Abstract
Objective:
Both delirium duration and delirium severity are associated with adverse patient outcomes. Serum biomarkers associated with delirium duration and delirium severity in intensive care unit (ICU) patients have not been reliably identified. We conducted our study to identify peripheral biomarkers representing systemic inflammation, impaired neuroprotection, and astrocyte activation associated with delirium duration, delirium severity, and in-hospital mortality.
Design:
Observational study.
Setting:
Three Indianapolis hospitals.
Patients:
321 critically ill delirious patients.
Interventions:
None
Measurements and Main Results:
We analyzed the associations between biomarkers collected at delirium onset and delirium/coma free days assessed through RASS/CAM-ICU, delirium severity assessed through CAM-ICU-7, and in-hospital mortality. After adjusting for age, gender, APACHE II score, Charlson comorbidity score, sepsis diagnosis and study intervention group, IL-6, 8, 10, TNF-alpha, CRP and S-100β levels in quartile 4 were negatively associated with delirium/coma free days by one week and 30-days post enrollment. IGF-1 levels in quartile 4 were not associated with delirium/coma free days at both time-points. IL-6, 8, 10, TNF-alpha, CRP and S-100β levels in quartile 4 were also associated with delirium severity by one week. At hospital discharge, IL-6, 8, and 10 retained the association but TNF-alpha, CRP, and S-100β lost their associations with delirium severity. IGF-1 levels in quartile 4 were not associated with delirium severity at both time-points. IL-8 and S-100β levels in quartile 4 were also associated with higher in-hospital mortality. IL-6, 10, TNF-alpha and IGF-1 were not found to be associated with in-hospital mortality.
Conclusions:
Biomarkers of systemic inflammation and those for astrocyte and glial activation were associated with longer delirium duration, higher delirium severity, and in-hospital mortality. Utility of these biomarkers early in delirium onset to identify patients at a higher risk of severe and prolonged delirium, and delirium related complications during hospitalization needs to be explored in future studies.
Keywords: delirium, coma, biomarkers, ICU, mortality
INTRODUCTION:
Delirium or acute brain failure is a complex neuropsychiatric syndrome characterized by acute and fluctuating changes in cognition and consciousness.1 Approximately 50% to 80% of critically ill mechanically ventilated patients have delirium for at least one day of their intensive care unit (ICU) or hospital stay.2–4 Not only does the presence of delirium portend adverse patient-related consequences; both delirium duration and severity have also been associated with mortality,3–6 although it remains unclear if the relationship is causal. At present, there are no reliable biomarkers associated with longer delirium duration and higher delirium severity in critically ill patients with delirium.
Current hypothetical models of delirium pathophysiology focus on systemic inflammation, loss of neuronal protection, and astrocyte and microglial activation.7 Systemic inflammation is common among ICU patients and may predispose to blood-brain barrier disruption, resulting in peripheral leukocyte infiltration into the central nervous system.8–11 The resultant neuro-inflammation promotes a state of cholinergic failure, predisposing to delirium.12 Similarly, depletion of neurotrophic factors such as insulin-like growth factor-1 (IGF-1), required for neuronal development and survival could destabilize neuronal integrity in the face of insults and may predispose to delirium or its adverse consequences.13–14 The neuro-inflammation activates microglia, which produce local pro-inflammatory cytokines, reactive oxygen species and activation of astrocytes.15,16 Astrocyte and glial activation can result in elevated S-100β levels, with the external manifestation of delirium.17,18
While prior studies have shown associations between systemic biomarkers of inflammation (IL-6, 8, C-reactive protein) and S-100β with delirium occurrence,19 the relationships between systemic inflammation, loss of neurotrophic factors, and astrocyte and glial activation with delirium duration, delirium severity, and downstream mortality have not been comprehensively evaluated in critically ill patients. We conducted a prospective, observational study with the hypothesis that peripheral biomarkers representing systemic inflammation, impaired neuroprotection, and astrocyte activation will be independently associated with delirium duration, delirium severity, and mortality in critically ill patients.
METHODS:
The Institutional Review Board of Indiana University approved the study. Informed consent was obtained from patient’s legally authorized representative.
Study Setting and Patient population:
Patients were included if they were admitted to the ICU services of three Indianapolis hospitals from March 2009–January 2015, enrolled in the Pharmacological Management of Delirium (PMD) trials,20,21 and have blood samples collected for biomarker analyses. PMD comprised two randomized trials that investigated the effectiveness of low dose haloperidol and deprescribing of anticholinergics and benzodiazepine in reducing delirium duration or severity. There were no significant differences in clinical outcomes between the intervention and control groups.20,21 The three hospitals included Eskenazi Health, a 457-bed public hospital with 22-bed medical/surgical ICU (MICU/SICU) and a 29-bed step-down ICU, Indiana University (IU) Health University Hospital, a 257-bed tertiary care hospital with 36 MICU and SICU beds and IU Health Methodist Hospital, an 802-bed tertiary care center with 65 MICU and SICU beds.
Inclusion criteria: 1) patients admitted to the ICU for ≥ 24 hours, 2) age ≥ 18 years, 3) screen positive for delirium based on the Richmond Agitation-Sedation Scale (RASS)22 and the Confusion Assessment Method for the ICU (CAM-ICU)23, 4) English-speaking and 5) have a blood draw for candidate biomarkers analyses at enrollment. Exclusion criteria: 1) history of severe mental illness, 2) severe cognitive impairment or severe dementia per electronic medical records, 3) alcohol-related delirium, 4) aphasic stroke, 5) pregnant or nursing, or 6) previously enrolled in the study.
Procedures and Data Collection:
Outcome Measures:
a). Delirium/coma free days:
Delirium/coma free days were defined as the number of days after enrollment a patient was alive and free of delirium or coma. Delirium/coma free days provide an estimate of duration of normal brain function free of coma and delirium and hence function as negative surrogate of delirium duration not confounded by coma or death. Delirium/coma free days as an outcome has been used previously in high impact studies and takes into account confounding by death and discharge.2, 24 The CAM-ICU and RASS have been validated in varied ICU populations and were used to detect delirium and coma throughout the hospital stay.22, 23, 25
b). Delirium Severity:
Delirium severity was assessed by the CAM-ICU-7,26 a seven-point rating scale (0–7), derived from the RASS and the CAM-ICU. The CAM-ICU-7 score ranges from 0–7; categorized as 0–2: no delirium, 3–5: mild to moderate delirium, and 6–7: severe delirium. Trained research assistants conducted twice-daily RASS, CAM-ICU, and CAM-ICU-7 assessments after 24 hours of ICU admission until patients’ hospital discharge or death.
Biomarkers:
The biomarkers were selected a-priori based on literature review27 and expert opinion and included inflammatory markers [Interleukin (IL)-6, 8, 10, tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP)], neuroprotection (IGF-1), and astrocyte and glial activation (S-100β). A venous blood sample was collected within 24 hours of enrollment between 9–11:00 am (Supplementary material).
Other data collection:
We collected demographics, prior cognition and functional status,28–30 reasons for admission, severity of illness,31 chronic comorbidities,32 mortality and medication dispensing data (Supplementary material).
Statistical Analysis:
All biomarkers with values below the detectable limit were imputed with the midpoint between 0 and the minimum detectable limit. Two time points were used for delirium/coma free days: one week post-randomization as this was the end of the haloperidol use in the intervention group, and day 30 post-randomization as this was the end of active delirium monitoring. Patients who died before one week or day 30 had their subsequent delirium/coma free days counted as 0. Patients who were discharged alive before day 8 or 30 had the remaining days counted as delirium/coma free. For delirium severity, average observed CAM-ICU-7 scores up to one week or at hospital discharge were used and no data extrapolation was done to fill the missing observations. We compared the distributions of biomarker values between the intervention and control groups using Wilcoxon rank sum tests. We used Spearman correlation coefficients to assess the relationship between biomarkers. To explore potential non-linear relationship between biomarkers and clinical outcomes and to minimize influence of potential outliers, we categorized each biomarker into quartile groups. Each biomarker’s association with the clinical outcomes was individually tested using models adjusting for age, gender, APACHE II score, Charlson comorbidity score, sepsis diagnosis, and study groups. Specifically, we used analysis of covariance (ANCOVA) models for delirium severity (through day 8 and discharge) and delirium/coma free days (day 8 and 30). Logistic regression was used for in-hospital mortality. We also examined the interaction terms between biomarker and intervention groups and found no significant interaction in any of the models. Since this was one of the earlier studies to determine the effects of multiple biomarkers of inflammation, neuro-protection, and astrocyte and glial activation with delirium outcomes, we did not adjust our results for multiple comparisons. We focused our analyses on identifying biomarkers with consistent associations to delirium outcomes. All analyses were conducted using SAS v9.4.
RESULTS:
The study cohort was comprised of 321 critically ill patients with delirium. Baseline characteristics are presented in Table 1. Supplementary Table 1 shows the median biomarker values by intervention groups. Besides IL-6 in the intervention arm of one PMD trial, no significant differences were found between the four groups. In addition, no significant interactions between biomarkers and any of the outcomes were found indicating the groups did not differ in the associations between biomarkers and delirium duration, delirium severity and mortality (Supplementary Table 2 provides delirium duration model at one week). Therefore, we decided to report analysis results in all patients. Table 2 provides Spearman’s correlations among biomarkers. IGF-1 negatively correlated with S-100β (p<0.0001) and IL-8 (p=0.002). IL-6, 8, 10, and TNF-alpha positively correlated with each other (p<0.0001). S-100β positively correlated with IL-6, 8, 10, and TNF-alpha (p<0.0001).
Table 1:
Baseline Patient Characteristics and Outcomes of the Study Population
| Variable | Cohort (n=321) |
|---|---|
| Age | 60 (52,69) |
| Female n (%) | 179 (55.8) |
| African-American n (%) | 155 (48.7) |
| Hispanic n (%) | 2 (0.6) |
| Education (years) | 12 (10, 12) |
| APACHEa II | 21 (15, 26) |
| Charlson Comorbidity Index | 3 (1, 5) |
| Activities of Daily Living (ADL) | 6 (5, 6) |
| Instrumental Activities of Daily Living (IADL) | 8 (3,8) |
| IQCODEb | 3 (3, 3.3) |
| Mechanically Ventilated n (%) | 196 (61.1) |
| ICU Location | |
| Medical ICU n (%) | 219 (68.2) |
| Surgical ICU n (%) | 76 (23.7) |
| Progressive/step-down ICU n (%) | 26 (8.1) |
| Diagnoses n (%) | |
| Acute Respiratory Failure and/or Sepsis | 167 (52) |
| Neurological conditionsc | 26 (8) |
| Othersd | 128 (40) |
| Biomarker values | |
| S-100β ng/ml | 0.11 (0.06, 0.21) |
| IL-6e pg/ml | 22.0 (7.0, 49.9) |
| IL-8 pg/ml | 27.1 (15.0, 58.8) |
| IL-10 pg/ml | 8.9 (2.5, 22.7) |
| IGF-1f ng/ml | 36.4 (26.1, 62.5) |
| C-Reactive Protein μg/ml | 30.9 (19.3, 40.6) |
| TNF-alphag pg/ml | 10.7 (5.8, 17.9) |
| Outcomes | |
| Delirium duration in days | 2 (0, 4) |
| Delirium/coma free days by one week | 5 (2, 7) |
| Delirium/coma free days by 30 days | 26 (19, 29) |
| Delirium severity by one week mean (SD) | 3.8 (1.6, 5.8) |
| Delirium severity by discharge mean (SD) | 2.8 (1.3, 4.6) |
| ICU length of stay (days) | 10 (5, 17) |
| Hospital length of stay (days) | 12 (8, 21) |
| ICU mortality n (%) | 30 (9.4) |
| In-hospital mortality n (%) | 35 (10.9) |
Acute Physiology and Chronic Health Evaluation Score
Informant Questionnaire on Cognitive Decline in the Elderly
Neurological conditions include traumatic brain injury, status epilepticus and cerebrovascular accidents
Others: Include trauma, cardiovascular and gastrointestinal diagnoses
Values are presented as median (interquartile ranges) unless otherwise specified
IL: Interleukin
IGF-1: Insulin-like growth factor-1
TNF: Tumor Necrosis Factor
Table 2:
Correlations among baseline biomarkers of Inflammation, neuro-protection, and astrocyte and glial activation
| Biomarkers | IGF-1 | IL10 | IL6 | IL8 | CRP | S-100β | TNF-alpha |
|---|---|---|---|---|---|---|---|
| IGF-1 | |||||||
| Correlation | 1.00 | −0.11 | −0.03 | −0.18 | 0.08 | −0.24 | −0.05 |
| P-value | 0.051 | 0.536 | 0.002 | 0.130 | <.0001 | 0.410 | |
| N | 320 | 319 | 319 | 319 | 319 | 317 | 319 |
| IL10 | |||||||
| Correlation | 1.00 | 0.43 | 0.46 | 0.04 | 0.27 | 0.40 | |
| P-value | <.0001 | <.0001 | 0.477 | <.0001 | <.0001 | ||
| N | 320 | 320 | 320 | 318 | 316 | 320 | |
| IL6 | |||||||
| Correlation | 1.00 | 0.55 | 0.42 | 0.20 | 0.52 | ||
| P-value | <.0001 | <.0001 | <.0001 | <.0001 | |||
| N | 320 | 320 | 318 | 316 | 320 | ||
| IL8 | |||||||
| Correlation | 1.00 | 0.20 | 0.29 | 0.60 | |||
| P-value | <.0001 | <.0001 | <.0001 | ||||
| N | 320 | 318 | 316 | 320 | |||
| CRP | |||||||
| Correlation | 1.00 | 0.04 | 0.41 | ||||
| P-value | 0.482 | <.0001 | |||||
| N | 319 | 316 | 318 | ||||
| S-100β | |||||||
| Correlation | 1.00 | 0.33 | |||||
| P-value | <.0001 | ||||||
| N | 317 | 316 | |||||
| TNF-alpha | |||||||
| Correlation | 1.00 | ||||||
| P-value | |||||||
| N | 320 | ||||||
IL: Interleukin, IGF-1: Insulin-like growth factor-1, CRP: C-reactive protein, TNF: Tumor Necrosis Factor
Delirium/coma free days:
After adjusting for age, gender, APACHE II score, Charlson comorbidity score, sepsis diagnosis and study intervention group, biomarkers of inflammation IL-6, 8, 10, and TNF-alpha in the highest quartile (Q4) were negatively associated with delirium/coma free days by one week [IL-6: β −2.08 (95% CI −2.99, −1.17, IL-8: β −1.88 (−2.80, −0.96), IL-10: β −1.75 (−2.70, −0.81), TNF-alpha: β −1.95 (−2.86, −1.04) (p<0.001)] and 30-days post enrollment [IL-6: β −5.61 (−8.39, −2.83, IL-8: β −6.08 (−8.88, −3.29), IL-10: β −5.43 (−8.30, −2.55), TNF-alpha: β −5.20 (−7.98, −2.43) (p<0.001)]. CRP and S-100β levels in Q4 were also negatively associated with delirium/coma free days by one week [CRP: β −1.55 (−2.49, −0.61) (p=0.001), S-100β: β −1.91 (−2.83, −0.98) (p<0.001)] and 30 days post-enrollment [CRP: β −4.89 (−7.74, −2.04) (p=0.001), S-100β: β −5.46 (−8.31, −2.61) (p=0.000)]. IGF-1 associations were not significant [one week: β −0.42 (−1.44, 0.60) (p=0.419)], 30 days: β 0.11 (−3.00, 3.22) (p=0.943)] (Figure 1 and Supplementary Table 3). To better interpret the above findings and assess the true relationship between biomarkers and delirium duration, we conducted further analyses focusing on biomarkers and delirium and coma duration separately among patients who survived the hospital stay. In separate models, IL-6, 8, 10, TNF-alpha, CRP, and S-100β levels in Q4 were associated with longer delirium duration by one week in patients who survived the hospital stay, whereas only IL-6, TNF-alpha, and CRP in Q4 were associated with coma duration by one week (Supplementary Tables 4 and 5). Similarly, the relationship between biomarkers and delirium/coma free days did not change when the analysis was limited to patients who survived the hospital stay (Supplementary Table 6).
Figure 1: Relationship between biomarkers of inflammation, neuro-protection and astrocyte activation with delirium/coma free days by one week and 30 days.
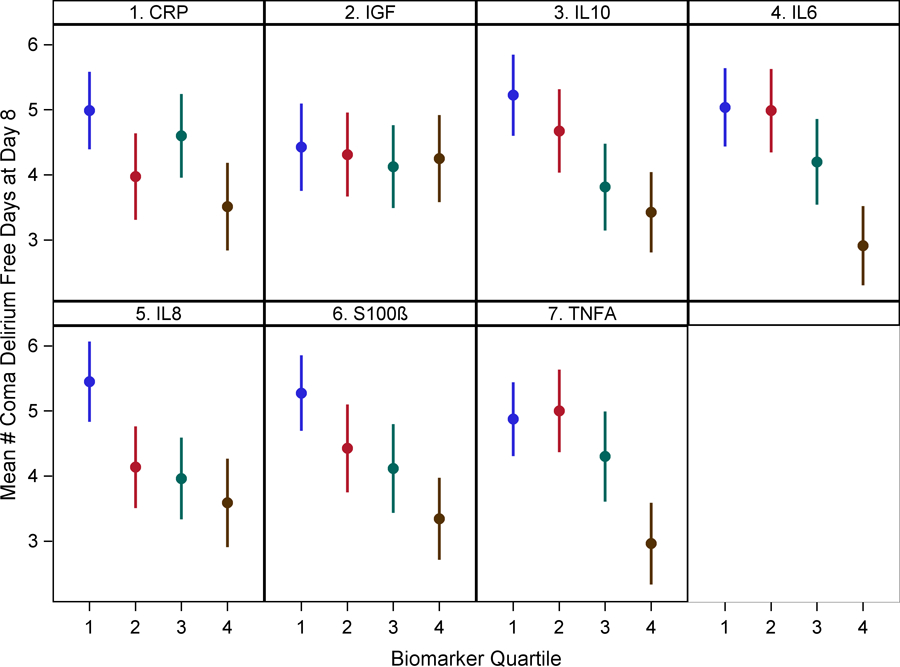
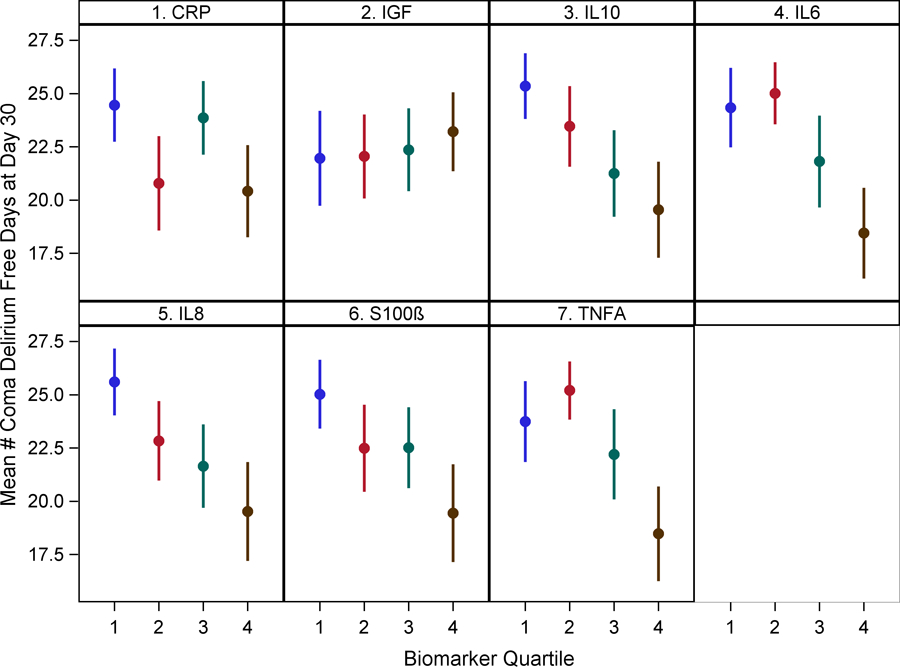
Dots represent group means and bars represent 95% confidence intervals.
Delirium Severity:
After adjusting for age, gender, APACHE II score, Charlson comorbidity score, sepsis diagnosis and study treatment group, IL-6, 8, 10, and TNF-alpha levels in Q4 were positively associated with higher mean delirium severity by one week [IL-6: β 1.45 ( 0.72, 2.17) (p<0.001), IL-8: β 1.21 (0.49, 1.94) (p=0.001), IL-10: β 1.02 (0.28, 1.77) (p=0.008), TNF-alpha: β 0.93 (0.20, 1.67) (p=0.013)] compared to those in Q1. Similar results were shown at hospital discharge except TNF-alpha lost significance [IL-6: β 0.75 (0.11, 1.39) (p=0.022), IL-8: β 0.65 (0.00, 1.29) (p=0.050), IL-10: β 0.90 (0.25, 1.56) (p=0.007), TNF-alpha: β 0.24 (−0.41, 0.88) (p=0.476)]. CRP levels in Q4 showed an association by one week [β 0.77 (0.02, 1.53) (p=0.045) but not at hospital discharge [β 0.29 (−0.38, 0.96) (p=0.396)]. S-100β levels in Q4 were also associated with higher mean delirium severity by one week [β 1.14 (0.41, 1.86) (p=0.002)] but became marginal at hospital discharge [β 0.56 (−0.09, 1.20) (p=0.09). IGF-1 levels were not associated with delirium severity at either time-points. (Figure 2 and Supplementary Table 7).
Figure 2: Relationship between biomarkers of inflammation, neuro-protection and astrocyte activation with mean delirium severity by one week and hospital discharge.
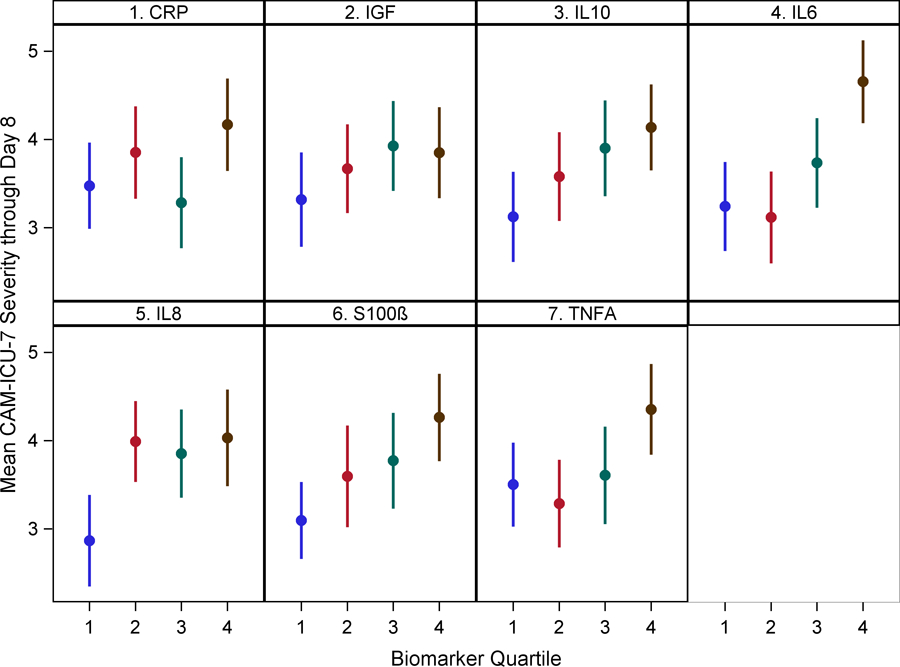
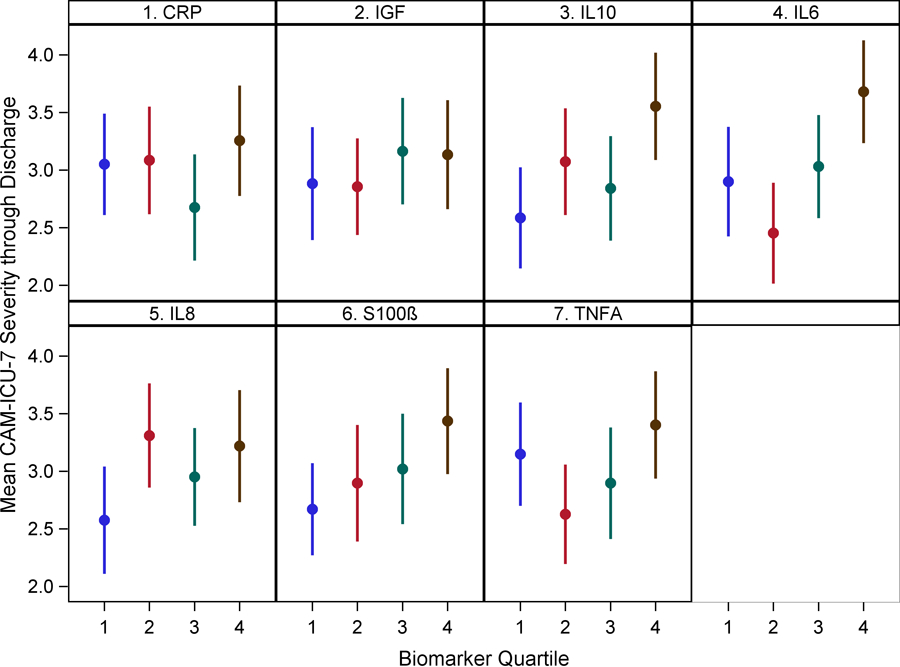
Dots represent group means and bars represent 95% confidence intervals.
Mortality:
IL-8 levels in Q4 were associated with higher in-hospital mortality in delirious patients [Odds Ratio (OR)=5.03 (1.50, 16.89), p=0.009] compared to those in Q1. Higher S-100β levels in Q4 were also associated with higher in-hospital mortality [OR=5.77 (1.52, 21.94) (p=0.010)]. IL-6, 10, TNF-alpha and IGF-1 were not found to be associated with in-hospital mortality (Figure 3 and Supplementary Table 8).
Figure 3: Relationship between biomarkers of inflammation, neuro-protection and astrocyte activation with in-hospital mortality.
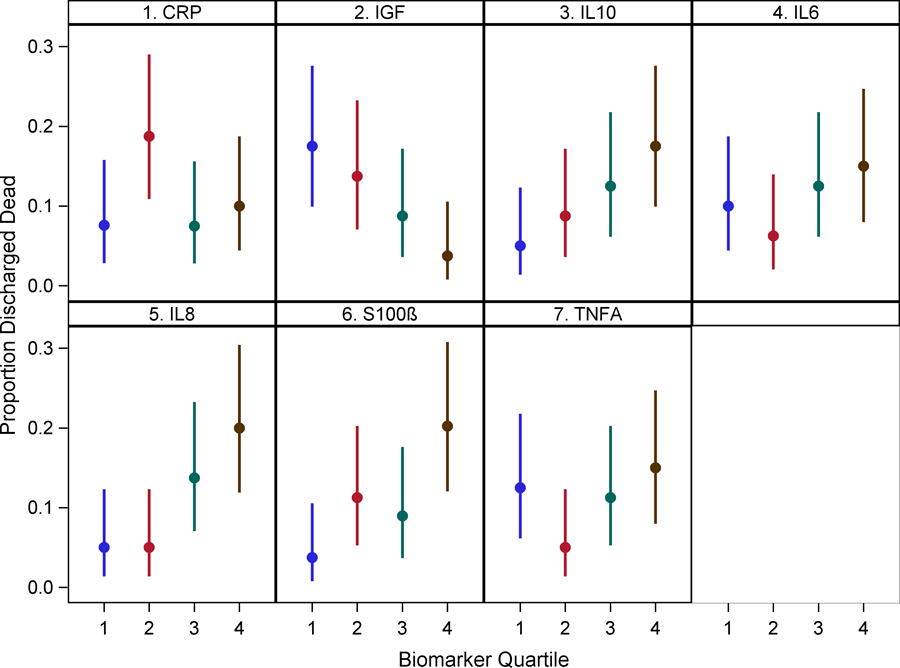
Dots represent group means and bars represent 95% confidence intervals.
Other Analyses (Excluding Neurologic Diagnoses, Delirium Severity Mediation Analysis and Analysis showing odds of hyperactive delirium):
We performed a sensitivity analysis to assess the relationship between S-100β and delirium/coma free days, delirium severity, and in-hospital mortality in critically ill patients without neurologic injury (Supplementary Table 9). This was performed to rule out the release of S-100β from overt neurologic damage secondary to diagnoses such as traumatic brain injury and ischemic and hemorrhagic cerebrovascular accidents. After excluding 26 patients, in the remaining sample of 295 subjects, S-100β levels in Q4 retained the negative association with delirium/coma free days by one week [β −2.08 (−3.04, −1.11) (p<0.001)], and by 30 days post enrollment [β −5.84 (−8.82, −2.85) (p<0.001)]. S-100β levels in Q4 were associated with higher mean delirium severity by one week [β 1.29 (0.54, 2.05) (p=0.001)], and by hospital discharge [β 0.72 (0.05, 1.39) (p=0.036)]. Additionally, higher S-100β levels in Q4 were associated with higher in-hospital mortality [OR=6.44 (1.67, 24.88) (p=0.007]. Supplementary Table 10 provides estimated associations between biomarkers and in-hospital mortality adjusting for delirium severity. Supplementary Table 11 shows that among patients with hypoactive delirium on enrollment, besides CRP, none of the other biomarkers were able to predict future hyperactive episodes.
DISCUSSION:
In this study, we found that higher levels of inflammatory markers and S-100β-a marker of astrocyte and glial activation-were associated with greater delirium duration. Prior literature has shown conflicting associations of cytokines with delirium in critically ill patients.33,34 While Alexander et al showed elevated IL-6 to be associated with ICU delirium;33 data from Ritter et al were inconclusive.34 Both the studies had small sample sizes and were primarily interested in delirium occurrence and not delirium duration. We focused on delirium duration to further characterize patients who have already developed delirium, a complication ubiquitous in the ICU. Our larger sample size and the consistent association of inflammatory cytokines with delirium duration increase the confidence in the findings.
S-100β has shown to be associated with delirium duration in critically ill patients in prior literature.35,36 While designing our study, we hypothesized that systemic inflammation would result in blood-brain barrier disruption with concomitant neuro-inflammation predisposing to astrocyte and glial activation manifesting through S-100β release. The combined increase in cytokines and S-100β in a large sample of delirious patients lends credibility to our hypothesis. As both biomarkers were collected at the same time point, we cannot state with certainty whether the S-100β release mediated the relationship. Future analysis with S-100β collected downstream will better clarify this scenario. The IGF-1 results showed no association with delirium duration, a finding consistent with prior literature.37
Delirium severity has not been routinely assessed in the ICU and our findings of the association between elevated IL-6, 8, 10, S-100β and delirium severity are novel and provide further insights into plausible biological mechanisms of inflammation and astrocyte activation contributing to delirium severity as well as delirium duration. As delirium severity is independently associated with adverse patient outcomes,26 early identification of delirious patients predisposed to higher delirium severity could allow focused application of limited ICU resources such as physical therapy directed mobility.
Both delirium duration and delirium severity have shown to be associated with increased mortality,3–6 but further risk stratification of patients with these conditions based on associated biomarkers have not been addressed. We showed that higher IL-8 and S-100β levels were associated with higher mortality among delirious patients. These results highlight the role of inflammation and astrocyte activation beyond delirium duration and severity and may explain mechanisms behind the downstream effects of delirium on mortality. It is possible that in conditions such as sepsis with systemic inflammation and release of S-100β from enteric glia due to shock, these biomarkers could be associated with mortality independent of delirium. In our patient population as shown through the mediation analysis, the relationships between inflammatory biomarkers and S-100β with mortality have been partially mediated through delirium severity pointing towards the downstream adverse effects of delirium. The seemingly protective effect of high IGF-1 levels in Q4 on mortality in the absence of any association with delirium duration and delirium severity could point towards a mechanism independent of the delirium pathway. This finding showed some similarities with prior evidence of IGF-1 and its relationship with mortality in animal models of sepsis.38
Associations of S-100β with mortality and other poor outcomes have been identified in traumatic brain injury and ischemic stroke subjects.39–41 Because of the potential of release of S-100β from overt neurologic injury scenarios, we performed a sensitivity analysis excluding patients with neurologic catastrophes and found that S-100β continue to retain its association with delirium duration, severity and mortality. This argues towards release of S-100β even in occult brain injury manifesting as delirium and extends the prognostic ability of the biomarker to the field of delirium. Even though our present study explores the associations between biomarkers and delirium duration and severity and may not provide a clinical tool applicable at the bedside immediately, our findings could have implications for both future research and clinical practice. One of the compelling reasons to study biomarkers associated with prolonged delirium is that they might provide us insight into the mechanisms that promote long-term cognitive impairment. Additionally, at present, the only modifiable risk factor for ICU acquired long term cognitive impairment is delirium duration. Understanding of mechanisms associated with delirium duration and severity, the association of delirium with long term cognitive impairment, and identification of vulnerable patients could lead to future preventive and therapeutic approaches with the potential to modify a subset of the population at risk for dementia in the long term.
Our study has several limitations. First, all of our subjects had delirium so we cannot compare our results with non-delirious subjects. As we were focused on biomarkers of delirium duration and severity, we designed the study to include only delirious patients and not non-delirious subjects. Second, our findings represent association and not causation and need further validation. Third, we analyzed the biomarkers at one time-point and hence cannot infer trends that could have been achieved by analyses at multiple time-points. Fourth, we did not collect CSF biomarkers, which could have reflected brain related disease pathology closely. Our study, however, has several strengths. This is the largest cohort of critically ill delirious patients with multiple biomarkers. We followed a well-outlined hypothetical model. We had a diverse patient population including females and African-Americans. All the relevant confounders were adjusted in the final model. We used reliable and validated sedation and delirium assessment tools. The relationship between biomarkers and clinical outcomes persists at all endpoints with stronger associations at earlier time points. We followed major requirements for reporting prognostic biomarker studies,42 including blinding in the evaluation of the prognostic biomarker to the outcome and vice versa, a prospective design, validated outcomes assessment tools, description of biomarker assay with appropriate reference, and inclusion of the whole length of patient’s stay in the hospital.
In conclusion, among critically ill patients with delirium, biomarkers of inflammation and astrocyte and glial activation are associated with longer delirium duration, higher delirium severity, and higher in-hospital mortality. The utility of these biomarkers should be expanded to longer-term outcomes such as ICU acquired cognitive impairment. Finally, studies are needed to assess whether these biomarkers are sensitive to delirium-focused interventions, which could lead us closer to developing personalized therapies for critically ill delirious patients.
Supplementary Material
Acknowledgments
Source of Funding: The study was supported by a grant from the National Institute on Aging (R01AG034205), awarded to Dr. Malaz Boustani. Dr. Khan’s work on the project was supported through a career development award from the National Institute on Aging (K23AG043476). Dr. Marcantonio’s effort was supported by grants R01AG044518 and K24AG035075 from the National Institute on Aging.
Role of the Funder/Sponsor: The National Institute on Aging had no role in the study design, data collection, analysis, data interpretation, and the decision to submit the paper for publication.
Footnotes
Work performed at: Indiana University School of Medicine, Indianapolis, IN
The authors declare no relevant financial interests related to this manuscript.
Copyright form disclosure: Dr. Khan’s institution received funding from the National Institutes of Health/National Institute on Aging. Drs. Khan, Perkins, Shekhar, Gao, Wang, Marcantonio, and Boustani received support for article research from the NIH. Dr. Perkins’ institution received funding from the NIH and CMS. Dr. Prasad disclosed that he is currently employed at Eli Lilly, which has no direct or indirect role or influence on the research findings presented here. Dr. Shekhar received research grants from the NIH, Department of Defense, Eli Lilly, Johnson & Johnson, and Astra Zeneca for unrelated research. Dr. Gao’s institution received funding from the NIH. Dr. Wang received funding from American Psychiatric Publishing (royalties). Dr. Boustani received funding from a R01 award and disclosed that he has ownership equity in two for profit companies, PPHM and RestUp; the products and services of the two companies are not related to the research activities of the published papers. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES:
- 1.American Psychiatric Association, DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). Arlington, VA, US: American Psychiatric Publishing, Inc. [Google Scholar]
- 2.Girard TD, Exline MC, Carson SS, et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. NEJM 2018. December 27;379 (26): 2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisani MA, Kong SYJ, Kasl SV, Murphy TE, Araujo KLB, Van Ness PH. Days of Delirium Are Associated with 1-Year Mortality in an Older Intensive Care Unit Population. American Journal of Respiratory and Critical Care Medicine, 2009. Vol 180 pp. 1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shehabi Y; Riker R; Bokesch P; Wisemandle W; Shintani A; Ely EW: Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Critical Care Medicine 2010. Volume 38(12): 2311–2318 [DOI] [PubMed] [Google Scholar]
- 5.Klouwenberg K, Zaal IJ, Spitoni C, et al. The attributable mortality of delirium in critically ill patients: a prospective cohort study. BMJ 2014. November 24;349:g6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcantonio E, Ta T, Duthie E, Resnick N. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. JAGS 200250: 850–857 [DOI] [PubMed] [Google Scholar]
- 7.Maldonado JR. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018. November;33(11):1428–1457 [DOI] [PubMed] [Google Scholar]
- 8.Bjornsson I, Thorsteinsson L, Gudmundsson KO, Jonsson H Jr, Gudmundsson S, Gudbjornsson B. Inflammatory cytokines in relation to adrenal response following total hip replacement. Scand J Immunol. 2007;65(1):99–105. [DOI] [PubMed] [Google Scholar]
- 9.Kragsbjerg P, Holmberg H, Vikerfors T. Serum concentrations of interleukin-6, tumor necrosis factor alpha, and C-reactive protein in patients undergoing major operations. Eur J Surg. 1995;161(1):17–22. [PubMed] [Google Scholar]
- 10.Hofer S, Bopp C, Hoerner C, et al. Injury of the blood brain barrier and up-regulation of ICAM-1 in polymicrobial sepsis. J Surg Res. 2008;146:276–281 [DOI] [PubMed] [Google Scholar]
- 11.Nishioku T, Sohgu S, Takata F. et al. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol. 2009;29(3):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63(7):764–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lackey BR, Gray SL, Hendricks DM. Actions and interactions of the IGF system in Alzheimer’s disease: review and hypothesis. Growth Horm IGF Res. 2000. February;10(1):1–13. [DOI] [PubMed] [Google Scholar]
- 14.Venters HD, Broussard SR, Zhou JH, et al. Tumor necrosis factor-alpha and insulin like growth factor-1 in the brain:is the whole greater than the sum of its parts? Journal of Neuroimmunology. 2001;119(2):151–65. [DOI] [PubMed] [Google Scholar]
- 15.Block ML, Zecca L, Hong JS. Microglia mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neuroscience. 2007; 8(1):57–69. [DOI] [PubMed] [Google Scholar]
- 16.Cardona AE, Li M, Liu L, Savarin C, Ransohoff RM. Chemokines in and out of the central nervous system: much more then chemotaxis and inflammation. J Leukoc Biol. 2008;84(3):587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto SS, Gottfried C, Mendez A, et al. Immunocontent and secretion of S100B in astrocyte cultures from different brain regions in relation to morphology, FEBS Letters. 2000;486:203–7. [DOI] [PubMed] [Google Scholar]
- 18.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family, Biochem. J. 2006;396:201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michels M, Michelon C, Damasio D et al. Biomarker Predictors of Delirium in Acutely Ill Patients: A Systematic Review. Journal of Geriatric Psychiatry and Neurology 2019. 1–18 [DOI] [PubMed]
- 20.Khan BA, Perkins AJ, Campbell NL, et al. Pharmacological Management of Delirium in the Intensive Care Unit: A Randomized Pragmatic Clinical Trial. J Am Geriatr Soci. 2019;67(5):1057–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell NL, Perkins AJ, Khan BA, et al. Deprescribing in the Pharmacological Management of Delirium: A Randomized Trial in the Intensive Care Unit. J Am Geriatr Soci. 2019;67(4):695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344 [DOI] [PubMed] [Google Scholar]
- 23.Ely EW, Inouye SK, Bernard GR, et al. : Delirium in mechanically ventilated patients: Validity and Reliability of the Confusion Assessment Method for the intensive care unit (CAM-ICU). JAMA 2001; 286:2703–2710 [DOI] [PubMed] [Google Scholar]
- 24.Van den Boogaard M, Slooter AJC, Brüggemann RJM et al. Effect of Haloperidol on Survival Among Critically Ill Adults With a High Risk of Delirium. The REDUCE Randomized Clinical Trial. JAMA. 2018;319(7):680–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitasova A, Kostalova M, Bednarik J et al. Poststroke delirium incidence and outcomes: validation of the confusion assessment method for the intensive care unit (CAM-ICU). Crit Care Med 2012;40:484–490 [DOI] [PubMed] [Google Scholar]
- 26.Khan BA, Perkins AJ, Gao E et al. The CAM-ICU-7 Delirium Severity Scale: A Novel Delirium Severity Instrument for Use in the ICU. Crit Care Med. 2017. May;45(5):851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan B, Zawahiri M, Campbell N, et al. Biomarkers for Delirium-A Review. Journal of American Geriatrics Society 2011. 59:S256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004. September;16(3):275–93. [DOI] [PubMed] [Google Scholar]
- 29.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of Adl: A standardized measure of biological and psychosocial function. J Am Med Assoc. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 30.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969; 9:179–186. [PubMed] [Google Scholar]
- 31.Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE – Acute physiology and chronic health evaluation: A physiologically based classification system. Crit Care Med 1981;9:591–597. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–38346. [DOI] [PubMed] [Google Scholar]
- 33.Alexander AS, Ren D, Ely EW et al. Interleukin-6 and apolipoprotein-E as predictors of acute brain dysfunction and survival in ICU patients. AJCC 2014; 23(1):49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritter C, Tomasi CD, Dal-Pizzol F et al. Inflammation biomarkers and delirium in critically ill patients. Critical Care 2014, 18 R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes CG, Pandharipande PP, Thompson JL, et al. Endothelial activation and blood brain barrier injury as risk factors for delirium in critically ill patients. Crit Care Med 2016; 44(9):e809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan BA, Farber MO, Campbell N,et al. S100 calcium binding protein B as a biomarker of delirium duration in the intensive care unit - an exploratory analysis. Int J Gen Med. 2013. December 2;6:855–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morandi A, Gunther ML, Pandharipande PP, et al. Insulin-like growth factor-1 and delirium in critically ill mechanically ventilated patients: A preliminary investigation. Int Psychogeriatr. 2011; 23 (7): 1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashare A, Nymon AB, Doerschug KC et al. Insulin-like growth factor-1 improves survival in sepsis via enhanced hepatic bacterial clearance. AJRCCM 2008. July 15; 178(2):149–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egea-Guerrero JJ, Murillo-Cabezas F, Gordillo-Escobar E, et al. S100B protein may detect brain death development after severe traumatic brain injury. Journal of Neurotrauma 2013; 30:1762–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shakeri M, Mahdkhah A, Panahi F. S-100B protein as post-traumatic biomarker for prediction of brain death in association with patient outcomes. Archives of Trauma Research 2013; 2(2):76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foerch C, Otto B, Neumann-Haefelin et al. Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke. 2004; 35 (9):2160–4 [DOI] [PubMed] [Google Scholar]
- 42.Kyzas PA, Denaxa-Kyza D, Ioannidis JPA. Quality of Reporting of Cancer Prognostic Marker Studies: Association with Reported Prognostic Effect. J Natl Cancer Inst 2007;99:236–43 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


