Graphical abstract
Figure 4 modified: A curves showing survival of participants with severe COVID-19 pneumonia treated with tocilizumab: B. Kaplan-meier survival curves in patients with severe COVID-19 stratified according to ventilation ststus at baseline. B. Incidence of bloodstream infections over the study period.

Keywords: COVID-19, tocilizumab, IL-6 inhibitors
Abbreviations: COVID-19, CoronaVirus Disease (COVID)-19; SARS-CoV-2, Severe acute respiratory syndrome-Coronavirus-2; ARDS, Acute respiratory distress syndrome; IQR, interquartile rate; CPAP, Continuous positive airway pressure; ICU, intensive care unit; sHLH, haemophagocytic lymphohistiocytosis
Abstract
Background
Tocilizumab, a humanized monoclonal antibody, targets IL-6 receptors blocking downstream pro-inflammatory effects of IL-6. In preliminary reports it was suggested to be beneficial in patients with severe COVID-19.
Methods
In this open-label prospective study we describe clinical characteristics and outcome of 51 patients hospitalized with confirmed and severe COVID-19 pneumonia treated with tocilizumab intravenously. All patients had elevated IL-6 plasma level (>40 pg/mL) and oxygen saturation <93% in ambient air. Clinical outcomes, oxygen support, laboratory data and adverse events were collected over a follow-up of 30 days.
Results
Forty-five patients (88%) were on high-flow oxygen supplementation, six of whom with invasive ventilation. From baseline to day 7 after tocilizumab we observed a dramatic drop of body temperature and CRP value with a significant increase in lymphocyte count (p<0.001). Over a median follow-up time of 34 days from tocilizumab, 34 patients (67%) showed an improvement in their clinical severity class; 31 were discharged; 17 (33%) showed a worsening of their clinical status, of these 14 died (27%). The mortality rate was significantly associated with mechanical ventilation at baseline (83.3% vs 20% of patients on non-invasive oxygen support; p=0.0001). The most frequent side effects were an increase of hepatic enzymes (29%), thrombocytopenia (14%), and serious bacterial and fungal infections (27%).
Conclusion
Tocilizumab exerts a rapidly beneficial effect on fever and inflammatory markers, although no significant impact on the clinical outcome can be inferred by our results. Critically ill patients seem to have a high risk of serious infections with this drug.
1. Introduction
From December 2019 when the first outbreak of COVID-19 disease was reported in Wuhan, China, to April 19, 2020 a pandemic diffusion of a newly identified Betacoronavirus named SARS-CoV-2 occurred worldwide reaching a global number exceeding 2,000,000 confirmed cases [1], [2], [3]. At the beginning of the epidemic in the western countries, Italy presented the highest number of diagnosed cases in Europe and is currently the third country in the world most hit by the epidemic with a total of 181,228 cases, mostly recorded in the Region of Lombardy (37%) [3,4].
The case-fatality rate (CFR) of COVID-19 is approximately 6.8% [5], [6], [7] showing a wide range among countries (from 0.7 to 13.2), likely due to the differences in the estimate of the actual number of infected subjects. The significant morbidity and mortality of COVID-19 urgently requires effective and safe treatments.
In about 6-29% of infected patients a life-threatening pneumonia rapidly evolving to acute respiratory distress syndrome (ARDS) and requiring invasive ventilation is observed [8], [9], [10], [11]. This severe clinical picture of COVID-19 has been associated with an hyperinflammatory state resembling a cytokine storm syndrome with release of high levels of proinflammatory cytokines including interleukin (IL) -6, tumour necrosis factor α (TNF-α), IL-12 granulocyte colony stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α [12,13].
Promising results of an immune-based approach to COVID-19 treatment were initially reported in China on small case series of patients with severe disease treated with tocilizumab [14,15], that is an IL-6 receptor blocker, licensed for rheumatoid arthritis [16], cytokine release syndrome [17,18] and idiopathic multicentric Castleman's disease [19]. Several multicentre, randomised controlled trials of tocilizumab are currently ongoing in patients with COVID-19 pneumonia and elevated IL-6 worldwide.
Pending the results of these studies we report here our experience with the off-label use of tocilizumab in 51 patients with severe COVID-19 infection hospitalized in Milan, Italy.
2. Patients and methods
From March 10 to March 23, 51 patients hospitalized at the Infection Disease ward (IDW) or intensive care unit (ICU) of Fatebenefratelli-Sacco Hospital in Milan with SARS-CoV-2 pneumonia were offered an off-label treatment access to tocilizumab. SARS-CoV-2 pneumonia was confirmed by nasopharyngeal swabs tested positive by real-time reverse-transcriptase-polymerase-chain-reaction (ELITe InGenius® system and the GeneFinder COVID-19 Plus RealAmp Kit assay; ELITechGroup, France) and by a chest X-ray showing the presence of interstitial alterations and/or consolidation(s). The protocol was approved in emergency by the Institutional Ethic Committee. A written informed consent was obtained from all the patients, except for those on mechanical ventilation in ICU, for whom we applied the urgency principle.
The inclusion criteria were: age ≥ 18 years, respiratory rate ≥ 30/minutes, SpO2 < 93% while breathing room air, PaO2/FiO2 < 250 mmHg, IL-6 plasma level > 40 pg/mL. The exclusion criteria included: pregnancy, neutrophil count < 500 cells/µL, platelets count < 50,000/µL, concomitant immunosuppressive therapies, active tuberculosis, concomitant bacterial or fungal systemic infections.
According to the drug protocol established in our Hospital, patients who gave their consent received initially an off-label treatment with lopinavir-ritonavir (400 mg and 100 mg, respectively) BID plus hydroxychloroquine 200 mg BID.
Patients who satisfied the inclusion criteria received tocilizumab intravenously either at fixed dose of 400 mg at T0 followed by 400 mg after 12 hours or 8 mg/kg at T0 followed by 8 mg/kg after 12 hours (in patients with body weight ≥ 60 Kg).
Following the Chinese Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infection, the severity of SARS-CoV-2 disease was classified into four grades: 1) mild, with slight clinical symptoms and no evidence of pneumonia; 2) moderate, with fever, respiratory symptoms and confirmed pneumonia; 3) severe, with any of the following: respiratory distress with RR> 30 times/minutes, oxygen saturation at rest <93% or PaO2/FiO2 < 300 mmHg; 4) critically severe, with any of the following: respiratory failure needing mechanical ventilation, shock, or a combination of other organ failures requiring intensive care [20].
2.1. IL-6 test
Interleukin-6 serum concentrations were assessed on the fully automated immunochemistry platform COBAS e601 (Roche Diagnostics) by the proprietary electrochemilunescent immunoassay (ref. 05109442190, lot 43676101).
2.2. Data collection
At enrolment the following data were collected: demographic data, concomitant diseases (and Charlson comorbidity index, CCI), concomitant medications, pre-treatment serum IL-6 levels. Moreover, clinical symptoms, fraction of inspired oxygen (FiO2), peripheral oxygen saturations, ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (P/F), white blood cell count, lymphocytes and platelets count, serum levels of d-dimer, lactate dehydrogenase (LDH), aspartate transaminase (AST), alanine transaminase (ALT), C-reactive protein (CRP) and creatinine were collected at baseline, day 1, day 3 and day 7 from the start of tocilizumab treatment.
Data were censored on April 19, 2020 and follow-up information was collected by telephone calls for patients who had been discharged. The primary endpoints were death or hospital discharge. Secondary endpoints included: 1) a change in the disease severity grade and the change of oxygen therapy support in a scale from the need of mechanical ventilation to oxygen-therapy weaning at different time-points from tocilizumab initiation. Finally, we analysed the changes in body temperature and blood examinations from baseline to day 7 after tocilizumab administration.
2.3. Statistical analysis
Descriptive analyses of the variables were expressed as median (interquartile range [IQR]), or number (%). Continuous variables were compared using a nonparametric test (Mann-Whitney test). For categorical variables the Fisher's exact test was used. Survival curves were calculated for overall survival according to Kaplan-Meier method and comparisons were made using the log rank test.
Competing risk survival analysis method was applied to estimate the cumulative incidence of improving over time from the tocilizumab administration. This method allows to handle death as competing event, avoiding overestimation and bias that occurs using the Kaplan-Meier method. Univariate and multivariable Cox proportional hazard regression was used to estimate hazard ratios for death after adjusting for age, gender, time between tocilizumab and onset of symptoms, basal IL-6 levels, invasive ventilation at the time of treatment.
The variations in body temperature, blood cells count, AST, ALT, LDH, CRP and D-dimer were assessed using a multivariable linear mixed effects regression model, and the SAS PROC MIXED procedure was used to correlate repeated measures. All the statistical analyses were made using SAS version 9.4 software, and differences with p values of <0.05 were considered statistically significant.
3. Results
From March 10 to March 23, 51 patients received at least one dose of tocilizumab, including 18 (35%) who received a fix dose of 400 mg for two doses 12 hours apart and 33 (65%) who initiated a schedule with 8 mg/Kg repeated after 12 hours. Two patients did not receive the second dose because of death and for the onset of rash, respectively. The baseline characteristics of patients are described in Table 1 . Forty-five patients (88%) were on high-flow oxygen supplementation, six of whom with invasive ventilation, and only 6 (11.7%) had oxygen support by FiO2 ≤ 50% or by nasal cannula. Most of the patients at baseline had a disease classified as severe (84%), or critically severe (12%).
Table 1.
Characteristics of the study population.
| Characteristic | Total (n=51) |
| Gender, n (%) Male | 40 (78.4) |
| Age, median years (IQR) | 60 (50-70) |
| Age >65 years, n (%) | 19 (37.2%) |
| Ward of hospitalization | |
| Infectious Diseases ward | 42 (82.4%) |
| Intensive care unit | 9 (17.6%) |
| Signs/symptoms at hospital admission, n (%) | |
| Fever | 38 (74.5) |
| Cough | 32 (62.7) |
| Dyspnoea | 28 (54.9) |
| Comorbidities, number, median (IQR) | 1 (0-2) |
| Charlson Comorbidity Index, median (IQR) | 2 (1-3) |
| Types of comorbidities, n (%) | |
| Cardiovascular diseases | 25 (49.0) |
| Hypertension | 15 (29.4) |
| Diabetes | 6 (11.8) |
| Chronic lung diseases | 5 (9.8) |
| Cancer* | 3 (5.9) |
| Rheumatologic diseases | 3 (5.9) |
| Others§ | 3 (5.9) |
| Time from hospitalization to baseline (days), median (IQR) | 3 (2-6) |
| Time from illness onset to baseline (days), median (IQR) | 12 (10-16) |
| Additional medications for COVID-19, n (%) | |
| Hydroxycloroquine | 50 (98) |
| Lopinavir/ritonavir | 42 (82) |
| Remdesivir | 24 (42) |
| Antibiotics | 39 (76) |
| Body temperature >37.5°C, n (%) | 39 (76) |
| Chest X-ray, n (%) | 51 (100) |
| No alterations | 1 (2.0) |
| Diffuse interstitial opacities⁎⁎ | 43 (84) |
| Consolidation(s) | 17 (33) |
| Pleural effusion | 1 (2.0) |
| Oxygen support therapy, n (%) | |
| None | 0 (0) |
| Nasal cannula | 3 (5.9) |
| Venturi-type mask | 20 (39.2) |
| Continuous positive airway pressure | 22 (43.1) |
| Invasive ventilation | 6 (11.8) |
| Disease severity†, n (%) | |
| Mild | 0 (0) |
| Moderate | 2 (3.9) |
| Severe | 43 (84.3) |
| Critically severe | 6 (11.8) |
| Laboratory examinations, median (IQR) | . |
| WBC count × 103/μL | 9.1 (6.5-10.9) |
| Lymphocyte count × 103/μL | 0.8 (0.6-1.1) |
| Neutrophil count × 103/μL | 7.3 (5.0-11.0) |
| Platelets × 103/μL | 230 (169-337) |
| Lactate dehydrogenase (IU/L) | 470 (400-561) |
| AST (IU/L) | 48 (36-69) |
| ALT (IU/L) | 39 (23-60) |
| C-reactive protein (mg/L) | 189 (138-268) |
| D-dimer (μg/L) | 1706 (860-5261) |
| Interleukin-6 (pg/mL) | 116 (65-180) |
IQR=interquartile range; WBC=white blood cell; CT= computed tomography; AST= aspartate aminotransferase; ALT= alanine aminotransferase.
All in remission;
HIV, chronic hepatitis C, previous tuberculosis;
CT-scan was performed in 9 patients and showed Multiple ground-glass opacities †As classified by Wu et al. [11].
Almost 50% of patients showed a concomitant cardiovascular disease, with arterial hypertension being the most frequent comorbidity (60%). Besides tocilizumab, during the hospital stay most of the patients were treated with either hydroxycloroquine (98%) associated with lopinavir/ritonavir in 84% of these cases, or remdesivir (45%). Furthermore, 76% of patients also received antibiotic therapy. The timing of concomitant anti-CoV-2 drugs is reported in Fig. 1 . The median interval time from the onset of symptoms to the start of treatment with tocilizumab was 12 days (IQR, 10-16).
Fig. 1.
Concomitant anti COVID-19 therapies administered during hospitalization for each patient. The point zero vertical bar represents the time of tocilizumab administration. The horizontal lines indicate the timing and duration of hydroxychloroquine (A), lopinavir-ritonavir (B) and remdesivir (C) administration for each patient.
3.1. Treatment outcomes
The patients were followed up for a median of 34 days (IQR 32-37) after receiving the first dose of tocilizumab; during this time 31 patients (61%) had been discharged, 14 (27%) had died and 6 (12%) were still hospitalized at censoring date.
The mortality rate over a 30-days observation period was 27%, including 5/6 patients (83%) who were receiving invasive ventilation at the time of tocilizumab treatment and 9 of 45 (20%) who were receiving noninvasive oxygen support. Fig. 2 shows the Kaplan Meier cumulative survival curve (A) and the survival curves stratified by invasive mechanical ventilation or non-invasive oxygen supplementation (B) (p=0.0001). Respiratory failure and ARDS was identified as the most frequent cause of death, although 4 patients had concomitant septic shock and multi-organ failure.
Fig. 2.
A. Kaplan-Meier curves showing survival of participants with severe COVID-19 pneumonia treated with tocilizumab; B. Survival curves in the cohort stratified according to ventilation status at baseline.
Results of the multivariable Cox proportional hazard model showed that only being on invasive mechanical ventilation at the time of treatment was independently associated with increased risk of death, adjusted HR 7.18 (CI 95% 2-25; p=0.002). Neither age and gender nor the interval between the onset of symptoms and tocilizumab administration resulted to significantly influence the clinical outcome.
3.2. Secondary outcomes
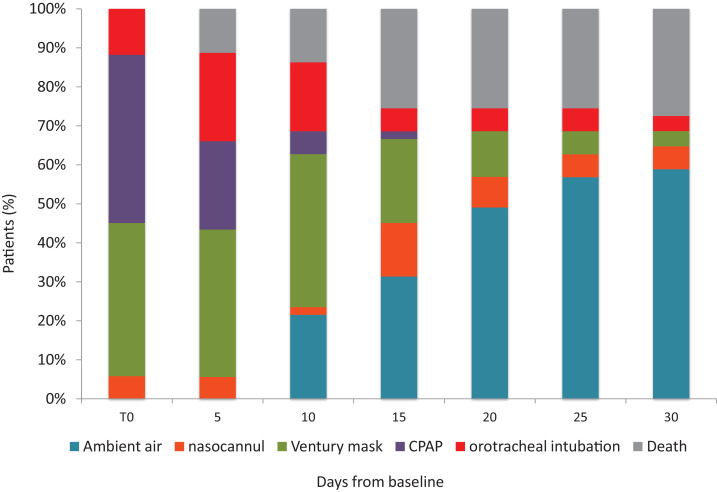
Over the follow-up time after tocilizumab administration, 34 patients (67%) showed an improvement in their clinical severity class; 17 (33%) showed a worsening of their clinical status, including the 14 patients who died. Fig. 3 describes the changes in oxygen-support at the different time points from tocilizumab administration. Overall, 34 of 51 (66,6%) patients reached a reduction in the intensity of oxygen therapy or were weaned off oxygen support. However, none of the 6 patients who were treated while receiving invasive mechanical ventilation have been extubated, and 16/51 (33%) patients did not change their status or needed an intensification of oxygen support, including 14 who eventually died.
Fig. 3.
Change in oxygen support, and deaths over the 30-days follow-up. T0 was the day on which tocilizumab was initiated. CPAP, Continuous positive airway pressure.
The cumulative incidence of improving over time from the tocilizumab administration is shown by a competing risk survival analysis and death is handled as competing event. Dashed lines define the 95% confidence interval range.
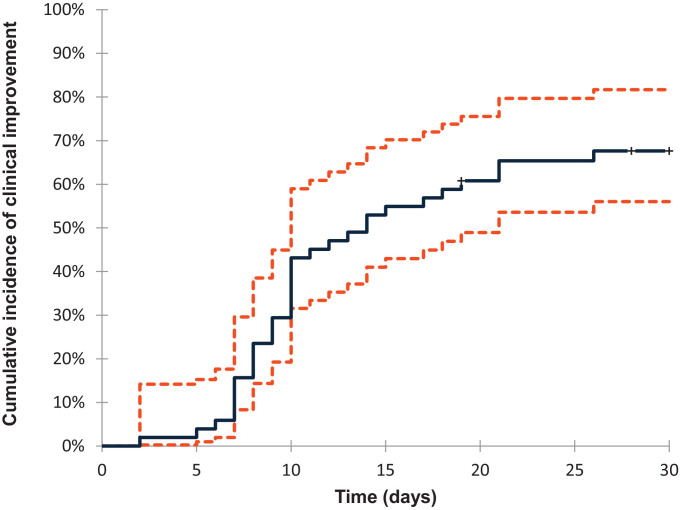
The cumulative incidence of clinical improvement from baseline to day 30 of follow up, as defined by either a decrease of 1 or more points in the severity class category or discharge, was 67% (95% confidence interval [CI], 56-82) and is reported in Fig. 4 .
Fig. 4.
Cumulative Incidence of clinical improvement from baseline to day 30.The cumulative incidence of improving over time from the tocilizumab administration is shown by a competing risk survival analysis and death is handled as competing event. Dashed lines define the 95% confidence interval range.
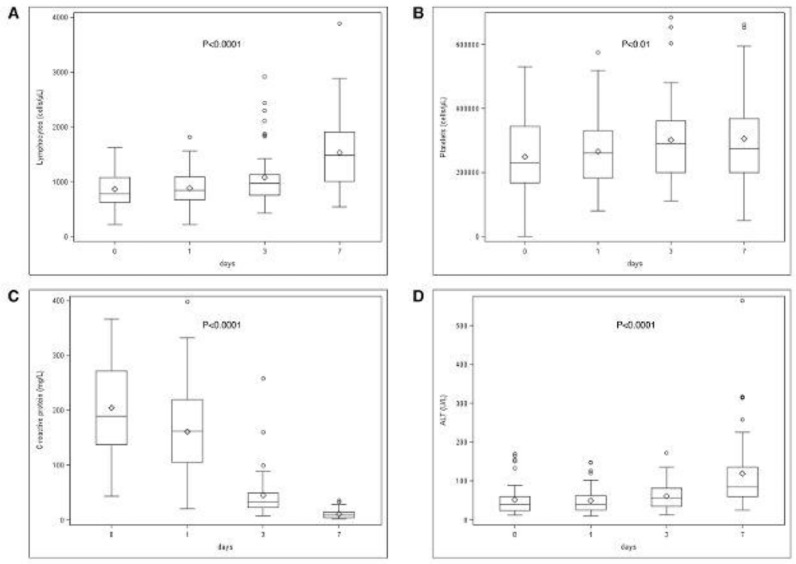
As for the other secondary endpoints (body temperature, blood cells counts, AST, ALT, LDH, CRP and D-dimer), data were recorded at baseline and day 1, 3, and 7 after the first dose of tocilizumab. The baseline values are reported in Table 1. We observed a dramatic drop of body temperature and CRP value, a significant increase in lymphocyte and platelet counts, and a significant increase in aminotransferases within day 7 (Fig. 5 ). Total white blood cells and neutrophils showed only a transitory decline at day 1 and 3 with no significant difference at day 7 (data not shown).
Fig. 5.
Changes in the values of lymphotytes (A), platelets (B), CRP (C) and ALT (D) after treatment with tocilizumab. The time points considered are: T0, the day of tocilizumab administration, day 1, day 3 and day 7 after treatment.
Total lymphocytes and CRP returned to normal values in 28 patients (55%), and 36 patients (71%), respectively. No change occurred for LDH and D-dimer values.
3.3. Safety
The most common adverse events were the increase of hepatic enzymes of at least 3 times above the normal values (29%), thrombocytopenia (14%), neutropenia (6%) and cutaneous rash (2%). Bacteremia emerged in 14 patients (27%), 86% of whom were hospitalized in ICU. Blood cultures gave a positive result in 31 episodes on 14 patients and the most frequently isolated pathogens were Enterococcus spp. (8), Carbapenemase-producing Klebsiella pneumoniae (5), extended spectrum beta-lactamase (ESBL)-producing Klebsiella pneumoniae (5), Candida spp. (3), Coagulase-negative Staphylococci (4), methicillin-resistant Staphylococcus aureus (3), Pseudomonas aeruginosa (1), ESBL producing E. coli (1), Enterobacter aerogenes (1). The median time from tocilizumab administration to the bacteremic event was 11 days (IQR 9-13).
4. Discussion
To the best of our knowledge, this is the largest cohort of patients with severe COVID-19 pneumonia treated in an off-label access with tocilizumab. It also analyzes the longest post-therapy follow-up so far.
Although at present the only recognized standard treatment of COVID-19 pneumonia is oxygen support and the management of ARDS, several therapeutic approaches have been proposed and tested including antivirals (lopinavir/ritonavir, hydroxychloroquine, remdesivir) [21], [22], [23], [24], [25] and anti-inflammatory drugs (corticosteroids, COX-2 inhibitors, (IL)-6 and (IL)-1 receptor antagonists) [26]. The use of tocilizumab, a recombinant humanized anti-human IL-6 receptor monoclonal antibody that binds both membrane bound IL-6 receptor (mIL6R) and soluble IL-6 receptor (sIL- 6R), was inferred from the observation that patients with severe COVID-19 might develop a hyper-inflammatory state resembling the cytokine release syndrome observed in secondary haemophagocytic lymphohistiocytosis (sHLH) for which tocilizumab is registered [16,17]. sHLH is a hyperinflammatory syndrome that leads to fatal hypercytokinaemia and multiorgan failure and shows features of inflammation similar to those observed in COVID 19 [10,27]: fever, highly increased ferritin level, decreased platelet counts, high erythrocyte sedimentation rate and high circulating IL-6, IL-2, IL-7, IL-10, granulocyte-colony stimulating factor (G-CSF), interferon-γ-inducible protein (IP10), monocyte chemoattractant protein (MCP1), macrophage inflammatory protein 1 alpha (MIP1A), and TNF-α. [28].
Several phase 2 and 3 randomized clinical trials with the use of tocilizumab in severe COVID-19 infection are ongoing in China, Europe and USA. To date, Xu et al. [14] reported encouraging preliminary results in 21 patients with severe COVID-19 pneumonia treated with tocilizumab in an open-label study conducted in China. Over a five-days follow-up, they observed an immediate improvement of all clinical symptoms: normalization of body temperature in all patients, reduction of oxygen requirements in 75% of cases, improvement of CT scan imaging in 90.5%, normalization of lymphocytes count in 52% of subjects, significant reduction of CRP values in 84%. No adverse events were reported and none of the patients died.
We report herein our experience regarding the off-label treatment with tocilizumab of the first 51 patients with severe and critical forms of COVID-19. Over a median follow-up of 34 days, 61% were discharged in good clinical conditions, 27% died and 12% are still hospitalized. A clinical improvement associated with reduced need of oxygen flows was observed in 67% of patients, while 33% worsened and the 30-days mortality was 27%, similar to that reported in Wuhan, China, on 201 patients hospitalized with severe disease where the overall a mortality was 22% and 66% in those on mechanical ventilation [11]. In agreement with Xu et al. a decrease in inflammatory symptoms and markers was rapidly seen in our patients associated with an increase in lymphocyte and platelet counts. Nevertheless, in the most critically ill patients and with a longer follow-up period, no clinical improvement or reduction of oxygen supplementation was seen and 5/6 patients receiving tocilizumab while mechanically ventilated died. Indeed, most of our deaths occurred between day 7 and day 21 of follow-up.
More recently, two small case series regarding patients with COVID-19 pneumonia treated with tocilizumab in China and Italy have been published [19,29]. Rapid improvement of respiratory conditions, fever and inflammation was observed in the Italian case report, although these three patients were moderately ill at dosing of tocilizumab. By contrast, a high mortality rate (40%) was shown in the series from China with the Authors concluding that tocilizumab did not modify the disease outcome in critically ill patients. However, these reports are not comparable mainly because of the different tocilizumab dosage used, the different concomitant medications and the characteristics of patients studied.
Additionally, we observed a number of both early or late adverse events. Among the early potentially drug-related side effects the most frequently seen were hypertransaminasemia (15/51), thrombocytopenia (7/51) and neutropenia (3/51), although the role of SARS-CoV-2 per se or of concomitant medications cannot be excluded. The late events were serious bacterial and fungal infections of the bloodstream. The latter occurred in 12/14 patients while in ICU. In particular, the three episodes of candidemia are concerning because of their life threatening nature and may indicate a severe underlying immunosuppression. Indeed, the use of tocilizumab in patients with rheumatoid arthritis is known to be associated with an increased risk of serious bacterial and fungal infections [30, 31] as a result of the blockade of IL-6 that may impair B-cell proliferation and T-cell differentiation and cytotoxicity, essential for immune control of infections [32]. This is particularly true for patients hospitalized in ICU, who are known to be at higher risk of bloodstream infections [33]. Indeed, we found mechanical ventilation at the time of tocilizumab therapy to be independently associated with the risk of death at multivariable analysis. A possible explanation of the different incidence of side effects, in particular infective events, between our study and previous reports might be the longer follow up of our observation, given the half-life of tocilizumab and the persistence of active drug levels for weeks after the infusion [34].
Our study has several limitations. First and foremost, the lack of a randomized control group, does not allow us to draw definitive conclusions. In addition, all our patients were treated with hydroxychloroquine, lopinavir/ritonavir or remdesivir, alone or in combination. The variety of treatment schedules used accounted for the absence of defined treatment protocols that reflects the first few weeks of the COVID-19 epidemic and represents an evident confounding factor in the analysis of potential therapeutic efficacy. However, in our cohort the high mortality rate was observed over a longer follow-up period than in other published, uncontrolled experiences with tocilizumab. In addition, this elevated mortality rate did not differ from what is reported in other cohorts of severely ill patients in whom different treatments other than tocilizumab were used [11]. Only randomized, controlled trials will clarify the efficacy of tocilizumab in the different severity categories of patients and the best timing of its administration and will identify the subgroup of patients for whom immunosuppression could be harmful.
In conclusion, tocilizumab seems to exert a rapidly beneficial effect on fever and inflammation, although no significant impact on the clinical outcome can be inferred by our results. Furthermore, special attention must be paid when treating critically ill patients with COVID-19, because of the high risk of life-threatening secondary infections we have documented in this fragile population.
Fundings
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interests
None.
Acknowledgements
We are grateful to Dr Alberto Dolci for dosing the basal IL-6 levels of the patients. We thank Mrs Anna Piccini for the art graphic support.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating, Research Team A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control (ECDC). https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases. [Accessed April 19, 2020].
- 4.Spiteri G, Fielding J, Diercke M, Campese C, Enouf V, Gaymard A, Bella A, Sognamiglio P, Sierra Moros MJ, Riutort AN, Demina YV, Mahieu R, Broas M, Bengnér M, Buda S, Schilling J, Filleul L, Lepoutre A, Saura C, Mailles A, Levy-Bruhl D, Coignard B, Bernard-Stoecklin S, Behillil S, van der Werf S, Valette M, Lina B, Riccardo F, Nicastri E, Casas I, Larrauri A, Salom Castell M, Pozo F, Maksyutov RA, Martin C, Van Ranst M, Bossuyt N, Siira L, Sane J, Tegmark-Wisell K, Palmérus M, Broberg EK, Beauté J, Jorgensen P, Bundle N, Pereyaslov D, Adlhoch C, Pukkila J, Pebody R, Olsen S, Ciancio BC. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9) doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Coronavirus Disease 2019 (COVID-19) Situation Report-90. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. [Accessed April 19, 2020].
- 6.Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, Sun W, Wang Y. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25757. Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020 Feb 28. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. 1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease. J Clin Invest. 2019;2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y. Effective treatment of severe COVID-19 patients with tocilizumab.chinaXiv:202003.00026v1. [DOI] [PMC free article] [PubMed]
- 15.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020:1–5. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaly L, Rosner I. Tocilizumab - a novel therapy for non-organ-specific autoimmune diseases. Best Pract Res Clin Rheumatol. 2012;26:157–165. doi: 10.1016/j.berh.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 17.United States Food and Drug Administration Press Release; April 19, 2020. FDA approval brings first gene therapy to the United States.https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm574058.htm Available from. [Accessed. [Google Scholar]
- 18.Riegler LL, Jones GP, Lee DW. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther Clin Risk Manag. 2019;15:323–335. doi: 10.2147/TCRM.S150524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimoto N, Honda O, Sumikawa H, Johkoh T, Aozasa K, Kanakura Y, Japanese MRA Study Group on MCD A long-term (5-year) sustained efficacy of tocilizumab for multicentric castleman's disease and the effect on pulmonary complications. Blood. 2007;110:646. doi: 10.1182/blood.V110.11.646.646. [DOI] [Google Scholar]
- 20.Xu YH, Dong JH, An WM, Lv XY, Yin XP, Zhang JZ, Dong L, Ma X, Zhang HJ, Gao BL. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020;80:394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 Mar 9: doi: 10.1093/cid/ciaa237. ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent JL, Gorham J, Taccone FS. Understanding pathways to death in patients with COVID-19. Lancet Respir Med 2020:S2213-2600 (20)30165-X. 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed]
- 23.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020 Mar 18: doi: 10.1056/NEJMoa2001282. NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020 Apr 10 doi: 10.1056/NEJMoa2007016. NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S, Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020 Mar 27;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seguin A, Galicier L, Boutboul D, Lemiale V, Azoulay E. Pulmonary Involvement in Patients With Hemophagocytic Lymphohistiocytosis. Chest. 2016;149:1294–1301. doi: 10.1016/j.chest.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. 1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Giambenedetto S, Ciccullo A, Borghetti A, Gambassi G, Landi F, Visconti E, et al. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J Med Virol 2020:0–2. 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed]
- 30.Lang VR, Englbrecht M, Rech J, Nüsslein H, Manger K, Schuch F. Risk of infections in rheumatoid arthritis patients treated with tocilizumab. Rheumatology. 2012;51:852–857. doi: 10.1093/rheumatology/ker223. [DOI] [PubMed] [Google Scholar]
- 31.Winthrop KL, Mariette X, Silva JT, Benamu E, Calabrese LH, Dumusc A. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors) Clin Microbiol Infect. 2018;24:S21–S40. doi: 10.1016/j.cmi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Acelas AL, de Abreu Almeida M, Engelman B, Cañon-Montañez W. Risk factors for health care–associated infection in hospitalized adults: systematic review and meta-analysis. Am J Infect Control. 2017;45:e149–e156. doi: 10.1016/j.ajic.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Pham T, Claudepierre P, Constantin A, de Bandt M, Fautrel B, Gossec L, Gottenberg JE, Goupille P, Guillaume S, Hachulla E, Masson C, Morel J, Puéchal X, Saraux A, Schaeverbeke T, Wendling D, Bruckert E, Pol S, Mariette X, Sibilia J. Club Rhumatismes et Inflammation (CRI). Tocilizumab: therapy and safety management. Joint Bone Spine. 2010;77 Suppl 1:100–101. doi: 10.1016/S1297-319X(10)70001-4. Jun. [DOI] [PubMed] [Google Scholar]