Abstract
Background
Proton pump inhibitor (PPI) use is widespread. There have been increasing concerns about overuse of high-dose PPIs for durations longer than clinically necessary.
Objective
To evaluate the impact of national education initiatives on reducing PPI use in Australia.
Design
Population-based, controlled interrupted time series analysis of PPI dispensing claims data for Australian adults from July 2012 to June 2018; we used statin dispensing as a control.
Interventions
A year-long educational initiative led by NPS MedicineWise (previously the National Prescribing Service) from April 2015. Simultaneously, Choosing Wisely released recommendations in April 2015 and May 2016. Both promoted review of prolonged PPI use and encouraged stepping down or ceasing treatment, where appropriate.
Measurements
We examined monthly changes in PPI (and statin) dispensing (stratified by high, standard and low tablet strength), rates of switching from higher to lower strength PPIs and rates of PPI (and statin) discontinuation.
Results
We observed 12 040 021 PPI dispensings to 579 594 people. We observed a sustained −1.7% (95% CI: −2.7 to −0.7%) decline in monthly dispensing of standard strength PPIs following the initiatives until the end of the study period. There were no significant changes in high or low strength PPI (or statin) dispensings, switching to lower strength PPIs, or PPI (and statin) treatment discontinuation.
Conclusion
Our findings suggest that these educational initiatives alone were insufficient in curbing overuse of PPIs on a national level. Concerted efforts with policy levers such as imposing tighter restrictions on subsidised use of PPIs may be more effective. Noting low strength esomeprazole is not publicly subsidised in Australia, availability of these preparations may also facilitate more appropriate practice
Keywords: pharmacoepidemiology, health services research, healthcare quality improvement, health policy
Introduction
In the past two decades, proton pump inhibitors (PPIs) have become the ubiquitous treatment for managing gastrointestinal-acid-related disorders globally.1–7 These disorders are widespread in Western societies with worldwide prevalence estimates for gastro-oesophageal reflux disease (GORD) ranging from 10% to 20%8 and 5% to 10% for peptic ulcer disease.9 PPIs are also commonly prescribed prophylactically to prevent stress ulcers and upper gastrointestinal bleeding, which may be induced by treatment with non-steroidal anti-inflammatory drugs or certain anticoagulant medicines.7 10 Contemporary prevalence estimates of PPI use range internationally from 7% to 16%2 4 5; it is estimated that 15% of Australian adults were dispensed at least one PPI in 2017.11
PPIs have an excellent safety profile when used for short periods; however, concerns are growing regarding their long-term use due to increased risks of bone fractures, vitamin B12 and magnesium deficiencies, and Clostridium difficile infection, especially in older people.12–14 Furthermore, recent evidence suggests an increased risk of mortality with prolonged PPI use, specifically mortality due to cardiovascular disease, chronic kidney disease and upper gastrointestinal cancer.15 The rapid rise in PPI use since their introduction to the market in the 1990s16 has prompted widespread trepidation about their overuse, particularly the use of higher dose PPIs for durations longer than clinically necessary.2 4 17 18
Contemporary clinical treatment guidelines for common gastro-intestinal-acid-related disorders recommend initial treatment with standard strength PPIs for 4–8 weeks.19–21 After initial treatment, if symptoms persist, guidelines recommend a step-down approach where patients continue PPIs on a lower dose and attempt to discontinue treatment. However, evidence suggests that real-world PPI use is discordant with these evidence-based guidelines.18 22 Reasons cited for this evidenced-practice discrepancy include patient preference for treatment, fears of rebound hypersecretion on discontinuation, practitioner time constraints and poor communication, particularly between primary care and in hospital prescribers.18 23–27
In an attempt to curb escalating and potentially inappropriate PPI use in Australia, two national initiatives were launched in 2015. NPS MedicineWise, an agency (previously the National Prescribing Service) established to improve the quality use of medicines as part of Australia’s National Medicines Policy,28 introduced a year-long, multifaceted educational programme focused on changing prescribers’ behaviour to align with guideline recommended care for PPIs. At the same time, Choosing Wisely Australia (hereafter referred to as Choosing Wisely) as part of a grassroots, physician-led international initiative aiming to identify and reduce low-value practices (also facilitated in Australia by NPS MedicineWise)29 published two recommendations relating to appropriate PPI prescribing.
In this study, we evaluated the impact of the NPS MedicineWise educational initiative and the Choosing Wisely recommendations on PPI use in Australia. Specifically, we estimated changes in PPI utilisation, following these initiatives, including dispensings, switching from higher to lower strength PPIs and discontinuation of PPI treatment among Australian adults.
Methods
Study setting and population
In Australia, all citizens and residents are entitled to subsidised prescription medicines via the Pharmaceutical Benefits Scheme (PBS). We used dispensing records from 1 July 2012 to 31 June 2018 for a random 10% sample of all PBS-eligible Australian residents. The PBS 10% sample is a standardised dataset provided by the Australian Government Department of Human Services for analytical use which captures PBS medicine dispensings.30 These de-identified, individual-level data include complete demographic information (gender and year of birth) and PBS dispensing records (date of dispensing, dispensed medicine, dispensed medicine strength, quantity dispensed, prescriber specialty). Private prescriptions (ie, not PBS subsidised) and over-the-counter medicine dispensings are not captured in the data.
We restricted our study to individuals aged ≥18 years with at least one dispensing of a PBS-listed medicine.
Medicines of interest
We included all strength and formulations of PBS-listed PPI medicines corresponding to the WHO Anatomical Therapeutic Chemical (ATC) classification code A02BC.31 These were as follows: omeprazole, pantoprazole, lansoprazole, rabeprazole and esomeprazole. We classified PPI tablet strengths as high, standard and low according to the guidelines by Australian Therapeutic Guidelines and the National Institute for Health and Care Excellence guideline (CG184; table 1).19 20 We excluded PPIs in combination with antibiotics (ATC code: A02BD) prescribed for the acute treatment of Helicobacter pylori infection.
Table 1.
Classifications and date each PPI medicine was made available as an over-the-counter medicine
| PPI medicine | ATC code | High strength | Standard strength | Low strength | Date medicine became available over the counter |
| Omeprazole | A02BC01 | – | 20 mg* | 10 mg | June 2016 |
| Pantoprazole | A02BC02 | – | 40 mg | 20 mg* | June 2015 |
| Lansoprazole | A02BC03 | – | 30 mg | 15 mg* | June 2016 |
| Rabeprazole | A02BC04 | – | 20 mg | 10 mg* | June 2016 |
| Esomeprazole | A02BC05 | 40 mg | 20 mg* | – | February 2016 |
*The strength of PPI medicine rescheduled to over-the-counter medicine (Therapeutic Goods Administration et al.)57
ATC, Anatomical Therapeutic Chemical; PPI, proton pump inhibitor.
We used statins as a control medicine in our evaluation to strengthen assumptions that any changes in PPI use were related to the interventions rather than extraneous factors concurrently affecting the dispensings of other medicines. We chose statins as they are used by similar populations to those using PPIs and the initiatives under study were unlikely to affect their use.32 We included the following PBS-listed statin medicines (ATC code C10AA): simvastatin, pravastatin, fluvastatin, atorvastatin and rosuvastatin.
Initiatives evaluated
In April 2015, NPS MedicineWise implemented a 12-month educational programme primarily targeting general practitioners (GPs) with the aim of changing PPI prescribing behaviour to better reflect best-practice guidelines. First, the programme targeted all GPs across Australia by mailing information and feedback about their own PPI prescribing compared with other GPs, based on nationwide claims data.
Second, this initiative delivered key messages on PPI prescribing to GPs, other health professionals (eg, pharmacists and nurses) and consumers through a number of active and passive interventions. A variety of resources and activities were made available through a centralised online portal accessed via the NPS MedicineWise website. Active interventions included a clinical e-audit, where GPs could upload 10 of their patients’ clinical information and receive immediate and dynamic patient-specific recommendations and a national case study, available to all health professionals, in which interactive clinical scenarios were presented with feedback and expert commentary related to PPI therapy. Completion of these activities was incentivised through professional development points, recognised by health professionals in Australia.33
Passive interventions included educational resources for health professionals on PPIs and the management of GORD, including an expert-led two-part video called PPIs: Too Much of a Good Thing, up-to-date evidenced-practice summary sheets, factsheets to display in a workplace and a downloadable symptomatic management pad to help create personalised PPI therapy plans with patients. In parallel, NPS MedicineWise and Choosing Wisely also developed online resources such as leaflets, factsheets and articles targeting consumers. These provided practical information for patients about the management of GORD and also promoted conversation between patients and practitioners.34 35
The following recommendations were released, in April 2015 and May 2016, as a part of the Choosing Wisely campaign and underpinned the key messages of the initiatives promoting appropriate PPI prescribing.
Do not use proton pump inhibitors (PPIs) long-term in patients with uncomplicated disease without regular attempts at reducing dose or ceasing.36
Do not continue prescribing long-term proton pump inhibitor (PPI) medication to patients without attempting to reduce the medication down to the lowest effective dose or cease the therapy altogether.37
By providing patient-specific feedback to prescribers and education to both health professionals and consumers, the components of each intervention aimed to facilitate consumer and prescriber interactions to enable best-practice prescribing. These inherently address some of the root causes of inappropriate PPI prescribing, for example, patient preference for treatment and/or fear of discontinuation.
Measures and statistical analysis
In this population-based, retrospective observational study, we examined three measures of PPI utilisation dispensings counts, discontinuation of treatment and switches to lower strength PPIs.
We defined discontinuation of treatment as the absence of PPI (or statin) dispensings within 90 days of the last dispensing. The discontinuation date was estimated by adding the expected duration of a dispensing to the last dispensing (ie, 30 days—median duration between dispensings).
We recorded switches to lower strength PPIs when a subsequent dispensing for a person was of a lower strength PPI (ie, dispensing of high strength PPI followed by a standard or low strength PPI dispensing). To capture people who had reinitiated treatment on a lower strength, we did not apply restrictions in time between dispensings.
The main study measures did not capture the stepping down of PPI treatment if people remained on the same tablet strength but reverted to less frequent intake for example, one tablet every other day. To explore whether this practice became more common after the initiatives, we conducted a supplementary analysis plotting the days between dispensings in the year before, during and after the NPS MedicineWise PPI programme and Choosing Wisely recommendations. We would expect to observe an increase in the time between dispensings after the initiatives had treatment been stepped down by less frequent intake.
In our analysis, we first described the above measures in the year before (April 2014–April 2015) and the year after (May 2016–May 2017) the NPS MedicineWise PPI programme and Choosing Wisely recommendations. We stratified each by strength and medicine. For discontinuation, this was stratified by the medicine first dispensed within the observed course of treatment. For switching, we stratified by medicine switched from.
Second, to assess the impact of the initiatives we calculated the following rates:
Monthly dispensing counts for PPIs (stratified by tablet strength) and statins (July 2012–June 2018).
Monthly rates of treatment discontinuation (PPIs and statins): calculated by dividing the number of discontinuations by the number of people treated within the month (we restricted the time period to January 2013 to May 2018 to ensure that we had enough time to ascertain treatment and discontinuation).
Monthly rates of switches to lower strength PPIs: calculated by dividing the number of switches to a lower strength PPI divided by the number of people dispensed high or standard strength PPIs within the month (we restricted the time period to January 2013–May 2018 to ensure that we had enough time to ascertain treatment and switching).
We used controlled interrupted times series analyses with seasonal autoregressive integrated moving average modelling for each of the above rates, so as to account for non-stationarity, seasonality and autocorrelation in the data. PBS dispensing data are commonly subject to seasonality; due to the effect of the PBS Safety Net.30 This can lead to stockpiling in the latter months of the year, resulting in increased rates of dispensing towards the end of the year and a subsequent fall early in the following year. We applied Box-Jenkins methodology to determine the best fitting models for each analysis.38 We used two intervention points: one in April 2015 marking the beginning of the NPS MedicineWise PPI programme and the release of the first Choosing Wisely PPI recommendation; and one for May 2016 marking the end of the programme with the release of the second Choosing Wisely PPI recommendation. We tested whether these initiatives resulted in a change in any of the three aforementioned rates following their implementation, including both a temporary shift (a sudden change only observed within the month of the intervention) and a level shift (a sudden, sustained change for the remainder of the study period).
Sensitivity analysis
From June 2015, standard and low strength PPIs became available over the counter in only 7-day pack sizes in Australia (table 1). Since our data did not capture over-the-counter medicines, we conducted a sensitivity analysis to explore whether any observed change in dispensings count or rate of discontinuation in the main analysis was related to people switching to over-the-counter PPIs. We restricted this analysis to concessional beneficiaries as they have less financial incentive than the general population to buy medicines over the counter, due to receiving an additional social security subsidy for prescribed medicines. Concessional beneficiaries comprise roughly 24% of Australia’s population39 40 and mainly include people aged above 65 years and/or people who receive benefits for disability or low income. We considered concessional beneficiaries as those for whom every dispensing during the study period attracted a concessional benefit.30
We conducted all analyses in SAS V.9.4 (SAS institute, Cary, NC, USA) and R V.3.43 (The R foundation for statistical computing, Vienna, Austria).
Data access approval
Data access was granted by the Australian Department of Human Services External Request Evaluation Committee (Approval Numbers: MI9829, MI10933). Direct access to the data and analytical files to other individuals or authorities is not permitted without the express permission of the approving human research ethics committees and data custodians.
Results
We observed 579 594 people who were dispensed at least one PPI medicine from July 2012 to June 2018, of which 55% were women and the median age was 57 years (IQR: 42–69 years). Over the study period, we observed a total of 12 040 021 dispensings of PPIs, the majority (82%) of which were prescribed by GPs. Standard strength dispensings accounted for 78%, high strength 17% and low strength 5% of all dispensings. Standard strength esomeprazole (20 mg) and standard strength pantoprazole (40 mg) were the most commonly dispensed PPIs each comprising approximately 25% of all PPI dispensing. In the year following the initiatives, 76% of switches to a lower strength PPI were from high strength esomeprazole (40 mg) to a standard strength PPI, while, respectively, 2.6% and 14.6% were from standard strength esomeprazole and standard strength pantoprazole to a low strength PPI (online supplementary table S1).
bmjqs-2019-009897supp001.pdf (579.3KB, pdf)
Impact of initiatives on PPI dispensing
Following the initiatives, from May 2016 until the end of the study period, we observed a sustained reduction in monthly PPI dispensings of −1.7% (95% CI: −2.9% to −0.4%), and no significant changes in statin dispensing (figure 1A, table 2).
Figure 1.
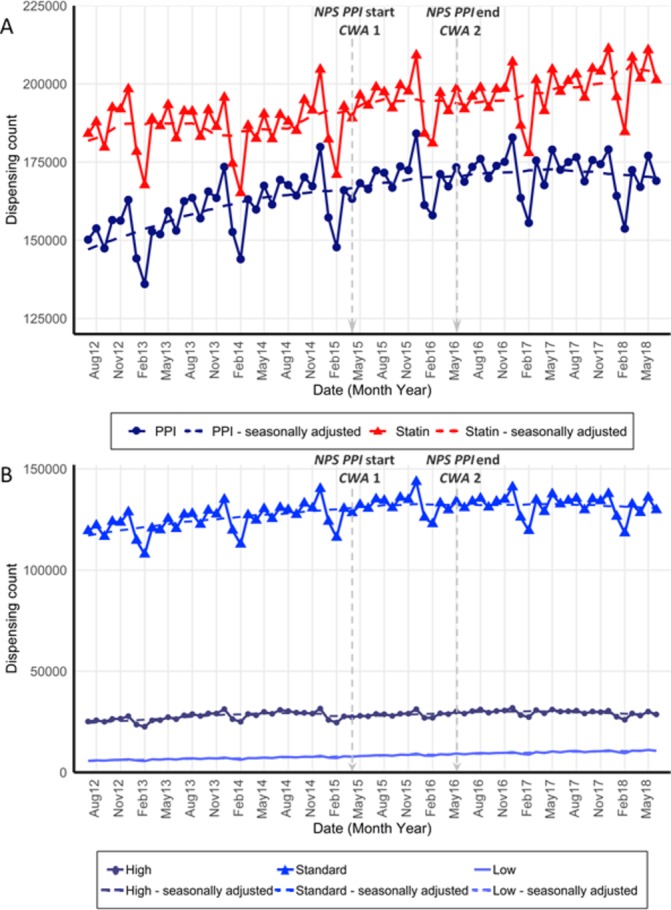
(A) Monthly unadjusted and seasonally adjusted (dashed line) dispensing counts of PPIs and statins; (B) dispensing counts of PPIs by strength: high, standard and low, from July 2012 to June 2018. NPS MedicineWise’s PPI program start and end and Choosing Wisely Australia recommendations 1 and 2 marked at April 2015 and may 2016. Standard strength PPI esomeprazole was made available over the counter in February 2016. PPIs, proton pump inhibitors
Table 2.
Change in the monthly PPI dispensing counts, rate of switching to a lower strength PPIs and rates of treatment discontinuation at each intervention point, estimated using ARIMA models adjusted for seasonality
| Seasonal ARIMA model specification* | Mean monthly dispensings† | Level shift‡ from April 2015 | Level shift from May 2016 | |
| % (95% CI) | % (95% CI) | |||
| Dispensing counts | ||||
| PPIs | (2,1,0) (0,1,0)12 | 164 361 | 0.6 (−0.7 to 2.0) | −1.7 (−2.9 to −0.4) |
| High strength | (1,1,0) (0,1,0)12 | 28 728 | −0.2 (−3.1 to 2.7) | 0.3 (−2.6 to 3.3) |
| Standard strength | (0,1,1) (0,1,0)12 | 128 156 | 1.2 (0.1 to 2.3) | −1.7 (−2.7 to −0.7) |
| Low strength | (0,1,1) (0,1,0)12 | 7481 | −0.1 (−2.6 to 2.5) | −0.8 (−2.9 to 1.4) |
| Statins (control) | (0,1,1) (0,1,1)12 | 187 407 | 1.0 (−1.1 to 3.3) | −1.6 (−3.7 to 0.5) |
| Switching to lower strength PPI | ||||
| (0,1,1) (1,0,0)12 | 3.0 (−1.6 to 7.7) | 0.2 (−3.8 to 4.4) | ||
| Treatment discontinuation | ||||
| PPIs | (2,1,0 1,1,0)12 | −0.1 (−0.4 to 0.2) | −0.1 (−0.4 to 0.2) | |
| Statins (control) | (1,0,0) (0,1,0)12 | 0 (−0.3 to 0.4) | 0.3 (0 to 0.6) | |
*ARIMA (p, d, q) x (P, D, Q)12 model where 12 indicates seasonal differencing at 12-month lag.
†Mean monthly dispensings in the year leading up to NPS MedicineWise programme in April 2015.
‡A sudden, sustained change for the remainder of the study period.
ARIMA, autoregressive integrated moving average;PPI, proton pump inhibitor.
This level change was driven by fewer dispensings (−1.7%, 95% CI: −2.7% to −0.7%) of standard strength PPIs from May 2016. We observed no significant changes in high or low strength PPI dispensings at either intervention point (figure 1B, table 2).
Impact of initiative on discontinuation of PPI use and switching to a lower strength PPI
We did not observe any significant temporary or level changes in the monthly rate of PPI (or statin) discontinuation at either intervention point (figure 2A, table 2). In addition, we did not observe any significant changes in rate of monthly switching from higher to lower strength PPIs at either intervention point (figure 2B, table 2). We observed a similar distribution of the time between dispensings in the year before, during and after the initiatives, suggesting that stepping down of PPI treatment by less frequent intake did not become more common after the initiatives (online supplementary figure S1).
Figure 2.
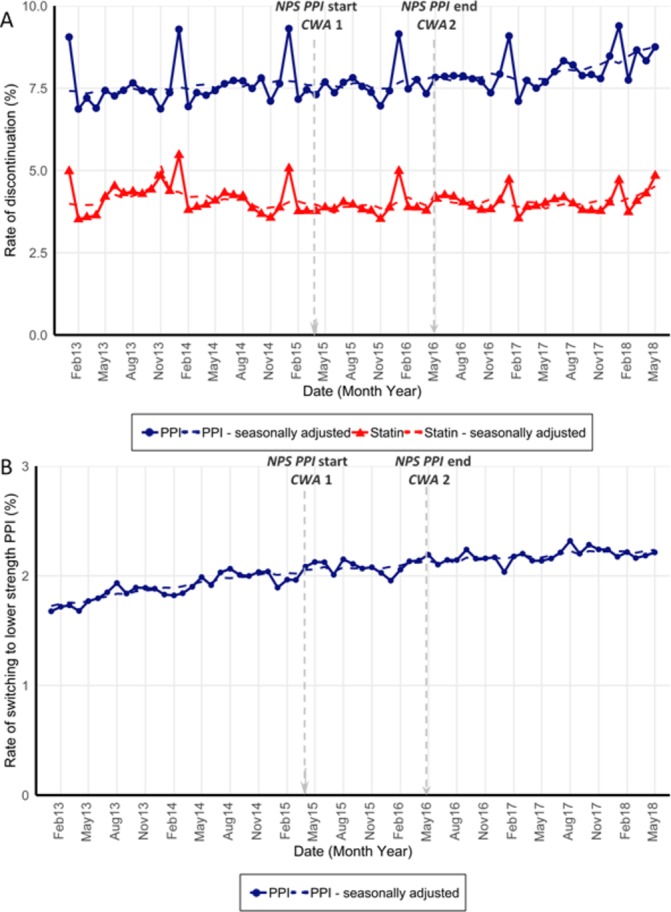
(A) Monthly unadjusted and seasonally adjusted (dashed line) rate (%) of PPI and statin discontinuation among those covered by treatment; (B) rate of switching to a lower strength PPI among those dispensed standard or high strength PPIs each month, from January 2013 to May 2018. NPS MedicineWise’s PPI program start and end and Choosing Wisely Australia recommendations 1 and 2 marked at April 2015 and May 2016. Standard strength PPI esomeprazole was made available over the counter in February 2016. PPI, proton pump inhibitor.
Sensitivity analysis
Concessional beneficiaries accounted for 46.4% (n=2 69 019) of our sample. Similar to our main analysis, we observed a reduction of −1.8% (95% CI: −2.9 to −0.7) in PPI dispensings from May 2016 until the end of the study period. However, we observed a simultaneous level shift in statin dispensing of −2.2% (95% CI: −3.6 to −0.7) (figure 3A and online supplementary table S2), suggesting there may be some confounding effects due to factors outside the initiatives under evaluation. We also observed no significant changes in rates of discontinuation of PPIs (or statins) at either intervention points (online supplementary table S2 and online supplementary figure S2).
Figure 3.

(A) Monthly unadjusted and seasonally adjusted (dashed line) concessional beneficiaries’ dispensing counts of PPIs and statins (B) concessional beneficiaries’ dispensing counts of PPIs by strength: high, standard and low, from July 2012 to May 2018. NPS MedicineWise’s PPI programme start and end and Choosing Wisely Australia recommendations 1 and 2 marked at April 2015 and May 2016. Standard strength PPI esomeprazole was made available over the counter in February 2016. PPI, proton pump inhibitors.
Discussion
The results of this population-based study demonstrate that the educational initiatives, led by NPS MedicineWise and Choosing Wisely, had a limited impact on PPI use on a national level. We observed only a small (1.7%) decline in PPI dispensings following the initiatives, corresponding to approximately 27 941 fewer than expected dispensings from May 2016 to June 2018. Despite the promotion of stepping down or discontinuing PPI treatment in these initiatives, we did not observe any changes in the rates of switching to lower PPI strengths or discontinuation of treatment. In fact, higher strength PPIs remained the dominant choice for Australians throughout the study period; low strength PPIs accounted for only 6% of PPI dispensing in the period following the initiatives.
Together, the two national initiatives provided a multifaceted approach to promote appropriate PPI prescribing, encouraging review of people using PPIs long-term and recommending individuals to step-down treatment, to lower dose or discontinue, where appropriate. The deprescribing of medicines is a well-known challenge.41 Nevertheless, a multitude of community-based interventions have been successful in influencing medicine prescribing on a local scale, in particular the deprescribing of antibiotics42 and sedative-hypnotics such as benzodiazepine.43 44 In contrast, there is relatively little evidence concerning nationwide initiatives implemented to reduce inappropriate prescribing.
Pratt et al 45 examined previous nationwide NPS MedicineWise PPI initiatives and found they increased the use of low strength PPIs and reduced the overall rate of PPI use among veterans in Australia. Differences in both the scope of initiatives, study design and population subgroup evaluated may explain the discrepancies between our findings and the Pratt et al study. Previous NPS MedicineWise initiatives included active components such as free educational outreach visits to GPs, which have been demonstrated to be successful,46 and complementary initiatives regarding appropriate PPI use directly targeted veterans.47 Our study examined more recent NPS MedicineWise and Choosing Wisely initiatives on a wider representative population of Australia using additional proxy measures such as switching and discontinuation to assess change in prescribing behaviour.
We did not observe any significant change in the rates of people switching from higher to lower strength PPIs or discontinuing treatment following the initiatives. Findings of a large UK primary care database study highlighted potentially inappropriate use by demonstrating that only 40% of new users treated with PPIs for over 12 months attempted to step down to a lower strength, with only 8.7% discontinuing PPI treatment.4 In the UK, the dissemination of nationwide clinical guidelines on PPIs in 2002 appeared to have little impact on GP prescribing behaviour.27 Additional investigation into the long-term use of PPIs in Australia is warranted to improve policy and education related to appropriate prescribing of these widespread medicines.
The passive nature of these interventions may have been insufficient or not well targeted to perturb the root causes of inappropriate PPI prescribing. While multifaceted educational interventions have been shown to be more effective than dissemination of guidelines alone in achieving evidence-based practice,48 49 additional barriers to change need to be addressed for the best chance of success.50 51 In Australia, restrictions are often placed around prescribing subsidised medicines to ensure use is cost-effective.30 Until May 2019, the PBS PPI restrictions did not align with treatment guidelines,52 53 this may have undermined the efforts of the national initiatives under study. Additionally, esomeprazole is the most commonly prescribed PPI in Australia,54 but interestingly no low strength formulation (ie, 10 mg tablets) of this PPI is publicly subsidised, with only 10 mg sachets available on the Australian market. We observed very few step-down attempts from standard strength esomeprazole in our data, indicating that the absence of a low strength esomeprazole may have discouraged the step down of PPIs to the lowest effective dose and potentially diminished the impact of the initiatives on low strength PPI uptake.
Our findings should be interpreted in context of with the study limitations. As an interrupted time series analysis cannot separate the effect of concurrent events, we conducted a control series design to adjust for time-varying confounders in interrupted time series analyses.55 Additionally, to account for the concurrent introduction of PPIs as over-the-counter medicines, we conducted a sensitivity analysis on concessional beneficiaries, who did not have a financial incentive to switch to over-the-counter PPI use. Our findings from this analysis were twofold. First, we observed a similar decline of PPI dispensing among concessional beneficiaries (1.8%) as among the total population (1.7%), indicating that this small decline was not affected by the recent availability of over-the-counter PPI medicines. Second, we observed a simultaneous shift in dispensing of statins, the control medicine among concessional beneficiaries, suggesting that the minimal decline in PPI dispensings may have been influenced by factors outside of the initiatives assessed.
The PBS 10% sample is a nationally representative sample of the adult Australian population and allows for the longitudinal capture of medicine use30; however, our results may underestimate true PPI use in the community, as private prescription claims, and over-the-counter medicine dispensings are not captured in our data. Another limitation of our study was our use of proxies rather than direct measures of the prescribing behaviours targeted in the interventions. We did not have information on exact dose prescribed and therefore used tablet strength as a proxy for dose. This may have underestimated the number of switches to lower doses as our proxy measure did not capture people who reduced dose by less frequent intake of the same tablet strength that is, from one tablet per day to every other day or two tablets to one per day. However, our supplementary analysis suggested that stepping down PPI treatment in this manner did not become more common after the initiatives. We were not privy to information regarding individuals’ clinical indications for PPI use or severity of symptoms underlying treatment, and we thus could not determine the appropriateness of the observed changes in PPI use. Lastly, we did not include measures to address the fidelity of the educational initiatives assessed in this study.
The results of this study reinforce that stakeholders should review, and realign if necessary, regulatory and administrative incentives to facilitate the uptake of evidence-based practice. To this end, the Australian government have increased the restrictions on the prescribing of subsidised PPI formulations, effective from 1 May 2019, that is, after the end of our study period. These new restrictions53 are intended to further promote the use of lower strength PPIs and to contain long-term treatment of higher strength PPIs to clinically appropriate cases.56 The effectiveness of these regulatory efforts remains to be assessed.
Conclusions
In this study, we have demonstrated that the national educational initiatives targeting prescribers and patients have had a limited impact on improving quality use of PPIs. Higher strength PPI dispensing continues to comprise most PPI use in Australia. It appears that educational initiatives working alone are unlikely to make the inroads required to curb overuse of PPIs; policy levers such as imposing tighter restrictions on subsidised use of PPIs in concert with education may be more effective. Furthermore, we believe that increased availability of low strength preparations, such as 10 mg esomeprazole tablets, could facilitate more appropriate management of PPI treatment.
Acknowledgments
We acknowledge Melisa Litchfield for her role in providing the data and gaining ethics approval. We further thank the Department of Human Services for providing the data.
Footnotes
Presented at: 35th International Conference on Pharmacoepidemiology & Therapeutic Risk Management, August 24-28, 2019, Philadelphia, PA, USA.
Correction notice: This article has been corrected since it was published. The order of two co-authors has changed ie, Sallie-Anne Pearson as the second author and Benjamin Daniels as the third author.
Contributors: All authors contributed to the study concept and design. SAP and HZ acquired the data. CB analysed the data with input from AS and BD. CB drafted the manuscript with the supervision of HZ and SAP. NB provided clinical insight throughout. All of the authors critically revised the manuscript for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This research is supported by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence in Medicines and Ageing (ID: 1060407) and a Cooperative Research Centre Project (CRC-P) Grant from the Australian Government Department of Industry, Innovation and Science (ID: CRC-P-439). Dr Zoega was supported by a Scientia Fellowship from UNSW Sydney. Dr Schaffer was supported by a NHMRC Early Career Fellowship (#1158763).
Disclaimer: SAP is a member of the Drug Utilisation Sub Committee of the Pharmaceutical Benefits Advisory Committee. The views expressed in this paper do not represent those of the Committee.
Competing interests: None declared.
Patient consent for publication: Not required. Individual consent for the release of these data has been waived according to the Australian Privacy Act of 1988.
Ethics approval: Our study was approved by the NSW Population and Health Services Research Ethics Committee (Approval Number: 2013/11/494).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available.
References
- 1. Fraeyman J, Van Hal G, Godman B, et al. The potential influence of various initiatives to improve rational prescribing for proton pump inhibitors and statins in Belgium. Expert Rev Pharmacoecon Outcomes Res 2013;13:141–51. 10.1586/erp.12.88 [DOI] [PubMed] [Google Scholar]
- 2. Hálfdánarson Óskar Ö, Pottegård A, Björnsson ES, et al. Proton-Pump inhibitors among adults: a nationwide drug-utilization study. Therap Adv Gastroenterol 2018;11:1756284818777943 10.1177/1756284818777943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishtala PS, Soo L. Proton pump inhibitors utilisation in older people in New Zealand from 2005 to 2013. Intern Med J 2015;45:624–9. 10.1111/imj.12757 [DOI] [PubMed] [Google Scholar]
- 4. Othman F, Card TR, Crooks CJ. Proton pump inhibitor prescribing patterns in the UK: a primary care database study. Pharmacoepidemiol Drug Saf 2016;25:1079–87. 10.1002/pds.4043 [DOI] [PubMed] [Google Scholar]
- 5. Pottegård A, Broe A, Hallas J, et al. Use of proton-pump inhibitors among adults: a Danish nationwide drug utilization study. Therap Adv Gastroenterol 2016;9:671–8. 10.1177/1756283X16650156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeng W, Finlayson AE, Shankar S, et al. Prescribing efficiency of proton pump inhibitors in China: influence and future directions. BMC Health Serv Res 2015;15:11 10.1186/s12913-014-0638-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver 2017;11:27–37. 10.5009/gnl15502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871–80. 10.1136/gutjnl-2012-304269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lanas A, Chan FKL. Peptic ulcer disease. The Lancet 2017;390:613–24. 10.1016/S0140-6736(16)32404-7 [DOI] [PubMed] [Google Scholar]
- 10. Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor Cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA 2018;320:2221–30. 10.1001/jama.2018.17242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Australian Commission on Safety and Quality in Health Care and Australian Institute of Health and Welfare Proton pump inhibitor medicines dispensing, 18 years and over. The third Australian atlas of healthcare variation. Sydney, NSW: ACSQHC, 2018: 117–30. [Google Scholar]
- 12. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected]. Am J Gastroenterol 2009;104:S27–S32. 10.1038/ajg.2009.49 [DOI] [PubMed] [Google Scholar]
- 13. Cao F, Chen CX, Wang M, et al. Updated meta-analysis of controlled observational studies: proton-pump inhibitors and risk of Clostridium difficile infection. J Hosp Infect 2018;98:4–13. 10.1016/j.jhin.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 14. Nehra AK, Alexander JA, Loftus CG, et al. Proton pump inhibitors: review of emerging concerns. Mayo Clin Proc 2018;93:240–6. 10.1016/j.mayocp.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 15. Xie Y, Bowe B, Yan Y, et al. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ 2019;365 10.1136/bmj.l1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollingworth S, Duncan EL, Martin JH. Marked increase in proton pump inhibitors use in Australia. Pharmacoepidemiol Drug Saf 2010;19:1019–24. 10.1002/pds.1969 [DOI] [PubMed] [Google Scholar]
- 17. Naunton M, Peterson GM, Bleasel MD. Overuse of proton pump inhibitors. J Clin Pharm Ther 2000;25:333–40. 10.1046/j.1365-2710.2000.00312.x [DOI] [PubMed] [Google Scholar]
- 18. Gadzhanova SV, Roughead EE, Mackson JM. Initiation and duration of proton pump inhibitors in the Australian veteran population. Intern Med J 2012;42:e68–73. 10.1111/j.1445-5994.2010.02259.x [DOI] [PubMed] [Google Scholar]
- 19. National Institute for Health and Care Excellence Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. NICE guideline (CG184), 2014. Available: www.nice.org.uk/guidance/cg184/chapter/1-recommendations [Accessed 27 Mar 2018]. [PubMed]
- 20. Australian Therapeutic Guidelines Disorders of the oesophagus. Therapeutic Guidelines Ltd: Victoria, 2018. [Google Scholar]
- 21. Australian Medicines Handbook Gastrointestinal Drugs - Proton pump inhibitors. Adelaide: Australian Medicines Handbook Pty Ltd, 2018. [Google Scholar]
- 22. Batuwitage BT, Kingham JGC, Morgan NE, et al. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J 2007;83:66–8. 10.1136/pgmj.2006.051151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Family Medicine Research Centre. Sand Abstract No. 241 from the beach program: proton pump inhibitor use among general practice patients. Sydney: FMRC University of Sydney, 2016. [Google Scholar]
- 24. Wermeling M, Himmel W, Behrens G, et al. Why do GPs continue inappropriate Hospital prescriptions of proton pump inhibitors? A qualitative study. Eur J Gen Pract 2014;20:174–80. 10.3109/13814788.2013.844787 [DOI] [PubMed] [Google Scholar]
- 25. Thompson W, Black C, Welch V, et al. Patient values and preferences surrounding proton pump inhibitor use: a scoping review. Patient 2018;11:17–28. 10.1007/s40271-017-0258-4 [DOI] [PubMed] [Google Scholar]
- 26. Haastrup P, Paulsen MS, Begtrup LM, et al. Strategies for discontinuation of proton pump inhibitors: a systematic review. Fam Pract 2014;31:625–30. 10.1093/fampra/cmu050 [DOI] [PubMed] [Google Scholar]
- 27. Wathen B, Dean T. An evaluation of the impact of NICE guidance on GP prescribing. Br J Gen Pract 2004;54:103–7. [PMC free article] [PubMed] [Google Scholar]
- 28. Weekes LM, Mackson JM, Fitzgerald M, et al. National prescribing service: creating an implementation arm for national medicines policy. Br J Clin Pharmacol 2005;59:112–6. 10.1111/j.1365-2125.2005.02231.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levinson W, Kallewaard M, Bhatia RS, et al. ‘Choosing Wisely’: a growing international campaign. BMJ Qual Saf 2015;24:167–74. 10.1136/bmjqs-2014-003821 [DOI] [PubMed] [Google Scholar]
- 30. Mellish L, Karanges EA, Litchfield MJ, et al. The Australian pharmaceutical benefits scheme data collection: a practical guide for researchers. BMC Res Notes 2015;8:634 10.1186/s13104-015-1616-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. WHO ATC structure and principles : Guidelines for ATC classification and DDD assignment. 21st edn Oslo: WHO Collaborating Centre for Drug Statistics Methodology, 2018: 54–5. [Google Scholar]
- 32. Lopez Bernal J, Cummins S, Gasparrini A. The use of controls in interrupted time series studies of public health interventions. Int J Epidemiol 2018;47:2082–93. 10.1093/ije/dyy135 [DOI] [PubMed] [Google Scholar]
- 33. NPS MedicineWise For health professionals: Connect with meaningful, evidence-based information, resources and data insights to inform your practice. Sydney: National Prescribing Service Ltd, 2019. [Google Scholar]
- 34. NPS MedicineWise Nps MedicineWise annual report 2014-2015. Sydney: National Prescribing Service Ltd, 2016. [Google Scholar]
- 35. Choosing Wisely Australia Choosing wisely in Australia 2017 report. NPS MedicineWise: Sydney, 2017. [Google Scholar]
- 36. Choosing Wisely Australia The Royal Australian College of general practitioners: tests, treatments and procedures clinicians and consumers should question. NPS MedicineWise: Sydney, 2015. [Google Scholar]
- 37. Choosing Wisely Australia Gastroenterological Society of Australia: tests, treatments and procedures clinicians and consumers should question. NPS MedicineWise: Sydney, 2016. [Google Scholar]
- 38. Shumway R, Stoffer D, Models A. Time series analysis and its applications- with R examples. 4th edn Switzerland: Springer International Publishing, 2017: 77–155. [Google Scholar]
- 39. Department of Social Services Dss payment demographic data Commonwealth of Australia: Canberra, 2019. Available: https://data.gov.au/data/dataset/cff2ae8a-55e4-47db-a66d-e177fe0ac6a0 [Accessed 6 Aug 2019].
- 40. Australian Bureau of Statistics Quarterly population estimates (Erp), by State/Territory, sex and age. Canberra: Commonwealth of Australia, 2019. [Google Scholar]
- 41. Ailabouni NJ, Nishtala PS, Mangin D, et al. Challenges and Enablers of deprescribing: a general practitioner perspective. PLoS One 2016;11:e0151066 10.1371/journal.pone.0151066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;20 10.1002/14651858.CD003539.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pollmann AS, Murphy AL, Bergman JC, et al. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol 2015;16 10.1186/s40360-015-0019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging 2018;35:493–521. 10.1007/s40266-018-0544-4 [DOI] [PubMed] [Google Scholar]
- 45. Pratt NL, Kalisch Ellett LM, Sluggett JK, et al. Use of proton pump inhibitors among older Australians: national quality improvement programmes have led to sustained practice change. Int J Qual Health Care 2017;29:75–82. 10.1093/intqhc/mzw138 [DOI] [PubMed] [Google Scholar]
- 46. Chhina HK, Bhole VM, Goldsmith C, et al. Effectiveness of academic detailing to optimize medication prescribing behaviour of family physicians. J Pharm Pharm Sci 2013;16:511–29. 10.18433/J3KK6C [DOI] [PubMed] [Google Scholar]
- 47. Roughead EE, Kalisch Ellett LM, Ramsay EN, et al. Bridging evidence-practice gaps: improving use of medicines in elderly Australian veterans. BMC Health Serv Res 2013;13:514 10.1186/1472-6963-13-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chauhan BF, Jeyaraman M, Mann AS, et al. Behavior change interventions and policies influencing primary healthcare professionals’ practice—an overview of reviews. Implementation Science 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prior M, Guerin M, Grimmer-Somers K. The effectiveness of clinical guideline implementation strategies - a synthesis of systematic review findings. J Eval Clin Pract 2008;14:888–97. 10.1111/j.1365-2753.2008.01014.x [DOI] [PubMed] [Google Scholar]
- 50. Grol R, Wensing M. What drives change? barriers to and incentives for achieving evidence-based practice. Med J Aust 2004;180(6 Suppl):S57–60. [DOI] [PubMed] [Google Scholar]
- 51. Fischer F, Lange K, Klose K, et al. Barriers and strategies in guideline Implementation—A scoping review. Health Care 2016;4:36 10.3390/healthcare4030036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pharmaceutical Benefits Advisory Committee March 2018 PBAC Outcomes - other matters. Canberra: Australian Government Department of Health, 2018. [Google Scholar]
- 53. NPS MedicineWise Proton pump inhibitors: PBS changes May 2019; Changes to the PBS listing criteria for PPIs, effective from 1 May 2019. Sydney: National Prescribing Service Ltd, 2019. [Google Scholar]
- 54. PBS Information Management Section Pbs expenditure and prescriptions 2017-18. Department of Health: Canberra, 2019. [Google Scholar]
- 55. Gasparrini A, Cummins S, Bernal JL. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2016;46:348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pharmaceutical Benefits Advisory Committee July 2018 PBAC Outcomes - other matters. Canberra: Australian Government Department of Health, 2018. [Google Scholar]
- 57. Therapeutic Goods Administration Scheduling delegate’s final decisions, March 2016. Canberra: Proton Pump Inhibitors. Commonwealth Department of Health, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjqs-2019-009897supp001.pdf (579.3KB, pdf)


