Sterically-hindered ligands 1 and 2 were shown to be particularly effective and a variety of electronically-diverse ortho-arylation products were obtained in high yields. In the same year, they reported a series of modified NHCs by changing the backbone from 5-membered imidazolidinylidenes to 6-membered tetrahydropyrimidinylidenes 6–10 (Scheme 2).89 These new NHCs were also found to be excellent ligands for the C(sp2)–H arylation of benzaldehydes. In 2008, the same group described that benzimidazolylidene ligands 11–13 were effective for the same transformation (Scheme 3).90 Pd–NHC complex 12 was shown to be the most efficient among the three complexes towards this aldehyde-directed C(sp2)–H arylation. In 2009, the Cetinkaya group designed three new imidazolidinylidene ligands featuring α-branching at the N-atom that could also promote this C–H arylation reaction (Scheme 4).91 Comparison studies showed that 15 and 16 exhibited higher reactivity than 14 in most cases.
Scheme 1.
Pd–NHC Catalyzed Aldehyde Directed C(sp2)–H Arylation by Cetinkaya
Scheme 2.
Pd–NHC Catalyzed Aldehyde Directed C(sp2)–H Arylation using Six-Membered NHC Ligands by Cetinkaya
Scheme 3.
Pd–NHC Catalyzed Aldehyde Directed C(sp2)–H Arylation using Benzimidazolium NHC Ligands by Cetinkaya
Scheme 4.
Pd–NHC Catalyzed Aldehyde Directed C(sp2)–H Arylation using Imidazolinium NHC Ligands by Cetinkaya
It is interesting to note that an in situ formation of Pd–NHC complexes led to significantly better results than the direct use of preformed Pd–NHC catalysts, which might indicate a non-innocent behavior of the base (Schemes 1–4). In this example, Cs2CO3 was demonstrated as the optimal base and 1,4-dioxane or DMF proved to be the most effective solvents.
2.1.2. Intramolecular Non-Directed C(sp2)–H/C–X Activation
In 2005, Fagnou and co-workers reported an efficient intramolecular C(sp2)–H arylation with aryl chlorides using hydrated [(IPr(Pd)(OAc)2] catalyst bearing carboxylate ligands (Scheme 5).92,93 The turnover number of this Pd–NHC catalyst was substantially improved through addition of IPr·HCl to the reaction. In this chemistry, deprotonation of the IPr·HCl salt in the presence of K2CO3 in DMA at 130 °C resulted in C(sp2)–H arylation in good to excellent yields.
Scheme 5.
Pd–NHC Catalyzed Intramolecular Non-Directed C(sp2)–H/C(sp2)–X Activation by Fagnou
A comparative study between different Pd(II)–NHC complexes in the intramolecular C(sp2)–H arylation with aryl chlorides was reported by Sefkow and co-workers in 2010 (Scheme 6).94 Thus, out of four complexes investigated, namely [(SIPr)Pd(cin)Cl] (cin = cinnamyl), [(IPr)PdCl2]2, [Pd-PEPPSI-SIPr] and [(IPr)2PdCl2], [(SIPr)Pd(cin)Cl] was the most reactive complex. In addition, the bis-NHC complex [(IPr)2PdCl2] was ineffective in promoting this C(sp2)–H arylation reaction. This protocol is quite effective for achieving intramolecular biaryl C–H/C–Cl coupling with easily handled Pd(II)–NHC complexes at low catalyst loadings.
Scheme 6.
Pd–NHC Catalyzed Intramolecular Non-Directed C(sp2)–H/C(sp2)–X Activation by Sefkov
2.1.3. Intermolecular Non-Directed C(sp2)–H/C–X Activation
The direct C–H activation of heterocycles and acidic arene C–H bonds represent important reactions catalyzed by Pd–NHC complexes.82,83 These reactions are classified by the type of heterocycle and bond undergoing the C–H activation process; however, it should be noted that several Pd–NHC complexes have been shown to be effective in activating various classes of heterocycles.
2.1.3.1. C(sp2)–H Activation in Heteroarenes
These reactions are categorized into 5-membered heterocycles with one heteroatom such as pyrroles, thiophenes, furans, indoles, benzofurans, benzothiophenes, and 5-membered heterocycles with two or more heteroatoms, such as imidazoles, oxazoles, thiazoles and their benzoderivatives, among others. It is worth noting that C(sp2)–H activations in heteroarenes are among the most successful reactions developed using NHC ancillary ligands to date. Among the key advantages of Pd–NHCs in this class of C–H activation reactions are high stability to the reaction conditions, high regioselectivity and beneficial functional group tolerance as compared to the more traditionally used tertiary phosphine ligands.
2.1.3.1.1. 5-Membered heterocycles with one heteroatom
The first example of a direct C(sp2)–H activation of heterocycles by Pd–NHC complexes was reported by Sames and co-workers in 2005 (Scheme 7).95 These researches established that the C3 C–H arylation of unprotected indole after deprotonation with MeMgCl in the presence of TMEDA proceeds with 67:1 C3/C2 selectivity using IMes ligand (cf. 14:1 C3/C2 selectivity using PPh3).
Scheme 7.
Pd–NHC Catalyzed Direct C3–H Arylation of Indole by Sames
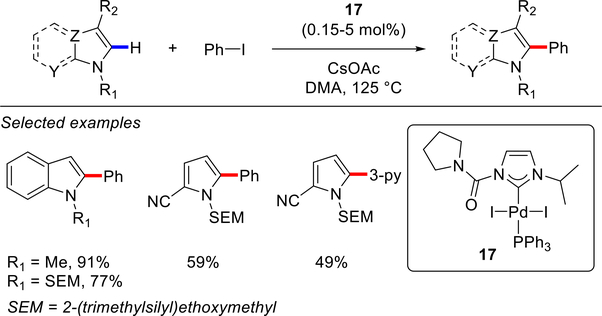
In 2006, the Sames96 and Sanford groups97 independently reported further examples of Pd–NHC catalyzed C(sp2)–H arylation of heterocycles (Schemes 8–9). Sames and co-workers developed a mixed [Pd(NHC)I2(PPh3)] complex 17 for C(sp2)–H arylation of SEM-protected (SEM = trimethylsilylethoxymethyl) azoles with aryl iodides in DMA at 125 °C (Scheme 8).96 A wide range of azoles, including pyrroles, indoles, imidazoles and imidazo[1,2-a]pyridines, was directly arylated in good to excellent yields (see also Scheme 26). The reaction was characterized by operational simplicity and stability of the Pd(II)–NHC pre-catalyst 17 to air- and moisture. At about the same time, Sanford and co-workers reported [Pd(IMes)(OAc)2], featuring carboxylate ligands, for the C(sp2)–H arylation of indoles and pyrroles using aryl iodonium salts (Scheme 9).97 A wide range of indoles and representative pyrroles were functionalized with Ar2I+BF4− in AcOH at 25 °C to give 2-arylindoles and 2-arylpyrroles in high yields. It is noteworthy that this reaction could be carried out under remarkably mild room temperature conditions and also appeared to be compatible with air and moisture.
Scheme 8.
Pd–NHC Catalyzed Direct C–H Arylation of Azoles by Sames
Scheme 9.
Pd–NHC Catalyzed Direct C–H Arylation of Indoles and Pyrroles by Sanford
Scheme 26.
Pd–NHC Catalyzed Direct C5–H Arylation of Imidazoles by Sames
The area of direct C(sp2)–H arylation of heterocycles by Pd–NHC complexes then remained dormant until 2010 when Özdemir and co-workers synthesized a series of Pd–NHC benzimidazolylidene complexes 18–21 bearing different N-side chains and employed them as catalysts for the direct C(sp2)–H arylation of furan, thiophene and thiazole derivatives with aryl bromides (Scheme 10, see also Schemes 27–28).98 Impressively, only 1.0 mol% of air-stable Pd(II)–NHC pre-catalysts was required for this transformation. This represented a major practical advantage over the procedures employing air-sensitive phosphine ligands. In addition, this procedure generates KOAc and HBr as major by-products instead of metal salts.
Scheme 10.
Pd–NHC Catalyzed Direct C–H Arylation of Furans, Thiophenes and Thiazoles by Özdemir
Scheme 27.
Pd–NHC Catalyzed Direct C–H Arylation of Benzothiazole by Özdemir
Scheme 28.
Pd–NHC Catalyzed Direct C–H Arylation of Benzothiazole and Benzoxazole by Arslan
In 2014, Akkoc and co-workers reported bis-Pd–NHC benzimidazolylidene complexes 23–25 for the direct C(sp2)–H C5 arylation of furans, thiophenes and thiazoles (Scheme 11).99 The procedure was compatible with both electron-deficient and electron-rich aryl halides as coupling partners. High catalytic activity was observed using 0.5 mol% of Pd–NHC complexes in DMA at 130 °C. The same group reported similar bis-Pd–NHC benzimidazolylidene complexes 26–30 with N-benzylic side-chains for the direct C(sp2)–H C5 arylation of furans, thiophenes and thiazoles at the C5 position (Scheme 12).100 Comparison of the reactivity demonstrated that Pd(II)–NHC 30 was the most efficient pre-catalyst.
Scheme 11.
Pd–NHC Catalyzed Direct C–H Arylation of Furans, Thiophenes and Thiazoles by Akkoc
Scheme 12.
Pd–NHC Catalyzed Direct C–H Arylation of Furans, Thiophenes and Thiazoles by Akkoc
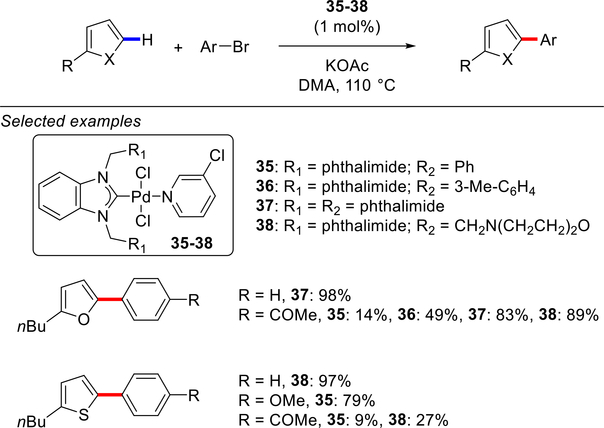
Subsequently, Cetinkaya and co-workers reported bis-Pd–NHC tetrahydropyrimidinylidene complexes 31–34 as catalysts for the direct C2 or C5 arylation of furans, thiophenes and thiazoles with aryl bromides (Scheme 13).101 High catalytic activity was observed using KOAc in DMA at 150 °C. More recently, Akkoc and co-workers reported a series of Pd–PEPPSI-type complexes 35–38 based on phthalimide-functionalized benzimidazolylidene scaffold for the direct C(sp2)–H arylation reactions of furans and thiophenes (Scheme 14).102 These complexes contain pyridine throw-away ligand and for some of these C–H arylations displayed good catalytic activity at 110 °C.
Scheme 13.
Pd–NHC Catalyzed Direct C–H Arylation of Furans, Thiophenes and Thiazoles using 6-Membered NHC Ligands by Cetinkaya
Scheme 14.
Pd–NHC Catalyzed Direct C–H Arylation of Furans and Thiophenes by Akkoc
In 2012, the Lee group reported that a highly electron-rich mixed [(NHC)Pd(PCy3)Cl2] complex 39 can be used as efficient catalyst for the C5–H arylation of furans, thiophenes and thiazoles with aryl chlorides (Scheme 15).103 By using PivOH as an additive (30 mol%) and K2CO3 as a base (1.5 equiv), the desired C–H arylated products were obtained in high to excellent yields in DMA at 110 °C.
Scheme 15.
Pd–NHC Catalyzed Direct C–H Arylation of Furans, Thiophenes and Pyrroles by Lee
Simultaneously, the Nolan group reported the use of [(NHC)Pd(cin)Cl] complexes as catalysts for the direct C–H arylation of thiophenes, benzothiophenes and imidazo[1,2-a]pyridines with aryl bromides (Scheme 16).104 The imidazolidinylidene complex [(SIPr)Pd(cin)Cl] proved to be the most efficient catalyst for this reaction. The C–H bond functionalization was achieved at very low catalyst loadings (0.01–0.10 mol%) in DMA at 140 °C.
Scheme 16.
Pd–NHC Catalyzed Direct C–H Arylation of Thiophenes and Imidazopyridines by Nolan
Subsequently, Grisi and co-workers reported a hydroxyl functionalized [(NHC)Pd(CH3CN)Cl2] complex 40 for the direct C(sp2)–H arylation of furans and thiophenes derivatives with aryl bromides (Scheme 17).105 The reaction afforded the C–H arylated products heterocycles in moderate to good yields and with very good functional group tolerance.
Scheme 17.
Pd–NHC Catalyzed Direct C–H Arylation of Furans and Thiophenes by Grisi
Substantial progress in the direct C–H arylation of heterocyles has been made by the Shao group using an imidazole-supported Pd–NHC complex, [(IPr)Pd(1-Me-im)Cl2] (Schemes 18–19, see also Schemes 34–35 and 44–45). In 2015, their reported the direct C(sp2)–H arylation of benzofurans with aryl chlorides in the presence of Cu2O and KOtBu in THF at 130 °C (Scheme 18).106 A wide range of C2-arylated benzofurans were obtained in moderate to high yields. Subsequently, they used the same Pd(II)–NHC complex for the direct C2–H arylation of thiophenes and benzothiophenes, furnishing the arylated products in good to excellent yields (Scheme 19).107 Thus, this work amply demonstrated that the class of NHC-Pd(II)-Im complexes can serve as efficient catalysts in the direct C(sp2)–H arylation with less reactive aryl chlorides.
Scheme 18.
Pd–NHC Catalyzed Direct C–H Arylation of Benzo[b]Furans by Shao
Scheme 19.
Pd–NHC Catalyzed Direct C–H Arylation of Thiophenes by Shao
Scheme 34.
Pd–NHC Catalyzed Direct C–H Arylation of Oxazoles by Shao
Scheme 35.
Pd–NHC Catalyzed Direct C–H Benzylation of Oxazoles by Shao
Scheme 44.
Pd–NHC Catalyzed Direct C–H Arylation of Imidazoles by Shao
Scheme 45.
Pd–NHC Catalyzed Direct C3–H Arylation of Imidazo[1,2-a]pyridines by Shao
The first comprehensive study on the direct C(sp2)–H arylation of pyrroles catalyzed by Pd–NHC complexes was reported by the Doucet group in 2013 (Scheme 20).108 They found that bis-NHC complexes 41–44 derived from the benzimidazolylidene scaffold were highly effective in the direct regioselective C2 or C5 arylation of a range of pyrrole derivatives using electron-deficient aryl chlorides. The reaction was carried out in the presence of KOAc in DMA at 150 °C. Bulky N-substituents on the NHC ligand were found to enhance the catalytic activity.
Scheme 20.
Pd–H Arylation of Pyrroles by Doucet
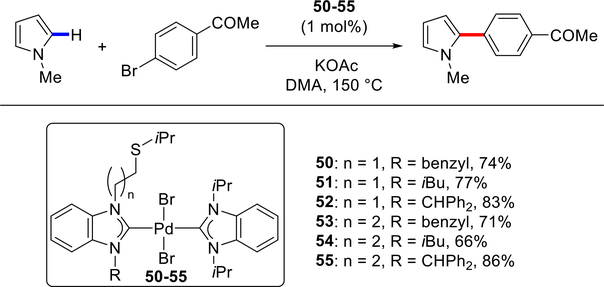
In 2014, the Huynh group reported the synthesis and application of indazolin-3-ylidene [(NHC)Pd(amine)Br2] complexes 45–49 with pendant tertiary amine side chains in the direct C(sp2)–H arylation of N-methylpyrrole (Scheme 21).109 Pd–NHC complex 46 (R = NEt2) was shown to be most effective and the reactivity order was 46 > 45 (R = Br) ≈ 47 (R = NPr2) ≈ 48 (R = NBu2) ≈ 49 (R = NiBu2). Simultaneously, the same group reported bis-benzimidazolylidene [(NHC)2PdBr2] complexes 50–55 with an alkyl thioether side chain in the direct C(sp2)–H arylation of N-methylpyrrole (Scheme 22).110 High catalytic activity was observed with both ethyl-thioisopropyl and propyl-thioisopropyl side chains (n = 1 or n = 2).
Scheme 21.
Pd–NHC Catalyzed Direct C–H Arylation of Pyrrole using Indazolin-3-ylidene Complexes by Huynh
Scheme 22.
Pd–H Arylation of Pyrrole using Benzimidazolin-2-ylidene Complexes by Huynh
In 2017, the Yigit group investigated perhydrobenzimidazolylidene bis-Pd–NHC complexes 56–60 in the direct C(sp2)–H arylation of pyrroles (Scheme 23).111 All complexes showed high catalytic activity using electronically-activated aryl chlorides as the coupling partners. Reactivity comparison studies demonstrated that the reactivity order of these bis-Pd–NHC complexes depends on the substitution of both coupling partners.
Scheme 23.
Pd–NHC Catalyzed Direct C–H Arylation of Pyrroles by Yigit
Subsequently, Özdemir and co-workers reported the synthesis of benzimidazolylidene Pd–PEPPSI-type complexes 61–65 and their application in the direct C(sp2)–H arylation of pyrrole derivatives (Scheme 24).112 These complexes displayed high catalytic activity in that even unactivated aryl chlorides such as chlorobenzene or 4-chlorotoluene were compatible as the coupling partners in DMA at 120 °C. Pd–NHC pre-catalyst 64 bearing a chelating MeO group showed the highest catalytic activity. In 2018, the same group reported a series of benzimidazolylidene bis-Pd–NHC complexes 66–70 with chelating N-side chains as catalysts in the direct C(sp2)–H arylation of pyrroles (Scheme 25).113 High catalytic performance was observed using unactivated aryl chlorides as the coupling partners. Precatalyst 67 featuring an unhindered N-benzyl group was found to be the most catalytically-active.
Scheme 24.
Pd–H Arylation of Pyrroles by Özdemir
Scheme 25.
Pd–NHC Catalyzed Direct C–H Arylation of Pyrroles by Özdemir
2.1.3.1.2. 5-Membered heterocycles with two or more heteroatoms
In general, the direct arylation of five-membered heterocycles with two or more heteroatoms is more facile than that of electron-rich heterocycles due to more acidic C(sp2)–H bonds.82,83 In 2006, the Sames group reported the first example of a direct C(sp2)–H arylation of imidazoles using Pd–NHC complexes (Scheme 26).96 The reaction was fully regio-selective for the C5 arylation in the presence of catalytic [Pd(NHC)(PPh3)I2] complex 17. The authors demonstrated that the use of Ag2CO3 as a base (2.0 equiv) significantly improved the yields as compared to CsOAc used for the direct C(sp2)–H arylation of electron-rich heterocycles (see Scheme 8). In 2010, Sames and co-workers utilized the same conditions for the direct C(sp2)–H monoarylation of N-SEM-imidazole (SEM = trimethylsilylethoxymethyl) at the C5 position in their studies en route to complex 2,4,5-triarylimidazoles (not shown).114
In 2009, the Özdemir group reported the synthesis and catalytic activity of benzimidazolylidene bis-Pd–NHC complexes 71–74 in the direct C(sp2)–H arylation of benzothiazole with aryl bromides (Scheme 27).115 The optimized reaction conditions utilized K3PO4 in NMP at 150 °C. The highest activity was observed with complex 74 featuring chelating MeO groups on the N-benzyl ring. Around the same time, Arslan and co-workers reported a related benzimidazolylidene Pd-bis-NHC complex 75 as an efficient catalyst for the direct C(sp2)–H arylation of benzoxazoles and benzothiazoles with aryl bromides in the presence of K3PO4 in NMP at 130 °C (Scheme 28).116
Subsequently, the Özdemir group reported the direct C(sp2)–H arylation benzothiazoles with aryl bromides using a bridged benzimidazolylidene Pd-bis-NHC complex 76 bearing a linked chain (Scheme 29).117 The arylation reactions could be accomplished in good to high yields using several functionalized aryl bromides in the presence of K3PO4 in NMP at 130 °C. The same group developed further analogues of the bridged complex 76 featuring different N-substitution of NHC ligands (Scheme 30).118 In particular, an increase in the catalytic activity in the direct C(sp2)–H arylation of benzothiazoles was realized by using the more sterically-hindered N-iPr group in the NHC framework.
Scheme 29.
Pd–NHC Catalyzed Direct C–H Arylation of Benzothiazole by Özdemir
Scheme 30.
Pd–NHC Catalyzed Direct C–H Arylation of Benzothiazole by Özdemir
More recently, Özdemir and co-workers reported another series of benzimidazolylidene Pd-bis-NHC complexes 81–84, which proved to be effective catalysts in the direct C(sp2)–H arylation of benzoxazoles and benzothiazoles with aryl bromides (Scheme 31).119 A diverse range of aryl bromides was successfully applied in this reaction in the presence of KOAc in DMA at 150 °C. The Pd–NHC complex bearing N-alkyl chain proved to be the least reactive in this catalyst series. In 2018, Özdemir and co-workers reported similar benzimidazolylidene Pd-bis-NHC complexes 85–88 for the direct C(sp2)–H arylation of thiazoles (Scheme 32).120 The reaction was regio-selective for the C5–H arylation. Both electron-deficient and electron-rich aryl bromides were shown to be excellent coupling partners.
Scheme 31.
Pd–NHC Catalyzed Direct C–H Arylation of Benzothiazole and Benzoxazole by Özdemir
Scheme 32.
Pd–NHC Catalyzed Direct C–H Arylation of Thiazole by Özdemir
In 2011, Hoarau and co-workers disclosed the direct C(sp2)–H arylation of oxazoles using Pd(OAc)2/IMes as an effective catalyst system (Scheme 33).121 The reaction was performed in the presence of Cs2CO3 in dioxane at 110 °C, giving facile access to 2,5-diaryloxazole-4-carboxylates in good to excellent yields and with high functional group tolerance. The product 2,5-diphenyloxazoles (DPO) and 1,4-bis(5-phenyloxazol-2-yl)benzenes (POPOP) constitute major components of commercial plastics and liquid scintillation mixtures. Thus, this efficient C–H arylation approach gave rapid access to novel sensors with unusual stokes shifts.
Scheme 33.
Pd–NHC Catalyzed Direct C–H Arylation of Oxazoles by Hoarau
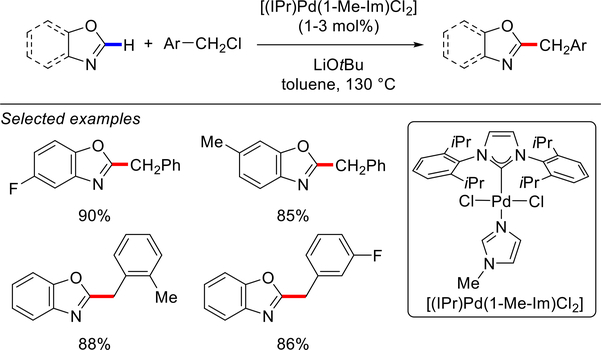
In 2014, the Shao group reported the direct C(sp2)–H arylation of benzoxazoles and oxazoles with aryl chlorides using their well-defined, air- and moisture-stable [Pd(IPr)(1-Me-im)Cl2] complex (Scheme 34).122 A variety of electron-deficient and electron-rich aryl chlorides were successfully utilized as the arylating reagents in the presence of excess LiOtBu (5 equiv) in toluene at 130 °C. In an interesting extension of this work, Shao and co-workers reported the direct C(sp2)–H benzylation of benzoxazoles and oxazoles with benzyl chlorides (Scheme 35).123 Similar catalytic protocol using lower catalyst loading of Pd– NHC was employed, and a broad range of substrates gave the desired C2-benzylated products in excellent yields, highlighting the utility of [Pd(NHC)(im)Cl2] complexes in the direct C(sp2)–H functionalization reactions.
In 2015, the Yang group described a chelating [Pd(NHC)Cl2] complex 89 containing N-2-pyrimidine and N-2-hydroxyalkyl side chains as an efficient catalyst for the direct C(sp2)–H arylation of benzoxazoles (Scheme 36).124 The optimized reaction conditions utilized LiOtBu as a base in DMF at 130 °C. This protocol was applied to a broad range of substrates and achieved TON of 40.
Scheme 36.
Pd–NHC Catalyzed Direct C–H Arylation of Benzoxazoles by Yang
In 2018, the Gandhi group developed N-4-dibenzofuryl-functionalized Pd–PEPPSI-type catalyst 90 for the direct C(sp2)–H arylation of benzoxazoles (Scheme 37).125 The C–H arylation was achieved in DMF at 120 °C. These N-dibenzofuryl Pd–NHC pre-catalysts also showed high activity in the Suzuki-Miyaura cross-coupling of aryl bromides.
Scheme 37.
Pd–NHC Catalyzed Direct C–H Arylation of Benzoxazoles by Gandhi
Recently, Li and co-workers reported an unsymmetrical pincer-type [Pd(NHC)(PPh2Ar)Cl2] carbene–nitrogen–phosphine (C–N–P) complex 91 for the direct C(sp2)–H arylation of benzoxazoles (Scheme 38).126 Notably, the direct C–H arylation with aryl bromides was accomplished with only 0.25–0.5 mol% catalyst loading at 90 °C. In addition, the authors showed that the PdCl2(κ2-CP) complex 91 is more reactive than the corresponding cationic [PdCl(κ2-CNP)]PF6 complex in this direct C(sp2)–H arylation.
Scheme 38.
Pd–NHC Catalyzed Direct C–H Arylation of Benzoxazoles by Li
In 2018, the Yang group reported Pd–PEPPSI-type complexes featuring (2-pyridyl)alkyl carboxylate ligands 92–95 as catalysts for the direct C(sp2)–H arylation of benzoxazoles and oxazoles with aryl bromides (Scheme 39).127 All complexes showed high activity in this transformation. Furthermore, the following order of reactivity was found: 92 (n = 1, IPr) > 93 (n = 1, SIPr) > 94 (n = 2, IPr) > 95 (n = 2, SIPr). At the same time, Yang and co-workers synthesized a series of Pd–PEPPSI complexes with (2-quinolinyl)alkyl carboxylate ligands 96–101 and evaluated their activity in the direct C(sp2)–H arylation of benzoxazoles (Scheme 40).128 These (2-quinolinyl)alkyl carboxylate Pd–NHC complexes proved to be similarly effective in the C–H arylation to (2-pyridyl)alkyl carboxylate pre-catalysts 92–95 (Scheme 39).127
Scheme 39.
Pd–NHC Catalyzed Direct C–H Arylation of Benzoxazoles by Yang
Scheme 40.
Pd–NHC Catalyzed Direct C–H Arylation of Benzoxazoles by Yang
Very recently, Yang reported a series of cationic Pd–NHC complexes featuring allyl and N-heterocyclic throw-away ligands 102–103 (Scheme 41).129 These catalysts showed high activity in the direct C(sp2)–H arylation of azoles and allowed for the C–H arylation of beznoxazoles to occur at 100 °C in DME.
Scheme 41.
Pd–NHC Catalyzed Direct C–H Arylation of Benzoxazoles by Yang
In 2011, the Lee group reported mixed [Pd(NHC)(PCy3)OAc2] complex 104 bearing electron-rich phosphine and carboxylate ligands for the regioselective efficient selective C5–H arylation of imidazoles at the C5 position with aryl halides (Scheme 42 cf. Scheme 15).130 Notably, this catalytic system allowed the use of electron-deficient and electron-rich aryl chlorides as coupling partners using KOAc as a base in DMA at 140 °C. In 2016, the same group reported well-defined Pd(0)–NHC complexes 105–108 with N-tethered phosphine-functionalized NHC ligands, wherein Pd(0) is stabilized by maleic anhydride (Scheme 43).131 The authors showed that complex 105, featuring six-membered chelate, is more effective in this direct C(sp2)–H arylation of imidazoles than the N-sterically-hindered 106 and the analogous complex 107 featuring a conformationally-restricted seven-membered chelate.
Scheme 42.
Pd–NHC Catalyzed Direct C5–H Arylation of Imidazoles by Lee
Scheme 43.
Pd–NHC Catalyzed Direct C–H Arylation of Imidazoles by Lee
In 2014, the Shao group developed an efficient method for the C(sp2)–H arylation of benzimidazoles and imidazoles at the C2 position catalyzed by [Pd(IPr)(1-Me-im)Cl2] (Scheme 44).132 A variety of activated and deactivated aryl and heteroaryl chlorides were successfully coupled using KOtBu as a base in toluene at 120 °C, providing access the 2-arylbenzimidazoles and 2-arylimidazoles in good to excellent yields. Subsequently, the same Pd(II)–NHC complex was found to be successful as a catalyst in the direct C(sp2)–H bond arylation of imidazo[1,2-a]pyridines with aryl chlorides (Scheme 45).133 These studies further demonstrate the utility of imidazole-supported Pd–NHCs in the direct C(sp2)–H arylation using challenging aryl chlorides.
In 2014, Huynh and co-workers reported dipalladium triazolediylidene complexes such as 109 featuring phosphines as ancillary ligands (Scheme 46).134 These Pd–triazolediylidene systems proved to be effective in the direct C5 arylation of 1-methylimidazole using aryl bromides and the complex 109 showed the highest reactivity.
Scheme 46.
Pd–NHC Catalyzed Direct C5–H Arylation of Imidazoles by Huynh
In 2016, the Liu group reported sterically-hindered tetra-arylimidazolylidene Pd(II)–NHC complexes as efficient pre-catalysts for the direct C(sp2)–H arylation of imidazoles at the C5 position (Scheme 47).135 The direct arylation could be conducted under aerobic conditions in the presence of pivalic acid (30 mol%) and K2CO3 as a mild base in DMA at 130 °C. Following their initial report, Liu and co-workers identified bulky BIAN-type Pd–NHC complexes (BIAN = bis(imino)acenaphthene) for the direct C(sp2)–H arylation of a broad range of azoles with aryl bromides (Scheme 48).136 These impressive reactions proceed efficiently with only 0.05–0.5 mol% catalyst loading and are performed under aerobic conditions. A wide variety of heterocycles, including imidazoles, thiazoles, isoxazoles and pyrazoles provided the direct C–H activation products in good to excellent yields.
Scheme 47.
Pd–NHC Catalyzed Direct C5–H Arylation of Imidazoles by Liu
Scheme 48.
Pd–NHC Catalyzed Direct C–H Arylation of Imidazoles, Thiazoles, Isoxazoles, Pyrazoles and Triazoles by Liu
In addition, the Lee group reported decarboxylative C(sp2)–H arylation of electron-deficient azoles using Pd(0)–NHC complex (not shown).137,138 This approach offers an alternative strategy to the direct C(sp2)–H arylation of heterocycles using Pd–NHC catalysts.
2.1.3.2. C(sp2)–H Activation in Arenes
In contrast to the C(sp2)–H bond functionalization of heterocycles, the direct C(sp2)–H activation of acidic arenes by Pd–NHC complexes remains largely underdeveloped.
In 2012, the Huynh group reported the first example of the direct C(sp2)–H arylation of pentafluorobenzene catalyzed by a Pd–NHC complex (Scheme 49).139 They found that a mixed benzimidazolylidene/triazolyldiylidene complex 112 showed high efficiency and good functional group tolerance in arylations using aryl bromides in the presence of K2CO3 in DMA at 120 °C.
Scheme 49.
Pd–NHC Catalyzed Direct C–H Arylation of Pentafluorobenzene by Huynh
Subsequently, they reported pyrazolylidene Pd–NHC complexes for the direct C(sp2)–H arylation of pentafluorobenzene (Scheme 50).140 The mixed pyrazolylidene/imidazolidinylidene complex 113, [Pd(NHC)(SIPr)Br2], proved to be the best for this arylation allowing for good functional group tolerance. The high σ-donating aptitude of the pyrazolin-5-ylidene ligand, which is beneficial for the C–H arylation, was confirmed by the HEP method (HEP = Huynh’s Electronic Parameter) using 13C NMR spectroscopy.41
Scheme 50.
Pd–NHC Catalyzed Direct C–H Arylation of Pentafluorobenzene by Huynh
In addition, Huynh and co-workers reported a series of bridged Pd–NHC complexes 114–118 featuring two different NHC ligands (Scheme 51).141 These complexes proved to be active in the direct C(sp2)–H arylation of pentafluorobenzene with 4-chlorobromobenzene. In general, it was found that Pd–NHC complexes containing more sterically-hindered N-substituents showed higher catalytic activity.
Scheme 51.
Pd–NHC Catalyzed Direct C–H Arylation of Pentafluorobenzene by Huynh
2.1.4. Hydroxyl Directed C(sp2)–H Activation/Alkenylation
The hydroxyl group has been successfully used as a directing group for the Pd–NHC catalyzed C(sp2)–H functionalization (Schemes 52–53, cf. Schemes 1–4, Section 2.1.1.)
Scheme 52.
Pd–NHC Catalyzed Synthesis of Benzofurans by Hydroxyl Directed C(sp2)–H Activation by Liu
Scheme 53.
Pd–NHC Catalyzed Hydroxyl Directed C(sp2)–H Oxidative Annulation by Lam
In 2011, Liu and co-workers group reported an oxidative phenol-directed C(sp2)–H activation/C–O cyclization catalyzed by the Pd(OAc)2/IPr system (Scheme 52).142 After extensive optimization it was found that the 4,5-diazafluoren-9-one additive (10 mol%) is critical in improving the reaction efficiency, presumably to facilitate regeneration of Pd(II). Based on labeling studies, the authors proposed that C–O reductive elimination is the rate-determining step in this transformation. This efficient method provides straightforward access to dibenzofurans from simple 2-arylphenols and highlights the capacity of Pd–NHC systems in oxidative C–H functionalization.
In 2013, Lam and co-workers reported a Pd–NHC catalyzed oxidative annulation of 2-aryl-1,3-dicarbonyls with alkynes (Scheme 53).143 Interestingly, the use of [Pd–PEPPSI–IPr] in the presence of Cu(OAc)2 in DMF at 120 °C afforded the corresponding spiroindenes by the 2-aryl C(sp2)–H activation, while a catalyst system based on [Ru(p-cym)Cl2]2 gave benzopyrans by the C(sp2)–H activation of the fused aromatic ring (not shown). This reaction demonstrates the capacity of Pd–NHC catalysts to promote C–H functionalization by weak coordination to enolate hydroxyl groups.
2.1.5. Carboxyl Directed C(sp2)–H Activation/Arylation
The synthesis of meta-substituted biaryls with Pd–NHC systems has been achieved by Larrosa and co-workers using carboxylic acid as a transient directing group followed by decarboxylation (Scheme 54).144 The optimized reaction conditions use [Pd–PEPPSI–IPr] (2 mol%) in the presence of Ag2CO3 and K2CO3 in AcOH at 150 °C. Using this protocol, a variety of readily available salicylic acids could be converted into meta-substituted phenols.
Scheme 54.
Pd–NHC Catalyzed Carboxylate Directed C(sp2)–H Arylation/Decarboxylation by Larrosa
In 2015, the same group reported that similar conditions could be used for the direct conversion of salicylaldehydes to meta-substituted phenols (Scheme 55).145 The authors found that [Pd–PEPPSI–IPr] provided higher yields than Pd(OAc)2 in this transformation. Mechanistically, the reaction involves the following steps: (1) aldehyde oxidation, (2) carboxylate directed C(sp2)–H arylation, (3) protodecarboxylation. Using the salicylaldehyde substrates resulted in decreased yields (cf. salicylic acids), which is likely due to one extra oxidation step in the sequence. This use of carboxylic acids and aldehydes as removable directing groups may facilitate future applications of Pd–NHC catalysis in regioselective C–H functionalization.
Scheme 55.
Pd–NHC Catalyzed Oxidation/Carboxylate Directed C(sp2)–H Arylation/Decarboxylation by Larrosa
2.1.6. Sulfoxide Directed C(sp2)–H Activation/Arylation
Very recently, the first example of a sulfoxide-directed Pd–NHC catalyzed C(sp2)–H activation was reported by Colobert and co-workers (Scheme 56).146 The use of chiral sulfoxides allows for atropo-selective biaryl synthesis through weak palladium coordination in the presence of various functional groups. Interestingly, the authors found that the system based on Pd(OAc)2 and IPr·HCl gave higher yields and diastereoselectivity than using IMes·HCl. Mechanistically, the authors proposed the reaction proceeds via Pd(II)/(IV) catalysis.
Scheme 56.
Pd–NHC Catalyzed Sulfoxide Directed C(sp2)–H Arylation by Colobert
2.1.7. C(sp2)–H Oxygenation/Halogenation by Pd(II)/(IV)
C–H bond functionalization via Pd(II)/(IV) cycle has become an attractive strategy in catalysis.77 In 2009, Arnold, Sanford and co-workers reported that a cyclometallated Pd–NHC alkoxide complex 119 is an effective catalyst for the selective pyridine-directed C(sp2)–H bromination (Scheme 57).147 Stoichiometric studies demonstrated that 119 undergoes oxidative addition of chloride ligands to give NHC-stabilized Pd(IV) alkoxide at −35 °C. Upon warming to 33 °C this complex underwent C–Cl forming reductive elimination from Pd(IV). Thus, this elegant mechanistic study set the stage for using NHCs as supporting ligands for C–H bond functionalization via Pd(II)/(IV) catalysis.
Scheme 57.
Pd–NHC Catalyzed C(sp2)–H Bromination by Arnold and Sanford
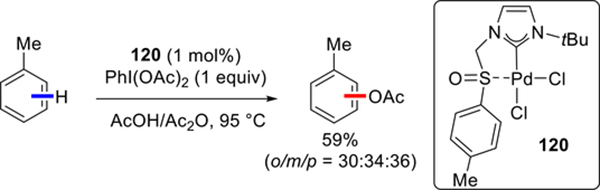
Recently, several halogenation and oxygenation reactions using Pd–NHC systems via Pd(II)/(IV) cycle were reported. An efficient Pd–NHC catalyzed C(sp2)–H acetoxylation of simple arenes via Pd(II)/(IV) cycle using sulfoxide-tethered [Pd(NHC)Cl2] complexes was achieved by the Cardenas group (Scheme 58).148 These novel Pd–NHC complexes feature sulfinyl group as a hemilabile moiety. The best catalytic activity was achieved with complex 120 bearing N-tBu side chain. Interestingly, replacement of the N-tBu side chain with less sterically-hindered N-Me or by aromatic N-Mes or NDIPP also afforded active complexes with comparable catalytic reactivity.
Scheme 58.
Pd–NHC Catalyzed C(sp2)–H Acetoxylation by Cardenas
Subsequently, the Choudhury group demonstrated that both C(sp2)–H acetoxylation and bromination can be accomplished using the same Pd–NHC complexes (Scheme 59).149 They found that chelating bis-NHC–Pd complexes 121–122 featuring N-benzylic tether display high catalytic activity in non-directed acetoxylation and pyridine-directed bromination using PhI(OAc)2 and NBS as oxidants, respectively, via Pd(II)/(IV) cycle. In 2017, the same group reported a simple PEPPSI-type [Pd(NHC)(py)I2] complex 123 for efficient C(sp2)–H acetoxylation, bromination and chlorination of arenes (Scheme 60).150 Kinetic studies suggested that a bimetallic Pd–Pd intermediate might be involved in the rate-determining C–H activation step. In 2017, in alternative approach to C(sp2)–H functionalization via Pd(II)/(IV) catalysis, Choudhury and co-workers disclosed a heterogenous Pd–NHC complex 124 supported on Ru(II)-terpyridine scaffold (Scheme 61).151 This complex was successfully applied to the ortho-selective C(sp2)–H bromination and chlorination of arenes using a range of directing groups. Furthermore, the complex could be recycled five times with minimal loss of catalytic activity.
Scheme 59.
Pd–NHC Catalyzed C(sp2)–H Acetoxylation and Bromination by Choudhury
Scheme 60.
Pd–NHC Catalyzed C(sp2)–H Acetoxylation and Halogenation by Choudhury
Scheme 61.
Pd–NHC Catalyzed C(sp2)–H Halogenation by Choudhury
The regioselective C(sp2)–H acetoxylation directed by pyridines was also achieved by the Wendt group in 2016 using [Pd–PEPPSI–IPr] as the preferred catalyst (Scheme 62).152 This complex showed better catalytic activity than Pd–PEPPSI complexes featuring SIPr, IMes, IPent and IAd ligands; however, it should be noted that all complexes were highly effective in the model acetoxylation of 2-phenylpyridine (77–93% yields). The scope of this reaction is broad and leads to a variety of monofunctionalized heterocycles in good to excellent yields. Several related systems for C(sp2)–H acetoxylation have been developed.153–155
Scheme 62.
Pd–NHC Catalyzed C(sp2)–H Acetoxylation by Wendt
It should also be mentioned that Wilton-Ely and co-workers reported a series of imidazolium-2-thiocarboxylate complexes [Pd(NHC·CS2)(PR3)] as effective pre-catalysts for pyridine directed C(sp2)–H chlorination via Pd(II)/(IV) catalysis.156
2.1.8. C(acyl)–H Activation
In 2012, Martin and co-workers reported an intramolecular C(sp2)–H acylation of aryl chlorides catalyzed by [Pd(2-Me-allyl)Cl]2/IAd (Scheme 63).157 The active Pd–NHC catalyst was prepared in situ by deprotonation of the IAd·HBF4 salt. The reaction gave functionalized benzocyclobutenones in high yields and with excellent functional group tolerance. Interestingly, the authors found that the addition of allyl ether (50 mol%) was crucial for achieving high yields. They proposed that allyl ether might stabilize the monoligated IAd–Pd(0) species, leading to high cyclization/reduction selectivity. Several other NHCs were tested, including IPr, SIPr, SIPr, IMes, ICy and IiPr, with IAd providing the best selectivity. This C(acyl)–H functionalization of aldehydes is related to C(acyl)–N and C(acyl)–O functionalizations of carboxylic acid derivatives by Pd–NHC complexes.55,158–160
Scheme 63.
Pd–NHC Catalyzed Intramolecular C(Acyl)–H/C(sp2)–Cl Arylation by Martin
2.2. C(sp3)–H Activation
Although the activation of C(sp3)–H bonds is significantly more challenging that of C(sp2)–H bonds,60,61 Pd–NHCs have been established as effective catalysts to perform this class of transformations. In general, the activation of C(sp3)–H bonds by Pd–NHCs can be categorized into the following classes: (1) oxidation of methane; (2) intramolecular C(sp3)–H/C–X functionalization by Pd(0)/(II) catalysis; (3) amide directed C(sp3)–H functionalization using acidic N–Ar auxiliary; (4) C(sp3)–H functionalization by Pd(II)/(IV) catalysis, and (5) allylic C(sp3)–H functionalization.
2.2.1. Methane Oxidation
Selective methane oxidation by Pd–NHC complexes has been pioneered by Strassner and co-workers. In 2002, they reported the first example of Pd–NHC complexes for the conversion of CH4 into MeOH via a C(sp3)–H activation pathway isolated as trifluoroacetic acid methyl ester (Scheme 64).161 They found that chelating Pd–NHC complexes 125–128 promoted the C(sp3)–H activation in the presence of K2S2O8 in CF3CO2H/(CF3CO)2O. In this C–H activation, the counterion had a significant impact on the activity, and the C–H activation products were not detected using iodide counterions. This interesting observation was explained by the lower basicity of iodide ligands, which could prevent opening of a free coordination site by halide protonation.
Scheme 64.
Pd–NHC Catalyzed C–H Oxidation of Methane by Strassner
To obtain further insights into this counterion effect, the Strassner group conducted a comparative study of different counterions in the C(sp3)–H activation (chloride, trifluoroacetate) (Scheme 65).162 Interestingly, all ligands except iodide (X = Cl, CF3CO2, Br) displayed comparable activity in the C–H activation. In this case, the X-ray structures showed similarly of the ground-state geometries of these Pd–NHC complexes, while DFT computations suggested the highest energy barrier for replacing the iodide ligand with trifluoroacetic acid.
Scheme 65.
Pd–NHC Catalyzed C–H Oxidation of Methane by Strassner
In addition, Strassner and co-workers investigated the effect of different bridge lengths in NHC ligands on methane oxidation (Scheme 66).163 They found that the ethylene-bridged Pd–NHC complex 132 was the most efficient catalyst in the C(sp3)–H activation of methane. The methylene-bridged complex 129 (n = 1) and butylene-bridged complex 133 (n = 4) showed similar reactivity, while the propylene-bridged complex 132 (n = 2) was less reactive. Furthermore, the Strassner group investigated the reactivity of chelating 2-pyrimidine-functionalized Pd–NHC complexes (Scheme 67).164 In this study, the cationic complex 140 featuring two NHC ligands coordinated to the metal center showed the highest reactivity in the C(sp3)–H activation of methane. On the basis of experimental and computational studies the authors proposed a mechanism involving Pd(II)/(IV) cycle with a palladium tetrahalogenido complex as the resting state and C(sp3)–H activation as the rate-determining step in the catalytic cycle (Scheme 68).165 These elegant reports from the Strassner group provide insight into the C(sp3)–H activation of methane and demonstrate the ability of Pd–NHC complexes in C–H activation of small molecules.166–169 In addition, Zhang reported DFT studies on related propane C–H activation.170
Scheme 66.
Pd–NHC Catalyzed C–H Oxidation of Methane by Strassner
Scheme 67.
Pd–NHC Catalyzed C–H Oxidation of Methane using Pyrimidine-Functionalized NHC Ligands by Strassner
Scheme 68.
Proposed Mechanism in Pd–NHC Catalyzed C–H Oxidation of Methane by Strassner
2.2.2. Non-Directed C(sp3)–H/C–X Activation
2.2.2.1. Intramolecular C(sp3)–H Activation
In 2010, the Wu group reported the synthesis of methylene-bridged polyarenes using a Pd(OAc)2/IPr system to effect the intramolecular benzylic C(sp3)–H activation (Scheme 69).171 Interestingly, the use of IPr·HCl as a ligand precursor showed similar efficiency to a combination of PCy3/pivalic acid (100 mol%), while PCy3 alone was less effective. This reaction provides a general procedure for the synthesis of fluorene derivatives in excellent yields. Mechanistically, it was proposed that C(sp3)–H activation is the rate-determining step.
Scheme 69.
Pd–NHC Catalyzed Non-Directed Benzylic C–H/C–X Arylation by Wu
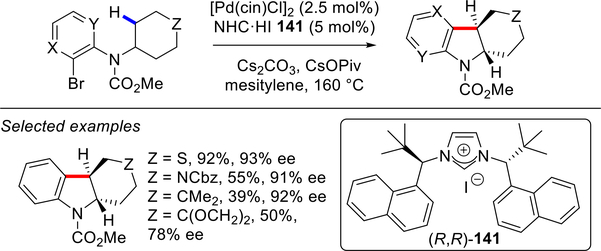
The Kündig group has made significant contributions to the area of intramolecular C(sp3)–H activation using chiral NHC ligands. In 2011, they successfully applied chiral imidazolylidene ligand 141 to the synthesis of fused indolines via intramolecular C(sp3)–H activation catalyzed by Pd–NHCs (Scheme 70).172 The active catalyst was generated in situ from the NHC salt and [Pd(cin)Cl]2. Examination of several related chiral ligands bearing 2-Me-benzyl and 2-MeO-benzyl N-substituents gave lower conversion and/or enantioselectivity. Despite high reaction temperature (140–160 °C), this reaction affords fused indolines with excellent enantioselectivities.
Scheme 70.
Pd–NHC Catalyzed Asymmetric C(sp3)–H/C–X Arylation by Kündig
In 2012, the same group reported the synthesis of 2- and 2,3-substituted indolines using the R,R enantiomer of 141 in most cases (Scheme 71).173,174 In this study, they also disclosed the regio-divergent synthesis of indolines by C(sp3)–H activation of either methyl or methylene groups; however, high asymmetric induction was observed only in the latter case. The reaction is distinguished by exceptionally high enantioselectivity in the synthesis of 2,3-disubstituted indolines.
Scheme 71.
Synthesis of Indolines by Pd–NHC Catalyzed Asymmetric C(sp3)–H/C–X Arylation by Kündig
In 2013, Kündig and co-workers reported an extension of the substrate scope of their asymmetric C(sp3)–H functionalization, including the effects of leaving and protecting groups (Scheme 72).175 Interestingly, these reactions are efficient with aryl bromides and iodides (77–94%), while aryl triflates and even chlorides provide promising reactivity (40–50%). Further, excellent enantio-selectivities are observed in all cases (90–98.5% ee). In terms of the N-protecting/activating group, various carbamates (CO2Me, CO2Et, CO2Bn) and amides (COiPr, COtBu) are well-tolerated giving high levels of enantio-induction. On the basis of computational studies, the authors proposed that the Concerted Metalation Deprotonation (CMD) mechanism step determines enantioselectivity, while ligand exchange from bromide to pivalate is rate-limiting.
Scheme 72.
Synthesis of Functionalized Fused Indolines by Pd–NHC Catalyzed Asymmetric C(sp3)–H/C–X Arylation by Kündig
In 2014, an efficient regiodivergent C(sp3)–H activation was reported by the Kündig group (Scheme 73).176 They established that using two bulky, chiral NHC ligands, namely R,R-141 and S,S-142, affords two different diastereoisomeric trans-2,3-substituted indolines. In contrast, when using enantiopure starting materials, this C–H activation reaction gives regioisomeric enantiomers depending on the catalyst structure (not shown). A comprehensive computational study provided support for the CMD mechanism in these C(sp3)–H activations.
Scheme 73.
Pd–NHC Catalyzed Regiodivergent Asymmetric C(sp3)–H/C–X Arylation by Kündig
Very recently, the Baudoin group reported enantioselective synthesis of (nor)illudalane sesquiterpenes using chiral NHC ligand 141 reported by Kündig (Scheme 74).177 In the model optimization system, they established that matching the amide chirality with the Pd–NHC catalyst is crucial to obtain high stereoselectivity in the reaction. This approach was successfully applied to the enantioselective synthesis of several natural products, demonstrating the potential of Pd–NHC catalyzed C(sp3)–H activation in complex natural product synthesis.
Scheme 74.
Pd–NHC Catalyzed Asymmetric C(sp3)–H/C–X Arylation and Application to the Synthesis of (Nor)illudalanes by Baudoin
A mechanistically related intramolecular benzylic C(sp3)–H activation was reported by Cramer and co-workers in the synthesis of 2-(trifluoromethyl)indoles from trifluoroacetimidoyl chlorides (Scheme 75).178 Ligand screening demonstrated that SIPr is the preferred NHC ligand for this C–H activation, while IPr proved to be completely ineffective. Interestingly, comparable efficiency in the model system was observed using PCy3; however, the Pd–NHC conditions proved to be more general in the scope investigation. Impressively, this reaction was readily performed on 2.5 g scale at 1.0 mol% catalyst loading.
Scheme 75.
Pd–NHC Catalyzed Benzylic C(sp3)–H/C–X Acylation by Cramer
In 2015, the Yang group reported Pd–NHC catalyzed intramolecular benzylic C(sp3)–H activation as a part of their tandem aminoalkylation methodology (Scheme 76).179 Mechanistically, the σ-alkyl-Pd(II) intermediate formed in the intramolecular aminopalladation step activates the benzylic C(sp3)–H bond to form a fused cyclopropane ring. The authors found that IPr in the presence of pivalic acid (30 mol%) and K2CO3 (1.1 equiv) in refluxing xylene under oxygen atmosphere was optimal for this tandem sequence. In contrast, with KOtBu instead of K2CO3 the σ-alkyl-Pd(II) intermediate underwent amide α-alkylation to give pyrolizidines (not shown). Non-NHC ligands, such as PCy3, pyridine or 1,10-phenanthroline were significantly less effective in promoting this reaction (0–45%).
Scheme 76.
Pd–NHC Catalyzed Tandem Aminopalladation/Benzylic C(sp3)–H Activation by Yang
2.2.2.2. Intermolecular C(sp3)–H Activation
In 2014, the Shao group reported intermolecular benzylic C(sp3)–H arylation of fluorenes with aryl chlorides using their [Pd(IPr)(1-Me-im)Cl2] catalyst (Scheme 77).180 KOtBu is the optimal base in dioxane at 120 °C. The reaction is efficient for the synthesis of arylated fluorenes in typically excellent yields using electron-rich, electron-poor and sterically-hindered aryl chlorides. This reaction demonstrates the capacity of Pd–NHCs in C(sp3)–H activation of weakly acidic C–H bonds.
Scheme 77.
Pd–NHC Catalyzed Intermolecular Benzylic C(sp3)–H/C–X Arylation by Shao
2.2.3. Amide Directed C(sp3)–H Activation
The Yu group has made major breakthroughs in Pd-catalyzed aliphatic C(sp3)–H activation of weakly coordinating substrates using acidic amide N-auxiliary.75 This extremely useful mode of catalysis is compatible with Pd–NHC systems (Schemes 78–79).
Scheme 78.
Pd–NHC Catalyzed Amide Directed C(sp3)–H Alkynylation by Yu
Scheme 79.
Pd–NHC Catalyzed Amide Directed C(sp3)–H Arylation of Piperidines by Yu
In 2013, they reported the direct β-alkynylation of C(sp3)–H bonds in N-acidic aliphatic amides catalyzed by [Pd(allyl)Cl]2 and sterically-bulky IAd in the presence of Cs2CO3 (Scheme 78).181 A library of NHC ligands was screened in the reaction optimization, and IAd·HBF4 exhibited the highest reactivity. Other NHC ligands, including SIiPr, SIMes, SItBu, SIAd, and phosphines, including PCy3, PiPr3, PtBu2Ph, XPhos, were less effective. This reaction tolerates a wide range of aliphatic amides, providing the β-C(sp3)–H activation products in high yields. Mechanistically, the authors proposed that the reaction invovles Pd(0)/(II) catalysis.
In 2016, Yu and co-workers reported Pd–NHC catalyzed C(sp3)–H arylation of piperidines directed by their weakly coordinating N-acidic amide auxiliary (Scheme 79).182 In this reaction, SIAd gave the best results, while SIMes, SIPr and IAd were less effective. The reaction was further extended to the direct C(sp3)–H arylation of tetrahydropyrans using SItBu as the preferred ligand (not shown). Based on stoichiometric experiments, the authors proposed a mechanism involving Pd(II)/(IV) catalysis. This reaction complements intramolecular C(sp3)–H activation methodology via Pd(0)/(II) catalysis reported by the Kündig group (Schemes 70–73).
2.2.4. C(sp3)–H Oxygenation/Halogenation by Pd(II)/(IV)
In contrast to C(sp2)–H activation, very few examples of the direct C(sp3)–H functionalization by the Pd(II)/(IV) mechanism using Sanford’s systems with Pd–NHCs have been reported.
In 2011, Hou and co-workers reported a Pd–NHC catalyzed oxidative trifluoroacetoxylation of unactivated methylenes (Scheme 80).183 They found that chelating bis-NHC–Pd complexes gave the mono-trifluoroacetoxylation products in high yields and with good C–H activation selectivity.
Scheme 80.
Pd–NHC Catalyzed C(sp3)–H Acetoxylation of Unactivated Methylenes by Hou
In 2010, Kraft and co-workers reported the synthesis of chelating Pd(IV)-bis-NHC complexes [Pd(NHC)CH2(NHC)Cl4] 146–147 and their application as chlorinating reagents for C(sp3)–H functionalization (Scheme 81).184 A mechanism involving cationic [(NHC)CH2(NHC)Pd(IV)Cl3]+ intermediate as chlorinating agent was proposed.
Scheme 81.
Pd–NHC Catalyzed Benzylic C(sp3)–H Chlorination by Kraft
2.2.5. Allylic C(sp3)–H Activation
The direct C(sp3)–H functionalization of allylic bonds proceeds more easily than unactivated C(sp3)–H bonds due to higher bond acidity;60,61 however, several challenges with Pd–NHC catalysis in terms of compatibility of the reaction conditions still need to be addressed.
In 2017, Yang and co-workers reported the direct allylic C(sp3)–H functionalization and alkylation with oxindoles using [Pd(IPr)(cin)Cl] complex in the presence of 2,5-DMBQ (2.0 equiv) and KOtBu (1.5 equiv) in CH2Cl2 at 60–80 °C (Scheme 82).185 Interestingly, investigation of different ligands showed that the reaction was inhibited when IPr was replaced by bis(sulfoxide) or 4,5-diaza-9-fluorenone. Furthermore, phosphoramidites and PCy3 instead of IPr gave the allyl activation product with lower yields and poor regioselectivity (1.1:1 to 3:2:1 vs. 16:1 with IPr). Mechanistically, the active [Pd(IPr)Cl(OAc)] complex is generated in situ from IPrHCl and Pd(OAc)2. The key allyl palladium intermediate [Pd(IPr)Cl(R3-allyl)] is formed via allylic C–H activation. In the next step, the nucleophilic attack of an enolate and reductive elimination affords the desired C–H activation product. The active [Pd(IPr)Cl(OAc)] catalyst is regenerated by oxidation with 2,5-DMBQ. The NHC–metal complex in this C–H activation is stable to the oxidative conditions and gives high C–H allylation regioselectivity (cf. bis(sulfoxide), 4,5-diaza-9-fluorenone, phosphines). This protocol is characterized by broad scope, good to excellent yields and exceptional regioselectivity. This direct allylic C(sp3)–H method was also applied to the direct synthesis of [Pd(IPr)(cin)Cl] from IPr·HCl, PdCl2 and allylbenzene.
Scheme 82.
Pd–NHC Catalyzed Allylic C(sp3)–H Activation/Allylation of Oxindoles by Yang
2.3. Miscellaneous
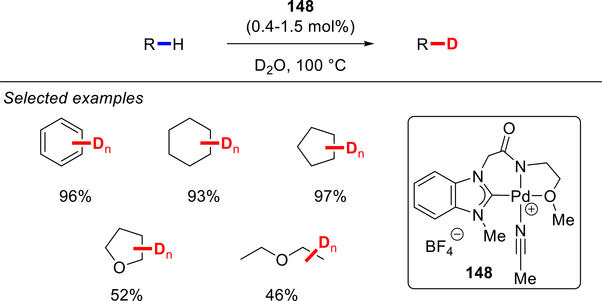
In 2009, Jung and co-workers reported a cationic tridentate benzimidazolylidene Pd–NHC system 148, which showed high activity in C–H/C–D exchange in D2O (Scheme 83).186 In this NHC template, the combined use of a strongly σ-donating NHC ligand, N-coordinating amide and hemilabile ether linkage, collectively weaken the interaction of D2O with the electrophilic Pd center, thus allowing for the high yielding C–H/C–D exchange of C(sp2)–H and C(sp3)–H bonds.
Scheme 83.
Pd–NHC Catalyzed C–H/C–D Exchange using D2O by Jung
In 2015, the same group disclosed C–H/C–D exchange in CF3CO2D catalyzed by a related neutral benzimidazolylidene Pd–NHC complex 149 (Scheme 84).187 In the presence of AgTFA (10 mol%), complex 149 proved to be significantly more reactive than simple Pd salts, including PdCl2, Pd(OAc)2, Pd(TFA)2, [Pd(MeCN)2Cl2] and [Pd(PPh3)2Cl2]. The studies by Jung demonstrate the potential of Pd–NHC complexes in H/D exchange reactions.
Scheme 84.
Pd–NHC Catalyzed C–H/C–D Exchange using CF3CO2D by Jung
In 2018, Strassner and co-workers reported chelating dicationic Pd–NHC complexes 150–152 featuring hemilabile N-2-pyrimidyl group and two carbene ligands coordinated to the metal center (Scheme 85).188 These complexes showed high activity in the electrophilic hydroarylation of alkynes by C(sp2)–H activation. Interestingly, N-steric bulk was found to disfavor the catalytic reactivity due to slower protodemetallation step. These results are promising and highlight the potential of Pd–NHC complexes in electrophilic C–H reactions.
Scheme 85.
Pd–NHC Catalyzed Hydroarylation of Alkynes by Strassner
3. Platinum–NHC Complexes
Although the first Pt–NHC complex was reported in 1973,189 there are few examples of Pt–NHC complexes in transition-metal-catalysis. Thus far, catalytic applications of Pt–NHCs have focused almost exclusively on hydroelement addition reactions, including hydrosilylation, hydroboration and hydroamination.190–195 Very few examples of C–H functionalizations using Pt–NHC complexes have been reported to date.
In 2006, Strassner and co-workers reported chelating methylene-bridged Pt–bis-NHC complexes 153–156 in the catalytic C–H activation of methane (Scheme 86).196 Complex 155 with electron-rich N-substituents was the most reactive; however, these Pt–NHCs showed inferior reactivity to Pd–NHCs under similar reaction conditions (cf. Schemes 64–66).
Scheme 86.
Pt–NHC Catalyzed C–H Activation of Methane by Strassner
In 2015, Chatani and co-workers reported [Pt(NHC)(dvtms)] complexes (dvtms = divinyltetramethyldisiloxane) for the catalytic C(sp2)–H borylation of arenes with B2pin2 (Scheme 87).197 A noteworthy feature of this protocol is selectivity for the sterically-hindered positions. A variety of congested arenes were converted to 2,6-disubstituted phenylboronic esters in moderate to good yields, providing complementary outcome to the well-established iridium-systems. Both IPr and ICy were found to be effective NHC ligands for this C–H borylation reaction. Furthermore, sensitive halides such as F, Cl and Br were intact during this C–H activation. Mechanistically, the authors demonstrated that dvtms dissociates from the metal center (Scheme 88).198 It was proposed that the coordinatively unsaturated Pt–NHC complex reacts with B2pin2 to give [Pt(NHC)(B2pin2)2], followed by C(sp2)–H activation via σ-bond metathesis or oxidative addition to give Pt(IV).
Scheme 87.
Pt-NHC Catalyzed C(sp2)–H Borylation by Chatani
Scheme 88.
Proposed Mechanism for the Pt-NHC Catalyzed C(sp2)–H Borylation
4. Rhodium–NHC Complexes
Following the first synthesis of a Rh–NHC complex in 1974,199 Rh–NHCs were among the earliest metal–NHC complexes to be successfully used in catalysis.200–203 Now, Rh–NHC complexes are routinely applied to determine electronic properties of NHCs through the synthesis of [Rh(NHC)(CO)2Cl] complexes and measurement of their CO stretching frequencies (TEP, Tolman electronic parameter).41,42 Thus, it is not surprising that, due to their common presence and ease of synthesis, Rh–NHC complexes have featured prominently in C–H functionalization reactions.
In 2004, an early demonstration of the high activity of Rh–NHC was reported by Nolan and co-workers in the intramolecular C–H activation of [Rh(ItBu)(coe)Cl]2 complex (coe = cyclooctene) (Scheme 89).204 It was found that this Rh(I) complex undergoes a solvent-dependent intramolecular C(sp3)–H bond activation process to give 159 through double C(sp3)–H activation in benzene or 160 through mono C(sp3)–H activation in hexane, respectively.
Scheme 89.
Solvent Dependent C–H Activation in [(ItBu)Rh(coe)Cl]2 Complex by Nolan
Around the same time, Bergman, Ellman and co-workers established a novel mechanism for the intramolecular C(sp2)–H functionalization of heterocycles with olefins proceeding through a Rh–NHC intermediate 161 (Scheme 90).205 The Rh(I) complex 161 was determined to be the resting state, while the carbene insertion was found to be the rate-determining step. Later, they conducted a detailed kinetic analysis and DFT studies for the intermolecular alkylation of heterocycles with alkenes and demonstrated the intermediacy of a related Rh(I) complex 163 formed through an intramolecular C(sp2)–H activation (Scheme 91).206–208
Scheme 90.
Intramolecular C–H Activation via a Rh–NHC Complex by Bergman and Ellman
Scheme 91.
Intermolecular C–H Activation via a Rh–NHC Complex by Bergman and Ellman
Currently, the major applications of Rh–NHC complexes in C–H activation include the following processes: (1) pyridine directed C(sp2)–H arylation and alkylation; (2) C(sp2)–H borylation; (3) two-fold C–H activation via Rh–NHC intermediates; (4) C(sp3)–H functionalization; and (5) C–H/C–D exchange.
4.1. C(sp2)–H Activation
4.1.1. Directed C(sp2)–H/C–X Activation
In 2009, Chang and co-workers reported a Rh–NHC catalyzed direct arylation of C(sp2)–H bonds in pyridines with aryl bromides (Scheme 92).209 They found that the catalytic activity was significantly increased by a combined use of IMes·HCl (3 mol%) and PCy3 (5 mol%) in the presence of NaOtBu as a stroichiometric base. Interestingly, when IMes·HCl was removed from the catalytic system or replaced by IPr·HCl, only traces of the C–H arylation product were formed. In contrast, PCy3 had a less pronounced effect on the coupling. A mechanism involving proton abstraction pathway by a bimetallic [Rh2(NHC)(OAc)4] species was proposed, while phosphine was suggested to stabilize the active complex. The active NHC-dirhodium(II) complex is generated from an [(IMes)Rh2(OAc)4(PCy3)] complex. Coordination of the nitrogen directing group and C–H activation assisted by tBuONa gives the five-membered rhodacycle intermediate. Oxidative addition to Rh(II) and reductive elimination generates the C–H arylation product and regenerates the active Rh(II) catalyst.
Scheme 92.
C(sp2)–H/C–X Arylation Catalyzed by NHC/Phosphine Rh Complexes by Chang
In 2011, the Chang group reported similar Rh–NHC conditions for the regioselective C(sp2)–H arylation of quinolines with aryl bromides at the C8 position (Scheme 93).210 NHC ligands were found to have a major effect on the C–H arylation, and IMes·HCl in combination with [Rh2(OAc)4] and stoichiometric NaOtBu gave the best results. In contrast, the reaction was suppressed when IMes·HCl was replaced by other ligands, including SIMes, IPr, IiPr, IAd and PPh3. This process allows for the rare regioselective synthesis of C8-arylquinoline derivatives in high yields.
Scheme 93.
Rh–NHC Catalyzed C(sp2)–H/C–X C8–Arylation of Quinolines by Chang
Subsequently, they described Rh–NHC catalyzed double C(sp2)–H hydroarylation of 2,2’-bipyridines by a rollover cyclometallation pathway (Scheme 94).211 Under the optimized conditions using Rh(acac)3 in the presence of IMes·HCl and NaOtBu, double functionalization was achieved in excellent yields with a variety of C–H activation substrates. Again, IMes was found to be significantly more effective than other ligands, including SIMes, SIPr, IPr, IiPr, ICy, IAd, and ItBu. The reaction was further extended to hydroarylation of alkynes under the same catalytic conditions (not shown). Mechanistically, it was proposed that Rh(I) complex, [Rh(NHC)(bipy)(OtBu)], re-enters the catalytic cycle after the first C–H activation step, driven by the strong trans-effect of the NHC ligand.
Scheme 94.
Rh–NHC Catalyzed Double C(sp2)–H Activation/Hydroarylation by Chang
In 2016, Wu and co-workers reported a cationic Rh–NHC complex 164 for the C(sp2)–H olefination of 4-aryl-1H-1,2,3-triazoles in the presence of Cu(OAc)2·H2O and KOAc (Scheme 95).212 A wide range of doubly C–H vinylated aryl-triazoles could be obtained in good yields in this reaction. The use of N,N-Me2-benzimidazolylidene NHC ligand resulted in significantly improved yields for this double C–H activation compared with [RhCp*Cl2]2. Furthermore, N-aliphatic α-branched substituents on the NHC ligand led to a decrease in catalytic activity.
Scheme 95.
Rh–NHC Catalyzed Double Triazole Directed C(sp2)–H Olefination by Wu
In 2017, Chatani and co-workers reported a Rh–NHC catalyzed C(sp2)–H arylation directed by oxazoline using aryl carbamates as arylating reagents and IMesMe as the NHC ligand (Scheme 96).213 The simultaneous activation of C–H and C–O bonds is very rare due to the low reactivity of these bonds. The key in this method was identification of a Rh-bis-NHC complex, while other Rh-catalysts such as [Rh(cod)Cl]2 and [Rh(C2H4)2Cl]2 were less effective. This double C–H/C–O activation was compatible with a wide range of functional groups under relatively mild reaction conditions in the presence of a carbonate base.
Scheme 96.
Rh–NHC Catalyzed Oxazoline Directed C(sp2)–H/C–O Arylation by Chatani
In 2018, the Li group reported a Rh(II)-catalyzed C(sp2)–H carboxylation of 2-pyridylphenols (Scheme 97).214 They found that IMes ligand performed well during the optimization study, while ICy and IiPr were less effective. Thus, combining [Rh2(OAc)4] and IMes·HCl in the presence of KOtBu in DMF promoted the direct C(sp2)–H carboxylation in 75% yield.
Scheme 97.
Rh–NHC Catalyzed Hydroxyl Directed C(sp2)–H Carboxylation by Li
Rh–NHC catalyzed C(sp2)–H activation has been successfully employed for the synthesis of boronic acid esters (Schemes 98–100). In 2014, Crudden and co-workers reported C(sp2)–H borylation of 2-arylpyridines using Rh(I)–NHC dimer, [Rh(IPr)(C2H4)Cl]2, as a catalyst and pinacol borane as a boron source at room temperature C–H borylation (Scheme 98).215 The authors also demonstrated that sequential borylation and arylation could be efficiently performed in a one-pot fashion. However, the scope of this reaction was rather limited.
Scheme 98.
Rh–NHC Catalyzed Pyridine Directed C(sp2)–H Borylation by Crudden
Scheme 100.
Rh–NHC Catalyzed Pyridine Directed C(sp2)–H Borylation by Wang
In 2018, Basle and co-workers reported a Rh(III)–NHC complex 165 featuring a pendant carboxylate group and Cp* ligand that showed high efficiency in the pyridine directed C(sp2)–H borylation under mild conditions (Scheme 99).216 Interestingly, the complex 165 was more reactive than the analogous Rh(III)–NHCs with iPr or Me groups in the NHC side-chain. Similarly, a mixture of [RhCp*Cl2]2 and IMes or [Cp*RhCl2]2 alone were found to be significantly less reactive, which highlights the importance of the NHC scaffold in 165. Importantly, the NHC ligand is readily accessible in a single step from Mes-NH2, L-leucine, formaldehyde and glyoxal according to the procedure by Mauduit and co-workers. The selective C(sp2)–H borylation reaction tolerated a broad range of functional groups.
Scheme 99.
Rh–NHC Catalyzed Pyridine Directed C(sp2)–H Borylation by Basle
Independently, Wang and co-workers developed a very mild protocol for Rh–NHC catalyzed C(sp2)–H borylation of 2-arylpyridines enabled by the combination of IPr·HBF4 and [Rh(cod)Cl]2 in the presence of KOtBu at room temperature (Scheme 100).217 Several other NHC showed high activity in this reaction, including SIMes, IMes and IPr; however, the NHC ligand is critical for the reaction with no product formed in its absence. Interestingly, even the simple IMe ligand (Scheme 100, box) is effective for promoting the model reaction in 78% yield. This methodology allows for C(sp2)–H borylation of a variety of 2-arylpyridines in good to excellent yields and shows excellent functional group tolerance (halides, ester, cyano). This reaction could also be performed well on a gram scale at 1.0 mol% catalyst loading.
4.1.2. C(acyl)–H Activation
In 2012, Sato and co-workers reported Rh(I)–NHC catalyzed intermolecular cycloaddition between 4-allenals and alkynes to construct eight-membered rings via C(acyl)–H activation (Scheme 101).218 The cationic complex formed in situ from [Rh(SIMes)(cod)Cl] gave the best performance in this transformation, while related IMes-based systems were less effective. Furthermore, phosphine-based catalysts, such as [RhCl(PPh3)3] and [Rh(dppe)]ClO4 were completely inactive for this transformation. The reaction showed good functional group compatibility for the synthesis of challenging eight-membered rings. This elegant study illustrates the potential of Rh(I)–NHC systems for C(acyl)–H bond activation.
Scheme 101.
Rh–NHC Catalyzed C(Acyl)–H Activation/Allene Addition by Sato
4.1.3. Two-Fold C–H/C–H Activation
The Choudhury group has pioneered two-fold cascade C–H activation/C–H annulation reactions driven by the formation of Rh–NHCs (Scheme 102–109).
Scheme 102.
Two-Fold C(sp2)–H Annulation via Rh–NHCs by Choudhury
Scheme 109.
C(sp2)–H Annulation via Thiazolium Rh–NHCs by Choudhury
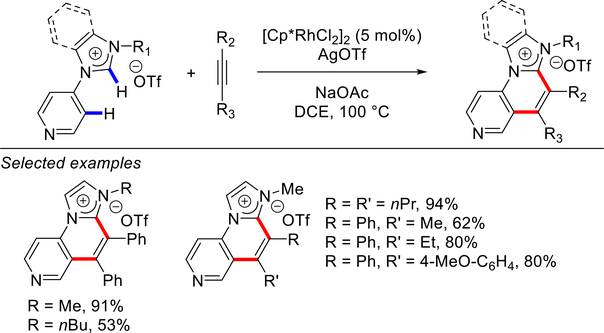
In 2014, they disclosed the first example of Rh-catalyzed annulations between N-arylimidazolium salts and alkynes using [RhCp*Cl2]2 (5 mol%) in the presence of AgOTf (3 equiv) and NaOAc as a base in CH2Cl2 at room temperature, leading to the formation of imidazo[1,2-a]quinoliniums (not shown).219 In 2015, the same group reported Rh-catalyzed double aromatic C(sp2)–H activation/C(sp2)–H annulation using similar reaction conditions in DCE at 100 °C (Scheme 102).220 This method furnishes densely functionalized polycyclic heteroaromatics based on the imidazo-quinolizinium scaffold. It is worth noting that the C(sp2)–H activation proceeds through normal/abnormal NHC ligands that direct these cascade C–H activation reactions.
At the same time, Choudhury and co-workers reported C(sp2)–H activation/C(sp2)–H annulation of electrophilic N-4-pyridyl-imidazolium salts (Scheme 103).221 These less reactive precursors required higher reaction temperature and afforded imidazo-naphthyridinium heterocycles in good to excellent yields. Subsequently, this C(sp2)–H activation methodology was further extended to olefinic C(sp2)–H bond activation/C(sp2)–H annulation, leading to a variety of imidazo-pyridinium scaffolds (Scheme 104).222 These examples demonstrate that aromatic, heteroaromatic and olefinic C(sp2)–H bonds can be activated in the cascade Rh–NHC annulation under similar catalytic conditions.
Scheme 103.
C(sp2)–H Annulation of Pyridine via Rh–NHCs by Choudhury
Scheme 104.
Vinylic C(sp2)–H Annulation via Rh–NHCs by Choudhury
In 2016, the Choudhury group reported C(sp2)–H activation/C(sp2)–H annulation of chelating 2-pyridylimidazolium salts catalyzed by [RhCp*Cl2]2 (3 mol%) in the presence of AgOTf (2.5 equiv) and NaOAc as a base in DCE at 110 °C (Scheme 105).223 In this protocol, the pyridine rollover C(sp2)–H activation was accomplished within the hemilabile [Rh(NHC)Rh(py)X2]+ complex, leading to imidazo-naphthyridinium heterocycles in good yields. In another cyclization mode, C(sp2)–H alkenylation at the abnormal Rh–NHC site produced 5-alkenylimidazoliums (not shown).223 The selectivity was controlled by sterics of 2-pyridylimidazolium precursors.
Scheme 105.
C(sp2)–H Annulation of Chelating Heterocycles via Rh–NHCs by Choudhury
Mechanistically, the authors proposed that the reaction involves the following steps: (1) rate-determining C–H activation of the imidazolium salt; (2) intramolecular aromatic C–H activation; (3) alkyne coordination; (4) alkyne insertion; and (5) reductive elimination (Scheme 106).224 Both C–H activation steps were proposed to proceed via CMD pathway.
Scheme 106.
Proposed Mechanism for C(sp2)–H Annulation via Rh–NHCs by Choudhury
In 2017, yet another Rh–NHC reaction pathway via C(sp2)–H activation/C–N annulation to give C–N ring expanded annulation was reported by Choudhury and co-workers (Scheme 107).225 The reaction was promoted by [RhCp*Cl2]2 (3 mol%) and Cu(BF4)2H2O (1.5 equiv) in MeOH at 140 °C. Key to this C–N annulation was the selection of an appropriate solvent/counterion combination as the use of Cu(OAc)2·H2O in toluene at 140 °C resulted in the typical C–C annulation (see Schemes 102–103).
Scheme 107.
C(sp2)–H/C–N Annulation via Rh–NHCs by Choudhury
The Choudhury group also showed that the normal Rh–NHC intermediates can be efficiently switched to abnormal Rh–NHCs by blocking the C2 position of the imidazole ring (Scheme 108).226 More recently, this C(sp2)–H activation/ C(sp2)–H annulation was achieved with N-arylthiazolium substrates instead of N-arylimidazoliums (Scheme 109).227,228 The reaction affords thiazolo-quinolinium heterocycles in good to excellent yields, and constitutes a rare example of thiazolin-2-ylidene metal–NHCs. Furthermore, an elegant 4-fold C(sp2)–H activation/C(sp2)–H annulation of bis-imidazolium substrates on pyridine and pyrazine backbones via rollover C–H activation with Rh–NHC intermediates has been recently reported by Choudhury and co-workers.229 Very recently, the strategy of generating metal–NHC intermediates in C–H annulation reactions has been extended to Co catalysis by the Choudhury group.230 The method demonstrates a potentially new approach to C(sp2)–H activation/annulation using protic NHC species by metallotropism-enabled C–H activation of azoles. More importantly, the development of this reaction suggests that the expensive Rh may be replaced by more sustainable and cost-effective 3d first-row transition metal-catalysts to enable two-fold cascade C–H activation/C–H annulation reactions by metal–NHC intermediates (see Section 8).
Scheme 108.
C(sp2)–H Annulation via Abnormal Rh–NHCs by Choudhury
Independently, You and co-workers reported a two-fold C(sp2)–H activation/C(sp2)–H annulation of N-arylimidazolium salts with electron-rich five-membered heterocycles by an overall quadruple C(sp2)–H activation sequence (Scheme 110).231 The catalytic system using RhCl3H2O (5 mol% in the presence of Cu(OAc)2·H2O (2 equiv) and CF3CO2H (2 equiv) in toluene at 140 °C was optimal for this transformation. The reaction showed broad substrate scope using benzofurans, thiophenes, furans, pyrroles and indoles as the coupling partners. The mechanism was proposed to involve a Rh(III)–NHC heteroarene C–H activation via a CMD pathway, followed by Rh(I)–Rh(III)–Rh(I) cycle with hydrogen evolution facilitated by CF3CO2H.
Scheme 110.
Two-Fold C(sp2)–H/C–H Arylation via Rh–NHCs by You
4.2. C(sp3)–H Activation
Several examples of Rh–NHC catalyzed C(sp3)–H functionalization have been achieved by a number of research groups.
In 2012, Sato and co-workers reported the intramolecular C(sp3)–H cyclopropanation of allenynes catalyzed by cationic [Rh(NHC)(cod)]ClO4 complexes (Scheme 111).232 The use of N-alkyl NHCs, such as IiPr or ICy, is crucial for this transformation as N-aromatic IMes resulted in inefficient cyclization, while bulky phosphines, such as tBuP3, gave low conversion. Mechanistically, the reaction proceeds via C(sp3)–H bond activation of the tert-butyl group by a cationic Rh(III) catalyst. On the basis of labelling studies, the authors proposed that the C(sp3)–H bond activation is rate-limiting. The reaction affords functionalized gem-dimethylcyclopropanes in high yields.
Scheme 111.
Rh–NHC Catalyzed Intramolecular C(sp3)–H Cyclopropanation by Sato
In 2014, Dong and co-workers reported α-ketone alkylation with olefins catalyzed by [Rh(coe)2Cl]2 (2.5 mol%) in the presence of IMes (5 mol%) and 7-azaindoline (25 mol%) (Scheme 112).233 This strategy employs secondary amine catalyst to generate a transient directing group in situ in a formal C(sp3)–H activation via enamine intermediates. This challenging transformation is broad in scope and could incorporate a large variety of functional groups, such as halogens, thioethers, carbonyls and free alcohols. The reaction is fully regio-selective for the less hindered α-position. Furthermore, various cyclic, acylic and aromatic ketones could be efficiently alkylated with simple terminal olefins, such as ethylene, propylene or styrene. Mechanistic studies revealed that reductive elimination is the rate-determining step in the catalytic cycle.234
Scheme 112.
Rh–NHC Catalyzed Ketone α-Alkylation by Dong
In an alternative approach to C(sp3)–H activation by Rh–NHC complexes, in 2016, Glorius and co-workers reported an enantioselective arylation of benzylic C(sp3)–H bonds with aryl bromides (Scheme 113).235 Using [RhCl(PPh3)3] as a pre-catalyst and a chiral unsymmetrical NHC salt 167, the reaction furnishes enantioenriched triarylmethanes in high yields and enantioselectivities. Mechanistic studies indicated that the Rh–NHC undergoes intramolecular C(sp3)–H activation of the ortho-methyl group, leading to a well-defined steric environment. This important study represents the first example of an efficient asymmetric induction by a chiral unsymmetrical NHC ligand.
Scheme 113.
Rh–NHC Catalyzed Benzylic C(sp3)–H Arylation by Glorius
4.3. C–H/C–D Exchange
The direct C–H/C–D exchange reactions have also been demonstrated using Rh–NHC complexes. In 2011, Oro and co-workers reported selective C–H/C–D exchange at the β-position of olefins catalyzed by Rh(III)–NHC complex 168 featuring a chelating 8-quinolinate ligand (Scheme 114).236 In this NHC scaffold, the bulky and strongly σ-donating IPr ligand controls the selective 2,1-olefin insertion. This deuteration reaction is performed in CD3OD, and results in excellent selectivity for the β-deuteration in a wide range of aromatic and aliphatic olefins.
Scheme 114.
Rh–NHC Catalyzed Vinylic C–H/C–D Exchange by Oro
In 2014, the same group reported a detailed structure-activity study on the effect of Rh(III)–NHC catalysts on vinylic deuteration (Scheme 115).237 In a series of Rh–NHC catalysts 168–172, the bulky and electron-rich SIPr ancillary ligand provided the best reactivity in comparison with IPr and IMes. Furthermore, the cationic complex 172 catalyzed the deuteration reaction most efficiently, consistent with a more facile olefin coordination and insertion in this complex. On the other hand, removal of the chloride ligand led to catalytically-inactive complexes.
Scheme 115.
Rh–NHC Catalyzed Vinylic Selective C–H/C–D Exchange by Oro
5. Iridium–NHC Complexes
In general, the progress in Ir–NHC catalyzed transformations has been slower than in using Pd–NHC and Rh–NHC complexes; however, Ir–NHC catalysts have gained significant attention in organic synthesis and several useful reactions have been developed.238–242 At present, the major applications of Ir–NHCs in C–H functionalization are in the following classes of transformations: (1) C(sp2)–H borylation; (2) C(sp2)–H silylation; (3) C–H/C–D exchange. In addition, the first example of a C(sp3)–H functionalization by Ir–NHCs has been established. These reactions together with the broad availability of Ir–NHC catalysts,242 which along with Rh–NHCs are commonly used for the determination of Tolman electronic parameter through [Ir(NHC)(CO)2Cl] complexes,41,42 provide a useful entry point to developing further C–H functionalizations catalyzed by Ir–NHC complexes.
In 2005, Nolan and co-workers reported an intramolecular C(sp3)–H activation within [Ir(ItBu)(coe)Cl]2 complex (Scheme 116).243 It was found that when the bulky ItBu ligand is reacted with the Ir(I) precursor, [Ir(coe)2Cl]2, double C–H activation will take place after 5 day at room temperature in C6H6 to give 173. The process could be stopped at the mono C–H activation by shortening the reaction time to 20 h, furnishing 174. DFT calculations suggested that the electrophilic iridium center is stabilized by π-donation from the chelating NHC ligands. Activation of Si–H bonds with 173 has been published.244
Scheme 116.
Time Dependent C–H Activation in [(ItBu)Ir(coe)Cl]2 Complex by Nolan
Independently, an intramolecular C(sp2)–H activation within [Ir(NHC)Cp*Cl2] complexes was observed by Peris and co-workers (Scheme 117).245 During the attempted synthesis of Ir–NHCs, they found that combining [IrCp*Cl2]2 with 1-benzyl-3-methylimidazolium iodide in the presence of Ag2O afforded the expected Ir–NHC complex 175, which underwent an intramolecular C(sp2)–H activation of the ortho-aromatic hydrogen, resulting in cyclometallated complex 176. This intramolecular C–H activation process was later extended to both intramolecular C(sp2)–H and C(sp3)–H activation by the Peris group (Scheme 118).246 These reactions proceed efficiently through Ag–NHC intermediates in an in situ process using NHC salts in the presence of Ag2O and [IrCp*Cl2]2. In both cases the C–H activation was promoted by the release of steric hindrance around the metal.
Scheme 117.
Intramolecular C–H Activation in [(NHC)IrCp*Cl2] Complex by Peris
Scheme 118.
Intramolecular C(sp2)–H and C(sp3)–H Activation in [(NHC)IrCp*I2] Complexes by Peris
5.1. C(sp2)–H Activation
Catalytic C(sp2)–H borylation and silylation have been widely used in organic synthesis because these methods allow for the introduction of useful boron and silicon functionality directly onto simple arenes.247
In 2006, the Herrmann group reported the first example of a direct C–H borylation of arenes catalyzed by Ir–NHC complexes (Scheme 119).248 The use of cationic Ir-bis-NHC complexes 181–185 allowed for the C(sp2)–H borylation using pinacolborane with good catalytic activity and high regioselectivity. Interestingly, even substrates containing sensitive halide groups could be functionalized in quantitative yields.
Scheme 119.
Ir–NHC Catalyzed C(sp2)–H Borylation by Herrmann
Subsequently, the same group disclosed an improved catalyst system for the C(sp2)–H borylation of arenes using cationic Ir–NHC complex 186 under microwave irradiation conditions (Scheme 120).249 The study of a counterion effect revealed that CF3CO2 was optimal for this C–H functionalization, while BF4, PF6, OTf and I gave lower reactivity. Under these conditions, a variety of disubstituted benzenes could be successfully converted into boronic esters with high regioselectivity.
Scheme 120.
Ir–NHC Catalyzed C(sp2)–H Borylation of Disubstituted Benzenes by Herrmann
In 2016, Chatani and co-workers reported an Ir–NHC system for the C(sp2)–H borylation of heterocycles and arenes using ICy as the NHC ligand, [Ir(OMe)(cod)]2 as the Ir precursor, and diisopropylaminoborane as the boron source (Scheme 121).250 Optimization studies using 1-methylindole demonstrated that other NHC ligands, including IPr, IMes and ItBu, as well as phosphines, including PPh3, PCy3, dppe, Xantphos and Xphos, were either ineffective or much less efficient. The developed reaction was compatible with several sensitive functional groups, providing the borylated products in good to high yields.
Scheme 121.
Ir–NHC Catalyzed C(sp2)–H Borylation of Heterocycles by Chatani
Direct C(sp2)–H silylation reactions have also been achieved using Ir(I)–NHC catalysts (Schemes 122–123). In 2013, Mashima and co-workers reported the direct dehydrogenative C(sp2)–H silylation of 2-arylpyridines and imines with triethylsilane in the presence of norbornene as the hydrogen acceptor using a permidine-derived Ir–NHC catalyst 187 (Scheme 122).251 Interestingly, the N-3,5-xylyl ring undergoes intramolecular ortho-C–H activation leading to a cyclometallated Ir–NHC catalyst, which increases steric hindrance around the metal and acts as a hydride acceptor. This methodology allows for the synthesis of a variety of ortho-silylated nitrogen-heterocycles in good to high yields.
Scheme 122.
Ir–NHC Catalyzed Pyridine-Directed C(sp2)–H Silylation by Mashima
Scheme 123.
Ir–NHC Catalyzed C(sp2)–H Silylation of Arenes by Oro
In 2017, Oro and co-workers reported dehydrogenative C(sp2)–H silylation of simple arenes and 2-arylpyridines using well-defined Ir(III)–NHC catalyst 188 in the presence of norbornene as the hydrogen acceptor (Scheme 123).252 In this catalyst scaffold, the use of labile pyridine ligands and the readily removable hydrides facilitates substrate coordination. The reaction provides access to aryl and heteroarylsilanes with good generality in high yields. Furthermore, this methodology is compatible with various silanes, including Et3SiH, Ph2MeSiH, PhMe2SiH, Ph3SiH and (EtO)3SiH, and could also be applied to the direct C–H double arylation of bis-hydrosilanes (not shown).
5.2. C(sp3)–H Activation
Only one example of the direct C(sp3)–H functionalization using Ir–NHC catalysts has been reported. In 2004, Sames and co-workers disclosed a catalyst system based on [Ir(cod)2Cl]2 and IPr in the presence of norbornene as hydrogen acceptor for the intramolecular C(sp3)–H alkylation at the α-position of pyrrolidines (Scheme 124).253 Interestingly, PCy3 was significantly less efficient in promoting this reaction. While only few examples were reported, the reaction was highly regioselective for the α-C(sp3)–H activation and could preserve the absolute stereochemistry of the starting pyrrolidine.
Scheme 124.
Ir–NHC Catalyzed Intramolecular C(sp3)–H Activation/Cyclization by Sames
5.3. C–H/C–D Exchange
Major contributions to the direct C–H/C–D exchange catalyzed by Ir–NHCs have been made by Kerr and co-workers. In 2007, Powell and co-workers reported the direct C–H/C–D exchange in several aromatic substrates using Ir–NHC complexes 189–192 based on IMe ligand (Scheme 125).254 Compared with the PCy3-based Crabtree catalyst, [Ir(PCy3)(py)cod]PF6, these novel Ir–NHCs showed promising reactivity; however, their activity was quite modest. Subsequently, a series of practical C–H/C–D exchange reactions catalyzed by Ir–NHC complexes has been developed. In 2008, the Kerr group reported a series of C–H/C–D exchange reactions of aromatic substrates catalyzed by Ir–NHC complexes 193–195, featuring a bulky IMes ligand (Scheme 126).255 In particular, they found that these mixed NHC-phosphine complexes show significantly higher catalytic activity than Crabtree’s catalyst, which has been commonly used as the standard for C–H/C–D exchange reactions.
Scheme 125.
Ir–NHC Mediated C–H/C–D Exchange by Powell
Scheme 126.
Ir–NHC Catalyzed C–H/C–D Exchange by Kerr
A practical advantage of Ir–NHC complex 193 featuring PPh3/IMes combination was demonstrated in the high and regioselective C–H/C–D exchange of Niclosamide, an anilide-based antiparasite drug (Scheme 127).256 In contrast, a lower incorporation (less than 15%) was observed using Crabtree’s catalyst.
Scheme 127.
Ir–NHC Catalyzed C–H/C–D Exchange in Niclosamide by Kerr
Kerr and co-workers reported a further variation in the catalyst design by replacing the phosphine ligand by chloride, resulting in neutral Ir–NHC complexes such as 196 (Scheme 128).257 These [Ir(NHC)(cod)Cl] complexes served as effective C–H/C–D exchange catalysts for labeling of simple arenes; however, their reactivity was limited to 5-membered metallacycles and gave lower levels of D-incorporation than cationic 193.
Scheme 128.
C–H/C–D Exchange Catalyzed by Neutral Ir–NHC Complexes by Kerr
The Kerr group demonstrated that this C–H/C–D exchange methodology is also compatible with alternative solvents under mild conditions (Scheme 129).258 Excellent levels of deuterium incorporation were observed in industrially-acceptable solvents, such as tBuOMe and 2-MeTHF, making this Ir–NHC catalysis potentially applicable in industrial settings. Simultaneously, the Kerr group reported a facile synthesis of cationic Ir–NHC complexes [Ir(IMes)(cod)(PR3)]PF6 (PR3 = PPh3, PBn3, PMe2Ph, PMePh2, P(OiPr)3) (not shown).259 Interestingly, all complexes were found to be catalytically-active, allowing for deuterium exchange in good to high yields. The authors also showed that these well-defined Ir–NHC complexes are air- and moisture-stable, and retain their activity over prolonged storage.
Scheme 129.
Ir–NHC Catalyzed C–H/C–D Exchange in Benign Solvents by Kerr
In 2014, Kerr and co-workers reported the first example of regioselective olefinic C(sp2)–H/C–D exchange catalyzed by Ir–NHCs (Scheme 130).260 Impressively, the use of [Ir(IMes)(cod)(PPh3)]PF6 catalyst 193 permitted β-regioselective C–H/C–D exchange of a range of Michael acceptors at 0.10 mol% catalyst loading at room temperature. Aromatic C(sp2)–H bonds remain intact in this C–H/C–D exchange.
Scheme 130.
Ir–NHC Catalyzed Vinylic C–H/C–D Exchange by Kerr
In 2015, Kerr and co-workers further expanded their Ir–NHC labeling methodology to the selective ortho-deuteration of primary benzenesulfonamides (Scheme 131).261 This reaction was found to be most effectively catalyzed by [Ir(IMesMe)(cod)Cl] complex 197. The IMesMe scaffold provided higher degree of deuterium incorporation than IXyl, IMes, SIMes and IPr, while the incorporation using ICy and IBn was negligible. The mechanism was proposed to involve activation of the [Ir(IMesMe)(cod)Cl] precatalyst via hydrogenative loss of cyclooctadiene to generate the active dideuteride complex [Ir(IMesMe)(D2)(S2)Cl], which is stabilized by solvent molecules. Substrate coordination and oxidative addition with a concomitant reductive elimination of deuterides affords the Ir(III) intermediate. Hydride fluxionality and the second oxidative addition/reductive elimination gives the C–H/C–D exchange product and regenerates the active Ir(III) catalyst. This transformation was compatible with a broad range of functional groups to access biologically relevant deuterated sulfonamides.
Scheme 131.
Ir–NHC Catalyzed C–H/C–D Exchange in Aryl Sulfonamides by Kerr
Similarly, the Kerr group conducted a comprehensive evaluation of their cationic [Ir(IMes)(cod)(PR3)]PF6 complexes 193–195 in the C–H/C–D exchange of pharmaceutically-relevant heterocycles, including arylpyrimidines, imidazoles, oxazoles, pyrazoles, isoxazoles and thiazoles (not shown).262 High levels of deuterium incorporation in moderate to excellent yields were observed in all examples examined. As a further extension of their studies, they reported the direct C–H/C–D exchange of aromatic esters using the cationic [Ir(IMes)(cod)(PPh)]PF6 catalyst 193 (Scheme 132).263 This reaction tolerated various alkyl benzoates, accommodating several sensitive functional groups.
Scheme 132.
Ir–NHC Catalyzed C–H/C–D Exchange in Aryl Esters by Kerr
Subsequently, the Kerr group reported a new class of Ir–NHC complexes 198–200 featuring a labile pyridine ligand (Scheme 133).264 In this family of [Ir(NHC)(cod)(py)]PF6 complexes, the SIMes-supported complex 200 was the most reactive. Along the same lines, they demonstrated that the more cationic Ir–NHC complexes bearing BArF counterion (cf. PF6, BArF = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate) are more reactive in promoting the C–H/C–D exchange of aromatic ketones, esters, amides and nitrobenzenes (not shown).265
Scheme 133.
C–H/C–D Exchange Catalyzed by Cationic [(NHC)Ir(cod)(py)] Complexes by Kerr
In 2016, the Kerr group exploited the effect of weakly coordinating BArF counterion in the selective ortho-directed C–H/C–D exchange of N-unprotected tetrazoles under basic conditions (Scheme 134).266 In this reaction, the cationic catalyst 201 proved to be significantly more reactive than its PF6 counterpart 193. Mechanistic investigations indicated that the C–H activation step involves a base-assisted CMD process. The utility of this reaction was exemplified in the direct C–H labeling of Valsartan, a top-selling angiotensin receptor blocker.
Scheme 134.
Ir–NHC Catalyzed C–H/C–D Exchange in Aryl Tetrazoles under Basic Conditions by Kerr
Following the C–H/C–D exchange strategy by Ir–NHC catalysis, in 2017, Kerr and co-workers disclosed the C2-selective deuteration of indoles and pyrroles directed by N-carbonyl groups (Scheme 135).267 Optimization studies revealed that the combination of their [Ir(IMes)(cod)(PPh3)]PF6 complex with N-COMe directing group provided optimal results, while the use of Crabrtee’s catalyst was much less efficient. This method represents a practical approach to labelling of indoles and pyrroles at the C2 position, including complex pharmaceuticals, such as Sumatriptan, a best-selling antimigraine drug.
Scheme 135.
Ir–NHC Catalyzed C–H/C–D Exchange in N-Heterocycles by Kerr
In an alternative process catalyzed by Ir–NHCs, the direct formyl C–H/C–D exchange was accomplished by the Kerr group (Scheme 136).268 They found that using the sterically-hindered [Ir(IPrMe)(cod)Cl] complex 202 allowed for the selective formyl-deuterium labeling of aromatic aldehydes. Notably, under the developed conditions, aromatic C–H/C–D exchange was not observed. The IPrMe-based catalyst showed slightly higher reactivity than its IMesMe counterpart. This methodology was shown to be compatible with a broad range of functional groups (halides, nitro, hydroxyl, benzyl), providing deuterated aldehydes in good to excellent yields.
Scheme 136.
Ir–NHC Catalyzed Formyl C–H/C–D Exchange in Aldehydes by Kerr
More recently, Kerr and co-workers reported the direct alkyl C(sp3)–H/C(sp3)–D exchange reaction of aliphatic amines at the α-position catalyzed by the cationic [Ir(IMes)(cod)(PPh3)]BArF complex 201 (Scheme 137).269 It was observed during the optimization studies that the cationic [Ir(IMes)(cod)(PPh3)]PF6 catalyst 193 gives lower deuterium incorporation, while the neutral [Ir(IMes)(cod)Cl]PF6 complex 196 was completely inefficient. This reaction allows for α-deuterium exchange in a broad range of biorelevant amines, including pharmaceuticals, such as an antidepressant, Mirtazapine, highlighting the utility of Ir–NHC complexes in various types of C–H/C–D reactions.270
Scheme 137.
Ir–NHC Catalyzed C(sp3)–H/C–D Exchange in Amines by Kerr
6. Ruthenium–NHC Complexes
Ru–NHC complexes are famous for their extraordinary activity in the field of olefin metathesis.271 Furthermore, several classes of robust and stable Ru–NHC complexes have been exploited as catalysts in hydrogenation, hydrosilylation and cyclopropanation reactions.272,273 Ru–NHCs have also been utilized as highly effective catalysts in C–H functionalization,71 in particular to promote the following types of transformations: (1) C(sp2)–H arylations; (2) redox-neutral C(sp2)–H annulations; and (3) oxidative C(sp2)–H annulations.
6.1. C(sp2)–H Activation
In 2008, Dixneuf and co-workers reported C(sp2)–H arylation of 2-arylpyridines with aryl bromides in the presence of Ru[(p-cym)Cl2]2 and tetrahydropyrimidinylidenes 203–205 (Scheme 138).274 They found that this Ru(II)–NHC catalytic system promotes the di-selective C–H arylation of 2-arylpyridines in good yields. The observed reactivity was in the following order: 203 ≈ 204 > 205, which suggested that electron-rich N-substituents render this C–H arylation more efficient. A mechanism involving proton abstraction by a cooperative action of the Ru–NHC catalyst and carbonate base was proposed.
Scheme 138.
Ru–NHC Catalyzed Direct C(sp2)–H/C–Br Arylation by Dixneuf
Subsequently, Özdemir and co-workers synthesized a series of well-defined, air- and moisture-stable Ru–NHC complexes 206–209 based on the imidazolidinylidene scaffold and evaluated their activity in (sp2)–H arylation of 2-arylpyridines with aryl chlorides (Scheme 139).275 They found that these [Ru(NHC)Cl2] complexes are highly active in the di-selective arylation of 2-arylpyridines. Catalyst comparison studies demonstrated that pre-catalyst 209 containing the N-4-methylbenzyl substituent was the most active in this series.
Scheme 139.
Ru–NHC Catalyzed Direct C(sp2)–H/C–Cl Arylation by Özdemir
In 2009, Özdemir, Bruneau and co-workers reported that ortho-xylyl bridged [Ru(NHC)2(PPh3)Cl2] complexes 210–213 catalyze C(sp2)–H arylation of 2-arylpyridines with aryl chlorides using a combination of KOAc (5 mol%) and K2CO3 (3 equiv) in NMP at 120 °C (Scheme 140).276 Excellent conversion was observed with all complexes, while complex 213 featuring N-pentamethylbenzyl group gave the highest di-/mono-arylation selectivity.
Scheme 140.
Ru–NHC Direct Catalyzed C(sp2)–H/C–Cl Arylation by Bruneau
The Özdemir group also disclosed the reactivity of a slightly modified series of [Ru(NHC)Cl2] complexes 214–217 based on the imidazolidinylidene ring (Scheme 141).277 These complexes contain modified N-benzyl groups and showed high efficiency in the di-selective C(sp2)–H arylation of 2-arylpyridines with aryl chlorides in the presence of Cs2CO3 (3 equiv) in NMP at 120 °C.
Scheme 141.
Ru–NHC Catalyzed Direct C(sp2)–H/C–Cl Arylation by Özdemir
In 2010, the same group reported the synthesis of related benzimidazolylidene [Ru(NHC)Cl2] complexes and demonstrated their reactivity in the di-selective C(sp2)–H arylation of 2-arylpyridines with more reactive aryl bromides (not shown).278 More recently, Özdemir and co-workers reported the mono-selective C(sp2)–H arylation of 2-arylpyridines with aryl chlorides using benzimidazolylidene [Ru(NHC)Cl2] complexes 218–221 under aqueous conditions (Scheme 142).279
Scheme 142.
Ru–NHC Catalyzed Monoselective C(sp2)–H/C–Cl Arylation by Özdemir
In 2010, Peris and co-workers reported [Ru(IBu)(p-cym)Cl2] 222 as a catalyst for the direct C(sp2)–H arylation of 2-arylpyridines with aryl bromides and aryl chlorides (Scheme 143).280 This catalyst together with its N-Me and N-nOct analogues showed high activity for the diarylation of 2-phenylpyridine and N-phenylpyrazole. In addition, 222 was also an effective catalyst for the nitrogen-directed C(sp2)–H deuteration in CD3OD. In 2014, the Peris group reported a pyrene-based bis-NHC-Ru complex 223 and demonstrated its high reactivity in the di-selective C(sp2)–H arylation and alkylation of 2-arylpyridines (Scheme 144).281 Interestingly, 223 was more reactive than [Ru[(p-cym)Cl2]2 in the sequential alkylation/arylation of 2-arylpyridines to give unsymmetrical 2-arylpyridines in good yields.
Scheme 143.
Ru–NHC Catalyzed Direct C(sp2)–H/C–X Arylation by Peris
Scheme 144.
Ru–NHC Catalyzed Direct C(sp2)–H/C–X Arylation using Pyrene Ligands by Peris
Around the same time, Peris and co-workers also reported hetero-bimetallic Ru(II)–Ir(III) complex based on triazolediylidene scaffold 224 and its homo-bimetallic Ru(II)–Ru(II) analogue 225 (Scheme 145).282 These complexes were applied to the tandem C(sp2)–H arylation/oxidation of 2-arylpyridines. Both complexes showed similar reactivity to the imidazolylidene Ru–NHC complex 222, while the reactivity of the analogous Ir–NHC 226 was not reported.
Scheme 145.
Ru–NHC Catalyzed Direct C(sp2)–H/C–X Arylation/Oxidation using Bimetallic Complexes by Peris
In 2016, the Ong group reported a Ru–NHC system for the C1 selective C(sp2)–H benzylation and alkylation of isoquinoline (Scheme 146).283 The reaction is carried out in the presence of [Ru(p-cym)Cl2]2 (2.5 mol%), IPr (10 mol%), AdCO2H (30 mol%) and K2CO3 leading to 1-substituted isoquinoline products in moderate to good yields. The alkylation mode could be switched from C(sp2)–H alkylation to oxidative dearomatization/N-alkylation by adding H2O. The proposed mechanism involves C(sp2)–H activation by CMD pathway, oxidative addition of an alkyl halide, and reductive elimination from the Ru(IV)–NHC complex.
Scheme 146.
Ru–NHC Catalyzed Direct C(sp2)–H/C–Cl Benzylation and Alkylation of Isoquinolione by Ong
In 2013, Zhao and co-workers reported an impressive example of Ru–NHC catalyzed formal [3+2] annulation between N–H ketimines and alkynes via ortho-C(sp2)–H activation at room temperature (Scheme 147).284 The key to this reaction is the use of NHCs as ancillary ligands to Ru as no reaction is observed using phosphines or in the absence of NHC. IPr is more effective than IMes in hexanes, while a switch in the reaction efficiency was observed in toluene. SIPr and SIMes were both ineffective. This mild protocol allows for the construction of complex indenamines with excellent functional group tolerance.
Scheme 147.
Ru–NHC Catalyzed Imine Directed C(sp2)–H Activation/Carbocyclization by Zhao
In 2018, the same group reported an intramolecular amide directed C(sp2)–H hydroarylation with alkenes catalyzed by a similar Ru–NHC system in the presence of acetic acid (2 equiv) in dioxane at 120 °C (Scheme 148).285 Acetic acid likely acts as a proton source in this hydroarylation. The reaction proceeds with excellent 5-exo-trig selectivity directed by the amide group. The utility of this reaction was showcased in a rapid synthesis of a progesterone receptor antagonist featuring oxindole ring.
Scheme 148.
Ru–NHC Catalyzed Amide Directed C(sp2)–H Activation/Hydroarylation by Zhao
In 2015, Wang and co-workers reported a Ru-catalyzed two-fold oxidative C(sp2)–H annulation that proceeds via Ru–NHCs (Scheme 149).286 This reaction is formally analogous to Rh-catalyzed annulations reported by Choudhury (cf. Schemes 102–110). The reaction of N-arylimidazolium salts with alkynes in the presence of [Ru(p-cym)Cl2]2, AgSbF6 and Cu(OAc)2 as a terminal oxidant gave a variety of benzoimidazoquinolizinium salts in good to excellent yields through the intermediacy of normal and abnormal Ru–NHCs. The mono-annulated products resulting from the cyclization of the more reactive C2–NHC could be isolated by reducing the catalyst loading. Several related reactions have been reported.287,288
Scheme 149.
Oxidative Two-Fold C(sp2)–H Annulation via Ru–NHCs by Wang
6.2. C(sp3)–H Activation
Ru–NHC catalyzed α-alkylation of ketones was achieved by Glorius and co-workers through a borrowing hydrogen mechanism (Scheme 150).289 This formal C(sp3)–H activation is catalyzed by a bis-NHC–Ru complex prepared in situ from the SINpEt precursor 227, identified earlier for arene hydrogenations, while only traces of the alkylation product were observed using ICy. This protocol demonstrated high selectivity for methylene C(sp3)–H bond activation, allowing to prepare a range of α-branched ketones, including a one-step synthesis of Donepezil, a top-selling anti-Alzeimer’s drug.
Scheme 150.
Ru–NHC Catalyzed Ketone α-Alkylation by Glorius
In 2017, Choudhury and co-workers reported a mixed NHC/bipyridine Ru(II)-complex 228 for the selective oxidation of benzylic C(sp3)–H bonds to ketones (Scheme 151).290 The reaction was conducted using NaIO4 as a mild oxidant. The NHC ligand was found to be stable under the oxidative conditions. No reaction was observed using [Ru(p-cym)Cl2]2 or RuCl3 instead of 228. In addition, several examples of an intramolecular C(sp3)–H bond activation within Ru–NHC complexes have been reported.291–293
Scheme 151.
Ru–NHC Catalyzed Benzylic C(sp3)–H Oxidation by Choudhury
7. Iron–NHC Complexes
Due to its broad availability as the fourth most abundant element in the Earth’s crust and very low toxicity, iron is an ideal metal for homogenous catalysis.294–296 However, despite recent important advances, the utilization of iron in homogeneous catalysis still lags behind precious metals and other earth-abundant 3d transition-metals.
The first example of an Fe–NHC complex was reported quite early, in 1969, by Öfele,297 while the first catalytic application was reported in 2000 by Grubbs and co-workers.298 Well-defined and in situ generated Fe–NHC complexes have been utilized in homogenous catalysis, in particular in reductions, reductive cyclizations and polymerizations.299–301 At present, several encouraging examples of Fe–NHC systems for C–H activation reactions exist.
7.1. C(sp2)–H Activation
In 2008, Tatsumi and co-workers reported intramolecular benzyl C(sp3)–H activation within the [Fe(NHC)Cp*Cl] complexes (NHC = IMes, IiPrMe) upon treatment with organolithiums (not shown).302 The resulting cyclometallated Fe–NHC complex enabled the direct C(sp2)–H activation of five-membered heterocycles, allowing their incorporation into the Fe–NHC complex to give [Fe(IiPrMe)Cp*Het] (Het = 2-thienyl, 2-furyl). Based on this study, in 2010, Tatsumi and co-workers disclosed a dehydrogenative C(sp2)–H borylation of furans and thiophenes with pinacolborane in in the presence of tert-butylethylene catalyzed by Fe–NHC complex [Fe(IMeMe)Cp*Me] 229 (Scheme 152).303 The reactions occurred regioselectively at the C2 or C5 positions of the furan and thiophene ring and showed relatively broad substrate scope. Mechanistically, C–H activation occurs at the Fe–NHC complex 229 with a concomitant loss of methane. The reaction with pinacolborane generates the final product.
Scheme 152.
Fe–NHC Catalyzed C(sp2)–H Borylation of Furans and Thiophenes by Tatsumi
In 2015, Yoshikai and co-workers reported an imine-directed C(sp2)–H alkylation of N-alkylindoles with vinylarenes at C2 position catalyzed by Fe–NHCs (Scheme 153).304 The active catalyst was generated in situ from Fe(acac)3 and SIXyl·HCl in the presence of CyMgCl. In addition to SIXyl, several other NHC ligands were active in this reaction, including IXyl, SIMes and SIPr, however, the use of phosphine ligands inhibited the model reaction. The reaction was also successful using internal alkynes, leading to C2-vinyl indoles. A mechanism involving oxidative addition of the C–H bond to a low-valent Fe–NHC complex, migratory insertion and reductive elimination was proposed.
Scheme 153.
Fe–NHC Catalyzed Imine Directed C(sp2)–H Alkylation of Indoles by Yoshikai
In 2017, Ackermann and co-workers disclosed an impressive example of highly enantioselective C(sp2)–H alkylation of N-alkylindoles with vinylarenes catalyzed by a chiral Fe–NHC complex based on imidazolidinylidene 230 (Scheme 154).305,306 The bulky group at the remote meta-position of the N-aryl scaffold was crucial to obtain high enantioselectivity. This transformation showed broad scope and good functional group tolerance, delivering C2-alkylated products with highly enantioselectivity.
Scheme 154.
Asymmetric Fe–NHC Catalyzed Imine Directed C(sp2)–H Alkylation of Indoles by Ackermann
7.2. C(sp3)–H Activation
In 2018, Che and co-workers reported an iron(III) porphyrin complex [Fe(NHC)(TDCPP)] (TDCPP = tetrakis(2,6-dichlorophenyl)porphyrin) 231 for the intramolecular C(sp3)–H amination of alkyl azides, affording pyrrolidines and piperidines in good to excellent yields (Scheme 155).307 In this catalyst design, the axial NHC ligand could facilitate the azide decomposition by stabilizing the iron-carbene intermediate. The authors proposed that the reaction is initiated by a thermal dissociation of one of the NHC ligands from 231. This complex reacts with an alkyl azide to give an NHC-stabilized iron-nitrene, followed by C(sp3)–H amination.
Scheme 155.
Fe–NHC Porphyrin Catalyzed Intramolecular C(sp3)–H Amination by Che
In addition, Sun and co-workers reported esterification of benzylic C(sp3)–H bonds catalyzed by an iron-imidazolinium system (Scheme 156).308 This reaction might proceed through Fe–NHC catalysis. This methodology showed very good tolerance, including sterically-hindered substrates, providing a useful alternative for the synthesis of benzyl esters. This transformation uses DTBP (DTBP = di-tert-butyl peroxide) as external oxidant and is ineffective without the imidazolium ligand. The same group reported a similar catalyst system for the allylic C(sp3)–H esterification (not shown).309 Intramolecular C(sp3)–H activation within bidentate and imido Fe(II)–NHC complexes have been reported.310,311
Scheme 156.
Esterification of Benzylic C(sp3)–H Bonds by Sun
8. Cobalt–NHC Complexes
In the past few years, C–H bond activation using cost-efficient Co catalysis has emerged as a powerful tool for the synthesis of complex molecules.312–314 In this context, Co–NHC complexes have played an important role in this emerging field. At present, the major advances in using Co–NHC complexes in C–H bond activation include the following classes of reactions: (1) pyridine-directed C(sp2)–H arylation reactions with phenols and aryl chlorides; (2) pyridine-directed C(sp2)–H alkenylation reactions; (3) imine-directed C(sp2)–H alkylation reactions with unactivated olefins; (4) imine directed C(sp2)–H alkylation reactions with alkyl halides; (5) pyridine-directed addition of C(sp2)–H bonds to imines and aziridines; (6) C(sp2)–H activation reactions of pivalphenone imine; and (7) tandem C(sp2)–H activation reactions via radical intermediates. In addition, the development of C(sp2)–H activation/annulation via protic Co–NHC species by metallotropism-enabled C–H activation of azoles should be noted (see Section 4.1.3).230
8.1. C(sp2)–H Activation
In 2012, Ackermann and co-workers reported one of the first examples of using inexpensive Co–NHC systems for the direct C(sp2)–H arylation of 2-arylpyridines with aryl sulfamates (Scheme 157).315 Extensive optimization studies demonstrated that IMes is the preferred NHC ligand for this transformation, while IPr, SIMes, SIPr, dppe, PCy3 and phenanthroline were less effective. Directing groups included 2-arylpyridines, 2-arylpyrimidines and N-2-pyridylindoles. In terms of electrophiles, the C–O activation occurred efficiently with aryl sulfamates, aryl carbamates and aryl phosphates. It was proposed that the reaction proceeds via a non-radical reaction mechanism. The mild reaction conditions and utilization of phenol derivatives as electrophiles in this methodology are noteworthy.
Scheme 157.
Co–NHC Catalyzed Pyridine Directed C(sp2)–H/C–O Arylation by Ackermann
Subsequently, Ackermann and co-workers reported a similar Co–NHC catalytic systems for the direct C(sp2)–H arylation of 2-arylpyridines with aryl chlorides (Scheme 158).316 They found that a combination of the inexpensive Co(acac)2 and IMes·HCl as the NHC precursor in the presence of CyMgCl (1.6 equiv) in DMPU proved to be highly effective for the arylation at room temperature. Again, IMes was identified as the key ligand, while significantly lower conversion was observed using IPr, SIPr and SIMes. A similar protocol using Co(acac)2 and IPr as the NHC ligand was also reported for C(sp2)–H arylation of 2-arylpyridines with alkyl chlorides (not shown).316
Scheme 158.
Co–NHC Catalyzed Pyridine Directed C(sp2)–H/C–Cl Arylation by Ackermann
In 2015, the Ackermann group reported a further extension of their Co–NHC methodology to the direct C(sp2)–H arylation of weakly coordinating secondary benzamides (Scheme 159).317 In this study, ICy was employed as the NHC ligand instead of IMes. Interestingly, replacing ICy by IPr or SIPr resulted in no reaction, while IMes, SIMes and PPh3 gave traces of the arylation product. Various benzamides were converted into biaryl products in good to high yields, including the synthesis of complex biaryls. This protocol was further extended to the direct C(sp2)–H arylation of 5-aryltetrazoles with aryl carbamates using IMes as the optimum NHC ligand (not shown).317
Scheme 159.
Co–NHC Catalyzed Amide Directed C(sp2)–H/C–Cl Arylation by Ackermann
In 2016, the Ackermann group reported a similar reaction for the C(sp2)–H arylation of 2-aryloxazolines with aryl chlorides (Scheme 160).318 This strongly chelating imidate group allowed for efficient C–H arylations at room temperature. Importantly, the reaction was ineffective without the NHC ligand, while IPr, IMes and ItBu gave lower conversion. It is worth noting that the oxazoline directing group could be readily converted into other functional groups, including alcohol, carboxylic acid, ester and amide.
Scheme 160.
Co–NHC Catalyzed Oxazoline Directed C(sp2)–H/C–Cl Arylation by Ackermann
In addition to C(sp2)–H arylations, in 2015, the Ackermann group achieved the direct C(sp2)–H olefination catalyzed by Co–NHC complexes (Scheme 161).319 A combination of CoI2 and IPr·HCl in the presence of CyMgCl in DMPU at 23 °C provided the best yields. Other ligands, including IMes, ICy, IAd were less effective, while phosphines PPh3, PCy3 and dppe completely inhibited the catalysis. A number of diverse alkenyl acetates and alkenyl phosphates proved effective as olefinating reagents, providing the C–H activation products in good to excellent yields. In 2017, Ackermann and co-workers reported triazolylidene ligands for the Co–NHC catalyzed olefins with alkenyl acetates at room temperature (Scheme 162).320 These NHCs offer an alternative to the typical imidazolylidene ligands in Co–NHC C(sp2)–H activations.
Scheme 161.
Co–NHC Catalyzed Pyridine Directed C(sp2)–H/C–O Alkenylation by Ackermann
Scheme 162.
Co–NHC Catalyzed Pyridine Directed C(sp2)–H/C–O Alkenylation using Triazolylidene Ligands by Ackermann
Major advances in the Co–NHC catalyzed C(sp2)–H activation have been made by Yoshikai and co-workers. In 2011, they reported a linear selective C(sp2)–H alkylation of 2-arylpyridines with styrenes catalyzed by a combination of CoBr2 and IMes·HCl in the presence of tBuCH2MgBr in THF at 60 °C (Scheme 163).321 Interestingly, replacing IMes with PCy3 allowed for switching the selectivity to access the branched products. High yields and excellent regioselectivities were observed for various styrenes using both catalytic systems. A mechanism involving oxidative addition of the C–H bond to a Co–NHC, styrene insertion and reductive elimination was proposed. In this study, the first example of an imine-directed C(sp2)–H activation catalyzed by Co–NHCs was also reported.
Scheme 163.
Co–NHC Catalyzed C(sp2)–H Alkylation by Yoshikai
Based on this study, Yoshikai and co-workers developed a Co–NHC catalyzed intramolecular C(sp2)–H alkylation of indoles directed by a C3 imine group (Scheme 164).322 The reaction affords dihydropyrroloindole A or tetrahydropyridoindole B products, controlled by the selection of the NHC ligand. Thus, SIMes favors the formation of 5-exo-cyclization products, while IPr leads to 6-endo-cyclization. Phosphines, including PPh3, dppe and DPEphos are ineffective in this cyclization, while IMes and SIPr give the 5-exo or 6-endo selectivity, respectively; however, afford the cyclization products in lower yields. Mechanistically, the olefin insertion step determines the regioselectivity in this reaction.
Scheme 164.
Intramolecular Co–NHC Catalyzed C(sp2)–H Alkylation by Yoshikai
Subsequently, the same group reported intermolecular C(sp2)–H alkylation of 3-iminoindoles with allyl arenes via a tandem olefin isomerization/C(sp2)–H alkylation pathway (Scheme 165).323 The reaction provides 1,1-diarylalkanes in good yields. IXyl was the best ligand for this transformation, affording significantly higher yields than IMes, IPr, as well as PPh3 and PCy3.
Scheme 165.
Co–NHC Catalyzed Alkene Isomerization/C(sp2)–H Alkylation by Yoshikai
In 2012, Yoshikai and co-workers reported the direct C(sp2)–H arylation of aromatic imines with aryl chlorides using Co–IMes catalytic system in the presence of tBuCH2MgBr in THF at 25 °C (Scheme 166, cf. Scheme 158).324 Optimization studies revealed that IPr, PPh3, PCy3, PEt3 and PMe3 were much less effective than IMes in this C–H arylation. This reaction affords the biaryl coupling products in good to high yields with excellent mono-arylation selectivity. The imine-directing group is readily removed by acidic work-up, providing a functional group equivalent to the carbonyl group.
Scheme 166.
Co–NHC Catalyzed Imine Directed C(sp2)–H/C–Cl Arylation by Yoshikai
In 2013, Yoshikai and co-workers disclosed the direct C(sp2)–H alkylation of aromatic imines with alkyl halides (Scheme 167).325 In this reaction, CoBr2 in a combination with imidazolidinylide, SIiPr, or benzimidazolylidene, iPr-bimy, provided the best yields. These C(sp2)–H alkylation reactions were accomplished in high yields and with excellent regioselectivity over a broad range of substrates. Linear and branched alkyl halides as well as cycloalkyl halides were shown to be effective coupling partners.326 The authors proposed that the reaction involves a single-electron transfer from C(sp2)–H activation cobaltacycle to the alkyl halide, followed by radical coupling.
Scheme 167.
Co–NHC Catalyzed Imine Directed C(sp2)–H/C–X Alkylation by Yoshikai
The Yoshikai group expanded their Co–NHC catalyzed C–H activation methodology to the direct addition of 2-arylpyridines to aldimines (Scheme 168).327 The combination of CoBr2, IPr·HCl and tBuCH2MgBr allowed for the C(sp2)–H alkylation of 2-arylpyridines with aromatic aldimines in THF at 60 ° C with goodfunctional group tolerance. It is worth noting that IMes could also promote this reaction; however, this ligand was significantly less effective than IPr. The reaction was proposed to proceed by a mechanism involving chelation-directed C(sp2)–H oxidative addition, nucleophilic addition of the cobaltacycle intermediate to aldimine and transmetallation of the cobalt amide.
Scheme 168.
Co–NHC Catalyzed Pyridine Directed C(sp2)–H Addition to Aldimines by Yoshikai
In 2014, Yoshikai and co-workers reported the direct C(sp2)–H alkylation of 2-arylpyridines with 2-arylaziridines (Scheme 169).328 In this system, CoCl2 is a catalyst, while IPr·HCl serves as a ligand precursor in the presence of tBuCH2MgBr in THF at room temperature. Phosphines are completely ineffective in this reaction, while IMes and SIPr gave lower yields. The reaction allows for a facile access to 1,1-diarylethanes featuring 2-amino moiety in moderate to good yields. The mechanism was proposed to involve a nucleophilic attack of the cobaltacycle intermediate on 2-arylazirdine, resulting in an overall alkylative ring opening process.
Scheme 169.
Co–NHC Catalyzed Pyridine Directed C(sp2)–H Addition to Aziridines by Yoshikai
The direct C(sp2)–H olefination of aryl imines with vinyl phosphates catalyzed by Co–NHC complexes was also reported by Yoshikai and co-workers (Scheme 170).329 In this reaction, they identified uncommon CyIEt as the best NHC ligand. Significantly lower yields were obtained with IMes, IPr, IEt and MeIEt. The utility of 2-alkenylacetophenones was exemplifies in their conversion to benzofulvenes, indoles and carbazoles (cf. Schemes 161–162).
Scheme 170.
Co–NHC Catalyzed Imine Directed C(sp2)–H/C–O Alkenylation by Yoshikai
The Yoshikai group also demonstrated that MeOTs could be an effective coupling partner in the C(sp2)–H alkylation of 2-arylpyridines and aryl imines catalyzed by Co–NHCs (Scheme 171).330 This methylation reaction was promoted by a benzo[d]imidazole-based N-alkyl NHC ligand, sBu-bimy. The mechanism of this apparent C–H/C–O activation involves an in situ conversion of MeOTs to the more active methylating reagent, MeBr.
Scheme 171.
Co–NHC Catalyzed Pyridine and Imine Directed C(sp2)–H/C–O Methylation by Yoshikai
The direct C(sp2)–H activation of pivalophenone imine represents another class of reactions that have been achieved with Co–NHC catalysis. In 2017, the Yoshikai group reported C(sp2)–H alkylation of pivalophenone imine using CoBr2 and SIiPr as the catalyst system (Scheme 172).331 The main advantage of this protocol is using the alkylated products of pivalphenone imine as nitrile surrogates that would be especially difficult to access by other C–H activation methods. Subsequently, Yoshikai and co-workers reported the direct C(sp2)–H olefination reactions using the same pivalophenone imine substrates (Scheme 173).332 The reaction provides straightforward access to 2-alkenylated pivalophenone imines in the presence of a bulky dipp-bimy NHC ligand. This NHC gave dramatically better yields than IPr, IMes, SIPr, SIMes and IEt, while PPh3 and PCy3 were completely ineffective in promoting the C–H alkenylation. A similar C(sp2)–H benzylation process using benzyl phosphates has been reported (not shown).333
Scheme 172.
Co–NHC Catalyzed Pivalophenone Imine Directed C(sp2)–H/C–Br Alkylation by Yoshikai
Scheme 173.
Co–NHC Catalyzed Pivalophenone Imine Directed C(sp2)–H/C–O Alkenylation by Yoshikai
C(sp2)–H activation catalyzed by Co–NHCs have been extended to tandem alkylations involving radical reactions. In 2018, Yoshikai and co-workers reported a tandem C(sp2)–H/C(sp3)–H coupling between aryl imines and N-2-bromobenzyl-protected secondary amines (Scheme 174).334 This reaction is promoted by the combination of CoBr2, SIPr·HCl and sBuMgBr in THF/DME at 0 °C and allows for the C(sp2)–H alkylation with aliphatic amines at the α position. In this system, the cyclometallated cobalt intermediate undergoes single-electron transfer to N-2-bromobenzylamine, promoting 1,5-hydrogen atom transfer. The mechanism involves generation of the low-valent organocobalt catalyst, cyclometallation with an aryl imine to give the cobaltacycle intermediate, single electron transfer (SET) and 1,5-hydrogen atom transfer (HAT) steps to furnish the α-aminoradical intermediate. Aryl-alkyl coupling gives the C–H activation product, and the catalyst is regenerated by transmetallation with Grignard reagent. The reaction allows for α-coupling of cyclic and alicyclic aliphatic amines with aromatic C–H bonds.
Scheme 174.
Co–NHC Catalyzed Imine Directed C(sp2)–H/C(sp3)–H Coupling by Yoshikai
More recently, the same group disclosed a tandem C(sp2)–H activation/ radical cyclization of bromoalkenes promoted by Co–NHC complexes (Scheme 175).335 In this reaction, the cobaltacycle intermediate formed after the C(sp2)–H activation step undergoes single-electron transfer to bromoalkene, triggering an intramolecular radical 5-exo-trig cyclization, which is followed by radical recombination and reductive elimination to give the final product. The imidazolidinylidene based NHC ligand 234 is the best promoter for this reaction, while SIiPr, SICy, IMes, IPr and iPr-bimy afforded lower yields of the desired product. The reaction is compatible with various functional groups on the imine and bromoalkene coupling partner. Furthermore, imines could be directly hydrolyzed to ketones or transformed to the cyano group via radical decomposition.
Scheme 175.
Co–NHC Catalyzed Imine Directed C(sp2)–H/ Tandem C–C Coupling by Yoshikai
In another direction utilizing Co–NHC complexes, in 2017, Li and co-workers reported a non-directed, decarboxylative C(sp2)–H /C(sp2) cross-coupling of benzothiazoles and benzoxazoles with activated carboxylic acids (Scheme 176).336 The reaction is catalyzed by a combination of CoBr2, IPr and Ag2CO3 in 2-fluorobenzotrifluoride at 160 °C. IPr gives higher yields than IMes, PCy3 or PPh3. The proposed mechanism involves a Co-catalyzed C(sp2)–H activation to give a Co(III)–ArX2 intermediate, which then reacts with aryl radical generated from the carboxylic acid in the presence of Ag2CO3, followed by reductive elimination. Although only highly reactive carboxylic acids capable of decarboxylation with Ag2CO3 serve as suitable substrates in this process, the scope of the reaction is quite broad and accommodates various electron-withdrawing substituents on the carboxylic acid component.
Scheme 176.
Co–NHC Catalyzed C(sp2)–H/Decarboxylative Arylation by Li
8.2. C(sp3)–H Activation
Several examples of intramolecular C(sp3)–H activation within low-valent Co(0)–NHC and Co(I)–NHC complexes have been reported.337–340 At present, these processes are limited to the synthesis of new Co–NHC complexes.
9. Nickel–NHC Complexes
In general, Ni-catalyzed transformations are highly attractive due to low price and availability of nickel, while facile oxidative addition and access to various oxidation states have enabled the development of a broad variety of catalytic methods using homogenous nickel catalysis.341,342 In this regard, Ni–NHC complexes have found applications in a numerous organic reactions, including activation of small molecules,343,344 cleavage of C–X bonds345,346 and formation of C–C and C–N bonds, among other applications.347,348 Recently, major advances have been made in using Ni–NHCs as catalysts for C–H functionalization reactions. In particular, Ni–NHCs have emerged as an attractive class of catalysts for the following C–H functionalizations: (1) remote C(sp2)–H alkylation and alkenylation reactions; (2) C(sp2)–H alkylation of heterocycles; (3) intramolecular C(sp2)–H annulation of nitrogen-heterocycles, including enantioselective processes; (4) direct C(sp2)–H arylation reactions; (5) C(sp2)–H borylation; and (6) C(acyl)–H amidation and arylation reactions.
9.1. C(sp2)–H Activation
In 2010, Nakao and co-workers reported C4 selective C(sp2)–H alkylation of pyridines with olefins catalyzed by a cooperative Ni–NHC/Lewis acid catalysis (Scheme 177).349 They proposed that conventional C2–H functionalization of pyridines would be diverted into C4–H functionalization by using a combination of a bulky Lewis acid and a bulky NHC ligand that would favor oxidative addition at the remote carbon through η2-substrate coordination to Ni(0)–NHC and η1-N-coordination to the Lewis acid. They found that IPr as the NHC ligand and MAD as the Lewis acid (MAD = 2,6-tBu2-4-Me-C6H2O)2AlMe) in the presence of Ni(cod)2 allowed for linear-selective alkylation of a wide range of pyridines with alkenes in toluene at 130 °C at the C4 position. In this system, only traces of the branched products and unselective alkylation are observed.
Scheme 177.
Ni–NHC Catalyzed C4 Selective C(sp2)–H Alkylation of Pyridines by Nakao
Independently, Ong and co-workers developed C4 selective C(sp2)–H alkenylation of pyridines with alkynes using a combination of Ni(cod)2, AlMe3 and chelating imidazolylidene ligand 235 (Scheme 178).350 This methodology is based on a similar concept to the C4-alkylation reported by Nakao and co-workers. In this case, the authors were able to directly observe and fully characterize by the X-ray crystallography the η2, η1-pyridine-Ni(0)–Al(III) intermediate prior to the C4–H activation step. The authors proposed that the hemilabile amino-side chain in 235 might stabilize the Ni(0)–NHC center. In agreement with this hypothesis, other NHC ligands, including IMes, SIMes and SIPr proved significantly less effective in the reaction. The proposed mechanism involves formation of the Ni–Al complex by the reaction of (NHC)Ni with pyridine and (NHC)AlMe3. Oxidative addition of the C–H bond gives the Ni–H intermediate. Alkyne insertion and reductive elimination gives the C–H activation product and regenerates the active catalyst. This methodology allows for the construction of C4-alkenylated pyridines with excellent C4 regioselectivity.
Scheme 178.
Ni–NHC Catalyzed C4 Selective C(sp2)–H Alkenylation of Pyridines by Ong
In 2015, Ong and co-workers reported C4 selective C(sp2)–H alkylation of pyridines by a catalyst-dependent switchable isomerization of allylarenes (Scheme 179).351 They found that the use of Ni(cod)2 in the presence of IPr and MAD resulted in a linear product from the insertion of allylarene, while a similar catalyst system based on NHC ligand 236 featuring a hemilabile dimethylamino side-chain promoted olefin isomerization prior to the insertion step. Interestingly, IMes was completely unselective in this reaction, while phosphines, such as PPh3 and PCy3, did not promote the reaction. The use of a more bulky N,N-dimethylamino ligand 236 (cf. N-tert-butyl ligand 235) resulted in higher yields. A mechanistic study on the regioselectivity of this reaction has been published.352
Scheme 179.
Ni–NHC Catalyzed Switchable C4 Selective C(sp2)–H Alkylation of Pyridines by Ong
The first example of a direct C2-selective C(sp2)–H alkylation of heterocycles catalyzed by Ni–NHCs was reported by Nakao and co-workers in 2010 (Scheme 180).353 It was found that using Ni(cod)2 in the presence of IMes as a ligand in hexane at 130 °C promoted the desired C2–H alkylation of various five-membered heterocycles with olefins to give branched alkylated products in high yields. The corresponding linear alkylation products were not detected using styrenes, while an example with aliphatic olefin (cf. Scheme 181)354 gave exclusively the linear product. Furthermore, other ligands, including IPr, PCy3 and PnBu3 gave only trace of the product. The reaction was successfully applied to the alkylation of various heterocycles, including C3-activated indoles, benzimidazole, benzofuran, benzothiazole, benzoxazole and oxazole.
Scheme 180.
Ni–NHC Catalyzed C2 Selective C(sp2)–H Alkylation of Heterocycles by Nakao
Scheme 181.
Ni–NHC Catalyzed C2 Linear Selective C(sp2)–H Alkylation of Heterocycles by Hartwig
Later, Nakao, Hartwig and co-workers were able to extend this direct C2-selective C(sp2)–H alkylation of heterocycles catalyzed by Ni–NHCs to the formation of linear products using indoles, benzofurans, furans and pyrroles as arylation substrates (Scheme 181).354 After optimization it was observed that a catalyst system based on electron-rich, sterically-demanding IPr*MeO or IPrMe NHC ligands worked best for this transformation. With these catalyst systems, various aliphatic olefins were coupled with excellent functional group tolerance and high linear:branched selectivity.
In 2017, Ackermann and co-workers reported a divergent C2-selective C(sp2)–H of five-membered heterocycles with allenes (Scheme 182).355 Interestingly, especially from the practical point of view, this reaction leads to C2-allylated products using the catalyst system generated from Ni(cod)2 and IPr in toluene at 100 °C, while the addition of NaOtBu (1.0 equiv) to the same catalyst system results in the selective formation of C2-vinyl products by olefin isomerization. This bifurcated C(sp2)–H activation pathway highlights the importance of selecting appropriate conditions for generating the active Ni–NHC catalyst. The reaction represents an effective way for constructing C2-allyl and C2-vinyl purine derivatives.
Scheme 182.
Ni–NHC Catalyzed C(sp2)–H Functionalization of Heterocycles with Allenes by Ackermann
In 2012, Ong and co-workers reported a switchable branched- or linear-selective C(sp2)–H alkylation of benzimidazoles with alkenes at the C2 position (Scheme 183).356,357 They discovered that the catalytic system using Ni(cod)2 in the presence of their amine-chelating NHC ligand 235 (see Scheme 178) in toluene at 100 °C furnished the branched products, while the addition of AlMe3 (10 mol%) completely switched the selectivity to linear products via cooperative Ni–Al bimetallic catalysis. The structure of a Lewis pair complex with Al coordinated to the nitrogen atom of the benzimidazole ring was confirmed by the X-ray crystallography. Compared with 235, IPr and IMes also showed excellent selectivity but were less efficient. Interestingly, MAD gave much lower selectivity than AlMe3.
Scheme 183.
Ni–NHC Catalyzed Switchable C2 Selective C(sp2)–H Alkylation of Heterocycles by Ong
Subsequently, the same group reported another example of a divergent C(sp2)–H functionalization of imidazoles in the reactions with allylarenes using Ni–NHCs (Scheme 184).358 Now, they found that the standard monometallic Ni–NHC catalysis protocol (Ni(cod)2/IMes, toluene, 100 °C) leads to the formation of branched C2–H functionalization products by alkene isomerization, while bimetallic Ni–Al catalysis (Ni(cod)2/IPr/AlMe3, toluene, 100 °C) affords linear C2–H functionalization adducts through steric control of the olefin insertion.
Scheme 184.
Ni–NHC Catalyzed Switchable C2 Selective C(sp2)–H Isomerization/Alkylation of Heterocycles by Ong
Meanwhile, Ong and co-workers established yet another regiodivergent C(sp2)–H functionalization of heterocycles by switching between monometallic Ni–NHC and bimetallic Ni–NHC–Al catalysis in the direct C3 or C5 alkenylation of imidazo[1,5-a]pyridines with alkynes (Scheme 185).359 In this instance, the standard Ni–NHC catalysis (Ni(cod)2/IPr, toluene) results in C–H alkenylation at the most acidic C3 position, while the addition of AlMe3 (60 mol%) switches the selectivity to C5–H alkenylation by Lewis acid coordination to the basic nitrogen atom. It is noteworthy that these switchable C(sp2)–H activation reactions proceed under very mild room temperature conditions, indicating a significant potential for late-stage functionalization of this class of heterocycles. The Ong group also reported that the direct C(sp2)–H alkenylation of imidazo[1,5-a]pyridines could occur selectively at the C8 position when the C5 position is blocked (Scheme 186).360 Interestingly, the C3 position remains intact under these cooperative Ni–NHC–Al conditions.
Scheme 185.
Ni–NHC Catalyzed Regiodivergent C(sp2)–H Alkenylation of Imidazo[1,5-a]pyridine by Ong
Scheme 186.
Ni–NHC Catalyzed C5 Selective C(sp2)–H Alkenylation of Imidazo[1,5-a]pyridine by Ong
In 2015, an extension of the Ni–NHC catalyzed C(sp2)–H functionalization to the coupling with cyclic dienes was realized by Ong and co-workers (Scheme 187).361 The catalyst system using Ni(cod)2 in the presence of IMes resulted in the formation of α-alkenyl products at the C2 position of various azoles through olefin isomerization, while replacing IMes with PCy3 fully switched the selectivity to give β-alkenyl C–H functionalization products. The authors demonstrated that the olefin isomerization using Ni(cod)2/PCy3 is much slower than with the Ni(cod)2/IMes catalyst under these reaction conditions. Interestingly, IPr and their hemilabile NHC ligand 235 were found to be ineffective in this transformation.
Scheme 187.
Ni–NHC Catalyzed C2 Selective C(sp2)–H Alkenylation of Heterocycles with Cyclodienes by Ong
The use of an abnormal NHC ligand in the Ni–NHC catalyzed C(sp2)–H alkylation of benzoxazoles with styrenes was reported by Mandal and co-workers (Scheme 188).362 This reaction proceeds in the presence of Ni(cod)2 and 237 in hexene at 80 °C and leads to a variety of 1,1-diarylethane products with excellent branched selectivity. Based on stoichiometric studies, the authors proposed that the mechanism involves oxidative addition of the C(sp2)–H heteroarene bond to give [Ni(NHC)(H)(Ar)] intermediate, olefin insertion and reductive elimination. The C–H activation step is likely facilitated by the more flexible steric environment of the abnormal NHC ligand.
Scheme 188.
Abnormal Ni–NHC Catalyzed C2 Selective Alkylation of Benzoxazoles by Mandal
Following their initial report on bimetallic Ni(0)–Al(III) catalysis for the alkylation of pyridines (Scheme 177),349 Nakao and co-workers developed several protocols for remote C(sp2)–H alkylation of arenes directed by the presence of a suitable Lewis basic functional group (Schemes 189–192). In 2016, the Nakao group disclosed the para-selective C(sp2)–H alkylation of benzamides and aromatic ketones promoted by a combination of Ni–NHC and MAD as a Lewis acid co-catalyst (Scheme 189).363 They found that in this system the use of a sterically-bulky and electron-rich NHC ligand 238 gave dramatically improved yields and regioselectivities than IPr or IPr*.364 Similarly, MAD proved to be better in terms of both yield and regioselectivity than AlMe3. This methodology could be used for para-selective C(sp2)–H alkylation of a wide range of N,N-disubstituted benzamides and aryl ketones in moderate to high yields and with excellent para-regioselectivity. Mechanistically, the reaction was proposed to involve coordination of the Lewis acid to the carbonyl group, which facilitates C4-selective C–H activation by weakening the para C–H bond and sterically-directing the Ni–NHC catalyst.
Scheme 189.
Ni–NHC Catalyzed p-Selective Alkylation of Benzamides and Ketones by Nakao
Scheme 192.
Ni–NHC Catalyzed p-Selective Alkylation of Sulfonylarenes by Nakao
In 2018, the same group reported meta- and para-selective C(sp2)–H alkylation of anilides by cooperative Ni–NHC–Al catalysis (Scheme 190).365 In this variation, meta-selective C(sp2)–H activation is achieved by the combined use of IPrMe (10 mol%) and MAD (100 mol%) in the presence of unhindered N-Me-N-COCy anilides, while the para-selective process exploits bulky N-anilide protecting groups and uses very similar reaction conditions to the alkylation of benzamides with sterically-demanding NHC ligand 238 (cf. Scheme 189). This highly challenging reaction affords moderate yields and typically high regioselectivity; however, the scope appears to be limited.
Scheme 190.
Ni–NHC Catalyzed Remote Alkylation of Anilides by Nakao
In addition, Nakao and co-workers reported C6-selective C(sp2)–H alkylation of benzofurans substituted with a sterically-hindered 2-carbamoyl moiety (Scheme 191).366 In this process, the bulky NHC ligand 238 (10 mol%) and MAD (100 mol%) were identified as the best combination to deliver C6 alkylated benzofurans in moderate yields and with high C6–H activation selectivity.
Scheme 191.
Ni–NHC Catalyzed C6 Selective Alkylation of Benzofurans by Nakao
Furthermore, the Nakao group disclosed para-selective C(sp2)–H alkylation of sulfonylarenes using the same bimetallic Ni–NHC–Al catalysis concept (Scheme 192).367 In this case, the C–H activation occurs under very similar conditions to the para-selective alkylation of benzamides using 238 (10 mol%) and MAD (40 mol%) in toluene at 150 °C (cf. Scheme 189). The main advantage of this protocol is facile removal of the sulfonyl group to access simple arenes, which was nicely demonstrated by the authors in the synthesis of meta-substituted dialkylbenezenes.
Intramolecular C(sp2)–H annulations catalyzed by Ni–NHC complexes have been successfully accomplished by Cramer and co-workers (Schemes 193–195). In 2015, the reported a regiodivergent annulation of pyridones by C(sp2)–H activation at the C6 position to form 1,6-annulated products via 6-endo or 5-exo cyclization mode in good to excellent yields (Scheme 193).368 They found that 6-endo- and 5-exo-cyclization regioselectivity could be switched by changing the catalyst system from Ni–NHC–Al bimetallic catalysis (Ni(cod)2/IPr/AlMe3) to Ni–Al catalysis (Ni(cod)2/AlMe3) under the same reaction conditions. A mechanism involving Ni(0)-catalyzed C–H insertion and irreversible hydroarylation was proposed. This methodology enables a straightforward way to construct medium-sized rings featuring [4.3.0] and [4.4.0] nitrogen-bridged heterocycles.
Scheme 193.
Intramolecular Ni–NHC Catalyzed C(sp2)–H Annulation of Pyridones by Cramer
Scheme 195.
Asymmetric Ni–NHC Catalyzed C(sp2)–H Annulation of Indoles by Cramer
In 2018, Cramer and co-workers extended this C(sp2)–H annulation methodology to the enantioselective cyclization of pyridines catalyzed by a new class of chiral Ni–NHC complexes (Scheme 194).369 They identified a bulky BIAN-based NHC ligand 239 featuring chiral N-Ar wingtips as optimal for promoting this C–H annulation in terms of reactivity and enantio-induction. The X-ray analysis of a related NHC ligand in the series (R = Ph, Scheme 194) indicated %Vbur of 43.5%, [Au(NHC)Cl] complex (Vbur = buried volume). This was significantly higher than the analogous [Ni(NHC)(Cp)Cl] complex (%Vbur = 35.7%), indicating flexibility of the ligand in adjusting to the steric environment. This novel class of ligands enabled efficient 6-endo-annulations under mild conditions in the presence of Ni(cod)2 and MAD (40 mol%) in toluene at 40 °C.
Scheme 194.
Asymmetric Ni–NHC Catalyzed C(sp2)–H Annulation of Pyridones by Cramer
More recently, the Cramer group achieved enantioselective C(sp2)–H annulations of N-tethered indoles and pyrroles using a similar catalyst system based on Ni(cod)2 and a chiral imidazolidinylidene ligand 240 (Scheme 195).370 An intriguing feature of this design is the identification of very bulky flanking groups on the N-aromatic wingtip to enhance enantioselectivity of the process. This class of SIPr-based ligands is well poised to find further use in C–H activation reactions. The process enables access to tetrahydropyridoindoles and tetrahydroindolizines in good yields and with excellent enantioselectivities.
Independently, the Sames group reported the intramolecular C(sp2)–H annulation of benzofurans with olefins catalyzed by Ni–NHC complexes in the context of synthetic studies on the Ibogamine alkaloids (Scheme 196).371 By using the combination of Ni(cod)2 and IMes in heptane at 130 °C, the intramolecular cyclization was achieved in moderate yields, while phosphine ligands were ineffective.
Scheme 196.
Intramolecular Ni–NHC Catalyzed C(sp2)–H Annulation by Sames
In 2015, Montgomery and co-workers identified the IMes-based Ni–NHC complex 241 as a highly active catalyst for the C(sp2)–H alkenylation of acidic C–H bonds in arenes and heteroarenes (Scheme 197).372 Remarkably, they found that the most commonly used throw-away ligand in Ni(0)–NHC catalysis, namely, 1,5-cyclooctadiene (cod), showed a significant inhibitory effect on the catalytic activity by the formation of inactive π-allyl complexes through hydride migration to Ni-bound cod. Thus, using the cod-free Ni(0)–NHC complex, [Ni(IMes)(1,5-hexadiene)], allowed to avoid the inactive π-allyl intermediates and resulted in high catalytic efficiency in this class of C(sp2)–H activation reactions. Complex 241 is prepared in a single step from NiCl2, IMes and allylmagnesium bromide.
Scheme 197.
Ni–NHC Catalyzed C(sp2)–H Alkenylation of Acidic C–H Bonds by Montgomery
The Chatani group demonstrated Ni–NHC catalysis in amide-directed C(sp2)–H activation reactions (Schemes 198–199). In 2016, they reported a NiBr2(dme)/SIMes system for the oxidative intermolecular annulation of arenes and alkynes enabled by the 8-aminoquinolinyl directing group (Scheme 198).373 They found that other ligands, including IMes, ICy, SICy and PCy3, were capable of promoting this reaction; however, these ligands were less efficient than IMes. Mechanistically, the reaction was proposed to involve a Ni(0)/(II) cycle with the C(sp2)–H activation step proceeding via a CMD pathway and the alkyne serving as the hydride acceptor. The same group also demonstrated that aromatic C(sp2)–H alkylation with challenging secondary alkyl halides directed by the 8-aminoquinolinyl group is facilitated by using a catalyst system based on Ni(OTf)2/IMesMe and PPh3 (Scheme 199).374 The use of an NHC ligand is critical for the success of this reaction.
Scheme 198.
Ni–NHC Catalyzed Amide Directed C(sp2)–H Oxidative Annulation by Chatani
Scheme 199.
Ni–NHC Catalyzed Amide Directed C(sp2)–H Alkylation by Chatani
The direct C(sp2)–H arylation of azoles is another class of C–H activation reactions that have been explored with Ni–NHC catalysis. In 2018, Ritleng and co-workers reported the direct C(sp2)–H arylation of benzothiazoles with aryl iodides catalyzed by cationic Ni–NHC complex 242 (Scheme 200).375 This complex displayed modest activity in the direct C–H arylation. The authors proposed that the Ni(II)–NHC pre-catalyst is activated by the dimerization of benzothiazole. In 2018, Ong and co-workers reported Ni(cod)2/IPr-catalyzed direct C(sp2)–H arylation of azoles with anisoles by a dual C–H/C–O activation pathway (Scheme 201).376 The reaction requires stoichiometric amounts of a Grignard reagent and the sterically-demanding o-TolMgBr gave the best results. This process is remarkably broad in scope and includes activated conjugated arenes as well as simple arenes as coupling partners. The authors proposed that the reaction is facilitated by a synergistic action of the Ni–NHC catalyst and the Grignard reagent to deprotonate the C2–H azole bond.
Scheme 200.
Ni–NHC Catalyzed Direct C(sp2)–H/C–I Arylation of Azoles by Ritleng
Scheme 201.
Ni–NHC Catalyzed Direct C(sp2)–H/C–O Arylation of Azoles by Ong
In addition, a non-directed C(sp2)–H borylation of arenes and heteroarens using Ni–NHC complexes has been achieved by Chatani and co-workers (Scheme 202).377 It was found that Ni(cod)2 as the catalyst and ICy·HCl as the ligand precursor gave optimal results. Other NHC ligands, including SICy, IPr, IMes, IiPr and ItBu were less effective, while PCy3 failed to deliver any product. The reaction enables the synthesis of a variety of C2-borylated indole derivatives in good yields. Furthermore, simple arenes, such as benzene, anisole and toluene, are also suitable substrates under the reaction conditions. The reaction was proposed to involve a heterogeneous nickel catalyst. Independently, Itami and co-workers reported that a catalytic system based on Ni(cod)2, IPr and CsF promotes C(sp2)-selective C–H borylation of toluene with B2pin2 (not shown).378
Scheme 202.
Ni–NHC Catalyzed C(sp2)–H Borylation by Chatani
Intramolecular ortho-N-aromatic C(sp2)–H activation within Ni(0)–NHC complex to give a catalytically-active cyclometallated Ni(II)–H for cycloisomerization of enynes has been reported by Louie and co-workers (not shown).379
9.2. C(acyl)–H Activation
The synthesis of acyl derivatives by C(acyl)–H activation has been reported using Ni–NHC systems (Schemes 203–204). In 2015, Dong and co-workers reported the direct conversion of aldehydes to amides using a combination of Ni(cod)2 (5 mol%), IPr or ItBu (5 mol%) and PhCOCF3 (1.1 equiv) as a mild oxidant (Scheme 203).380 This protocol allows for the dehydrogenative synthesis of a variety of amides from aliphatic and aromatic aldehydes and amines. The mechanism was proposed to involve the following steps: (1) coordination of Ni–NHC to PhCOCF3 and RCHO carbonyls; (2) oxidative addition of the C(acyl)–H bond; (3) hydride transfer to give Ni–alkoxide; (4) ligand exchange; (5) reductive elimination. This methodology is also applicable to the direct coupling of aldehydes with alcohols, providing an alternative way to construct hindered esters. Independently, Gu and co-workers reported the synthesis of aryl and alkyl ketones by the direct coupling of aldehydes with aryl and alkyl boronic esters catalyzed by Ni–NHCs (Scheme 204).381 The reaction is promoted by a very similar catalytic system using Ni(cod)2 (5 mol%), IPr (6 mol%) and CF3COCF3 (1.5 equiv) as the oxidant. Other NHC ligands, including SIPr and ItBu were less effective, while PCy3 failed to produce the coupling product. The reaction is compatible with a variety of aryl aldehydes and aryl and alkyl boronic esters, affording the desired ketone products under mild conditions.
Scheme 203.
Ni–NHC Catalyzed C(Acyl)–H Amidation by Dong
Scheme 204.
Ni–NHC Catalyzed C(Acyl)–H Arylation/Alkylation by Ge
10. Copper–NHC Complexes
The first homoleptic Cu–NHC complex, [Cu(IMes)2(OTf)], was isolated in 1993 by Arduengo.382 In 1994, Raubenheimer and co-workers synthesized and characterized the first monocarbene Cu–NHC complex, [Cu(NHC)Cl]2 (NHC = 3,4,5-trimethylthiazole).383 After these seminal studies, great success has been achieved in using Cu–NHC complexes as catalysts to promote organic transformations.384–386 At present, Cu–NHC have emerged, as particularly efficient catalysts for C(sp2)–H and C(sp)–H carboxylation reactions using carbon dioxide, thus representing a very attractive method for incorporating this C1 building block into organic molecules. Further examples of C–H activation reactions promoted by Cu–NHCs include the following classes of reactions: (1) C(sp2)–H arylation reactions; (2) C(sp2)–H allylations, including enantioselective allylations; (3) C(sp2)–H amination and thiolation; and (4) enantioselective C(sp)–H allylation.
10.1. C(sp2)–H Activation
The first examples of C(sp2)–H carboxylation catalyzed by Cu–NHCs were reported independently by the Hou and Nolan groups in 2010 (Schemes 205–206).387,388
Scheme 205.
Cu–NHC Catalyzed C(sp2)–H Carboxylation by Hou
Scheme 206.
Cu–NHC Catalyzed C(sp2)–H Carboxylation by Nolan
Hou and co-workers developed C(sp2)–H carboxylation of benzoxazoles with CO2 at atmospheric pressure using [(IPr)CuCl] (5 mol%) in THF at 80 °C (Scheme 205).387 The carboxylic acid products were isolated after esterification with iodohexane. Several other catalysts were tested, and SIPr, IMes and SIMes proved to be less efficient. Importantly, the well-defined [Cu(IPr)Cl] gave higher yields that the catalyst prepared in situ from Cu(OAc)2 and IPr·HCl. The reaction worked best with benzoxazole derivatives, while lower yielding examples of C(sp2)–H carboxylation of benzimidazole and 1,3,4-oxadiazole were also reported. The mechanism involving formation of the catalytically active [Cu(IPr)(OtBu)] complex and deprotonation of the most acidic C–H was proposed. First, the active [(IPr)Cu(OtBu)] complex reacts with the substrate to give the organocopper species [(IPr)Cu(Ar)] through Cu-mediated C–H activation. Reversible CO2 insertion affords the carboxylate complex [(IPr)Cu(ArCO2)] stabilized by N-coordination. Ligand exchange and esterification with an alkyl halide furnishes the desired product and regenerates the active [Cu(IPr)(OtBu)] catalyst.
Independently, Nolan and co-workers reported C(sp2)–H carboxylation of acidic arenes and heteroarenes catalyzed by [Cu(IPr)(OH)] (Scheme 206).388 In this system, the combination of [Cu(IPr)(OH)] (3 mol%) and CsOH in THF at 65 °C gave the optimal performance. The desired carboxylic acids were isolated directly after acidification of the reaction mixture. Furthermore, other Cu–NHC catalysts, including catalysts based on SIPr, IMes and SIMes NHC ligands, were less effective. The reaction showed excellent selectivity and broad substrate scope, including benzoxazole, benzothiazole, oxazole and acidic hydrocarbons.
In 2012, the Hou group expanded their C(sp2)–H carboxylation methodology to the direct carboxylation of benzoxazoles and benzothiazoles by using triazolylidene complex 243 (Scheme 207).389 This [Cu(TPr)Cl] complex was shown to be more effective than [Cu(IPr)Cl] in the carboxylation of benzoxazole. In general, the protocol allowed for the synthesis of C2-carboxylated benzoxazoles and benzothiazoles in excellent yields. The high catalytic activity of 243 likely results from the strong σ-donation of the triazolylidene ligand.
Scheme 207.
Cu–NHC Catalyzed C(sp2)–H Carboxylation using Triazolylidine Complexes by Hou
Subsequently, several variations of the C(sp2)–H carboxylation catalyzed by Cu–NHCs have been reported (Schemes 208–210). In 2013, Li and co-workers reported morpholine-functionalized [Cu(NHC)Cl] catalyst 244 for the direct C(sp2)–H carboxylation of benzoxazoles with CO2 at atmospheric pressure (Scheme 208).390 The main advantage of this pH-responsive Cu–NHC catalyst is that the authors could recycle the catalyst four times, while maintaining high activity. Independently, Hong and co-workers reported bifunctional NHC ligand 245 for the Cu–NHC catalyzed carboxylation of benzoxazoles (Scheme 209).391 This catalytic system gave the carboxylated products in higher yields than using [Cu(IPr)Cl] and showed excellent functional group tolerance. In an alternative approach to C(sp2)–H carboxylation, Hou and co-workers reported a tandem process relying on deprotonative alumination of arenes with iBu3Al(TMP)Li followed by carboxylation catalyzed by [Cu(IPr)Cl] (Scheme 210).392 The reaction is characterized by excellent yields and broad functional group tolerance using benzamides, benzonitriles, anisoles and acidic heterocycles as carboxylation substrates. A mechanism involving transmetallation of [Cu(IPr)(OtBu)] with organoaluminium to give aryl–Cu(IPr) was proposed. In addition, DFT study on the mechanism of C(sp2)–H carboxylation reactions promoted by Cu–NHC complexes has been published.393 These reactions allow for the incorporation of carbon dioxide under mild conditions.394–401
Scheme 208.
Cu–NHC Catalyzed C(sp2)–H Carboxylation using pH-Responsive Ligands by Li
Scheme 210.
Cu–NHC Catalyzed C(sp2)–H Deprotonative ortho-Carboxylation by Hou
Scheme 209.
Cu–NHC Catalyzed C(sp2)–H Carboxylation using Bifunctional Ligands by Hong
In 2014, Cazin and co-workers reported a cooperative Cu–NHC/Pd–NHC system for the C(sp2)–H arylation of acidic arenes (Scheme 211).402 In this design, [Cu(ItBu)Cl] promotes C(sp2)–H activation to form aryl-Cu(NHC) species after in situ conversion to the catalytically active [Cu(ItBu)(OH)]. In the cooperative cycle, Pd–NHC generates Ar–Pd(NHC)–Cl intermediate through the standard oxidative addition to Pd(0)–NHC. Transmetallation of [Cu(ItBu)(Ar)] with [(SIPr)Pd(Ar)Cl] and reductive elimination generates the biaryl product. This transformation shows broad scope with respect to fluorinated arenes and aryl halides, and also includes examples of acidic heterocycles as the C(sp2)–H activation coupling partners.
Scheme 211.
Cu–NHC/Pd–NHC Catalyzed C(sp2)–H/C–X Arylation by Cazin
In 2016, Chang and co-workers reported divergent C(sp2)–H alkenylation and allylation of acidic arenes and heteroarenes catalyzed by Cu–NHCs (Scheme 212).403 Interestingly, they found that C(sp2)–H activation of arenes with allyl halides promoted by [Cu(IiPr)Cl] in the presence of NaOtBu in THF at 60 °C resulted in the formation of vinyl products through C(sp2)–H allylation/double bond migration pathway, while the same catalytic system in benzene at 25 °C gave C(sp2)–H allylated arenes with excellent selectivity. Furthermore, this catalytic system was extended to electron-rich heteroarenes using Me2EtCONa as a base. A mechanism involving formation of Ar–Cu(NHC), oxidative addition of allyl halide to give π-allyl–Cu–Ar(NHC)(X), and reductive elimination was proposed.
Scheme 212.
Cu–NHC Catalyzed C(sp2)–H Alkenylation and Allylation by Chang
Independently, Sawamura and co-workers reported enantioslective C(sp2)–H allylation of azoles with allyl phosphates catalyzed by Cu–NHC complexes (Scheme 213).404 The reaction enables enantioselective construction of quaternary stereocenters with good substrate scope, excellent enantioselectivity and branched:linear selectivity. The catalyst system based on a chelating N-2-napthol ligand 246 gave the best yield and enantioselectivity. Interestingly, a dramatic decrease in the reaction efficiency was observed when the hydroxyl group in the ligand was protected as a methyl ether, while no conversion occurred using NHC ligands without the oxygen atom, including analogues of 246, IMes and SIMes.
Scheme 213.
Enantioselective Cu–NHC Catalyzed C(sp2)–H Allylation by Sawamuara
In 2016, Chang and co-workers disclosed direct C(sp2)–H amidation of acidic arenes and heteroarenes with BocNNaCl and related carbamates catalyzed by Cu–NHCs (Scheme 214).405 This mild amidation protocol allows for the direct amidation of fluorobenzenes, azoles and quinoline-N-oxides in the presence of [Cu(IiPr)Cl] and NaOtBu in THF at 25 °C. Other NHC ligands, including SIiPr, IMes, SIMes, IPr, SIPr and ICy were less effective than IiPr in this transformation. A proposed mechanism involves the following steps: (1) formation of the catalytically-active [Cu(IiPr)(OtBu)]; (2) C(sp2)–H activation to give Ar–Cu(NHC); (3) oxidative insertion of BocNaN–Cl; (4) Ar group transfer to carbamate; and (5) ligand exchange.
Scheme 214.
Cu–NHC Catalyzed C(sp2)–H Amidation by Chang
In an alternative approach to forming C–heteroatom bonds by Cu–NHC catalysis, Fukuzawa and co-workers demonstrated an oxidative thiolation of azoles via direct C(sp2)–H activation (Scheme 215).406 In the optimization study, they found that [Cu(IPr)Cl] is more catalytically-active than [Cu(IMes)Cl], [Cu(TPr)Cl]) and [Cu(TMes)Cl]; however, all complexes afforded the desired product in high yields. This methodology provides a straightforward access to diverse 2-thiobenzothiazoles.
Scheme 215.
Cu–NHC Catalyzed C(sp2)–H Oxidative Thiolation by Fukuzawa
In addition, the direct C(sp2)–H arylation of caffeine with aryl iodides catalyzed by Cu–NHC complexes was reported by Ong and co-workers (Scheme 216).407 They found that a system based on their hemilabile amino-tethered NHC scaffold 247 is an excellent catalyst for the direct arylation of a wide range of caffeine derivatives providing the C–H arylation products in good to high yields.
Scheme 216.
Cu–NHC Catalyzed C(sp2)–H/C–I Arylation of Caffeine by Ong
10.2. C(sp)–H Activation
Activation of terminal alkynes through copper acetylides represents another class of reactions catalyzed by Cu–NHC complexes. In general, the activation of terminal alkynes is facile due to increased C(sp)–H bond acidity. The use of copper-acetylides in organic synthesis has been recently reviewed.408
In 2010, Zhang and co-workers reported the direct carboxylation of terminal alkynes using Cu–NHCs and carbon dioxide at atmospheric pressure (Scheme 217).409 The main challenge associated with this transformation is the stability of the product propiolic acids to decarboxylation due to their low stability under high temperature conditions. The authors found that a catalyst system based on poly-NHC ligand 248 and CuCl efficiently promotes this carboxylation in DMF at ambient conditions. The most significant advantage of this approach is the functional group tolerance towards a wide range of substrates, mild reaction conditions and compatibility with deactivated alkynes.
Scheme 217.
Cu–NHC Catalyzed C(sp)–H Carboxylation by Zhang
Around the same time, Lu and co-workers reported a tandem process for the synthesis of allylic 2-alkynoates via C(sp)–H carboxylation catalyzed by Cu–NHCs as a key step (Scheme 218).410 They identified that after the C(sp)–H carboxylation step using [Cu(IPr)Cl] under the standard conditions, the resulting Cu-carboxylates could be alkylated directly with allyl halides, thus providing a step-economical approach to alkynoate esters. Interestingly, CuCl and [Cu(IMes)Cl] proved to be significantly less effective in this reaction, highlighting the key importance of a well-defined and sterically-hindered Cu–NHC complex.
Scheme 218.
Cu–NHC Catalyzed C(sp)–H Carboxylation/Allylation by Lu
In another illustrative example of functionalizing terminal alkynes catalyzed by Cu–NHC complexes, Sawamura and co-workers reported enantioselective C(sp)–H allylation with alkenyl phosphates (Scheme 219).411 The success of this reaction relied on the development of a new chiral NHC ligand 249 featuring a chelating hydroxyl group on the N-aromatic side-chain. Mechanistically, the authors proposed that the alkoxide coordinates to the catalytically-active Cu–NHC species as an anionic ligand, leading to the formation of a well-defined chiral environment. This methodology delivers skipped enynes with excellent branched:linear selectivity and very good functional group tolerance. Additional examples of C(sp)–H functionalization via Cu–NHC acetylides have been published.412–416
Scheme 219.
Enantioselective Cu–NHC Catalyzed C(sp)–H Allylation by Sawamura
10.3. C(sp3)–H Activation
In 2006, Perez and co-workers reported an example of C(sp3)–H functionalization with ethyl diazoacetate catalyzed by [Cu(IPr)Cl] (Scheme 220).417 This carbene insertion reaction gave a mixture of C–H activation of primary and secondary C(sp3)–H bonds, favoring the branched product. Interestingly, this regioselectivity is completely reversed using Au–NHC complexes (see Scheme 225).
Scheme 220.
Intermolecular Cu–NHC Catalyzed C(sp3)–H Alkylation by Perez
Scheme 225.
Au–NHC Catalyzed C(sp3)–H Alkylation by Perez
11. Silver–NHC Complexes
The first Ag–NHC complex, [Ag(IMes)2(OTf)], was reported by Arduengo in 1993.382 In the following years, Ag–NHC complexes have emerged as common NHC transfer reagents for the synthesis of other metal–NHC complexes by transmetallation.418,419 Catalytic applications of Ag–NHCs remain relatively underdeveloped compared to other metals.420–422 At present, examples of C–H bond functionalization catalyzed by Ag–NHC complexes are limited to C(sp)–H carboxylation reactions.
In 2012, Zhang and co-workers reported carboxylation of terminal alkynes catalyzed by poly-NHC–Ag complex 250 (Scheme 221).423 This system showed high catalytic activity in C(sp)–H carboxylation under ambient conditions. The substrate scope is comparable to that observed using a related poly-NHC–Cu complex (cf. Scheme 217). The proposed mechanism involves formation of a silver acetylide intermediate. The complex could be recycled five times without a loss of catalytic activity. More recently, a related carboxylation of terminal alkynes catalyzed by N-benzylic Ag–NHCs was reported by Zhou and co-workers (not shown).424 Catalytic C–H carboxylation reactions of terminal alkynes have been recently reviewed.425
Scheme 221.
Ag–NHC Catalyzed C(sp)–H Carboxylation by Zhang
12. Gold–NHC Complexes
Although the first Au–NHC complex was isolated in 1973,426 only recently Au–NHC complexes have been reported as catalysts and dynamic intermediates in organic synthesis.427–430 Thus far, only few examples of C–H functionalizations catalyzed by Au–NHC complexes have been reported.
12.1. C(sp2)–H Activation
In 2010, Nolan and co-workers reported a rare example of C(sp2)–H functionalization catalyzed by Au–NHC complexes (Scheme 222).431 They found that the direct C–H carboxylation of arenes and heteroarenes using [Au(IPr)OH] as the catalyst proceeded with high yields and excellent regioselectivity for arenes with acidic C–H bonds (pKa values of around 30 and lower). The optimized system involves catalytic Au–NHC (3 mol%), KOH (1.05 equiv) and CO2 (1.5 bar) in THF at 20 °C. It is noteworthy that this methodology tolerates various five- and six-membered heterocycles, including oxazole, isoxazole, thiazole, imidazole, pyrazine, triazine as well as chloro- and fluoroarenes, efficiently delivering the carboxylic acid products under mild conditions. A mechanism involving protonolysis of Au–NHC to give [(NHC)Au–Ar], nucleophilic addition to CO2, and exchange with KOH to give arene carboxylate was proposed.
Scheme 222.
Au–NHC Catalyzed C(sp2)–H Carboxylation by Nolan
Another application of Au–NHC complexes in C(sp2)–H activation was reported by the Perez group (Scheme 223). They demonstrated that [Au(IPr)Cl] catalyzes C(sp2)-selective C–H carbene insertion of α-functionalized ethyl diazoacetates to alkylbenzenes.432 These reactions give a mixture of o/m/p-functionalized products; however, high selectivity for the insertion into the aromatic C–H bond (cf. aliphatic or Buchner reaction) using this catalytic system was observed.
Scheme 223.
Au–NHC Catalyzed C(sp2)–H Alkylation by Perez
12.2. C(sp3)–H Activation
In 2012, Che and co-workers reported oxidative C(sp3)–H cyanation of N-aryltetrahydroisoquinolines using cationic Au(III)–NHC complex 251 under light irradiation conditions (Scheme 224).433 In this design, the strongly σ-donating NHC ligand increases the luminescence efficiency of Au(III). The oxidative cyanation was successful in the presence of sensitive halide functional groups, highlighting the mild conditions for oxidative generation of iminium ions by this approach.
Scheme 224.
Au–NHC Catalyzed Oxidative C(sp3)–H Cyanation of Amines by Che
Important studies further expanding the utility of Au–NHC complexes in aliphatic C–H functionalization by carbene insertion were published by Perez and co-workers. In their first contribution, they demonstrated the C(sp3)–H alkylation of toluene catalyzed by [Au(IPr)Cl] (not shown).434 Subsequently, they found that the same catalyst system promotes the branched-selective C–H functionalization of alkanes with ethyl diazoacetate (Scheme 225 cf. Scheme 220).417 Mechanism and selectivity of these carbene insertion reactions have been investigated by DFT methods.435
13. Conclusions and Outlook
As demonstrated in this review, tremendous advances have taken place using transition-metal–NHC complexes to promote C–H functionalization reactions. The unique electronic and steric properties of NHC ligands, in particular, their greater σ-donation than phosphine ligands and capacity to form well-defined complexes with diverse transition-metals, combined with the stability to oxidative conditions, have enabled the development of an array of novel C–H functionalization reactions that are impossible with other ligands and resulted in significant improvements of the existing C–H functionalization methods. The progress using NHC ligands has been remarkable with both precious metals and 3d transition-metals, which highlights the generality of the beneficial effect of this ligand platform. An especially exciting direction has been the development of asymmetric C–H functionalizations promoted by chiral NHC ligands, C–H activations at very low catalyst loading, C–H functionalization with typically inert electrophiles, divergent C–H functionalization and functionalization of unreactive C(sp3)–H bonds.
Despite significant progress, there are numerous challenges that need to be addressed. (1) First, compared with phosphines, much fewer NHC ligands are commercially available. This needs to be addressed so that the beneficial impact of NHC ligands is available to all researchers and facilitates screening of the reaction conditions in a rapid and modular fashion. (2) Second, there is scarcity of types of NHC ligands that have been tested in C–H activation reactions. Elegant mechanistic studies have already demonstrated that significant improvements in σ-donation and π-accepting properties of NHC ligands can be achieved by electronic and steric modification of the carbene scaffold.24,25 The discovery of new NHC ligands is likely to facilitate further progress in the field. (3) Third, the method of generation of the active catalyst needs to routinely explored. The most desirable is the use of well-defined NHC–metal complexes because this eliminates the complexation step and leads to the modularity of catalysis.23,54,55 (4) Fourth, structure-activity studies between the ligand structure (%Vbur, steric maps) and electronic properties (σ-donation, π-acceptance) and catalytic activity should be routinely performed. Many of these values are already available in the literature41–56 and all new ligands should be identified in a similar fashion. An example should be taken from Pd-catalyzed cross-coupling reactions, which have now reached the desired level of maturity using well-defined Pd–NHC systems through NHC ligand design and further improvements in both the reaction conditions and throw-away ligand modifications. (5) Fifth, mechanistic studies that provide insight into the role of NHC ligands in C–H activation reactions should become more common. Ultimately, better mechanistic understanding would lead to the design and discovery of improved catalyst systems that could find widespread application in various areas of organic chemistry and catalysis.
In summary, it is clear that the use of transition-metal–NHCs significantly expands the portfolio of C–H activation methods and has the potential to address the challenges in this field by designing even more efficient NHC ligands and catalytic systems. Such approaches using NHC ligands should be routinely leveraged by researchers involved in developing new C–H activation reactions.
15. Acknowledgements
Rutgers University, the NSF (CAREER CHE-1650766) and the NIH (1R35GM133326) are gratefully acknowledged for support. Acknowledgement is made to the donors of the American Chemical Society Petroleum Research Fund for partial support to Q.Z. (58841-DNI1). For work conducted by the Nolan group, VLAIO (SBO project CO2PERATE), and the Special Research Fund (BOF) of Ghent University (starting and senior project grants) are gratefully acknowledged.
Biography
Qun Zhao received his B.Sc. from Wuhan University of Technology in 2009. After a short experience in industry, he moved to Fudan University, and received his M.Sc. degree in 2014. Subsequently, he joined Fluorine Team of IRCOF in France, studying C–H activation and fluorine chemistry with Professors Tatiana Besset, Thomas Poisson, Jean-Philippe Bouillon and Xavier Pannecoucke, where he received his Ph.D. degree in 2017. In 2018, he joined the research group of Professor Michal Szostak at Rutgers University as a postdoctoral fellow. His research interests are focused on transition-metal-catalysis and C–H activation reactions.
Guangrong Meng was born in Shandong Province, P.R. of China, and received his B.Sc. degree from Dalian Medical University in 2011. He received his M.Sc. from Fudan University in 2014. He completed his Ph.D. at Rutgers University in 2019 where he worked under the supervision of Professor Michal Szostak. Currently, he is a post-doctoral fellow in the group of Professor Jin-Quan Yu at The Scripps Research Institute. His research interests are focused on transition-metal-catalysis and C–H activation reactions.
Steven P. Nolan received his B.Sc. in Chemistry from the University of West Florida and his Ph.D. from the University of Miami where he worked under the supervision of Professor Carl D. Hoff. After a postdoctoral stay with Professor Tobin J. Marks at Northwestern University, he joined the Department of Chemistry of the University of New Orleans in 1990. In 2006, he joined the Institute of Chemical Research of Catalonia (ICIQ). In early 2009, he joined the School of Chemistry at the University of St Andrews. In 2015 he moved to the Ghent University. His research interests include organometallic chemistry and catalysis. His group has studied N-heterocyclic carbenes and their role in catalysis since 1998.
Michal Szostak received his Ph.D. from the University of Kansas with Professor Jeffrey Aubé in 2009. After postdoctoral stints at Princeton University with Prof. David MacMillan and at the University of Manchester with Prof. David Procter, in 2014, he joined the faculty at Rutgers University. His research group is focused on the development of new synthetic methodology based on transition-metal catalysis, transition-metal-mediated free-radical chemistry, and application to the synthesis of biologically active molecules.
Footnotes
The authors declare no competing financial interests.
16. References
- (1).Nolan SP N-Heterocyclic Carbenes in Synthesis; 1st Ed, Wiley: New York, 2006. [Google Scholar]
- (2).Nolan SP N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; 1st Ed, Wiley-VCH: Mannheim, 2014. [Google Scholar]
- (3).Nolan S; Cazin C N-Heterocyclic Carbenes in Catalytic Organic Synthesis; 1st Ed, Thieme: Stuttgart, 2017. [Google Scholar]
- (4).Glorius F N-Heterocyclic Carbenes in Transition Metal Catalysis; 1st Ed., Springer: Berlin, 2007. [Google Scholar]
- (5).Huynh HV The Organometallic Chemistry of N-Heterocyclic Carbenes; 1st Ed, John Wiley & Sons: Hoboken, 2017. [Google Scholar]
- (6).Díez-González S N-Heterocyclic Carbenes: From Laboratory to Curiosities to Efficient Synthetic Tools; 1st Ed, Royal Society of Chemistry: London, 2010. [Google Scholar]
- (7).Díez-González S N-Heterocyclic Carbenes: From Laboratory to Curiosities to Efficient Synthetic Tools; 2st Ed, Royal Society of Chemistry: London, 2016. [Google Scholar]
- (8).Soleilhavoup M; Bertrand G Cyclic (Alkyl)(Amino) Carbenes (Caacs): Stable Carbenes on the Rise. Acc. Chem. Res. 2014, 48, 256–266. [DOI] [PubMed] [Google Scholar]
- (9).Hopkinson MN; Richter C; Schedler M; Glorius F An Overview of N-Heterocyclic Carbenes. Nature 2014, 510, 485. [DOI] [PubMed] [Google Scholar]
- (10).Peris E Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2017, 118, 9988–10031. [DOI] [PubMed] [Google Scholar]
- (11).Visbal R; Gimeno MC N-Heterocyclic Carbene Metal Complexes: Photoluminescence and Applications. Chem. Soc. Rev. 2014, 43, 3551–3574. [DOI] [PubMed] [Google Scholar]
- (12).Liu WK; Gust R Metal N-Heterocyclic Carbene Complexes as Potential Antitumor Metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [DOI] [PubMed] [Google Scholar]
- (13).Fevre M; Pinaud J; Gnanou Y; Vignolle J; Taton D N-Heterocyclic Carbenes (NHCs) as Organocatalysts and Structural Components in Metal-Free Polymer Synthesis. Chem. Soc. Rev. 2013, 42, 2142–2172. [DOI] [PubMed] [Google Scholar]
- (14).Crabtree RH Abnormal, Mesoionic and Remote N-Heterocyclic Carbene Complexes. Coord. Chem. Rev. 2013, 257, 755–766. [Google Scholar]
- (15).Wang FJ; Liu LJ; Wang WF; Li SK; Shi M Chiral NHC-Metal-Based Asymmetric Catalysis. Coord. Chem. Rev. 2012, 256, 804–853. [Google Scholar]
- (16).Velazquez HD; Verpoort F N-Heterocyclic Carbene Transition Metal Complexes for Catalysis in Aqueous Media. Chem. Soc. Rev. 2012, 41, 7032–7060. [DOI] [PubMed] [Google Scholar]
- (17).Valente C; Calimsiz S; Hoi KH; Mallik D; Sayah M; Organ MG The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [DOI] [PubMed] [Google Scholar]
- (18).Knappke CEI; Imami A; Jacobi von Wangelin A Oxidative N-Heterocyclic Carbene Catalysis. ChemCatChem 2012, 4, 937–941. [Google Scholar]
- (19).Grossmann A; Enders D N-Heterocyclic Carbene Catalyzed Domino Reactions. Angew. Chem. Int. Ed. 2012, 51, 314–325. [DOI] [PubMed] [Google Scholar]
- (20).Cohen DT; Scheidt KA Cooperative Lewis Acid/N-Heterocyclic Carbene Catalysis. Chem. Sci 2012, 3, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bugaut X; Glorius F Organocatalytic Umpolung: N-Heterocyclic Carbenes and Beyond. Chem. Soc. Rev. 2012, 41, 3511–3522. [DOI] [PubMed] [Google Scholar]
- (22).Nair V; Menon RS; Biju AT; Sinu CR; Paul RR; Jose A; Sreekumar V Employing Homoenolates Generated by NHC Catalysis in Carbon-Carbon Bond-Forming Reactions: State of the Art. Chem. Soc. Rev. 2011, 40, 5336–5346. [DOI] [PubMed] [Google Scholar]
- (23).Fortman GC; Nolan SP N-Heterocyclic Carbene (NHC) Ligands and Palladium in Homogeneous Cross-Coupling Catalysis: A Perfect Union. Chem. Soc. Rev. 2011, 40, 5151–5169. [DOI] [PubMed] [Google Scholar]
- (24).Melaimi M; Soleilhavoup M; Bertrand G Stable Cyclic Carbenes and Related Species Beyond Diaminocarbenes. Angew. Chem. Int. Ed. 2010, 49, 8810–8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Schuster O; Yang LR; Raubenheimer HG; Albrecht M Beyond Conventional N-Heterocyclic Carbenes: Abnormal, Remote, and Other Classes of NHC Ligands with Reduced Heteroatom Stabilization. Chem. Rev. 2009, 109, 3445–3478. [DOI] [PubMed] [Google Scholar]
- (26).Diez-Gonzalez S; Marion N; Nolan SP N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [DOI] [PubMed] [Google Scholar]
- (27).Corberan R; Mas-Marza E; Peris E Mono-, Bi- and Tridentate N-Heterocyclic Carbene Ligands for the Preparation of Transition-Metal-Based Homogeneous Catalysts. Eur. J. Inorg. Chem. 2009, 1700–1716. [Google Scholar]
- (28).Nair V; Vellalath S; Babu BP Recent Advances in Carbon-Carbon Bond-Forming Reactions Involving Homoenolates Generated by NHC Catalysis. Chem. Soc. Rev. 2008, 37, 2691–2698. [DOI] [PubMed] [Google Scholar]
- (29).Mata JA; Poyatos M; Peris E Structural and Catalytic Properties of Chelating Bis- and Tris-N-Heterocyclic Carbenes. Coord. Chem. Rev. 2007, 251, 841–859. [Google Scholar]
- (30).Marion N; Díez-González S; Nolan SP N-Heterocyclic Carbenes as Organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2988–3000. [DOI] [PubMed] [Google Scholar]
- (31).Kuhl O The Chemistry of Functionalised N-Heterocyclic Carbenes. Chem. Soc. Rev. 2007, 36, 592–607. [DOI] [PubMed] [Google Scholar]
- (32).Enders D; Niemeier O; Henseler A Organocatalysis by N-Heterocyclic Carbenes. Chem. Rev. 2007, 107, 5606–5655. [DOI] [PubMed] [Google Scholar]
- (33).Arnold PL; Pearson S Abnormal N-Heterocyclic Carbenes. Coord. Chem. Rev. 2007, 251, 596–609. [Google Scholar]
- (34).Scott NM; Nolan SP Stabilization of Organometallic Species Achieved by the Use of N-Heterocyclic Carbene (NHC) Ligands. Eur. J. Inorg. Chem. 2005, 1815–1828. [Google Scholar]
- (35).Cavallo L; Correa A; Costabile C; Jacobsen H Steric and Electronic Effects in the Bonding of N-Heterocyclic Ligands to Transition Metals. J. Organomet. Chem. 2005, 690, 5407–5413. [Google Scholar]
- (36).Peris E; Crabtree RH Recent Homogeneous Catalytic Applications of Chelate and Pincer N-Heterocyclic Carbenes. Coord. Chem. Rev. 2004, 248, 2239–2246. [Google Scholar]
- (37).Crudden CM; Allen DP Stability and Reactivity of N-Heterocyclic Carbene Complexes. Coord. Chem. Rev. 2004, 248, 2247–2273. [Google Scholar]
- (38).Cesar V; Bellemin-Laponnaz S; Gade LH Chiral N-Heterocyclic Carbenes as Stereodirecting Ligands in Asymmetric Catalysis. Chem. Soc. Rev. 2004, 33, 619–636. [DOI] [PubMed] [Google Scholar]
- (39).Cavell KJ; McGuinness DS Redox Processes Involving Hydrocarbylmetal (N-Heterocyclic Carbene) Complexes and Associated Imidazolium Salts: Ramifications for Catalysis. Coord. Chem. Rev. 2004, 248, 671–681. [Google Scholar]
- (40).Herrmann WA N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [DOI] [PubMed] [Google Scholar]
- (41).Huynh HV Electronic Properties of N-Heterocyclic Carbenes and Their Experimental Determination. Chem. Rev. 2018, 118, 9457–9492. [DOI] [PubMed] [Google Scholar]
- (42).Nelson DJ; Nolan SP Quantifying and Understanding the Electronic Properties of N-Heterocyclic Carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [DOI] [PubMed] [Google Scholar]
- (43).Vummaleti SVC; Nelson DJ; Poater A; Gomez-Suarez A; Cordes DB; Slawin AMZ; Nolan SP; Cavallo L What Can NMR Spectroscopy of Selenoureas and Phosphinidenes Teach Us about the π-Accepting Abilities of N-Heterocyclic Carbenes? Chem. Sci 2015, 6, 1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Meng G; Kakalis L; Nolan SP; Szostak M A Simple 1H NMR Method for Determining the σ-Donor Properties of N-Heterocyclic Carbenes. Tetrahedron Lett. 2019, 60, 378–381. [Google Scholar]
- (45).Liske A; Verlinden K; Buhl H; Schaper K; Ganter C Determining the π-Acceptor Properties of N-Heterocyclic Carbenes by Measuring the 77Se NMR Chemical Shifts of Their Selenium Adducts. Organometallics 2013, 32, 5269–5272. [Google Scholar]
- (46).Comas-Vives A; Harvey JN How Important Is Backbonding in Metal Complexes Containing N-Heterocyclic Carbenes? Structural and Nbo Analysis. Eur. J. Inorg. Chem. 2011, 5025–5035. [Google Scholar]
- (47).Wolf S; Plenio H Synthesis of (NHC)Rh(cod)Cl and (NHC)RhCl(CO)2 Complexes–Translation of the Rh-into the Ir-Scale for the Electronic Properties of NHC Ligands. J. Organomet. Chem. 2009, 694, 1487–1492. [Google Scholar]
- (48).Rosen EL; Varnado CD; Tennyson AG; Khramov DM; Kamplain JW; Sung DH; Cresswell PT; Lynch VM; Bielawski CW Redox-Active N-Heterocyclic Carbenes: Design, Synthesis, and Evaluation of Their Electronic Properties. Organometallics 2009, 28, 6695–6706. [Google Scholar]
- (49).Jacobsen H; Correa A; Poater A; Costabile C; Cavallo L Understanding the M (NHC)(NHC= N-Heterocyclic Carbene) Bond. Coord. Chem. Rev. 2009, 253, 687–703. [Google Scholar]
- (50).Gusev DG Donor Properties of a Series of Two-Electron Ligands. Organometallics 2009, 28, 763–770. [Google Scholar]
- (51).Kelly RA III; Clavier H; Giudice S; Scott NM; Stevens ED; Bordner J; Samardjiev I; Hoff CD; Cavallo L; Nolan SP Determination of N-Heterocyclic Carbene (NHC) Steric and Electronic Parameters Using the [(NHC)Ir(CO)2Cl] System. Organometallics 2007, 27, 202–210. [Google Scholar]
- (52).Diez-Gonzalez S; Nolan SP Stereoelectronic Parameters Associated with N-Heterocyclic Carbene (NHC) Ligands: A Quest for Understanding. Coord. Chem. Rev. 2007, 251, 874–883. [Google Scholar]
- (53).Gómez-Suárez A; Nelson DJ; Nolan SP Quantifying and Understanding the Steric Properties of N-Heterocyclic Carbenes. Chem. Commun. 2017, 53, 2650–2660. [DOI] [PubMed] [Google Scholar]
- (54).Marion N; Nolan SP Well-Defined N-Heterocyclic Carbenes-Palladium (II) Precatalysts for Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [DOI] [PubMed] [Google Scholar]
- (55).Shi S; Nolan SP; Szostak M Well-Defined Palladium(II)-NHC (NHC = N-Heterocyclic Carbene) Precatalysts for Cross- Coupling Reactions of Amides and Esters by Selective Acyl CO–X (X = N, O) Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. [DOI] [PubMed] [Google Scholar]
- (56).Clavier H; Nolan SP Percent Buried Volume for Phosphine and N-Heterocyclic Carbene Ligands: Steric Properties in Organometallic Chemistry. Chem. Commun. 2010, 46, 841–861. [DOI] [PubMed] [Google Scholar]
- (57).Kühl O Sterically Induced Differences in N-Heterocyclic Carbene Transition Metal Complexes. Coord. Chem. Rev. 2009, 253, 2481–2492. [Google Scholar]
- (58).Wuertz S; Glorius F Surveying Sterically Demanding N-Heterocyclic Carbene Ligands with Restricted Flexibility for Palladium-Catalyzed Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1523–1533. [DOI] [PubMed] [Google Scholar]
- (59).Díez-González S; Nolan SP Stereoelectronic Parameters Associated with N-Heterocyclic Carbene (NHC) Ligands: A Quest for Understanding. Coord. Chem. Rev. 2007, 251, 874–883. [Google Scholar]
- (60).Yu JQ Science of Synthesis: Catalytic Transformations via C−H Activation; 1st Ed, Thieme: Stuttgart, 2015. [Google Scholar]
- (61).Gensch T; Hopkinson M; Glorius F; Wencel-Delord J Mild Metal-Catalyzed C–H Activation: Examples and Concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. [DOI] [PubMed] [Google Scholar]
- (62).Miao J; Ge H Recent Advances in First-Row-Transition-Metal-Catalyzed Dehydrogenative Coupling of C(sp3)–H Bonds. Eur. J. Org. Chem. 2015, 2015, 7859–7868. [Google Scholar]
- (63).Daugulis O; Roane J; Tran LD Bidentate, Monoanionic Auxiliary-Directed Functionalization of Carbon–Hydrogen Bonds. Acc. Chem. Res. 2015, 48, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Chen Z; Wang B; Zhang J; Yu W; Liu Z; Zhang Y Transition Metal-Catalyzed C–H Bond Functionalizations by the Use of Diverse Directing Groups. Org. Chem. Front. 2015, 2, 1107–1295. [Google Scholar]
- (65).Davies HML; Morton D Recent Advances in C–H Functionalization. J. Org. Chem. 2016, 81, 343–350. [DOI] [PubMed] [Google Scholar]
- (66).Rouquet G; Chatani N Catalytic Functionalization of C(sp2)-H and C(sp3)-H Bonds by Using Bidentate Directing Groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [DOI] [PubMed] [Google Scholar]
- (67).Rossi R; Bellina F; Lessi M; Manzini C Cross-Coupling of Heteroarenes by C−H Functionalization: Recent Progress Towards Direct Arylation and Heteroarylation Reactions Involving Heteroarenes Containing One Heteroatom. Adv. Synth. Catal. 2014, 356, 17–117. [Google Scholar]
- (68).Giri R; Thapa S; Kafle A Pd-Catalysed, Directed C-H Coupling with Organometallics. Adv. Synth. Catal. 2014, 365, 1395–1411. [Google Scholar]
- (69).Wencel-Delord J; Glorius F C–H Bond Activation Enables the Rapid Construction and Late-Stage Diversification of Functional Molecules. Nat. Chem. 2013, 5, 369. [DOI] [PubMed] [Google Scholar]
- (70).Yamaguchi J; Yamaguchi AD; Itami K C−H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2012, 51, 8960–9009. [DOI] [PubMed] [Google Scholar]
- (71).Arockiam PB; Bruneau C; Dixneuf PH Ruthenium (II)-Catalyzed C–H Bond Activation and Functionalization. Chem. Rev. 2012, 112, 5879–5918. [DOI] [PubMed] [Google Scholar]
- (72).Yeung CS; Dong VM Catalytic Dehydrogenative Cross-Coupling: Forming Carbon−Carbon Bonds by Oxidizing Two Carbon−Hydrogen Bonds. Chem. Rev. 2011, 111, 1215–1292. [DOI] [PubMed] [Google Scholar]
- (73).Wencel-Delord J; Droege T; Liu F; Glorius F Towards Mild Metal-Catalyzed C–H Bond Activation. Chem. Soc. Rev. 2011, 40, 4740–4761. [DOI] [PubMed] [Google Scholar]
- (74).McMurray L; O’Hara F; Gaunt MJ Recent Developments in Natural Product Synthesis Using Metal-Catalysed C–H Bond Functionalisation. Chem. Soc. Rev. 2011, 40, 1885–1898. [DOI] [PubMed] [Google Scholar]
- (75).Engle KM; Mei T-S; Wasa M; Yu J-Q Weak Coordination as a Powerful Means for Developing Broadly Useful C–H Functionalization Reactions. Acc. Chem. Res. 2011, 45, 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Yu J-Q, Shi ZJ Topics in Current Chemistry: C−H Activation; 1st Ed, Springer: Berlin, 2010. [Google Scholar]
- (77).Lyons TW; Sanford MS Palladium-Catalyzed Ligand-Directed C−H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Kulkarni AA; Daugulis O Direct Conversion of Carbon-Hydrogen into Carbon-Carbon Bonds by First-Row Transition-Metal Catalysis. Synthesis 2009, 2009, 4087–4109. [Google Scholar]
- (79).Giri R; Shi B-F; Engle KM; Maugel N; Yu J-Q Transition Metal-Catalyzed C–H Activation Reactions: Diastereoselectivity and Enantioselectivity. Chem. Soc. Rev. 2009, 38, 3242–3272. [DOI] [PubMed] [Google Scholar]
- (80).Colby DA; Bergman RG; Ellman JA Rhodium-Catalyzed C−C Bond Formation Via Heteroatom-Directed C−H Bond Activation. Chem. Rev. 2009, 110, 624–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Chen X; Engle KM; Wang DH; Yu JQ Palladium (II)-Catalyzed C−H Activation/C−C Cross-Coupling Reactions: Versatility and Practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Ackermann L; Vicente R; Kapdi AR Transition-Metal-Catalyzed Direct Arylation of (Hetero) Arenes by C−H Bond Cleavage. Angew. Chem. Int. Ed. 2009, 48, 9792–9826. [DOI] [PubMed] [Google Scholar]
- (83).Alberico D; Scott ME; Lautens M Aryl−Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev. 2007, 107, 174–238. [DOI] [PubMed] [Google Scholar]
- (84).Shilov AE; Shul’pin GB Activation of C−H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. [DOI] [PubMed] [Google Scholar]
- (85).Sambiagio C; Schönbauer D; Blieck R; Dao-Huy T; Pototschnig G; Schaaf P; Wiesinger T; Farooq Zia M; Wencel-Delord J; Besset T et al. A Comprehensive Overview of Directing Groups Applied in Metal-Catalysed C–H Functionalisation Chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Gandeepan P; Müller T; Zell D; Cera G; Warratz S; Ackermann L 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452. [DOI] [PubMed] [Google Scholar]
- (87).Herrmann WA; Elison M; Fischer J; Köcher C; Artus GR Metal Complexes of N-Heterocyclic Carbenes—a New Structural Principle for Catalysts in Homogeneous Catalysis. Angew. Chem. Int. Ed. 1995, 34, 2371–2374. [Google Scholar]
- (88).Gürbüz N; Özdemir I; Çetinkaya B Selective Palladium-Catalyzed Arylation(s) of Benzaldehyde Derivatives by N-Heterocarbene Ligands. Tetrahedron Lett. 2005, 46, 2273–2277. [Google Scholar]
- (89).Özdemir I; Demir S; Çetinkaya B Use of Tetrahydropyrimidinium Salts for Highly Efficient Palladium-Catalyzed Cross-Coupling Reactions of Aryl Bromides and Chlorides. Tetrahedron 2005, 61, 9791–9798. [Google Scholar]
- (90).Dogan O; Gurbuz N; Özdemir I; Çetinkaya B Palladium N-Heterocyclic-Carbene-Catalyzed ortho-Arylation of Benzaldehyde Derivatives. Heteroat. Chem. 2008, 19, 569–574. [Google Scholar]
- (91).Dogan O; Gurbuz N; Özdemir I; Çetinkaya B New Functionalized N-Heterocyclic Carbene Ligands for Arylation of Benzaldehydes. J. Heterocycl. Chem. 2009, 46, 186–190. [Google Scholar]
- (92).Campeau L-C; Thansandote P; Fagnou K High-Yielding Intramolecular Direct Arylation Reactions with Aryl Chlorides. Org. Lett. 2005, 7, 1857–1860. [DOI] [PubMed] [Google Scholar]
- (93).Lafrance M; Lapointe D; Fagnou K Mild and Efficient Palladium-Catalyzed Intramolecular Direct Arylation Reactions. Tetrahedron 2008, 64, 6015–6020. [Google Scholar]
- (94).Coy ED; Cuca LE; Sefkow M Pd–NHC Catalyzed Biaryl Coupling by Direct C–H Activation—A Novel Strategy for the Synthesis of Dibenzocyclooctane Lignans. Synth. Commun. 2010, 41, 41–51. [Google Scholar]
- (95).Lane BS; Brown MA; Sames D Direct Palladium-Catalyzed C-2 and C-3 Arylation of Indoles: a Mechanistic Rationale for Regioselectivity. J. Am. Chem. Soc. 2005, 127, 8050–8057. [DOI] [PubMed] [Google Scholar]
- (96).Toure BB; Lane BS; Sames D Catalytic C-H Arylation of SEM-Protected Azoles with Palladium Complexes of NHCs and Phosphines. Org. Lett. 2006, 8, 1979–1982. [DOI] [PubMed] [Google Scholar]
- (97).Deprez NR; Kalyani D; Krause A; Sanford MS Room Temperature Palladium-Catalyzed 2-Arylation of Indoles. J. Am. Chem. Soc. 2006, 128, 4972–4973. [DOI] [PubMed] [Google Scholar]
- (98).Özdemir I; Gok Y; Ozeroglu O; Kaloglu M; Doucet H; Bruneau C N-Heterocyclic Carbenes: Useful Ligands for the Palladium-Catalysed Direct C5 Arylation of Heteroaromatics with Aryl Bromides or Electron-Deficient Aryl Chlorides. Eur. J. Inorg. Chem. 2010, 2010, 1798–1805. [Google Scholar]
- (99).Akkoc S; Gok Y; Akkurt M; Tahir MN Catalytic Activities in the Direct C5 Arylation of Novel Palladium N-Heterocyclic Carbene Complexes Containing Benzimidazol-2-Ylidene Nucleus. Inorg. Chim. Acta. 2014, 413, 221–230. [Google Scholar]
- (100).Akkoç S; Gök Y Catalytic Activities in Direct Arylation of Novel Palladium N-Heterocyclic Carbene Complexes. Appl. Organomet. Chem. 2014, 28, 854–860. [Google Scholar]
- (101).Karaca EO; Gurbuz N; Özdemir I; Doucet H; Sahin O; Buyukgungor O; Çetinkaya B Palladium Complexes with Tetrahydropyrimidin-2-Ylidene Ligands: Catalytic Activity for the Direct Arylation of Furan, Thiophene, and Thiazole Derivatives. Organometallics 2015, 34, 2487–2493. [Google Scholar]
- (102).Akkoç S; Gök Y; İlhan İÖ; Kayser V N-Methylphthalimide-Substituted Benzimidazolium Salts and PEPPSI Pd–NHC Complexes: Synthesis, Characterization and Catalytic Activity in Carbon–Carbon Bond-Forming Reactions. Beilstein J. Org. Chem. 2016, 12, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Ghosh D; Lee HM Efficient Pd-Catalyzed Direct Arylations of Heterocycles with Unreactive and Hindered Aryl Chlorides. Org. Lett. 2012, 14, 5534–5537. [DOI] [PubMed] [Google Scholar]
- (104).Martin AR; Chartoire A; Slawin AMZ; Nolan SP Extending the Utility of Pd(NHC)(cinnamyl)Cl Precatalysts: Direct Arylation of Heterocycles. Beilstein J. Org. Chem. 2012, 8, 1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Mariconda A; Grisi F; Costabile C; Falcone S; Bertolasi V; Longo P Synthesis, Characterization and Catalytic Behaviour of a Palladium Complex Bearing a Hydroxy-Functionalized N-Heterocyclic Carbene Ligand. New J. Chem. 2014, 38, 762–769. [Google Scholar]
- (106).Yin SC; Zhou Q; Zhao XY; Shao LX N-Heterocyclic Carbene-Palladium(II)-1-Methylimidazole Complex Catalyzed Direct C-H Bond Arylation of Benzo[b]Furans with Aryl Chlorides. J. Org. Chem. 2015, 80, 8916–8921. [DOI] [PubMed] [Google Scholar]
- (107).Yin SC; Zhou Q; He QW; Li SW; Qian PC; Shao LX N-Heterocyclic Carbene-Pd(II)-1-Methylimidazole Complex Catalyzed C-H Bond Arylation of (Benzo)Thiophenes with Aryl Chlorides. Tetrahedron 2017, 73, 427–431. [Google Scholar]
- (108).Özdemir I; Gürbüz N; Kaloğlu N; Doğan Ö; Kaloğlu M; Bruneau C; Doucet H N-Heterocyclic Carbene–Palladium Catalysts for the Direct Arylation of Pyrrole Derivatives with Aryl Chlorides. Beilstein J. Org. Chem. 2013, 9, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Bernhammer JC; Singh H; Huynh HV Amine-Functionalized Indazolin-3-Ylidene Complexes of Palladium (II) by Postmodification of a Single Precursor. Organometallics 2014, 33, 4295–4301. [Google Scholar]
- (110).Bernhammer JC; Huynh HV Benzimidazolin-2-Ylidene Complexes of Palladium (II) Featuring a Thioether Moiety: Synthesis, Characterization, Molecular Dynamics, and Catalytic Activities. Organometallics 2014, 33, 1266–1275. [Google Scholar]
- (111).Yiğit B; Gürbüz N; Yiğit M; Dağdeviren Z; Özdemir İ Palladium (II) N-Heterocyclic Carbene Complexes as Catalysts for the Direct Arylation of Pyrrole Derivatives with Aryl Chlorides. Inorg. Chim. Acta. 2017, 465, 44–49. [Google Scholar]
- (112).Kaloglu N; Kaloglu M; Tahir MN; Arici C; Bruneau C; Doucet H; Dixneuf PH; Çetinkaya B; Özdemir I Synthesis of N-Heterocyclic Carbene-Palladium-PEPPSI Complexes and Their Catalytic Activity in the Direct C-H Bond Activation. J. Organomet. Chem. 2018, 867, 404–412. [Google Scholar]
- (113).Kaloglu M; Kaloglu N; Özdemir I Direct C-H Bond Arylation of C2-Blocked Pyrrole with Aryl Halides Using Palladium(II)-N-Heterocyclic Carbene Catalysts. ChemistrySelect 2018, 3, 5600–5607. [Google Scholar]
- (114).Joo JM; Toure BB; Sames D C-H Bonds as Ubiquitous Functionality: a General Approach To Complex Arylated Imidazoles via Regioselective Sequential Arylation of All Three C-H Bonds and Regioselective N-Alkylation Enabled by SEM-Group Transposition. J. Org. Chem. 2010, 75, 4911–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Dogan O; Gurbuz N; Özdemir I; Çetinkaya B; Sahin O; Buyukgungor O Synthesis, Characterization and Catalytic Activity of Novel N-Heterocyclic Carbene-Palladium Complexes. Dalton Trans. 2009, 7087–7093. [DOI] [PubMed] [Google Scholar]
- (116).Arslan H; Özdemir I; Vanderveer D; Demir S; Çetinkaya B Synthesis and Characterization of N-Heterocyclic Carbene Palladium Complex and Its Application on Direct Arylation of Benzoxazoles and Benzothiazoles with Aryl Bromides. J. Coord. Chem. 2009, 62, 2591–2599. [Google Scholar]
- (117).Özdemir I; Arslan H; Demir S; Vanderveer D; Çetinkaya B Synthesis, Characterization and Catalytic Properties of Cis-Dibromo{1,1’-Di[3,4,5-Trimethoxybenzyl]-3,3’-Butylenedibenzimidazol-2, 2’-Diylidene}Palladium (II). Inorg. Chem. Commun. 2011, 14, 672–675. [Google Scholar]
- (118).Demir S; Özdemir I; Arslan H; Vanderveer D Butylene Linked Palladium N-Heterocyclic Carbene Complexes: Synthesis and Catalytic Properties. J. Organomet. Chem. 2011, 696, 2589–2593. [Google Scholar]
- (119).Kaloglu M; Kaloglur N; Özdemir I Direct CH Bond Activation of Benzoxazole and Benzothiazole with Aryl Bromides Catalyzed by Palladium(II)-N-Heterocyclic Carbene Complexes. Chin. J. Chem. 2018, 36, 837–844. [Google Scholar]
- (120).Kaloglu M; Özdemir I Palladium(II)-N-Heterocyclic Carbene-Catalyzed Direct C2-or C5-Arylation of Thiazoles with Aryl Bromides. Tetrahedron 2018, 74, 2837–2845. [Google Scholar]
- (121).Verrier C; Fiol-Petit C; Hoarau C; Marsais F DPO and POPOP Carboxylate-Analog Sensors by Sequential Palladium-Catalysed Direct Arylation of Oxazole-4-Carboxylates. Org. Biomol. Chem. 2011, 9, 6215–6218. [DOI] [PubMed] [Google Scholar]
- (122).Shen XB; Zhang Y; Chen WX; Xiao ZK; Hu TT; Shao LX Direct C-H Bond Arylation of (Benzo)Oxazoles with Aryl Chlorides Catalyzed by N-Heterocyclic Carbene-Palladium(II)-1-Methylimidazole Complex. Org. Lett. 2014, 16, 1984–1987. [DOI] [PubMed] [Google Scholar]
- (123).Ji YY; Zhang Y; Hu YY; Shao LX N-Heterocyclic Carbene-Pd(II)-1-Methylimidazole Complex Catalyzed C-H Bond Benzylation of (Benzo)Oxazoles with Benzyl Chlorides. Tetrahedron 2015, 71, 6818–6823. [Google Scholar]
- (124).Yang L; Yuan J; Mao P; Guo Q Chelating Palladium Complexes Containing Pyridine/Pyrimidine Hydroxyalkyl Di-Functionalized N-Heterocyclic Carbenes: Synthesis, Structure, and Catalytic Activity towards C–H Activation. RSC Adv. 2015, 5, 107601–107607. [Google Scholar]
- (125).Karthik S; Gandhi T Dibenzofuran and Dibenzothiophene Based Palladium(II)/NHC Catalysts - Synthesis and Applications in C-C Bond Formation. New J. Chem. 2018, 42, 15811–15819. [Google Scholar]
- (126).Li Y; Yu X; Wang Y; Fu H; Zheng X; Chen H; Li R Unsymmetrical Pincer N-Heterocyclic Carbene–Nitrogen–Phosphine Chelated Palladium (II) Complexes: Synthesis, Structure, and Reactivity in Direct C(sp2)–H Arylation of Benzoxazoles. Organometallics 2018, 37, 979–988. [Google Scholar]
- (127).Chen W; Yang J Synthesis of N-Heterocyclic Carbene-PdCl-[(2-Pyridyl) Alkyl Carboxylate] Complexes and Their Catalytic Activities towards Arylation of (Benzo) Oxazoles with Aryl Bromides. J. Organomet. Chem. 2018, 872, 24–30. [Google Scholar]
- (128).Yang J (N-Heterocyclic Carbene)PdCl(N-Heterocyclic Carboxylate) Complexes: Synthesis and Catalytic Activities towards Arylation of Benzoxazoles with Aryl Halides. Appl. Organomet. Chem. 2018, 32, 4394. [Google Scholar]
- (129).Yang J Mixed N-Heterocycles/N-Heterocyclic Carbene Palladium (II) Allyl Complexes as Precatalysts for Direct Arylation of Azoles with Aryl Bromides. Tetrahedron 2019, 75, 2182–2187. [Google Scholar]
- (130).Kumar PV; Lin WS; Shen JS; Nandi D; Lee HM Direct C5-Arylation Reaction Between Imidazoles and Aryl Chlorides Catalyzed by Palladium Complexes with Phosphines and N-Heterocyclic Carbenes. Organometallics 2011, 30, 5160–5169. [Google Scholar]
- (131).Lee JY; Shen JS; Tzeng RJ; Lu IC; Lii JH; Hu CH; Lee HM Well-Defined Palladium(0) Complexes Bearing N-Heterocyclic Carbene and Phosphine Moieties: Efficient Catalytic Applications in the Mizoroki-Heck Reaction and Direct C-H Functionalization. Dalton Trans. 2016, 45, 10375–10388. [DOI] [PubMed] [Google Scholar]
- (132).Gu ZS; Chen WX; Shao LX N-Heterocyclic Carbene-Palladium(II)-1-Methylimidazole Complex-Catalyzed Direct C–H Bond Arylation of (Benz)imidazoles with Aryl Chlorides. J. Org. Chem. 2014, 79, 5806–5811. [DOI] [PubMed] [Google Scholar]
- (133).Liu QX; He BY; Qian PC; Shao LX N-Heterocyclic Carbene-Palladium(II)-1-Methylimidazole Complex Catalyzed Direct C-H Bond Arylation of Imidazo 1,2-A Pyridines with Aryl Chlorides. Org. Biomol. Chem. 2017, 15, 1151–1154. [DOI] [PubMed] [Google Scholar]
- (134).Guo S; Huynh HV Dinuclear Triazole-Derived Janus-Type N-Heterocyclic Carbene Complexes of Palladium: Syntheses, Isomerizations, and Catalytic Studies toward Direct C5-Arylation of Imidazoles. Organometallics 2014, 33, 2004–2011. [Google Scholar]
- (135).He XX; Li YW; Ma BB; Ke ZF; Liu FS Sterically Encumbered Tetraarylimidazolium Carbene Pd-PEPPSI Complexes: Highly Efficient Direct Arylation of Imidazoles with Aryl Bromides under Aerobic Conditions. Organometallics 2016, 35, 2655–2663. [Google Scholar]
- (136).Hu LQ; Deng RL; Li YF; Zeng CJ; Shen DS; Liu FS Developing Bis(imino)Acenaphthene-Supported N-Heterocyclic Carbene Palladium Precatalysts for Direct Arylation of Azoles. Organometallics 2018, 37, 214–226. [Google Scholar]
- (137).Nandi D; Jhou YM; Lee JY; Kuo BC; Liu CY; Huang PW; Lee HM Pd(0)-Catalyzed Decarboxylative Coupling and Tandem C-H Arylation/Decarboxylation for the Synthesis of Heteroaromatic Biaryls. J. Org. Chem. 2012, 77, 9384–9390. [DOI] [PubMed] [Google Scholar]
- (138).Gomez-Herrera A; Nahra F; Wu JF; Izquierdo F; Brill M; Cazin CSJ; Nolan SP Synthesis of Di-Substituted Alkynes via Palladium-Catalyzed Decarboxylative Coupling and C-H Activation. ChemistrySelect 2019, 4, 5–9. [Google Scholar]
- (139).Yuan D; Huynh HV 1,2,3-Triazolin-5-Ylidenes: Synthesis of Hetero-Bis(Carbene) Pd(II) Complexes, Determination of Donor Strengths, and Catalysis. Organometallics 2012, 31, 405–412. [Google Scholar]
- (140).Bernhammer JC; Huynh HV Pyrazolin-5-Ylidene Palladium (II) Complexes: Synthesis, Characterization, and Application in the Direct Arylation of Pentafluorobenzene. Organometallics 2012, 31, 5121–5130. [Google Scholar]
- (141).Yuan D; Huynh HV Hetero-Dicarbene Complexes of Palladium (II): Syntheses and Catalytic Activities. Organometallics 2014, 33, 6033–6043. [Google Scholar]
- (142).Xiao B; Gong T-J; Liu Z-J; Liu J-H; Luo D-F; Xu J; Liu L Synthesis of Dibenzofurans via Palladium-Catalyzed Phenol-Directed C–H Activation/C–O Cyclization. J. Am. Chem. Soc. 2011, 133, 9250–9253. [DOI] [PubMed] [Google Scholar]
- (143).Dooley JD; Reddy Chidipudi S; Lam HW Catalyst-Controlled Divergent C–H Functionalization of Unsymmetrical 2-Aryl Cyclic 1,3-Dicarbonyl Compounds with Alkynes and Alkenes. J. Am. Chem. Soc. 2013, 135, 10829–10836. [DOI] [PubMed] [Google Scholar]
- (144).Luo J; Preciado S; Larrosa I Salicylic acids as readily available starting materials for the synthesis of meta-substituted biaryls. Chem. Commun. 2015, 51, 3127–3130. [DOI] [PubMed] [Google Scholar]
- (145).Luo J; Preciado S; Araromi SO; Larrosa I A Domino Oxidation/Arylation/Protodecarboxylation Reaction of Salicylaldehydes: Expanded Access to meta-Arylphenols. Chem. Asian J 2015, 11, 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Dherbassy Q; Wencel-Delord J; Colobert F Atroposelective Arylation of Biaryls by CH Activation. Tetrahedron 2018, 74, 6205–6212. [Google Scholar]
- (147).Arnold PL; Sanford MS; Pearson SM Chelating N-Heterocyclic Carbene Alkoxide as a Supporting Ligand for PdII/IV C−H Bond Functionalization Catalysis. J. Am. Chem. Soc. 2009, 131, 13912–13913. [DOI] [PubMed] [Google Scholar]
- (148).Tato F; GarcƖá -DomƖnguez AS; Ć árdenas DJ Palladium-Catalyzed Acetoxylation of Arenes by Novel Sulfinyl N-Heterocyclic Carbene Ligand Complexes. Organometallics 2013, 32, 7487–7494. [Google Scholar]
- (149).Desai SP; Mondal M; Choudhury J Chelating Bis-N-Heterocyclic Carbene-Palladium(II) Complexes for Oxidative Arene C-H Functionalization. Organometallics 2015, 34, 2731–2736. [Google Scholar]
- (150).Mondal M; Choudhury J Structure-Activity Comparison in Palladium-N-Heterocyclic Carbene (NHC) Catalyzed Arene C-H Activation-Functionalization. J. Mol. Catal. A: Chem. 2017, 426, 451–457. [Google Scholar]
- (151).Mondal M; Joji J; Choudhury J Designing a Heterogeneous Pd(II)-NHC-Based C-H Activation Catalyst On a Self-Supported Coordination Polymer Platform. Chem. Commun. 2017, 53, 3185–3188. [DOI] [PubMed] [Google Scholar]
- (152).Bolbat E; Wendt OF Ligand Control in Selective C-H Oxidative Functionalization Using Pd-PEPPSI-Type Complexes. Eur. J. Org. Chem. 2016, 2016, 3395–3400. [Google Scholar]
- (153).Majeed MH; Shayesteh P; Wallenberg LR; Persson AR; Johansson N; Ye L; Schnadt J; Wendt OF Polymer-Supported Palladium (II) Carbene Complexes: Catalytic Activity, Recyclability, and Selectivity in C−H Acetoxylation of Arenes. Chem. Eur. J. 2017, 23, 8457–8465. [DOI] [PubMed] [Google Scholar]
- (154).Majeed MH; Shayesteh P; Persson AR; Wallenberg LR; Schnadt J; Wendt OF A Pd(II)-Carbene Complex with Anthracene Side-Arms for π-Stacking on Reduced Graphene Oxide (RGO): Activity towards Undirected C–H Oxygenation of Arenes. Eur. J. Inorg. Chem. 2018, 2018, 4742–4746. [Google Scholar]
- (155).Yuan N; Majeed MH; Bajnóczi ÉG; Persson AR; Wallenberg LR; Inge AK; Heidenreich N; Stock N; Zou X; Wendt OF In Situ XAS Study of the Local Structure and Oxidation State Evolution of Palladium in a Reduced Graphene Oxide Supported Pd (II) Carbene Complex During an Undirected C–H Acetoxylation Reaction. Catal. Sci. Technol. 2019, 9, 2025–2031. [Google Scholar]
- (156).Champion MJ; Solanki R; Delaude L; White AJ; Wilton-Ely JD Synthesis and Catalytic Application of Palladium Imidazol(in)ium-2-Dithiocarboxylate Complexes. Dalton Trans. 2012, 41, 12386–12394. [DOI] [PubMed] [Google Scholar]
- (157).Flores-Gaspar A; Gutiérrez-Bonet Á; Martin R N-Heterocyclic Carbene Dichotomy in Pd-Catalyzed Acylation of Aryl Chlorides via C–H Bond Functionalization. Org. Lett. 2012, 14, 5234–5237. [DOI] [PubMed] [Google Scholar]
- (158).Lei P; Meng G; Szostak M General Method for the Suzuki-Miyaura Cross-Coupling of Amides Using Commercially Available, Air- and Moisture-Stable Palladium/NHC (NHC = N-Heterocyclic Carbene) Complexes. ACS Catal. 2017, 7, 1960–1965 [Google Scholar]
- (159).Lei P; Meng G; Shi S; Ling Y; An J; Szostak R; Szostak M Suzuki–Miyaura Cross-Coupling of Amides and Esters at Room Temperature: Correlation with Barriers to Rotation around C–N and C–O Bonds. Chem. Sci. 2017, 8, 6525–6530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Karthik S; Gandhi T Palladium(II)/N-Heterocyclic Carbene-Catalyzed Direct C-H Acylation of Heteroarenes with N-Acylsaccharins. Org. Lett. 2017, 19, 5486–5489. [DOI] [PubMed] [Google Scholar]
- (161).Muehlhofer M; Strassner T; Herrmann WA New Catalyst Systems for the Catalytic Conversion of Methane into Methanol. Angew. Chem. Int. Ed. 2002, 41, 1745–1747. [DOI] [PubMed] [Google Scholar]
- (162).Strassner T; Muehlhofer M; Zeller A; Herdtweck E; Herrmann WA The Counterion Influence on the CH-Activation of Methane by Palladium (II) Biscarbene Complexes–Structures, Reactivity and DFT Calculations. J. Organomet. Chem. 2004, 689, 1418–1424. [Google Scholar]
- (163).Ahrens S; Zeller A; Taige M; Strassner T Extension of the Alkane Bridge In BisNHC-Palladium-Chloride Complexes. Synthesis, Structure, and Catalytic Activity. Organometallics 2006, 25, 5409–5415. [Google Scholar]
- (164).Meyer D; Taige MA; Zeller A; Hohlfeld K; Ahrens S; Strassner T Palladium Complexes with Pyrimidine-Functionalized N-Heterocyclic Carbene Ligands: Synthesis, Structure and Catalytic Activity. Organometallics 2009, 28, 2142–2149. [Google Scholar]
- (165).Munz D; Meyer D; Strassner T Methane CH Activation by Palladium Complexes with Chelating Bis(NHC) Ligands: a DFT Study. Organometallics 2013, 32, 3469–3480. [Google Scholar]
- (166).Munz D; Strassner T On the Mechanism of the Palladium Bis(NHC) Complex Catalyzed CH Functionalization of Propane: Experiment and DFT Calculations. Chem. Eur. J. 2014, 20, 14872–14879. [DOI] [PubMed] [Google Scholar]
- (167).Munz D; Strassner T Catalytic Hydrocarbon Oxidation by Palladium-Bis-NHC-Complexes. Top. Catal. 2014, 57, 1372–1376. [Google Scholar]
- (168).Munz D; Strassner T Alkane C-H Functionalization and Oxidation with Molecular Oxygen. Inorg. Chem. 2015, 54, 5043–5052. [DOI] [PubMed] [Google Scholar]
- (169).Meyer D; Strassner T CH-Activation of Methane-Synthesis of an Intermediate? J. Organomet. Chem. 2015, 784, 84–87. [Google Scholar]
- (170).Ma D; Zhang C Propane CH Activation by Palladium Complexes Bearing Ligands with Charge-Shift Bonding Characteristics: a DFT Study. Comput. Theor. Chem. 2017, 1115, 30–36. [Google Scholar]
- (171).Hsiao CC; Lin YK; Liu CJ; Wu TC; Wu YT Synthesis of Methylene-Bridge Polyarenes through Palladium-Catalyzed Activation of Benzylic Carbon-Hydrogen Bond. Adv. Synth. Catal. 2010, 352, 3267–3274. [Google Scholar]
- (172).Nakanishi M; Katayev D; Besnard C; Kündig EP Fused Indolines by Palladium-Catalyzed Asymmetric C-C Coupling Involving an Unactivated Methylene Group. Angew. Chem. Int. Ed. 2011, 50, 7438–7441. [DOI] [PubMed] [Google Scholar]
- (173).Katayev D; Nakanishi M; Burgi T; Kündig EP Asymmetric C(sp3)-H/C(Ar) Coupling Reactions. Highly Enantio-Enriched Indolines via Regiodivergent Reaction of a Racemic Mixture. Chem. Sci. 2012, 3, 1422–1425. [Google Scholar]
- (174).Nakanishi M; Katayev D; Besnard C; Kündig EP Palladium-NHC Catalyzed Enantioselective Synthesis of Fused Indolines via Inert C(sp3)-H Activation. Chimia 2012, 66, 241–243. [DOI] [PubMed] [Google Scholar]
- (175).Larionov E; Nakanishi M; Katayev D; Besnard C; Kündig EP Scope and Mechanism of Asymmetric C(sp3)-H/C(Ar)-X Coupling Reactions: Computational and Experimental Study. Chem. Sci. 2013, 4, 1995–2005. [Google Scholar]
- (176).Katayev D; Larionov E; Nakanishi M; Besnard C; Kündig EP Palladium–N-Heterocyclic Carbene (NHC)-Catalyzed Asymmetric Synthesis of Indolines through Regiodivergent C(sp3)-H Activation: Scope and DFT Study. Chem. Eur. J. 2014, 20, 15021–15030. [DOI] [PubMed] [Google Scholar]
- (177).Melot R; Craveiro MV; Bürgi T; Baudoin O Divergent Enantioselective Synthesis of (nor) Illudalane Sesquiterpenes via Pd0-Catalyzed Asymmetric C (sp3)–H Activation. Org. Lett. 2019, 21, 812–815. [DOI] [PubMed] [Google Scholar]
- (178).Pedroni J; Cramer N 2-(Trifluoromethyl)Indoles via Pd(0)-Catalyzed C(sp3)-H Functionalization of Trifluoroacetimidoyl Chlorides. Org. Lett. 2016, 18, 1932–1935. [DOI] [PubMed] [Google Scholar]
- (179).Du W; Gu Q; Li Z; Yang D Palladium (II)-Catalyzed Intramolecular Tandem Aminoalkylation via Divergent C(sp3)–H Functionalization. J. Am. Chem. Soc. 2015, 137, 1130–1135. [DOI] [PubMed] [Google Scholar]
- (180).Ji YY; Lu LL; Shi YC; Shao LX Direct C−H Bond Arylation of Fluorenes with Aryl Chlorides Catalyzed by N-Heterocyclic Carbene-Palladium(II)-1-Methylimidazole Complex and Further Transformation of the Products in a One-Pot Procedure. Org. Biomol. Chem. 2014, 12, 8488–8498. [DOI] [PubMed] [Google Scholar]
- (181).He J; Wasa M; Chan KS; Yu J-Q Palladium(0)-Catalyzed Alkynylation of C(sp3)–H Bonds. J. Am. Chem. Soc. 2013, 135, 3387–3390. [DOI] [PubMed] [Google Scholar]
- (182).Ye S; Yang W; Coon T; Fanning D; Neubert T; Stamos D; Yu JQ N-Heterocyclic Carbene Ligand-Enabled C(sp3)−H Arylation of Piperidine and Tetrahydropyran Derivatives. Chem. Eur. J. 2016, 22, 4748–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Hou X-F; Wang Y-N; Göttker-Schnetmann I N-Heterocyclic Carbene Palladium Catalyzed Regioselective Oxidative Trifluoroacetoxylation of Unactivated Methylene sp3 C–H Bonds in Linear Alkyl Esters. Organometallics 2011, 30, 6053–6056. [Google Scholar]
- (184).Mccall AS; Wang H; Desper JM; Kraft S Bis-N-Heterocyclic Carbene Palladium (IV) Tetrachloride Complexes: Synthesis, Reactivity, and Mechanisms of Direct Chlorinations and Oxidations of Organic Substrates. J. Am. Chem. Soc. 2011, 133, 1832–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Hu R-B; Wang C-H; Ren W; Liu Z; Yang S-D Direct Allylic C–H Bond Activation to Synthesize [Pd(η3-cin)(IPr)Cl] Complex: Application in the Allylation of Oxindoles. ACS Catal. 2017, 7, 7400–7404. [Google Scholar]
- (186).Lee JH; Yoo KS; Park CP; Olsen JM; Sakaguchi S; Prakash GKS; Mathew T; Jung KW An Air/Water-Stable Tridentate N-Heterocyclic Carbene-Palladium(II) Complex: Catalytic C-H Activation of Hydrocarbons via Hydrogen/Deuterium Exchange Process in Deuterium Oxide. Adv. Synth. Catal. 2009, 351, 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Giles R; Ahn G; Jung KW H-D Exchange in Deuterated Trifluoroacetic Acid via Ligand-Directed NHC-Palladium Catalysis: a Powerful Method for Deuteration of Aromatic Ketones, Amides, and Amino Acids. Tetrahedron Lett. 2015, 56, 6231–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Schroeter F; Cisarova I; Soellner J; Herdtweck E; Strassner T Electron- Poor Hemilabile Dicationic Palladium NHC Complexes-Synthesis, Structure and Catalytic Activity. Dalton Trans. 2018, 47, 16638–16650. [DOI] [PubMed] [Google Scholar]
- (189).Cardin DJ; Çetinkaya B; Çetinkaya E; Lappert MF Carbene Complexes. Part I. Electron-Rich Olefins as a Source of Carbene Complexes of Platinum(II) and Palladium(II); and Some Experiments with (CF3)2CN2. J. Chem. Soc., Dalton Trans. 1973, 514–522. [Google Scholar]
- (190).Hu JJ; Li F; Hor TA Novel Pt (II) Mono-and Biscarbene Complexes: Synthesis, Structural Characterization and Application in Hydrosilylation Catalysis. Organometallics 2009, 28, 1212–1220. [Google Scholar]
- (191).Berthon-Gelloz G; Schumers J-M; De Bo G; Markó IE Highly β-(E)-Selective Hydrosilylation of Terminal and Internal Alkynes Catalyzed by a (IPr)Pt(Diene) Complex. J. Org. Chem. 2008, 73, 4190–4197. [DOI] [PubMed] [Google Scholar]
- (192).De Bo G; Berthon-Gelloz G; Tinant B; Markó IE Hydrosilylation of Alkynes Mediated by N-Heterocyclic Carbene Platinum (0) Complexes. Organometallics 2006, 25, 1881–1890. [Google Scholar]
- (193).Lillo V; Mata JA; Segarra AM; Peris E; Fernandez E The Active Role of NHC Ligands in Platinum-Mediated Tandem Hydroboration–Cross Coupling Reactions. Chem. Commun. 2007, 2184–2186. [DOI] [PubMed] [Google Scholar]
- (194).Bernhammer JC; Huynh HV Platinum (II) Complexes with Thioether-Functionalized Benzimidazolin-2-Ylidene Ligands: Synthesis, Structural Characterization, and Application in Hydroelementation Reactions. Organometallics 2013, 33, 172–180. [Google Scholar]
- (195).Zhang R; Xu Q; Mei L.-y.; Li S.-k.; Shi M A N-Heterocyclic Carbene (NHC) Platinum Complex as Pre-Catalyst for the Intramolecular Hydroamination of Olefins with Secondary Alkylamines and Oxidative Amination of ω-Alkenic Amines. Tetrahedron 2012, 68, 3172–3178. [Google Scholar]
- (196).Ahrens S; Strassner T Detour-Free Synthesis of Platinum-Bis-NHC Chloride Complexes, Their Structure and Catalytic Activity in the CH Activation of Methane. Inorg. Chim. Acta. 2006, 359, 4789–4796. [Google Scholar]
- (197).Furukawa T; Tobisu M; Chatani N C–H Functionalization at Sterically Congested Positions by the Platinum-Catalyzed Borylation of Arenes. J. Am. Chem. Soc. 2015, 137, 12211–12214. [DOI] [PubMed] [Google Scholar]
- (198).Furukawa T; Tobisu M; Chatani N C–H Borylation by Platinum Catalysis. Bull. Chem. Soc. Jpn. 2017, 90, 332–342. [Google Scholar]
- (199).Çetinkaya B; Dixneuf P; Lappert MF Carbene Complexes. Part VIII. Chromium(0), Iron(0), Rhodium(I), Iridium(I), Nickel(II), Palladium(II), Platinum(II), and Gold(I) Mono- and Oligo-Carbene Species from Electron-Rich Olefins. J. Chem. Soc., Dalton Trans. 1974, 1827–1833. [Google Scholar]
- (200).Lappert MF; Maskell RK Homogeneous Catalysis: VIII. Carbene-Transition-Metal Complexes as Hydrosilylation Catalysts. J. Organomet. Chem. 1984, 264, 217–228. [Google Scholar]
- (201).Praetorius JM; Crudden CM N-Heterocyclic Carbene Complexes of Rhodium: Structure, Stability and Reactivity. Dalton Trans. 2008, 4079–4094. [DOI] [PubMed] [Google Scholar]
- (202).Gil W; Trzeciak A N-Heterocyclic Carbene–Rhodium Complexes as Catalysts for Hydroformylation and Related Reactions. Coord. Chem. Rev. 2011, 255, 473–483. [Google Scholar]
- (203).Lee J; Hahm H; Kwak J; Kim M New Aspects of Recently Developed Rhodium (N-Heterocyclic Carbene)-Catalyzed Organic Transformations. Adv. Synth. Catal. 2019, 361, 1479–1499. [Google Scholar]
- (204).Dorta R; Stevens ED; Nolan SP Double C−H Activation in a Rh−NHC Complex Leading to the Isolation of a 14-Electron Rh (III) Complex. J. Am. Chem. Soc. 2004, 126, 5054–5055. [DOI] [PubMed] [Google Scholar]
- (205).Tan KL; Bergman RG; Ellman JA Intermediacy of an N-Heterocyclic Carbene Complex in the Catalytic C−H Activation of a Substituted Benzimidazole. J. Am. Chem. Soc. 2002, 124, 3202–3203. [DOI] [PubMed] [Google Scholar]
- (206).Wiedemann SH; Lewis JC; Ellman JA; Bergman RG Experimental and Computational Studies On the Mechanism of N-Heterocycle C−H Activation by Rh (I). J. Am. Chem. Soc. 2006, 128, 2452–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Lewis JC; Bergman RG; Ellman JA Rh(I)-Catalyzed Alkylation of Quinolines and Pyridines via C-H Bond Activation. J. Am. Chem. Soc. 2007, 129, 5332–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Lewis JC; Bergman RG; Ellman JA Direct Functionalization of Nitrogen Heterocycles via Rh-Catalyzed C-H Bond Activation. Acc. Chem. Res. 2008, 41, 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Kim M; Kwak J; Chang S Rhodium/N-Heterocyclic Carbene Catalyzed Direct Intermolecular Arylation of sp2 and sp3 C-H Bonds with Chelation Assistance. Angew. Chem. Int. Ed. 2009, 48, 8935–8939. [DOI] [PubMed] [Google Scholar]
- (210).Kwak J; Kim M; Chang S Rh(NHC)-Catalyzed Direct and Selective Arylation of Quinolines at the 8-Position. J. Am. Chem. Soc. 2011, 133, 3780–3783. [DOI] [PubMed] [Google Scholar]
- (211).Kwak J; Ohk Y; Jung Y; Chang S Rollover Cyclometalation Pathway in Rhodium Catalysis: Dramatic NHC Effects in the C-H Bond Functionalization. J. Am. Chem. Soc. 2012, 134, 17778–17788. [DOI] [PubMed] [Google Scholar]
- (212).Zhao S; Wu F; Ma Y; Chen W; Liu M; Wu H Enhancement of N-Heterocyclic Carbenes On Rhodium Catalyzed Olefination of Triazoles. Org. Biomol. Chem. 2016, 14, 2550–2555. [DOI] [PubMed] [Google Scholar]
- (213).Tobisu M; Yasui K; Aihara Y; Chatani N C-O Activation by a Rhodium Bis(N-Heterocyclic Carbene) Catalyst: Aryl Carbamates as Arylating Reagents in Directed C-H Arylation. Angew. Chem. Int. Ed. 2017, 56, 1877–1880. [DOI] [PubMed] [Google Scholar]
- (214).Cai Z; Li S; Gao Y; Li G Rhodium (II)-Catalyzed Aryl C−H Carboxylation of 2-Pyridylphenols with CO2. Adv. Synth. Catal. 2018, 360, 4005–4011. [Google Scholar]
- (215).Keske EC; Moore BD; Zenkina OV; Wang R; Schatte G; Crudden CM Highly Selective Directed Arylation Reactions via Back-to-Back Dehydrogenative C–H Borylation/Arylation Reactions. Chem. Commun. 2014, 50, 9883–9886. [DOI] [PubMed] [Google Scholar]
- (216).Thongpaen J; Schmid TE; Toupet L; Dorcet V; Mauduit M; Baslé O Directed ortho C–H Borylation Catalyzed Using Cp* Rh (III)–NHC Complexes. Chem. Commun. 2018, 54, 8202–8205. [DOI] [PubMed] [Google Scholar]
- (217).Zhong L; Zong Z-H; Wang X-C N-Heterocyclic Carbene Enabled Rhodium-Catalyzed ortho C(sp2)-H Borylation at Room Temperature. Tetrahedron 2019, 75, 2547–2552. [Google Scholar]
- (218).Oonishi Y; Hosotani A; Sato Y Construction of Monocyclic Eight-Membered Rings: Intermolecular Rhodium (I)-Catalyzed [6+2] Cycloaddition of 4-Allenals with Alkynes. Angew. Chem. Int. Ed. 2012, 51, 11548–11551. [DOI] [PubMed] [Google Scholar]
- (219).Ghorai D; Choudhury J Exploring a Unique Reactivity of N-Heterocyclic Carbenes (NHC) in Rhodium(III)-Catalyzed Intermolecular C–H Activation/Annulation. Chem. Commun. 2014, 50, 15159–15162. [DOI] [PubMed] [Google Scholar]
- (220).Ghorai D; Choudhury J Rhodium (III)–N-Heterocyclic Carbene-Driven Cascade C–H Activation Catalysis. ACS Catal. 2015, 5, 2692–2696. [Google Scholar]
- (221).Thenarukandiyil R; Choudhury J Rhodium(III)-Catalyzed Activation and Functionalization of Pyridine C-H Bond by Exploring a Unique Double Role of “N-Heterocyclic Carbene-Pyridyl” Ligand Platform. Organometallics 2015, 34, 1890–1897. [Google Scholar]
- (222).Thenarukandiyil R; Thrikkykkal H; Choudhury J Rhodium(III)-Catalyzed Nonaromatic sp2 C-H Activation/Annulation Using NHC as a Directing and Functionalizable Group. Organometallics 2016, 35, 3007–3013. [Google Scholar]
- (223).Ghorai D; Dutta C; Choudhury J Switching of “Rollover Pathway” in Rhodium(III)-Catalyzed C-H Activation of Chelating Molecules. ACS Catal. 2016, 6, 709–713. [Google Scholar]
- (224).Thenarukandiyil R; Gupta SK; Choudhury J Unraveling the Competition of Two C-H and Two M-C Bonds in Guiding the Mechanism of Rhodium(III)-Catalyzed C-H Activation-Annulation. ACS Catal. 2016, 6, 5132–5137. [Google Scholar]
- (225).Thenarukandiyil R; Dutta C; Choudhury J Switching of Reaction Pathway From C-C Rollover to C-N Ring-Extension Annulation. Chem. Eur. J. 2017, 23, 15529–15533. [DOI] [PubMed] [Google Scholar]
- (226).Dutta C; Ghorai D; Choudhury J To “Rollover” or Not? Stereoelectronically Guided C-H Functionalization Pathways From Rhodium-Abnormal NHC Intermediates. ACS Omega 2018, 3, 1614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (227).Dutta C; Sainaba AB; Choudhury J Annulating Thiazolium Cations via a Direct Double C-H Activation Strategy: Rh-N,S-Heterocyclic Carbene Is the Key. Chem. Commun. 2019, 55, 854–857. [DOI] [PubMed] [Google Scholar]
- (228).Dutta C; Choudhury J C-H Activation-Annulation on the N-Heterocyclic Carbene Platform. RSC Adv 2018, 8, 27881–27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (229).Karak P; Dutta C; Dutta T; Koner AL; Choudhury J Orchestrated Catalytic Double Rollover Annulation: Rapid Access to N-Enriched Cationic and Neutral PAHs. Chem. Commun. 2019, 55, 6791–6794. [DOI] [PubMed] [Google Scholar]
- (230).Dutta C; Rana SS; Choudhury J Leveraging Metallotropism-Enabled Substrate Activation in Cobalt-Catalyzed Annulation Chemistry: Protic NHC Template Is the Key. ACS Catal. 2019, 9, 10674–10679. [Google Scholar]
- (231).She Z; Wang Y; Wang D; Zhao Y; Wang T; Zheng X; Yu Z-X; Gao G; You J Two-Fold C−H/C−H Cross-Coupling Using RhCl3· 3H2O as the Catalyst: Direct Fusion of N-(Hetero) Arylimidazolium Salts and (Hetero) Arenes. J. Am. Chem. Soc. 2018, 140, 12566–12573. [DOI] [PubMed] [Google Scholar]
- (232).Oonishi Y; Kitano Y; Sato Y C–H Bond Activation Triggered by Formation of Metallacycles: Rhodium(I)-Catalyzed Cyclopropanation/Cyclization of Allenynes. Angew. Chem. Int. Ed. 2012, 51, 7305–7308. [DOI] [PubMed] [Google Scholar]
- (233).Mo F; Dong G Regioselective Ketone α-Alkylation with Simple Olefins via Dual Activation. Science 2014, 345, 68–72. [DOI] [PubMed] [Google Scholar]
- (234).Dang Y; Qu S; Tao Y; Deng X; Wang Z-X Mechanistic Insight into Ketone α-Alkylation with Unactivated Olefins via C–H Activation Promoted by Metal–Organic Cooperative Catalysis (MOCC): Enriching the MOCC Chemistry. J. Am. Chem. Soc. 2015, 137, 6279–6291. [DOI] [PubMed] [Google Scholar]
- (235).Kim JH; Gressies S; Boultadakis-Arapinis M; Daniliuc C; Glorius F Rh(I)/NHC*-Catalyzed Site- and Enantioselective Functionalization of C(sp3)-H Bonds toward Chiral Triarylmethanes. ACS Catal. 2016, 6, 7652–7656. [Google Scholar]
- (236).Di Giuseppe A; Castarlenas R; Pérez-Torrente JJ; Lahoz FJ; Polo V; Oro LA Mild and Selective H/D Exchange at the β Position of Aromatic α-Olefins by N-Heterocyclic Carbene–Hydride–Rhodium Catalysts. Angew. Chem. Int. Ed. 2011, 50, 3938–3942. [DOI] [PubMed] [Google Scholar]
- (237).Di Giuseppe A; Castarlenas R; Pérez-Torrente JJ; Lahoz FJ; Oro LA Hydride-Rhodium (III)-N-Heterocyclic Carbene Catalysts for Vinyl-Selective H/D Exchange: a Structure–Activity Study. Chem. Eur. J. 2014, 20, 8391–8403. [DOI] [PubMed] [Google Scholar]
- (238).Takeuchi R; Kezuka S Iridium-Catalyzed Formation of Carbon-Carbon and Carbon-Heteroatom Bonds. Synthesis 2006, 2006, 3349–3366. [Google Scholar]
- (239).Suzuki T Organic Synthesis Involving Iridium-Catalyzed Oxidation. Chem. Rev. 2011, 111, 1825–1845. [DOI] [PubMed] [Google Scholar]
- (240).Iglesias M; Oro LA A Leap Forward in Iridium–NHC Catalysis: New Horizons and Mechanistic Insights. Chem. Soc. Rev. 2018, 47, 2772–2808. [DOI] [PubMed] [Google Scholar]
- (241).Sipos G; Dorta R Iridium Complexes with Monodentate N-Heterocyclic Carbene Ligands. Coord. Chem. Rev. 2018, 375, 13–68. [Google Scholar]
- (242).Chianese AR; Li X; Janzen MC; Faller JW; Crabtree RH Rhodium and Iridium Complexes of N-Heterocyclic Carbenes via Transmetalation: Structure and Dynamics. Organometallics 2003, 22, 1663–1667. [Google Scholar]
- (243).Scott NM; Dorta R; Stevens ED; Correa A; Cavallo L; Nolan SP Interaction of a Bulky N-Heterocyclic Carbene Ligand with Rh (I) and Ir (I). Double C−H Activation and Isolation of Bare 14-Electron Rh (III) and Ir (III) Complexes. J. Am. Chem. Soc. 2005, 127, 3516–3526. [DOI] [PubMed] [Google Scholar]
- (244).Fortman GC; Jacobsen H; Cavallo L; Nolan SP Catalytic Deuteration of Silanes Mediated by N-Heterocyclic Carbene-Ir(III) Complexes. Chem. Commun. 2011, 47, 9723–9725. [DOI] [PubMed] [Google Scholar]
- (245).Corberán R; Sanaú M; Peris E Highly Stable Cp*−Ir (III) Complexes with N-Heterocyclic Carbene Ligands as C−H Activation Catalysts for the Deuteration of Organic Molecules. J. Am. Chem. Soc. 2006, 128, 3974–3979. [DOI] [PubMed] [Google Scholar]
- (246).Corberan R; Sanau M; Peris E Aliphatic and Aromatic Intramolecular C-H Activation On Cp*Ir(NHC) Complexes. Organometallics 2006, 25, 4002–4008. [Google Scholar]
- (247).Hartwig JF Borylation and Silylation of C–H Bonds: A Platform for Diverse C–H Bond Functionalizations. Acc. Chem. Res. 2012, 45, 864–873. [DOI] [PubMed] [Google Scholar]
- (248).Frey GD; Rentzsch CF; Von Preysing D; Scherg T; Mühlhofer M; Herdtweck E; Herrmann WA Rhodium and Iridium Complexes of N-Heterocyclic Carbenes: Structural Investigations and Their Catalytic Properties in the Borylation Reaction. J. Organomet. Chem. 2006, 691, 5725–5738. [Google Scholar]
- (249).Rentzsch CF; Tosh E; Herrmann WA; Kühn FE Iridium Complexes of N-Heterocyclic Carbenes in C–H Borylation Using Energy Efficient Microwave Technology: Influence of Structure, Ligand Donor Strength and Counter Ion On Catalytic Activity. Green Chem. 2009, 11, 1610–1617. [Google Scholar]
- (250).Tobisu M; Igarashi T; Chatani N Iridium/N-Heterocyclic Carbene-Catalyzed C-H Borylation of Arenes by Diisopropylaminoborane. Beilstein J. Org. Chem. 2016, 12, 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (251).Choi G; Tsurugi H; Mashima K Hemilabile N-Xylyl-N-Methylperimidine Carbene Iridium Complexes as Catalysts for C–H Activation and Dehydrogenative Silylation: Dual Role of N-Xylyl Moiety for ortho-C–H Bond Activation and Reductive Bond Cleavage. J. Am. Chem. Soc. 2013, 135, 13149–13161. [DOI] [PubMed] [Google Scholar]
- (252).Rubio-Pérez L; Iglesias M; Munárriz J; Polo V; Passarelli V; Pérez-Torrente JJ; Oro LA A Well-Defined NHC–Ir (III) Catalyst for the Silylation of Aromatic C–H Bonds: Substrate Survey and Mechanistic Insights. Chem. Sci. 2017, 8, 4811–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Deboef B; Pastine SJ; Sames D Cross-Coupling of sp3 C-H Bonds and Alkenes: Catalytic Cyclization of Alkene-Amide Substrates. J. Am. Chem. Soc. 2004, 126, 6556–6557. [DOI] [PubMed] [Google Scholar]
- (254).Powell ME; Elmore CS; Dorff PN; Heys JR Investigation of Isotopic Exchange Reactions Using N-Heterocyclic Iridium (I) Complexes. J. Labelled Compd. Rad. 2007, 50, 523–525. [Google Scholar]
- (255).Brown JA; Irvine S; Kennedy AR; Kerr WJ; Andersson S; Nilsson GN Highly Active Iridium(I) Complexes for Catalytic Hydrogen Isotope Exchange. Chem. Commun. 2008, 1115–1117. [DOI] [PubMed] [Google Scholar]
- (256).Nilsson GN; Kerr WJ The Development and Use of Novel Iridium Complexes as Catalysts for ortho-Directed Hydrogen Isotope Exchange Reactions. J. Labelled Compd. Rad. 2010, 53, 662–667. [Google Scholar]
- (257).Cochrane AR; Irvine S; Kerr WJ; Reid M; Andersson S; Nilsson GN Application of Neutral Iridium(I) N-Heterocyclic Carbene Complexes in ortho-Directed Hydrogen Isotope Exchange. J. Labelled. Compd. Rad. 2013, 56, 451–454. [DOI] [PubMed] [Google Scholar]
- (258).Cochrane A; Idziak C; Kerr W; Mondal B; Paterson LC; Tuttle T; Andersson S; Nilsson G Practically Convenient and Industrially-Aligned Methods for Iridium-Catalysed Hydrogen Isotope Exchange Processes. Org. Biomol. Chem. 2014, 12, 3598–3603. [DOI] [PubMed] [Google Scholar]
- (259).Brown JA; Cochrane AR; Irvine S; Kerr WJ; Mondal B; Parkinson JA; Paterson LC; Reid M; Tuttle T; Andersson S; Nilsson GN The Synthesis of Highly Active Iridium(I) Complexes and Their Application in Catalytic Hydrogen Isotope Exchange. Adv. Synth. Catal. 2014, 356, 3551–3562. [Google Scholar]
- (260).Kerr WJ; Mudd RJ; Paterson LC; Brown JA Iridium(I)-Catalyzed Regioselective C-H Activation and Hydrogen-Isotope Exchange of Non-Aromatic Unsaturated Functionality. Chem. Eur. J. 2014, 20, 14604–14607. [DOI] [PubMed] [Google Scholar]
- (261).Kerr WJ; Reid M; Tuttle T Iridium-Catalyzed C-H Activation and Deuteration of Primary Sulfonamides: an Experimental and Computational Study. ACS Catal. 2015, 5, 402–410. [Google Scholar]
- (262).Atzrodt J; Derdau V; Kerr WJ; Reid M; Rojahn P; Weck R Expanded Applicability of Iridium(I) NHC/Phosphine Catalysts in Hydrogen Isotope Exchange Processes with Pharmaceutically-Relevant Heterocycles. Tetrahedron 2015, 71, 1924–1929. [Google Scholar]
- (263).Devlin J; Kerr WJ; Lindsay DM; Mccabe TJD; Reid M; Tuttle T Iridium-Catalysed ortho-Directed Deuterium Labelling of Aromatic Esters-An Experimental and Theoretical Study on Directing Group Chemoselectivity. Molecules 2015, 20, 11676–11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (264).Cross PWC; Herbert JM; Kerr WJ; Mcneill AH; Paterson LC Isotopic Labelling of Functionalised Arenes Catalysed by Iridium(I) Species of the (COD)Ir(NHC)(Py)PF6 Complex Class. Synlett 2016, 27, 111–115. [Google Scholar]
- (265).Kerr WJ; Mudd RJ; Owens PK; Reid M; Brown JA; Campos S Hydrogen Isotope Exchange with Highly Active Iridium(I) NHC/Phosphine Complexes: a Comparative Counterion Study. J. Labelled Compd. Rad. 2016, 59, 601–603. [DOI] [PubMed] [Google Scholar]
- (266).Kerr WJ; Lindsay DM; Reid M; Atzrodt J; Derdau V; Rojahn P; Weck R Iridium-Catalysed ortho-H/D and H/T Exchange under Basic Conditions: C-H Activation of Unprotected Tetrazoles. Chem. Commun. 2016, 52, 6669–6672. [DOI] [PubMed] [Google Scholar]
- (267).Kerr WJ; Lindsay DM; Owens PK; Reid M; Tuttle T; Campos S Site-Selective Deuteration of N-Heterocycles via Iridium-Catalyzed Hydrogen Isotope Exchange. ACS Catal. 2017, 7, 7182–7186. [Google Scholar]
- (268).Kerr WJ; Reid M; Tuttle T Iridium-Catalyzed Formyl-Selective Deuteration of Aldehydes. Angew. Chem. Int. Ed. 2017, 56, 7808–7812. [DOI] [PubMed] [Google Scholar]
- (269).Kerr WJ; Mudd RJ; Reid M; Atzrodt J; Derdau V Iridium-Catalyzed Csp3-H Activation for Mild and Selective Hydrogen Isotope Exchange. ACS Catal. 2018, 8, 10895–10900. [Google Scholar]
- (270).Atzrodt J; Derdau V; Kerr WJ; Reid M C-H Functionalisation for Hydrogen Isotope Exchange. Angew. Chem. Int. Ed. 2018, 57, 3022–3047. [DOI] [PubMed] [Google Scholar]
- (271).Vougioukalakis GC; Grubbs RH Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts. Chem. Rev. 2009, 110, 1746–1787. [DOI] [PubMed] [Google Scholar]
- (272).Dragutan V; Dragutan I; Delaude L; Demonceau A NHC–Ru Complexes—Friendly Catalytic Tools for Manifold Chemical Transformations. Coord. Chem. Rev. 2007, 251, 765–794. [Google Scholar]
- (273).Gnanamgari D; Sauer EL; Schley ND; Butler C; Incarvito CD; Crabtree RH Iridium and Ruthenium Complexes with Chelating N-Heterocyclic Carbenes: Efficient Catalysts for Transfer Hydrogenation, β-Alkylation of Alcohols, and N-Alkylation of Amines. Organometallics 2008, 28, 321–325. [Google Scholar]
- (274).Özdemir I; Demir S; Çetinkaya B; Gourlaouen C; Maseras F; Bruneau C; Dixneuf PH Direct Arylation of Arene C−H Bonds by Cooperative Action of NHCarbene−Ruthenium (II) Catalyst and Carbonate via Proton Abstraction Mechanism. J. Am. Chem. Soc. 2008, 130, 1156–1157. [DOI] [PubMed] [Google Scholar]
- (275).Yaşar S; Doǧan Ö; Oezdemir I; Çetinkaya B Ruthenium N-Heterocyclic–Carbene Catalyzed Diarylation of Arene C–H Bond. Appl. Organomet. Chem. 2008, 22, 314–318. [Google Scholar]
- (276).Özdemir I; Demir S; Gurbuz N; Çetinkaya B; Toupet L; Bruneau C; Dixneuf PH Synthesis, Characterization and Catalytic Activity of New N-Heterocyclic Bis(Carbene)Ruthenium Complexes. Eur. J. Inorg. Chem. 2009, 1942–1949. [Google Scholar]
- (277).Demir S; Özdemir I; Çetinkaya B Synthesis and Catalytic Properties of Novel Ruthenium N-Heterocyclic-Carbene Complexes. J. Organomet. Chem. 2009, 694, 4025–4031. [Google Scholar]
- (278).Demir S; Özdemir I; Sahin O; Çetinkaya B; Buyukgungor O Synthesis and Catalytic Activity of Novel Benzimidazolinylidene-Ruthenium(II) Complexes. Synlett 2010, 496–500. [Google Scholar]
- (279).Kaloglu N; Özdemir I; Gurbuz N; Arslan H; Dixneuf PH Ruthenium(η6,η1-Arene-CH2-NHC) Catalysts for Direct Arylation of 2-Phenylpyridine with (Hetero)Aryl Chlorides in Water. Molecules 2018, 23, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (280).Prades A; Poyatos M; Peris E (η6-Arene)Ruthenium(N-Heterocyclic Carbene) Complexes for the Chelation-Assisted Arylation and Deuteration of Arylpyridines: Catalytic Studies and Mechanistic Insights. Adv. Synth. Catal. 2010, 352, 1155–1162. [Google Scholar]
- (281).Gonell S; Peris E Pyrene-Based Mono- and Di-N-Heterocyclic Carbene Ligand Complexes of Ruthenium for the Preparation of Mixed Arylated/Alkylated Arylpyridines. ACS Catal. 2014, 4, 2811–2817. [Google Scholar]
- (282).Sabater S; Mata JA; Peris E Heterobimetallic Iridium-Ruthenium Assemblies through an Ambidentate Triazole-Diylidene Ligand: Electrochemical Properties and Catalytic Behavior in a Cascade Reaction. Organometallics 2012, 31, 6450–6456. [Google Scholar]
- (283).Wang TH; Lee WC; Ong TG Ruthenium-Mediated Dual Catalytic Reactions of Isoquinoline via C-H Activation and Dearomatization for Isoquinolone. Adv. Synth. Catal. 2016, 358, 2751–2758. [Google Scholar]
- (284).Zhang J; Ugrinov A; Zhao P Ruthenium(II)/N-Heterocyclic Carbene Catalyzed [3+2] Carbocyclization with Aromatic N-H Ketimines and Internal Alkynes. Angew. Chem. Int. Ed. 2013, 52, 6681–6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (285).Kilaru P; Acharya SP; Zhao P A Tethering Directing Group Strategy for Ruthenium-Catalyzed Intramolecular Alkene Hydroarylation. Chem. Commun. 2018, 54, 924–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (286).Li R; Hu Y; Liu R; Hu R; Li B; Wang B Ruthenium (II)-Catalyzed Oxidative Annulation Reactions of Arylimidazolium Salts via N-Heterocyclic Carbene-Directed C–H Activation. Adv. Synth. Catal. 2015, 357, 3885–3892. [Google Scholar]
- (287).Ma C; Ai C; Li Z; Li B; Song H; Xu S; Wang B Synthesis and Alkyne Insertion Reactions of NHC-Based Cyclometalated Ruthenium (II) Complexes. Organometallics 2014, 33, 5164–5172. [Google Scholar]
- (288).Xie XK; Huynh HV Cyclometallated Ruthenium(II) Complexes with Ditopic Thienyl NHC-Ligands: Syntheses and Alkyne Annulations. Org. Chem. Front. 2015, 2, 1598–1603. [Google Scholar]
- (289).Schlepphorst C; Maji B; Glorius F Ruthenium-NHC Catalyzed Alpha-Alkylation of Methylene Ketones Provides Branched Products through Borrowing Hydrogen Strategy. ACS Catal. 2016, 6, 4184–4188. [Google Scholar]
- (290).Gupta SK; Choudhury J A Mixed N-Heterocyclic Carbene/2,2-Bipyridine-Supported Robust Ruthenium(II) Oxidation Precatalyst for Benzylic C-H Oxidation. ChemCatChem 2017, 9, 1979–1984. [Google Scholar]
- (291).Zhang C; Zhao Y; Li B; Song H; Xu S; Wang B The Intramolecular sp2 and sp3 C–H Bond Activation of (p-Cymene) Ruthenium (II) N-Heterocyclic Carbene Complexes. Dalton Trans. 2009, 5182–5189. [DOI] [PubMed] [Google Scholar]
- (292).Burling S; Mas-Marzá E; Valpuesta JE; Mahon MF; Whittlesey MK Coordination, Agostic Stabilization, and C−H Bond Activation of N-Alkyl Heterocyclic Carbenes by Coordinatively Unsaturated Ruthenium Hydride Chloride Complexes. Organometallics 2009, 28, 6676–6686. [Google Scholar]
- (293).Ogata K; Inomata S; Fukuzawa S-I Position-Selective Intramolecular Aromatic C–H Bond Activation of 1,2,3-Triazol-5-Ylidene (tzNHC) Ligands in (p-Cymene) Ruthenium (II) Complexes. Dalton Trans. 2013, 42, 2362–2365. [DOI] [PubMed] [Google Scholar]
- (294).Fürstner A Iron Catalysis in Organic Synthesis: A Critical Assessment of What It Takes To Make This Base Metal a Multitasking Champion. ACS Cent. Sci. 2016, 2, 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (295).Bauer I; Knölker HJ Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. [DOI] [PubMed] [Google Scholar]
- (296).Piontek A; Bisz E; Szostak M Iron-Catalyzed Cross-Coupling in the Synthesis of Pharmaceuticals: In Pursuit of Sustainability. Angew. Chem. Int. Ed. 2018, 57, 11116–11128. [DOI] [PubMed] [Google Scholar]
- (297).Ofele K Nucleophilic Cyclic Carbenes as Complex Ligands on Transition Metals. Angew. Chem. Int. Ed. 1969, 8, 916–917. [Google Scholar]
- (298).Louie J; Grubbs RH Highly Active Iron Imidazolylidene Catalysts for Atom Transfer Radical Polymerization. Chem. Commun. 2000, 1479–1480. [Google Scholar]
- (299).Ingleson MJ; Layfield RA N-Heterocyclic Carbene Chemistry of Iron: Fundamentals and Applications. Chem. Commun. 2012, 48, 3579–3589. [DOI] [PubMed] [Google Scholar]
- (300).Bezier D; Sortais JB; Darcel C N-Heterocyclic Carbene Ligands and Iron: An Effective Association for Catalysis. Adv. Synth. Catal. 2013, 355, 19–33. [Google Scholar]
- (301).Riener K; Haslinger S; Raba A; Högerl MP; Cokoja M; Herrmann WA; Kühn FE Chemistry of Iron N-Heterocyclic Carbene Complexes: Syntheses, Structures, Reactivities, and Catalytic Applications. Chem. Rev. 2014, 114, 5215–5272. [DOI] [PubMed] [Google Scholar]
- (302).Ohki Y; Hatanaka T; Tatsumi K C−H Bond Activation of Heteroarenes Mediated by a Half-Sandwich Iron Complex of N-Heterocyclic Carbene. J. Am. Chem. Soc. 2008, 130, 17174–17186. [DOI] [PubMed] [Google Scholar]
- (303).Hatanaka T; Ohki Y; Tatsumi K C–H Bond Activation/Borylation of Furans and Thiophenes Catalyzed by a Half-Sandwich Iron N-Heterocyclic Carbene Complex. Chem. Asian J. 2010, 5, 1657–1666. [DOI] [PubMed] [Google Scholar]
- (304).Wong MY; Yamakawa T; Yoshikai N Iron-Catalyzed Directed C2-Alkylation and Alkenylation of Indole with Vinylarenes and Alkynes. Org. Lett. 2015, 17, 442–445. [DOI] [PubMed] [Google Scholar]
- (305).Loup J; Zell D; Oliveira JCA; Keil H; Stalke D; Ackermann L Asymmetric Iron-Catalyzed C-H Alkylation Enabled by Remote Ligand Meta-Substitution. Angew. Chem. Int. Ed. 2017, 56, 14197–14201. [DOI] [PubMed] [Google Scholar]
- (306).Loup J; Parchomyk T; Lülf S; Demeshko S; Meyer F; Koszinowski K; Ackermann L Mössbauer and Mass Spectrometry Support for Iron (II) Catalysts in Enantioselective C–H Activation. Dalton Trans. 2019, 48, 5135–5139. [DOI] [PubMed] [Google Scholar]
- (307).Shing KP; Liu Y; Cao B; Chang XY; You T; Che CM N-Heterocyclic Carbene Iron (III) Porphyrin-Catalyzed Intramolecular C(sp3)–H Amination of Alkyl Azides. Angew. Chem. Int. Ed. 2018, 57, 11947–11951. [DOI] [PubMed] [Google Scholar]
- (308).Lu B; Zhu F; Sun HM; Shen Q Esterification of the Primary Benzylic C–H Bonds with Carboxylic Acids Catalyzed by Ionic Iron(III) Complexes Containing an Imidazolinium Cation. Org. Lett. 2017, 19, 1132–1135. [DOI] [PubMed] [Google Scholar]
- (309).Lu B; Zhu F; Wang D; Sun H; Shen Q Iron-Catalyzed Esterification of Allylic sp3 C–H Bonds with Carboxylic Acids: Facile Access to Allylic Esters. Tetrahedron Lett. 2017, 58, 2490–2494. [Google Scholar]
- (310).Blom B; Tan G; Enthaler S; Inoue S; Epping JD; Driess M Bis-N-Heterocyclic Carbene (NHC) Stabilized η6-Arene Iron (0) Complexes: Synthesis, Structure, Reactivity, and Catalytic Activity. J. Am. Chem. Soc. 2013, 135, 18108–18120. [DOI] [PubMed] [Google Scholar]
- (311).Cheng J; Liu J; Leng X; Lohmiller T; Schnegg A; Bill E; Ye S; Deng L A Two-Coordinate Iron (II) Imido Complex with NHC Ligation: Synthesis, Characterization, and Its Diversified Reactivity of Nitrene Transfer and C–H Bond Activation. Inorg. Chem. 2019, 58, 7634–7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (312).Yu DG; Gensch T; de Azambuja F; Vásquez-Céspedes S; Glorius F Co(III)-Catalyzed C–H Activation/Formal SN-Type Reactions: Selective and Efficient Cyanation, Halogenation, and Allylation. J. Am. Chem. Soc. 2014, 136, 17722–17725. [DOI] [PubMed] [Google Scholar]
- (313).Moselage M; Li J; Ackermann L Cobalt-Catalyzed C-H Activation. ACS Catal. 2016, 6, 498–525. [Google Scholar]
- (314).Yoshino T; Matsunaga S (Pentamethylcyclopentadienyl)cobalt(III)-Catalyzed C-H Bond Functionalization: From Discovery to Unique Reactivity and Selectivity. Adv. Synth. Catal. 2017, 359, 1245–1262. [Google Scholar]
- (315).Song W; Ackermann L Cobalt-Catalyzed Direct Arylation and Benzylation by C–H/ C–O Cleavage with Sulfamates, Carbamates, and Phosphates. Angew. Chem. Int. Ed. 2012, 51, 8251–8254. [DOI] [PubMed] [Google Scholar]
- (316).Punji B; Song WF; Shevchenko GA; Ackermann L Cobalt-Catalyzed CH Bond Functionalizations with Aryl and Alkyl Chlorides. Chem. Eur. J. 2013, 19, 10605–10610. [DOI] [PubMed] [Google Scholar]
- (317).Li J; Ackermann L Cobalt-Catalyzed C-H Arylations with Weakly-Coordinating Amides and Tetrazoles: Expedient Route to Angiotensin-II-Receptor Blockers. Chem. Eur. J. 2015, 21, 5718–5722. [DOI] [PubMed] [Google Scholar]
- (318).Mei RH; Ackermann L Cobalt-Catalyzed C-H Functionalizations by Imidate Assistance with Aryl and Alkyl Chlorides. Adv. Synth. Catal. 2016, 358, 2443–2448. [Google Scholar]
- (319).Moselage M; Sauermann N; Richter SC; Ackermann L CH Alkenylations with Alkenyl Acetates, Phosphates, Carbonates, and Carbamates by Cobalt Catalysis at 23 °C. Angew. Chem. Int. Ed. 2015, 54, 6352–6355. [DOI] [PubMed] [Google Scholar]
- (320).Sauermann N; Loup J; Kootz D; Yatham VR; Berkessel A; Ackermann L Triazolylidene Ligands Allow Cobalt-Catalyzed C-H/C-O Alkenylations at Ambient Temperature. Synthesis 2017, 49, 3476–3484. [Google Scholar]
- (321).Gao K; Yoshikai N Regioselectivity-Switchable Hydroarylation of Styrenes. J. Am. Chem. Soc. 2011, 133, 400–402. [DOI] [PubMed] [Google Scholar]
- (322).Ding Z; Yoshikai N Cobalt-Catalyzed Intramolecular Olefin Hydroarylation Leading to Dihydropyrroloindoles and Tetrahydropyridoindoles. Angew. Chem. Int. Ed. 2013, 52, 8574–8578. [DOI] [PubMed] [Google Scholar]
- (323).Yamakawa T; Yoshikai N Alkene Isomerization–Hydroarylation Tandem Catalysis: Indole C2-Alkylation with Aryl-Substituted Alkenes Leading to 1,1-Diarylalkanes. Chem. Asian J. 2014, 9, 1242–1246. [DOI] [PubMed] [Google Scholar]
- (324).Gao K; Lee PS; Long C; Yoshikai N Cobalt-Catalyzed ortho-Arylation of Aromatic Imines with Aryl Chlorides. Org. Lett. 2012, 14, 4234–4237. [DOI] [PubMed] [Google Scholar]
- (325).Gao K; Yoshikai N Cobalt-Catalyzed ortho Alkylation of Aromatic Imines with Primary and Secondary Alkyl Halides. J. Am. Chem. Soc. 2013, 135, 9279–9282. [DOI] [PubMed] [Google Scholar]
- (326).Gao K; Yamakawa T; Yoshikai N Cobalt-Catalyzed Chelation-Assisted Alkylation of Arenes with Primary and Secondary Alkyl Halides. Synthesis 2014, 46, 2024–2039. [Google Scholar]
- (327).Gao K; Yoshikai N Cobalt-Catalyzed Arylation of Aldimines via Directed C-H Bond Functionalization: Addition of 2-Arylpyridines and Self-Coupling of Aromatic Aldimines. Chem. Commun. 2012, 48, 4305–4307. [DOI] [PubMed] [Google Scholar]
- (328).Gao K; Paira R; Yoshikai N Cobalt-Catalyzed ortho-C-H Alkylation of 2-Arylpyridines via Ring-Opening of Aziridines. Adv. Synth. Catal. 2014, 356, 1486–1490. [Google Scholar]
- (329).Lee PS; Xu WG; Yoshikai N Directed C-H Alkenylation of Aryl Imines with Alkenyl Phosphates Promoted by a Cobalt-N-Heterocyclic Carbene Catalyst. Adv. Synth. Catal. 2017, 359, 4340–4347. [Google Scholar]
- (330).Sun Q; Yoshikai N Cobalt-Catalyzed Directed ortho-Methylation of Arenes with Methyl Tosylate. Org. Chem. Front. 2018, 5, 2214–2218. [Google Scholar]
- (331).Xu WG; Yoshikai N Pivalophenone Imine as a Benzonitrile Surrogate for Directed C-H Bond Functionalization. Chem. Sci. 2017, 8, 5299–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (332).Xu WG; Yoshikai N Cobalt-Catalyzed Directed C-H Alkenylation of Pivalophenone N-H Imine with Alkenyl Phosphates. Beilstein J. Org. Chem. 2018, 14, 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (333).Laskar RA; Yoshikai N Cobalt-Catalyzed, N–H Imine-Directed Arene C–H Benzylation with Benzyl Phosphates. J. Org. Chem. 2019, doi: 10.1021/acs.joc.9b01775. [DOI] [PubMed] [Google Scholar]
- (334).Sun Q; Yoshikai N Cobalt-Catalyzed C(sp2)-H/C(sp3)-H Coupling via Directed C-H Activation and 1,5-Hydrogen Atom Transfer. Org. Chem. Front. 2018, 5, 582–585. [Google Scholar]
- (335).Sun Q; Yoshikai N Cobalt-Catalyzed Tandem Radical Cyclization/C-C Coupling Initiated by Directed C-H Activation. Org. Lett. 2019, 21, 5238–5242. [DOI] [PubMed] [Google Scholar]
- (336).Li Y; Qian F; Wang M; Lu H; Li G Cobalt-Catalyzed Decarboxylative C–H (Hetero) Arylation for the Synthesis of Arylheteroarenes and Unsymmetrical Biheteroaryls. Org. Lett. 2017, 19, 5589–5592. [DOI] [PubMed] [Google Scholar]
- (337).Mo Z; Chen D; Leng X; Deng L Intramolecular C(sp3)–H Bond Activation Reactions of Low-Valent Cobalt Complexes with Coordination Unsaturation. Organometallics 2012, 31, 7040–7043. [Google Scholar]
- (338).Mo Z; Liu Y; Deng L Anchoring of Silyl Donors on a N-Heterocyclic Carbene through the Cobalt-Mediated Silylation of Benzylic C–H Bonds. Angew. Chem. Int. Ed. 2013, 52, 10845–10849. [DOI] [PubMed] [Google Scholar]
- (339).Zhang L; Liu Y; Deng L Three-Coordinate Cobalt (IV) and Cobalt (V) Imido Complexes with N-Heterocyclic Carbene Ligation: Synthesis, Structure, and Their Distinct Reactivity in C–H Bond Amination. J. Am. Chem. Soc. 2014, 136, 15525–15528. [DOI] [PubMed] [Google Scholar]
- (340).Gao Y; Chen Q; Leng X; Deng L Cyclometallation Reactions of a Three-Coordinate Cobalt(I) Complex Bearing a Nonsymmetric N-Heterocyclic Carbene Ligand. Dalton Trans. 2019, 48, 9676–9683. [DOI] [PubMed] [Google Scholar]
- (341).Tasker SZ; Standley EA; Jamison TF Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (342).Ananikov VP Nickel: The “Spirited Horse” of Transition Metal Catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar]
- (343).Dible BR; Sigman MS Unusual Reactivity of Molecular Oxygen with π-Allylnickel (N-Heterocyclic Carbene) Chloride Complexes. J. Am. Chem. Soc. 2003, 125, 872–873. [DOI] [PubMed] [Google Scholar]
- (344).Lee CH; Laitar DS; Mueller P; Sadighi JP Generation of a Doubly Bridging CO2 Ligand and Deoxygenation of CO2 by an (NHC)Ni (0) Complex. J. Am. Chem. Soc. 2007, 129, 13802–13803. [DOI] [PubMed] [Google Scholar]
- (345).Schaub T; Döring C; Radius U Efficient Nickel Mediated Carbon–Carbon Bond Cleavage of Organonitriles. Dalton Trans. 2007, 1993–2002. [DOI] [PubMed] [Google Scholar]
- (346).Schaub T; Backes M; Radius U Unusual Nickel-Mediated C–S Cleavage of Alkyl and Aryl Sulfoxides. Chem. Commun. 2007, 2037–2039. [DOI] [PubMed] [Google Scholar]
- (347).Prakasham A; Ghosh P Nickel N-Heterocyclic Carbene Complexes and Their Utility in Homogeneous Catalysis. Inorg. Chim. Acta. 2015, 431, 61–100. [Google Scholar]
- (348).Banach Ł; Guńka PA; Zachara J; Buchowicz W Half-Sandwich Ni(II) Complexes [Ni(Cp)(X)(NHC)]: From an Underestimated Discovery to a New Chapter in Organonickel Chemistry. Coord. Chem. Rev. 2019, 389, 19–58. [Google Scholar]
- (349).Nakao Y; Yamada Y; Kashihara N; Hiyama T Selective C-4 Alkylation of Pyridine by Nickel/Lewis Acid Catalysis. J. Am. Chem. Soc. 2010, 132, 13666–13668. [DOI] [PubMed] [Google Scholar]
- (350).Tsai CC; Shih WC; Fang CH; Li CY; Ong TG; Yap GPA Bimetallic Nickel Aluminun Mediated Para-Selective Alkenylation of Pyridine: Direct Observation of Ƞ2, Ƞ1-Pyridine Ni(0)-Al(III) Intermediates Prior to C-H Bond Activation. J. Am. Chem. Soc. 2010, 132, 11887–11889. [DOI] [PubMed] [Google Scholar]
- (351).Lee WC; Chen CH; Liu CY; Yu MS; Lin YH; Ong TG Nickel-Catalysed Para-CH Activation of Pyridine with Switchable Regioselective Hydroheteroarylation of Allylarenes. Chem. Commun. 2015, 51, 17104–17107. [DOI] [PubMed] [Google Scholar]
- (352).Singh V; Nakao Y; Sakaki S; Deshmukh MM Theoretical Study of Nickel-Catalyzed Selective Alkenylation of Pyridine: Reaction Mechanism and Crucial Roles of Lewis Acid and Ligands in Determining the Selectivity. J. Org. Chem. 2017, 82, 289–301. [DOI] [PubMed] [Google Scholar]
- (353).Nakao Y; Kashihara N; Kanyiva KS; Hiyama T Nickel-Catalyzed Hydroheteroarylation of Vinylarenes. Angew. Chem. Int. Ed. 2010, 49, 4451–4454. [DOI] [PubMed] [Google Scholar]
- (354).Schramm Y; Takeuchi M; Semba K; Nakao Y; Hartwig JF Anti-Markovnikov Hydroheteroarylation of Unactivated Alkenes with Indoles, Pyrroles, Benzofurans, and Furans Catalyzed by a Nickel–N-Heterocyclic Carbene System. J. Am. Chem. Soc. 2015, 137, 12215–12218. [DOI] [PubMed] [Google Scholar]
- (355).Nakanowatari S; Muller T; Oliveira JCA; Ackermann L Bifurcated Nickel-Catalyzed Functionalizations: Heteroarene C-H Activation with Allenes. Angew. Chem. Int. Ed. 2017, 56, 15891–15895. [DOI] [PubMed] [Google Scholar]
- (356).Shih WC; Chen WC; Lai YC; Yu MS; Ho JJ; Yap GPA; Ong TG The Regioselective Switch for Amino-NHC Mediated C–H Activation of Benzimidazole via Ni–Al Synergistic Catalysis. Org. Lett. 2012, 14, 2046–2049. [DOI] [PubMed] [Google Scholar]
- (357).Chen WC; Lai YC; Shih WC; Yu MS; Yap GPA; Ong TG Mechanistic Study of a Switch in the Regioselectivity of Hydroheteroarylation of Styrene Catalyzed by Bimetallic Ni-Al through C-H Activation. Chem. Eur. J. 2014, 20, 8099–8105. [DOI] [PubMed] [Google Scholar]
- (358).Lee WC; Wang CH; Lin YH; Shih WC; Ong TG Tandem Isomerization and C-H Activation: Regioselective Hydroheteroarylation of Allylarenes. Org. Lett. 2013, 15, 5358–5361. [DOI] [PubMed] [Google Scholar]
- (359).Yu MS; Lee WC; Chen CH; Tsai FY; Ong TG Controlled Regiodivergent C-H Bond Activation of Imidazo 1,5-A Pyridine via Synergistic Cooperation Between Aluminum and Nickel. Org. Lett. 2014, 16, 4826–4829. [DOI] [PubMed] [Google Scholar]
- (360).Liu CY; Chen YH; Chen CH; Yu MS; Tsai FY; Ong TG Selective C(8)-H Activation of Imidazopyridines Mediated by Cooperative Nickel-Aluminum Catalysis. Synthesis 2016, 48, 2781–2788. [Google Scholar]
- (361).Lee WC; Shih WC; Wang TH; Liu YH; Yap GPA; Ong TG Nickel Promoted Switchable Hydroheteroarylation of Cyclodienes via C-H Bond Activation of Heteroarenes. Tetrahedron 2015, 71, 4460–4464. [Google Scholar]
- (362).Vijaykumar G; Jose A; Vardhanapu PK; Mandal SK Abnormal-NHC-Supported Nickel Catalysts for Hydroheteroarylation of Vinylarenes. Organometallics 2017, 36, 4753–4758. [Google Scholar]
- (363).Okumura S; Tang SW; Saito T; Semba K; Sakaki S; Nakao Y Para-Selective Alkylation of Benzamides and Aromatic Ketones by Cooperative Nickel/Aluminum Catalysis. J. Am. Chem. Soc. 2016, 138, 14699–14704. [DOI] [PubMed] [Google Scholar]
- (364).Okumura S; Ebara T; Semba K; Nakao Y Synthesis of N-Heterocyclic Carbene Ligands for Site-Selective C–H Alkylation by Cooperative Nickel/Aluminum Catalysis. Heterocycles 2019, 99, 1128–1144. [Google Scholar]
- (365).Okumura S; Komine T; Shigeki E; Semba K; Nakao Y Site-Selective Linear Alkylation of Anilides by Cooperative Nickel/Aluminum Catalysis. Angew. Chem. Int. Ed. 2018, 57, 929–932. [DOI] [PubMed] [Google Scholar]
- (366).Okumura S; Nakao Y Aluminum-Mediated C6-Selective C-H Alkylation of 2-Carbamoylbenzofuran by Nickel Catalysis. Asian J. Org. Chem. 2018, 7, 1355–1357. [Google Scholar]
- (367).Okumura S; Nakao Y Para-Selective Alkylation of Sulfonylarenes by Cooperative Nickel/Aluminum Catalysis. Org. Lett. 2017, 19, 584–587. [DOI] [PubMed] [Google Scholar]
- (368).Donets PA; Cramer N Ligand-Controlled Regiodivergent Nickel-Catalyzed Annulation of Pyridones. Angew. Chem. Int. Ed. 2015, 127, 643–647. [DOI] [PubMed] [Google Scholar]
- (369).Diesel J; Finogenova AM; Cramer N Nickel-Catalyzed Enantioselective Pyridone C-H Functionalizations Enabled by a Bulky N-Heterocyclic Carbene Ligand. J. Am. Chem. Soc. 2018, 140, 4489–4493. [DOI] [PubMed] [Google Scholar]
- (370).Diesel J; Grosheva D; Kodama S; Cramer N A Bulky Chiral N-Heterocyclic Carbene Nickel Catalyst Enables Enantioselective C-H Functionalizations of Indoles and Pyrroles. Angew. Chem. Int. Ed. 2019, 58, 11044–11048. [DOI] [PubMed] [Google Scholar]
- (371).Kruegel AC; Rakshit S; Li XG; Sames D Constructing Iboga Alkaloids via C-H Bond Functionalization: Examination of the Direct and Catalytic Union of Heteroarenes and Isoquinuclidine Alkenes. J. Org. Chem. 2015, 80, 2062–2071. [DOI] [PubMed] [Google Scholar]
- (372).Nett AJ; Zhao W; Zimmerman PM; Montgomery J Highly Active Nickel Catalysts for C–H Functionalization Identified through Analysis of Off-Cycle Intermediates. J. Am. Chem. Soc. 2015, 137, 7636–7639. [DOI] [PubMed] [Google Scholar]
- (373).Castro LCM; Obata A; Aihara Y; Chatani N Chelation-Assisted Nickel-Catalyzed Oxidative Annulation via Double C-H Activation/Alkyne Insertion Reaction. Chem. Eur. J. 2016, 22, 1362–1367. [DOI] [PubMed] [Google Scholar]
- (374).Aihara Y; Wuelbern J; Chatani N The Nickel(II)-Catalyzed Direct Benzylation, Allylation, Alkylation, and Methylation of C-H Bonds in Aromatic Amides Containing an 8-Aminoquinoline Moiety as the Directing Group. Bull. Chem. Soc. Jpn. 2015, 88, 438–446. [Google Scholar]
- (375).Cardoso BDP; Bernard-Schaaf J-M; Shahane S; Veiros LF; Chetcuti MJ; Ritleng V Displacement of Η5-Cyclopentadienyl Ligands From Half-Sandwich C, C-(NHC-Cyanoalkyl)–Nickel (II) Metallacycles: Further Insight into the Structure of the Resulting Cp-Free Nickelacycles and a Catalytic Activity Study. Dalton Trans. 2018, 47, 1535–1547. [DOI] [PubMed] [Google Scholar]
- (376).Wang TH; Ambre R; Wang Q; Lee WC; Wang PC; Liu YH; Zhao LL; Ong TG Nickel-Catalyzed Heteroarenes Cross Coupling via Tandem C-H/C-O Activation. ACS Catal. 2018, 8, 11368–11376. [Google Scholar]
- (377).Furukawa T; Tobisu M; Chatani N Nickel-Catalyzed Borylation of Arenes and Indoles via C–H Bond Cleavage. Chem. Commun. 2015, 51, 6508–6511. [DOI] [PubMed] [Google Scholar]
- (378).Zhang H; Hagihara S; Itami K Aromatic C–H Borylation by Nickel Catalysis. Chem. Lett. 2015, 44, 779–781. [Google Scholar]
- (379).Tekavec TN; Louie J Nickel-Catalyzed Cycloisomerization of Enynes: Catalyst Generation via C–H Activation of Carbene Ligands. Tetrahedron 2008, 64, 6870–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (380).Whittaker AM; Dong VM Nickel-Catalyzed Dehydrogenative Cross-Coupling: Direct Transformation of Aldehydes into Esters and Amides. Angew. Chem. Int. Ed. 2015, 54, 1312–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (381).Gu LJ; Jin C; Zhang HT Nickel N-Heterocyclic Carbene Catalyzed C–C Bond Formation: a New Route to Aryl Ketones. Chem. Eur. J. 2015, 21, 8741–8744. [DOI] [PubMed] [Google Scholar]
- (382).Arduengo AJ III; Dias HR; Calabrese JC; Davidson F Homoleptic Carbene-Silver (I) and Carbene-Copper (I) Complexes. Organometallics 1993, 12, 3405–3409. [Google Scholar]
- (383).Raubenheimer HG; Cronje S; Olivier PJ; Toerien JG; van Rooyen PH Synthesis and Crystal Structure of a Monocarbene Complex of Copper. Angew. Chem. Int. Ed. 1994, 33, 672–673. [Google Scholar]
- (384).Gaillard S; Cazin CS; Nolan SP N-Heterocyclic Carbene Gold (I) and Copper (I) Complexes in C–H Bond Activation. Acc. Chem. Res. 2011, 45, 778–787. [DOI] [PubMed] [Google Scholar]
- (385).Egbert JD; Cazin CSJ; Nolan SP Copper N-Heterocyclic Carbene Complexes in Catalysis. Catal. Sci. Technol. 2013, 3, 912–926. [Google Scholar]
- (386).Lazreg F; Nahra F; Cazin CS Copper–NHC Complexes in Catalysis. Coord. Chem. Rev. 2015, 293, 48–79. [Google Scholar]
- (387).Zhang L; Cheng J; Ohishi T; Hou Z Copper-Catalyzed Direct Carboxylation of C–H Bonds with Carbon Dioxide. Angew. Chem. Int. Ed. 2010, 49, 8670–8673. [DOI] [PubMed] [Google Scholar]
- (388).Boogaerts IIF; Fortman GC; Furst MRL; Cazin CSJ; Nolan SP Carboxylation of N-H/C-H Bonds Using N-Heterocyclic Carbene Copper(I) Complexes. Angew. Chem. Int. Ed. 2010, 49, 8674–8677. [DOI] [PubMed] [Google Scholar]
- (389).Inomata H; Ogata K; Fukuzawa S-I; Hou Z Direct C–H Carboxylation with Carbon Dioxide Using 1,2,3-Triazol-5-Ylidene Copper (I) Complexes. Org. Lett. 2012, 14, 3986–3989. [DOI] [PubMed] [Google Scholar]
- (390).Wang W; Zhang G; Lang R; Xia C; Li F Ph-Responsive N-Heterocyclic Carbene Copper (I) Complexes: Syntheses and Recoverable Applications in the Carboxylation of Arylboronic Esters and Benzoxazole with Carbon Dioxide. Green Chem. 2013, 15, 635–640. [Google Scholar]
- (391).Park D-A; Ryu JY; Lee J; Hong S Bifunctional N-Heterocyclic Carbene Ligands for Cu-Catalyzed Direct C–H Carboxylation with CO2. RSC Adv. 2017, 7, 52496–52502. [Google Scholar]
- (392).Ueno A; Takimoto M; Wylie WNO; Nishiura M; Ikariya T; Hou ZM Copper-Catalyzed Formal C-H Carboxylation of Aromatic Compounds with Carbon Dioxide through Arylaluminum Intermediates. Chem. Asian J. 2015, 10, 1010–1016. [DOI] [PubMed] [Google Scholar]
- (393).Ariafard A; Zarkoob F; Batebi H; Stranger R; Yates BF DFT Studies On the Carboxylation of the C–H Bond of Heteroarenes by Copper (I) Complexes. Organometallics 2011, 30, 6218–6224. [Google Scholar]
- (394).Zhang L; Hou Z N-Heterocyclic Carbene (NHC)–Copper-Catalysed Transformations of Carbon Dioxide. Chem. Sci. 2013, 4, 3395–3403. [Google Scholar]
- (395).Tsuji Y; Fujihara T Carbon Dioxide as a Carbon Source in Organic Transformation: Carbon–Carbon Bond Forming Reactions by Transition-Metal Catalysts. Chem. Commun. 2012, 48, 9956–9964. [DOI] [PubMed] [Google Scholar]
- (396).Yang L; Wang H Recent Advances in Carbon Dioxide Capture, Fixation, and Activation by Using N-Heterocyclic Carbenes. ChemSusChem 2014, 7, 962–998. [DOI] [PubMed] [Google Scholar]
- (397).Liu Q; Wu L; Jackstell R; Beller M Using Carbon Dioxide as a Building Block in Organic Synthesis. Nat. Commun. 2015, 6, 5933–5948. [DOI] [PubMed] [Google Scholar]
- (398).Wang S; Du G; Xi C Copper-Catalyzed Carboxylation Reactions Using Carbon Dioxide. Org. Biomol. Chem. 2016, 14, 3666–3676. [DOI] [PubMed] [Google Scholar]
- (399).Tortajada A; Juliá-Hernández F; Börjesson M; Moragas T; Martin R Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide. Angew. Chem. Int. Ed. 2018, 57, 15948–15982. [DOI] [PubMed] [Google Scholar]
- (400).Hong J; Li M; Zhang J; Sun B; Mo F C−H Bond Carboxylation with Carbon Dioxide. ChemSusChem 2019, 12, 6–39. [DOI] [PubMed] [Google Scholar]
- (401).Yang Y; Lee J-W Toward Ideal Carbon Dioxide Functionalization. Chem. Sci. 2019, 10, 3905–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (402).Lesieur M; Lazreg F; Cazin CS A Cooperative Pd–Cu System for Direct C–H Bond Arylation. Chem. Commun. 2014, 50, 8927–8929. [DOI] [PubMed] [Google Scholar]
- (403).Xie W; Chang S [Cu(NHC)]-Catalyzed C−H Allylation and Alkenylation of Both Electron-Deficient and Electron-Rich (Hetero) Arenes with Allyl Halides. Angew. Chem. Int. Ed. 2016, 55, 1876–1880. [DOI] [PubMed] [Google Scholar]
- (404).Ohmiya H; Zhang H; Shibata S; Harada A; Sawamura M Construction of Quaternary Stereogenic Carbon Centers through Copper-Catalyzed Enantioselective Allylic Alkylation of Azoles. Angew. Chem. Int. Ed. 2016, 55, 4777–4780. [DOI] [PubMed] [Google Scholar]
- (405).Xie W; Yoon JH; Chang S (NHC)Cu-Catalyzed Mild C-H Amidation of (Hetero)Arenes with Deprotectable Carbamates: Scope and Mechanistic Studies. J. Am. Chem. Soc. 2016, 138, 12605–12614. [DOI] [PubMed] [Google Scholar]
- (406).Inomata H; Toh A; Mitsui T; Fukuzawa S-I N-Heterocyclic Carbene Copper (I) Complex-Catalyzed Direct C–H Thiolation of Benzothiazoles. Tetrahedron Lett. 2013, 54, 4729–4731. [Google Scholar]
- (407).Huang HJ; Lee WC; Yap GPA; Ong TG Synthesis and Characterization of Amino-NHC Coinage Metal Complexes and Application for C-H Activation of Caffeine. J. Organomet. Chem. 2014, 761, 64–73. [Google Scholar]
- (408).Adeleke AF; Brown APN; Cheng LJ; Mosleh KAM; Cordier CJ Recent Advances in Catalytic Transformations Involving Copper Acetylides. Synthesis 2017, 49, 790–801. [Google Scholar]
- (409).Yu D; Zhang Y Copper- and Copper-N-Heterocyclic Carbene-Catalyzed C-H Activating Carboxylation of Terminal Alkynes with CO2 at Ambient Conditions. Proc. Natl. Acad. Sci. 2010, 107, 20184–20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (410).Zhang W-Z; Li W-J; Zhang X; Zhou H; Lu X-B Cu (I)-Catalyzed Carboxylative Coupling of Terminal Alkynes, Allylic Chlorides, and CO2. Org. Lett. 2010, 12, 4748–4751. [DOI] [PubMed] [Google Scholar]
- (411).Yang L; Yuan Y; Wang H; Zhang N; Hong S Theoretical Insights into Copper (I)–NHC-Catalyzed C–H Carboxylation of Terminal Alkynes with CO2: the Reaction Mechanisms and the Roles of NHC. RSC Adv. 2014, 4, 32457–32466. [Google Scholar]
- (412).Harada A; Makida Y; Sato T; Ohmiya H; Sawamura M Copper-Catalyzed Enantioselective Allylic Alkylation of Terminal Alkyne Pronucleophiles. J. Am. Chem. Soc. 2014, 136, 13932–13939. [DOI] [PubMed] [Google Scholar]
- (413).Correia CA; Mcquade DT; Seeberger PH Copper (I)/N-Heterocyclic Carbene (NHC)-Catalyzed Addition of Terminal Alkynes to Trifluoromethyl Ketones for Use in Continuous Reactors. Adv. Synth. Catal. 2013, 355, 3517–3521. [Google Scholar]
- (414).Czerwiński P; Molga E; Cavallo L; Poater A; Michalak M NHC–Copper (I) Halide-Catalyzed Direct Alkynylation of Trifluoromethyl Ketones on Water. Chem. Eur. J. 2016, 22, 8089–8094. [DOI] [PubMed] [Google Scholar]
- (415).Czerwiński P; Michalak M NHC-Cu (I)-Catalyzed Friedlander-Type Annulation of Fluorinated O-Aminophenones with Alkynes on Water: Competitive Base-Catalyzed Dibenzo [b,f][1, 5] Diazocine Formation. J. Org. Chem. 2017, 82, 7980–7997. [DOI] [PubMed] [Google Scholar]
- (416).Jin L; Hao W; Xu J; Sun N; Hu B; Shen Z; Mo W; Hu X N-Heterocyclic Carbene Copper-Catalyzed Direct Alkylation of Terminal Alkynes with Non-Activated Alkyl Triflates. Chem. Commun. 2017, 53, 4124–4127. [DOI] [PubMed] [Google Scholar]
- (417).Fructos MR; De Frémont P; Nolan SP; Díaz-Requejo MM; Pérez PJ Alkane Carbon−Hydrogen Bond Functionalization with (NHC) MCl Precatalysts (M= Cu, Au; NHC = N-Heterocyclic Carbene). Organometallics 2006, 25, 2237–2241. [Google Scholar]
- (418).Wang HM; Lin IJ Facile Synthesis of Silver (I)−Carbene Complexes. Useful Carbene Transfer Agents. Organometallics 1998, 17, 972–975. [Google Scholar]
- (419).Su H-L; Pérez LM; Lee S-J; Reibenspies JH; Bazzi HS; Bergbreiter DE Studies of Ligand Exchange in N-Heterocyclic Carbene Silver (I) Complexes. Organometallics 2012, 31, 4063–4071. [Google Scholar]
- (420).Garrison JC; Youngs WJ Ag(I) N-Heterocyclic Carbene Complexes: Synthesis, Structure, and Application. Chem. Rev. 2005, 105, 3978–4008. [DOI] [PubMed] [Google Scholar]
- (421).Ramírez J; Corberán R; Sanaú M; Peris E; Fernandez E Unprecedented Use of Silver (I) N-Heterocyclic Carbene Complexes for the Catalytic Preparation of 1,2-Bis (Boronate) Esters. Chem. Commun. 2005, 3056–3058. [DOI] [PubMed] [Google Scholar]
- (422).Lin IJB; Vasam CS Preparation and Application of N-Heterocyclic Carbene Complexes of Ag(I). Coord. Chem. Rev. 2007, 251, 642–670. [Google Scholar]
- (423).Yu D; Tan MX; Zhang Y Carboxylation of Terminal Alkynes with Carbon Dioxide Catalyzed by Poly(N-Heterocyclic Carbene)-Supported Silver Nanoparticles. Adv. Synth. Catal. 2012, 354, 969–974. [Google Scholar]
- (424).Li S; Sun J; Zhang Z; Xie R; Fang X; Zhou M Carboxylation of Terminal Alkynes with CO2 Using Novel Silver N-Heterocyclic Carbene Complexes. Dalton Trans. 2016, 45, 10577–10584. [DOI] [PubMed] [Google Scholar]
- (425).Manjolinho F; Arndt M; Gooßen K; Gooßen LJ Catalytic C–H Carboxylation of Terminal Alkynes with Carbon Dioxide. ACS Catal. 2012, 2, 2014–2021. [Google Scholar]
- (426).Minghetti G; Bonati F Bis(Carbene) Complexes of Gold(I) and Gold(III) J. Organomet. Chem. 1973, 54, C62–C63. [Google Scholar]
- (427).Lin IJ; Vasam CS Review of Gold (I) N-Heterocyclic Carbenes. Can. J. Chem. 2005, 83, 812–825. [Google Scholar]
- (428).Marion N; Nolan SP N-Heterocyclic Carbenes in Gold Catalysis. Chem. Soc. Rev. 2008, 37, 1776–1782. [DOI] [PubMed] [Google Scholar]
- (429).Raubenheimer HG; Cronje S Carbene Complexes of Gold: Preparation, Medical Application and Bonding. Chem. Soc. Rev. 2008, 37, 1998–2011. [DOI] [PubMed] [Google Scholar]
- (430).Nolan SP The Development and Catalytic Uses of N-Heterocyclic Carbene Gold Complexes. Acc. Chem. Res. 2010, 44, 91–100. [DOI] [PubMed] [Google Scholar]
- (431).Boogaerts IIF; Nolan SP Carboxylation of C-H Bonds Using N-Heterocyclic Carbene Gold(I) Complexes. J. Am. Chem. Soc. 2010, 132, 8858. [DOI] [PubMed] [Google Scholar]
- (432).Rivilla I; Gómez-Emeterio BP; Fructos MR; Díaz-Requejo MM; Pérez PJ Exclusive Aromatic Vs Aliphatic C–H Bond Functionalization by Carbene Insertion with Gold-Based Catalysts. Organometallics 2011, 30, 2855–2860. [Google Scholar]
- (433).To WP; Tong GSM; Lu W; Ma C; Liu J; Chow ALF; Che CM Luminescent Organogold (III) Complexes with Long-Lived Triplet Excited States for Light-Induced Oxidative C-H Bond Functionalization and Hydrogen Production. Angew. Chem. Int. Ed. 2012, 51, 2654–2657. [DOI] [PubMed] [Google Scholar]
- (434).Fructos MR; Belderrain TR; De Frémont P; Scott NM; Nolan SP; Díaz-Requejo MM; Pérez PJ A Gold Catalyst for Carbene-Transfer Reactions From Ethyl Diazoacetate. Angew. Chem. Int. Ed. 2005, 44, 5284–5288. [DOI] [PubMed] [Google Scholar]
- (435).Fructos MR; Besora M; Braga AAC; Díaz-Requejo MM; Maseras F; Pérez PJ Mechanistic Studies on Gold-Catalyzed Direct Arene C–H Bond Functionalization by Carbene Insertion: The Coinage-Metal Effect. Organometallics 2017, 36, 172–179. [Google Scholar]