Abstract
Background and Aims
Incidence of paediatric inflammatory bowel disease [IBD] in Canada is among the highest worldwide, and age of onset may be decreasing. In a multicentre nationwide inception cohort study, we examined variation in phenotype of IBD throughout the paediatric age spectrum.
Methods
Children aged ≥2 years [y] and <17y [A1 age at diagnosis], with new onset IBD, were systematically evaluated at sites of the Canadian Children IBD Network. Prospectively recorded phenotypic data were compared between age groups.
Results
Among 1092 children (70% Caucasian; 64% Crohn’s disease [CD], 36% ulcerative colitis/inflammatory bowel disease unclassified [UC/IBD-U]; median age 13 y, interquartile range [IQR] 11–15 y), 210 [19%] were diagnosed before the age of age 10 y [Paris A1a] and 43 [4%] before age 6 y (very-early-onset [VEO-IBD]). CD was less common in younger children [42%, 56%, 66%, respectively, of VEO-IBD, A1a; A1b]. Colon-only IBD [UC/IBDU or CD-colon] was present in 81% of VEO-IBD and 65% of A1a; ileal disease increased progressively, reaching plateau at age 10 y. CD location was ileocolonic [L3] in 53% overall. Ileitis [L1] increased with age [6% of VEO-IBD; 13% of A1a; 21% of A1b], as did stricturing/penetrating CD [4% of A1a; 11% of A1b]. At all ages UC was extensive [E3/E4] in >85%, and disease activity moderate to severe according to Physician’s Global Assessment [PGA] and weighted Paediatric Crohn’s Disease Activity Index/Paediatric Ulcerative Colitis Activity Index [wPCDAI/PUCAI] in >70%. Heights were modestly reduced in CD [mean height z score -0.30 ± 1.23], but normal in UC/IBD-U.
Conclusions
Paris classification of age at diagnosis is supported by age-related increases in ileal disease until age 10 years. Other phenotypic features, including severity, are similar across all ages. Linear growth is less impaired in CD than in historical cohorts, reflecting earlier diagnosis.
Keywords: IBD, phenotype, paediatrics
1. Introduction
Inflammatory bowel disease [IBD] has become a global disease, and the incidence and prevalence of both paediatric- and adult-onset IBD in Canada remain among the highest worldwide.1 Incidence reached 9.68/105 (95% confidence interval [CI], 9.11–10.25] children under age 16 years in Canada in 2010, second only to Norway in this age group.2,3 International population-based studies suggest that the incidence may have stabilised among adults in Western countries, but Canadian data indicate a continued increase in children,4 suggesting that age of onset is decreasing. The Canadian Children Inflammatory Bowel Disease Network, a joint partnership of the Canadian Institutes of Health Research [CIHR] and the Childhood Intestinal and Liver Disorders [Ch.I.L.D.] Foundation was established to address the high burden of paediatric-onset IBD in Canada.
Distinct phenotypic features observed in children with Crohn’s disease [CD] and ulcerative colitis [UC] are recognizsd by the Paris modification of the Montreal classification of IBD.5 Children diagnosed at age <2 years were deliberately excluded from the Paris classification, in recognition of infantile-onset chronic IBD being more likely due to one of many monogenic disorders of immune regulation, now increasingly identified.5 Whereas the Montreal designation of IBD diagnosis before age 17 years as A1 was arbitrary, the Paris division of A1 disease into A1a [diagnosis <10 years] and A1b [diagnosis ≥10 and <17 years] was based on variation in spectrum of IBD localisation with age, specifically the relative rarity of any ileal disease before age 10 years.
The predominance of colon-only IBD, including UC, colonic CD, and IBD-unclassified [IBD-U], among children diagnosed before age 10 years is well recognized,6 but variations with age in other phenotypic features have seldom been rigorously and prospectively evaluated. Moreover the term ‘very-early-onset-IBD’ [VEO-IBD], usually implying diagnosis before age 6 years, has come into common usage without convincing evidence that these very young patients, [excluding those manifesting IBD as infants] have any distinct phenotypic features. We undertook to examine the variation in IBD type, location, behaviour, and severity across the entire paediatric age spectrum after infancy in a Canada-wide multicentre prospectively accrued inception cohort study of new-onset IBD.
2. Methods
2.1. Setting and participants
Children and adolescents from age 2 up to 17 years, with new-onset IBD, were prospectively recruited into an inception cohort study at 12 participating academic paediatric IBD centres across Canada, where care is provided until transition to adult gastroenterologists at age 18 years. Comprehensive baseline and longitudinal phenotypic and demographic data, including ethnicity and family history of IBD, were recorded using standardised case report forms [CRFs], anonymised and entered into a central database registry (Research Electronic Data Capture [REDCap] database), hosted at SickKids Hospital, Toronto, Canada. REDCap is a secure, web-based application designed to support data capture for research studies.7 Clinical site directors, all paediatric gastroenterologists with a clinical focus in IBD, were responsible for approving diagnostic label of type of IBD as CD or UC using conventional clinical, endoscopic, and histological criteria. The designation IBD-U is applied in the setting of colonic IBD with feature[s] suggestive of both UC and CD, anticipating that a clearer impression of CD or UC may be verified over time. A ‘Diagnostic Features’ case report form based on consensus guidance from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition [NASPGHAN]8 was designed to specifically capture atypical features [e.g. relative rectal sparing, caecal patch] that need not deter from a diagnosis of UC, and features highly suggestive of CD. Training in application of UC versus CD versus IBD-U labels based on NASPGHAN working group guidelines8 was provided in the context of network meetings and teleconferences, before study initiation and subsequently.
2.2. Study design
This was a cross-sectional study which included all inception cohort patients diagnosed between April 1, 2014 and December 31, 2017. All data were prospectively recorded. Patients were categorised according to age at diagnosis in accordance with Paris classification: A1a [diagnosis before age 10 years], and A1b [diagnosis at age ≥10 years and <17 years]. Baseline phenotypic characteristics, including IBD type, location, and clinical and endoscopic severity, were compared between the two groups. Children diagnosed before age 6 years, arbitrarily defined as VEO-IBD, were additionally compared with older A1a patients.
2.3. Description of IBD phenotype
Disease phenotype at diagnosis was categorised according to the Paris classification, basing location on macroscopic findings observed via colonoscopy, upper endoscopy, and magnetic resonance imaging of the small bowel.5 Patients classified as IBD-U were combined with UC patients for analyses unless, in follow-up, CD was the preferred diagnostic label. As per Paris classification, macroscopic Crohn’s disease confined to the distal one-third of the ileum ± limited caecal involvement is defined as L1; isolated colitis as L2; and ileocolonic disease as L3. Additional or isolated upper gastrointestinal tract involvement is designated L4a when proximal to the ligament of Treitz and L4b if distal to the ligament of Treitz but proximal to the distal one-third of the ileum. Perianal fistulising disease [p] denotes the presence of fistula[s] and/or abscesses. Disease behaviour is classified as: B1 inflammatory [non-stricturing, non-penetrating]; B2 stricturing; B3 internal penetrating; or B2B3 if both stricturing and internal penetrating complications co-exist. Disease extent in UC/IBD-U is designated as: E1 when limited macroscopically to the rectum [proctitis]; E2 for visible colonic inflammation extending no further than the splenic flexure; E3 for disease extending past the splenic flexure but not past the hepatic flexure; and E4 for colitis extending past the hepatic flexure.
Disease activity at baseline was assessed using the weighted Paediatric Crohn’s Disease Activity Index [wPCDAI]9,10 for CD patients or Paediatric Ulcerative Colitis Activity Index [PUCAI]11 for UC/IBD-U patients. Physician Global Assessment [PGA] of disease activity at time of diagnosis was recorded as mild, moderate, or severe. Endoscopic findings were recorded at the local site using the Mayo endoscopic score12 for UC/IBD-U and Simplified Endoscopic Severity Score for CD [SES-CD].13 Training in application of endoscopic measures was repeatedly provided at Network investigator meetings. Histological features including presence of granuloma[s] in mucosal biopsies, routinely taken from multiple sites throughout the colon, terminal ileum, and upper gastrointestinal tract, and as reported by the local site paediatric pathologists, were noted. Heights and other anthropometric measures at presentation were converted to age- and gender-adjusted standard deviation scores [z-scores] using Centers for Disease Control reference data.14
2.4. Statistical analyses
Normally distributed continuous variables were described as means and standard deviations [ ± SD], and non-normally distributed continuous variables as medians and interquartile ranges [IQR]. Categorical variables were expressed as frequency and proportions. Continuous variables were compared with analysis of variance [ANOVA] or the Kruskal-Wallis test, independent sample Student’s t test, or Mann-Whitney test, as appropriate. Categorical variables were compared with the Pearson chi square test or Fisher’s exact test, where cell counts were less than 5. Statistical significance was defined as two-tailed p-value <0.05. All analyses were performed using IBM® SPSS® Statistics Version 24 [Armonk, NY:IBM Corp.].
Ethical considerations: the study protocol was approved by the research ethics boards of each participating institution. Children and their parents or legal guardians provided informed assent and consent for enrolment.
3. Results
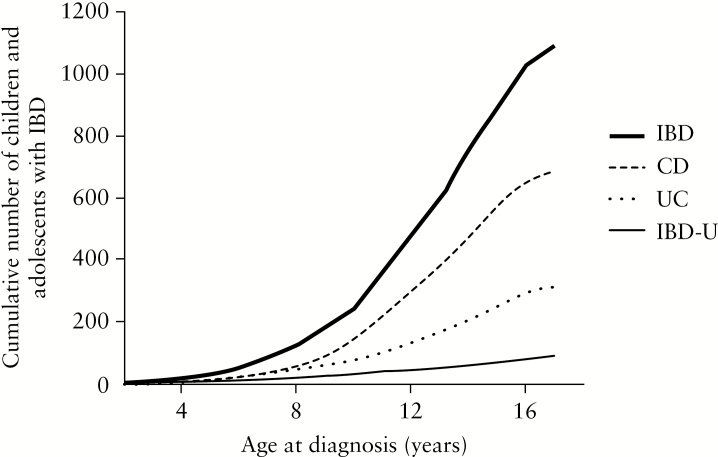
Between April 2014 and December 2017, 1092 Canadian children [57%] male, median age 13 years [y] [IQR 11–15 y] with new-onset IBD were enrolled and had baseline data uploaded into the network REDCAP database before data lock. A total of 210 [19%] were diagnosed before age 10 years [A1a], and only 43 [4%] under age 6 years [VEO-IBD]. The cumulative number of patients with newly diagnosed IBD is graphed according to age at diagnosis in Figure 1. Seventy three percent of the pan-Canadian cohort was of Caucasian ethnicity; South Asians [12.6%] constituted the second most prevalent ethnic group, followed by East Asians [2.0%] and Blacks [2.0%]. A total of 164 [15%] of all children reported having a first-degree relative [parent and/or sibling] affected with IBD.
Figure 1.
Cumulative number of patients with newly diagnosed inflammatory bowel disease [IBD] in the cohort is graphed according to age at diagnosis. Rate of rise in numbers increases after age 10 years, and more sharply for Crohn’s disease.
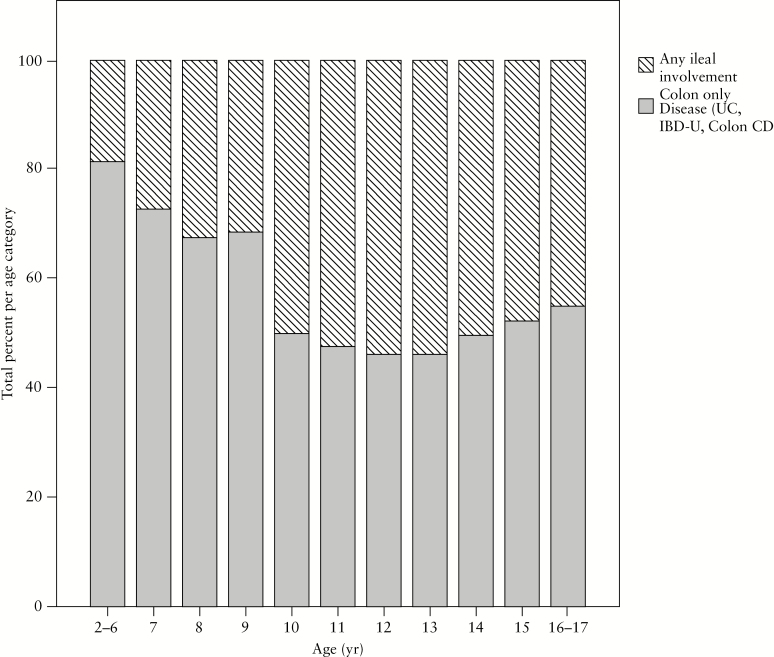
In the overall cohort, IBD type was initially classified as CD in 687 [62%], UC in 316 [30%], and IBD-U in 89 [8%]. Of those initially designated IBD-U, 39 [44%] were considered within the first year of follow-up to have UC, 39 [44%] still retained the IBD-U label, and 11 [12%] were classified as having CD. The initial provisional label of IBD-U was applied more commonly among the younger children [13% among all A1a; 7% among A1b children; p = 0.001]. Among all children with new-onset IBD, the proportion with CD increased with age at presentation [56% of A1a vs 66% of A1b; p = 0.002; 43% of VEO-IBD vs 59% among those >6 y but <10 y; p = 0.002]. Disease localised to the colon only [i.e. UC/IBD-U or isolated colonic CD] was more prevalent among children diagnosed before age 10 years versus later in childhood or adolescence [67% vs 51%, respectively; p < 0.001], and 81% of children diagnosed before age 6 years had colon-only IBD. As shown in Figure 2, there was a progressive increase with age in the percentage of children with any ileal disease present at diagnosis until age 10 years, after which the percentage remained stable. Colon-only disease [UC/IBD-U or colonic CD] also predominated in South Asians when compared with Caucasians [65% vs 35%, respectively; p = 0.009], whereas CD overall was less common in South Asians versus Caucasians [49% vs 64%. Respectively; p = 0.004].
Figure 2.
Among paediatric patients with newly diagnosed inflammatory bowel disease [IBD], isolated colonic disease [including ulcerative colitis, Crohn’s colitis, and IBD-unclassified] predominates in the youngest children. Presence of ileal involvement in Crohn’s disease becomes progressively more common with increasing age at diagnosis, reaching the frequency observed in older children and adolescents by age 10 years.
Symptoms present at diagnosis are summarised in Table 1. Symptoms differed significantly between CD and UC/IBD-U but, within these, IBD types were similar between age groups [Supplementary Table 1a, available as Supplementary data at ECCO-JCC online]. Eleven [12%] of the children initially labelled as IBD-U, but considered in follow-up to have CD, were included in the CD cohort. The duration of symptoms before diagnosis was longer in those with CD compared with those with UC/IBD-U [4.7 months, IQR 2.2–10.6 months, vs 2.9 months, IQR: 1.3–6.5 months, respectively; p <0.001].
Table 1.
Symptoms at time of presentation in Canadian children and adolescents with new-onset inflammatory bowel disease [IBD]. Comparisons are made between children determined to have Crohn’s disease and those with ulcerative colitis or IBD-unclassified.
| Percentage of patients with symptom present at time of diagnosis | Crohn’s disease [n = 698] | Ulcerative colitis/inflammatory bowel disease unclassified [n = 394] | p-value |
|---|---|---|---|
| Abdominal pain | 88% [a major symptom in 51%] | 81% [a major symptom in 32%] | 0.17 |
| 0.002 | |||
| Non-bloody diarrhoea | 30% | 5% | <0.001 |
| Bloody diarrhoea | 40% [a major symptom in 18%] | 87% [a major symptom in 60%] | <0.001 |
| <0.001 | |||
| Linear growth impairment | 22% | 3% | <0.001 |
| Any weight loss | 71% | 62% | 0.004 |
| Perianal lesion[s] [skin tags/fissures/fistula[s]/ abscess[es] | 27% | 2% | <0.001 |
| Extra-intestinal manifestation[s] [joint inflammation/skin lesions] | 15% | 1.2% | <0.001 |
| Oral ulcers | 29% | 9% | <0.001 |
| Fevers | 28% | 11% | <0.001 |
3.1. Phenotypic features of new-onset paediatric Crohn’s disease
Among the 698 children and adolescents with new-onset CD [59% male, median age 13 years, IQR 11–15 years], 17% were diagnosed before age 10 years [A1a] and only 2.6% before age 6 years [VEO-IBD]. The phenotypic features of CD and initial treatment in the paediatric cohort overall, and among patients categorised by age at diagnosis, are summarised in Table 2. The predominance of males versus females in paediatric CD was consistent across all ages. The majority of paediatric CD patients had moderately to severely active disease at presentation, as judged by both PGA and wPCDAI. Disease activity was judged to be mild in only one-quarter of children, regardless of age. The median wPCDAI in the overall cohort was 55 [IQR 35–75], with no significant differences across the age spectrum. Moderate to severe endoscopic activity [SES-CD >7] at diagnostic ileocolonoscopy was evident in 78% overall.
Table 2.
Demographic and phenotypic features at time of diagnosis with paediatric-onset Crohn’s disease. Comparisons are made between younger [A1a] and older [A1b] children as defined by Paris classification and between those with arbitrarily designated very-early-onset IBD [VEO-IBD] and others in A1a cohort.
| Overall cohort A1 [2 to <17y] [n = 698] | A1a <10y [n = 117] | A1b [10 to <17y] [n = 581] | p-value [A1a vs A1b] | VEO-IBD [<6y] [n = 19] | >6 to <10y [n = 98] | p-value [<6y vs 6-10y] | |
|---|---|---|---|---|---|---|---|
| Age in yearsa | 12.9 [10.9, 14.8] | 8.4 [7.1, 9.2] | 13.7 [11.9, 15.1] | <0.001 | 4.9 [3.8, 5.3] | 8.8 [7.7, 9.3] | <0.001 |
| Gender [% male] | 59% | 61% | 59% | 0.60 | 58% | 61% | 0.79 |
| Disease activity as presentation | |||||||
| wPCDAIa | 55 [35,75] | 50 [30,72] | 55 [35,75] | 0.43 | 53 [36,67] | 50 [30,73] | 0.75 |
| PGA mild | 26% | 33% | 24% | 0.61 | 28% | 34% | 0.24 |
| PGA moderate | 44% | 44% | 44% | 61% | 41% | ||
| PGA severe | 30% | 23% | 32% | 11% | 25% | ||
| Disease location | |||||||
| L1 | 20% | 12%* | 21% | 0.002 | 6% | 13% | 0.25 |
| L2 | 23% | 36%* | 20% | 53% | 33% | ||
| L3 | 57% | 52% | 56% | 41% | 54% | ||
| Any L4ab/L4bb | 27% | 33% | 26% | 0.18 | Unknownc | 33% | - |
| Perianal fistulising disease | 16% | 12% | 17% | 0.08 | 6% | 13% | 0.69 |
| Disease behaviour | |||||||
| Inflammatory [B1] | 87% | 96% | 85% | 0.001 | 94% | 97% | 0.58 |
| Stricturing [B2] | 9% | 3% | 11% | 6% | 2% | ||
| Penetrating [B3] | 1% | 0% | 3% | 0% | 1% | ||
| Stricturing and penetrating [B2B3] | 3% | 1% | 1% | 0% | 0% | ||
| Endoscopic severityd | |||||||
| Mild [SES-CD 3–6] | 22% | 24% | 21% | 0.75 | 23% | 24% | 0.48 |
| Moderate [SES-CD 7–15] | 35% | 33% | 36% | 46% | 30% | ||
| Severe [SES-CD ≥ 16] | 43% | 43% | 43% | 31% | 46% | ||
| Linear growth at presentation | |||||||
| Height z score [mean ± SD] | -0.3 ± 1.23 | -0.04 ± 1.03 | -0.28 ± 1.27 | 0.32 | -0.15 ± 0.87 | -0.44 ± 1.05 | 0.27 |
| Initial induction therapy | |||||||
| EEN | 35% | 30% | 36% | 0.27 | 21% | 32% | 0.36 |
| Corticosteroids | 31% | 37% | 30% | 0.14 | 42% | 35% | 0.60 |
| Anti-TNFα | 13% | 9% | 14% | 0.06 | 5% | 9% | 0.58 |
| 5-ASA/SASP | 10% | 14% | 9% | 0.12 | 26% | 12% | 0.05 |
| Other | 11% | 10% | 11% | 0.89 | 6% | 12% | 0.43 |
wPCDAI, Weighted Paediatric Crohn’s Disease Activity Index; PGA, Physician’s Global Assessment; SES-CD, Simplified Endoscopic Severity Score for CD; SD, standard deviation; EEN, exclusive enteral nutrition; anti-TNFα, anti-tumour necrosis factor alpha; 5-ASA/SASP,5-aminosalicylic acid or sulphasalazine; MRE, magnetic resonance enterography..
aValues are presented as median [interquartile range].
b11% of CD cohort did not undergo MRE; small bowel disease proximal to distal ileum [L4b] therefore not evaluated.
cMRE not performed among any children aged <6 years.
dSES-CD data missing in 4%.
Ileocolonic [L3] disease was the predominant localisation of CD among A1b and A1a patients. As is evident in Table 2, isolated colonic CD [L2] was more common in younger versus older children, and conversely disease confined to the distal ileum +/- caecum more common in older children. Of the CD cohort, 27% had proximal small bowel involvement [L4b, i.e. macroscopic disease proximal to the distal third of ileum] identified by magnetic resonance enterography [MRE], but in only 8 [1.2%] was it present without terminal ileal and/or colonic disease. L4b disease was more common in children with any distal ileal involvement [L1 or L3 CD], than in those with Crohn’s colitis [L2] [30% vs 9%; p <0.001]. Among the 96% of children evaluated by upper gastrointestinal [GI] endoscopy, macroscopic involvement characterised by at least small ulcers was present in the stomach in 31% of children, in the duodenum in 24%, and in the oesophagus in 12.5%.
As shown in Table 2, CD was inflammatory [non-stricturing/non-penetrating] [B1] at presentation in 87% of the cohort. Complicated disease [stricturing or penetrating] was more common in children with isolated ileal [L1] compared with ileocolonic [L3] and colonic only [L2] CD [23% vs 12.6% vs 5% respectively; p <0.001], and hence less common in younger children. Perianal fistulising CD was present in overall 16% [n = 106], nine of whom had isolated perianal disease without macroscopic luminal disease; 44 [48%] of 106 had multiple perianal fistulas. We found no association between perianal disease and complicated luminal disease behaviour at diagnosis, nor with disease location [L1:12%, L2:15%, L3:18%; p = 0.38].
Granulomas were present in pre-treatment ileocolonic and/or upper gastrointestinal mucosal biopsies in 51% of all children with CD. The prevalence of granulomatous inflammation was similar across all age categories. Mucosal granulomas were found in 60 [62%] of 106 patients with perianal disease compared with 50% of those without; p = 0.03.
Mean height z-score for all children newly diagnosed with CD was -0.30 ± 1.23 [95% confidence interval of the point estimate -0.39 to -0.20], reduced compared with age- and gender-matched standard populations. There were no differences in mean height z-scores across the age spectrum, nor between mean height z-scores of males and females in the cohort, -0.28 ± 1.30 and -0.33 ± 1.13, respectively; p = 0.65.
3.2. Phenotypic features of new-onset paediatric ulcerative colitis and IBD-U
Among the 392 children and adolescents with new-onset UC or IBD-U [52% male, median age 13 years, IQR 10–15 years], 24% were diagnosed before age 10 years [A1a], and 6% before age 6 years. The phenotypic features of UC/IBD-U and its initial treatment in the paediatric cohort overall, and among patients categorised by age at diagnosis, are summarised in Table 3. Colitis was consistently extensive [E3/E4] across all ages. Upper endoscopy was performed in 86% of the UC/IBD-U cohort. Macroscopic gastric involvement characterised by at least small ulcers was observed in only 6%, but any macroscopic gastritis was reported in 25%, and histological gastritis in overall 58%. Small ulcers in the duodenum were noted in 4%, any duodenal macroscopic inflammation in 8%, and histological duodenitis in overall 27%. Small oesophageal ulcers were reported in 1.2%, any macroscopic inflammation in 3.6%, and histological oesophagitis in overall 4.8%. As evident in Table 3, the spectrum of clinical disease activity judged by PUCAI and PGA was similar among younger and older patients, but endoscopic appearance of colitis assessed by Mayo score was more often severe in older patients. In keeping with the prevalence of moderate or severe disease at presentation in the paediatric UC/IBD-U cohort overall, first therapy was with corticosteroids in 62% and with oral 5-amonisalicylates [ASA] or sulphasalazine in 34%, without significant variation by age category. Patients initially labelled IBD-U, however, had overall milder colitis than those confidently considered to have UC from the outset [see Supplementary Table 2, available as Supplementary data at ECCO-JCC online]. Heights measured among the UC/IBD-U cohort at presentation were comparable to age- and gender-matched standard populations. Mean height z-score at diagnosis of the UC/IBD-U cohort overall was 0.11 ± 1.14, [95% confidence interval of the estimate -0.01 to +0.22], comparable to age- and gender-matched healthy peers.
Table 3.
Demographic and phenotypic features at time of diagnosis with paediatric ulcerative colitis/inflammatory bowel disease unclassified [IBD-U]. Comparisons are made between younger [A1a] and older [A1b] children as defined by Paris classification and between those with arbitrarily designated very-early-onset IBD [VEO-IBD] and others in A1a cohort.
| Overall cohort A1 [2–<17 years] [n = 394] | A1a <10 years [n = 93] | A1b [10–<17 years] [n = 301] | p-value A1a vs A1b | VEO-IBD [<6 years] [n = 24] | >6–<10 years [n = 69] | p-value [<6 vs 6–10 years] | |
|---|---|---|---|---|---|---|---|
| Age in years, median [IQR] | 13 [10, 15] | 7.6 [5.9, 8.7] | 14 [12, 16] | <0.001 | 4.4 [3.3, 5.4] | 8.2 [7.0, 8.9] | <0.001 |
| Gender [% male] | 52% | 48% | 54% | 0.33 | 63% | 44% | 0.11 |
| Extent | |||||||
| E1 | 7% | 4% | 7% | 0.45 | 0% | 5% | 0.45 |
| E2 | 6% | 7% | 6% | 4% | 8% | ||
| E3/E4 | 87% | 89% | 87% | 96% | 87% | ||
| Disease activity at presentation | |||||||
| PUCAI | 50 [35, 65] | 50 [40,65] | 50 [35, 70] | 0.53 | 45 [40, 60] | 50 [40, 60] | 0.97 |
| PGA mild | 24% | 30% | 23% | 0.39 | 26% | 30% | 0.69 |
| PGA moderate | 42% | 40% | 42% | 48% | 38% | ||
| PGA severe | 34% | 30% | 35% | 26% | 32% | ||
| Mayo endoscopic score | |||||||
| 1 [mild] | 14% | 18% | 12% | 0.02 | 10% | 19% | 0.01 |
| 2 [moderate] | 41% | 52% | 39% | 80% | 42% | ||
| 3 [severe] | 45% | 30% | 49% | 10% | 39% | ||
| Linear growth at presentation | |||||||
| Height z score [mean ± SD] | 0.11 ± 1.14 | -0.14 ± 1.09 | 0.19 ± 1.15 | 0.02 | -0.06 ± 1.26 | -0.20 ± 1.04 | 0.67 |
| Initial induction therapy | |||||||
| Corticosteroids | 61% | 61% | 62% | 0.70 | 58% | 62% | 0.64 |
| 5-ASA/SASP | 34% | 35% | 34% | 0.95 | 42% | 32% | 0.46 |
| Anti-TNFα | 3% | 2% | 3% | 0.79 | 0% | 3% | 0.40 |
| Other | 2% | 2% | 2% | 0.85 | 0% | 3% | 0.55 |
PUCAI, Paediatric Ulcerative Colitis Activity Index; PGA, Physician’s Global Assessment; SD, standard deviation; anti-TNFα, anti-tumour necrosis factor alpha; 5-ASA/SASP,5-aminosalicylic acid or sulphasalazine.
4. Discussion
In this contemporary multicentre prospective inception cohort, we have rigorously characterised the phenotypic variation of IBD across the paediatric age spectrum in a country where incidence is among the highest worldwide. Although not a population-based study, Canadian practice patterns are such that paediatric IBD care is provided predominantly by paediatric gastroenterologists at academic centres, who initially evaluate and then subsequently manage the breadth of paediatric UC and CD, rather than acting solely as tertiary referral centres.
A spectrum of disease severity at first presentation was seen in our cohort overall, and across the range of paediatric ages. ‘Mild’ paediatric CD and UC are, unfortunately, relatively uncommon, but do occur. The percentages of children with mild, moderate, and severe UC at diagnosis [respectively 24%, 42%, 34%] are similar to those recently reported in the UC PROTECT study [24%, 48%, 29%], involving 428 prospectively enrolled children and adolescents recruited at 27 sites in the USA and Canada.15
The median ages at diagnosis of CD and UC/IBD-U in our cohort indicate that paediatric CD, particularly, still develops predominantly in pre-adolescent or adolescent patients. Our study, like the Paris classification, excluded patients less than 2 years of age, recognising that severe infantile-onset chronic IBD, rather than being complex CD or UC, is more likely a monogenic disorder of immune regulation, now increasingly identified by targeted gene or whole exome sequencing combined with functional studies.16
The predominance of colon-only IBD of any type [UC or isolated colonic CD or IBD-U] in younger children, which was the justification for the Paris designation of A1a versus A1b, is confirmed by our data. The younger the child at diagnosis, the rarer the presence of ileal involvement, but in our study from age 10 years onward, the proportion of newly diagnosed patients with isolated colonic disease appears not to drop further. The EUROKIDS registry6 and a North American multicentre registry17 made similar observations comparing children diagnosed before age 10 years with older children, but variation in prevalence of colon-only disease across the entire spectrum of A1a IBD was not specifically examined.
The proportion of newly diagnosed paediatric IBD patients with CD increases steadily with age. The predominance of CD versus UC in this inception cohort overall has been observed in most paediatric studies. Population-based Canadian health administrative data from 1999 to 2010 reported the incidence of paediatric CD to be 6.5/105 compared with 2.4/105 for paediatric UC.4 Prevalence was estimated to be 25.5/105 and 10.7/105 children for CD and UC, respectively. A paediatric population-based study in France reported a mean incidence of 3.2/105 for CD and 1.1/105 for UC.18 Incidences of CD and UC were, respectively, 4.72/105 and 2.32/105 in a 2007–2009 Hungarian paediatric inception cohort.19 The predominance of CD in paediatric IBD stands in contrast to adult epidemiological data.1,19
Other features known to distinguish paediatric- from adult-onset IBD were re-affirmed across the spectrum of ages in this pan-Canadian paediatric inception cohort. The male predominance in paediatric CD observed in this study, as with the French EPIMAD study male to female ratio of 1.2,18 is in contrast to the greater prevalence of females among adults with CD.20,21 CD occurring in children, like UC, is often cited as also being more extensive compared with adult-onset CD, but observations are biased by differing diagnostic practices. Upper endoscopy is routinely performed in paediatric patients with suspected CD evaluated by paediatric gastroenterologists.22 This is less often the case in adult practice, which means co-existent upper tract involvement may more often be unrecognised. Of note, we have reported percentages of patients with upper tract ulceration, whereas often proximal macroscopic involvement has been less precisely defined. Ileocolonic [L3] disease was the predominant type of CD in our cohort overall, consistent with the 53–63% reported in numerous paediatric studies.6,23–26 Corresponding percentages in adult cohorts are often lower, but the large French EPIMAD registry reported up to 58% ileocolonic disease in adults, excluding those diagnosed after age 65 years, where phenotype, like that in our youngest children, is characteristically isolated colonic CD.27
The explanation for the rarity with which ileitis is diagnosed in younger children is unknown, but may relate to diagnostic delay and/or to true variation with age in susceptibility to development of chronic small intestinal inflammation.28,29 In comparison with colitis, symptoms of isolated ileitis are more subtle, often limited to abdominal cramps without significant diarrhoea or haematochezia, and may therefore come to attention later.29 However, the number of Peyer’s patches, formations of lymphoid follicles occurring in the distal ileum and linked to the development of chronic ileal inflammation, increases during childhood and reaches a peak in adolescence before involution, correlating with the age-related occurrence of ileal CD.30–32
Extensive colonic involvement [E3/E4], as herein observed in paediatric UC/IBD-U across the paediatric age spectrum, is recognised consistently as a characteristic of childhood-onset colitis.19 Extensive colitis and the rarity of ileal inflammation in younger children with CD combine to make differentiation of type of IBD challenging. This is evidenced by the greater prevalence of ‘IBD-U’ labelling among the younger patients of our cohort. Moreover, in comparison with patients classified as UC from the outset, those considered initially as IBD-U had milder colitis both clinically and endoscopically, as has been previously noted.33 The endoscopic patchiness of very mild colitis may lead to uncertainty of type of IBD. Certainly, provisional labelling as IBD-U may avoid inappropriate treatment in situations where E3/E4 UC and colonic CD respond differently. How often CD confined initially to the colon extends with age to the ileum has not been well studied in large cohorts, but extension has been suggested to occur more often in children than in adults.19,34
Terminal ileitis [L1], as originally described by Crohn,35 was relatively common in Canadian A1b patients. In contrast, L1 disease was much less common even in older children in a previously well-characterised Scottish paediatric cohort, perhaps in part reflecting the rarity in Scotland of CD-associated polymorphisms in the NOD2/CARD15 gene.19,24,36,37 NOD2/CARD15 is strongly associated with ileal CD,38,39 but the prevalence of the polymorphisms conferring susceptibility varies worldwide.37 Isolated L4b disease was rarely observed in our cohort, consistent with the Belgian and EUROkids registries, where 2–4% of patients were diagnosed with isolated L4b disease.6,25 Among paediatric patients of any age with distal ileal or ileocolonic CD, we identified co-existent proximal ileal and/or jejunal disease [L4b] by MRE in close to one-third. Mode of small bowel imaging will significantly alter the perceived prevalence of CD in the proximal small bowel, MRE being superior to traditional oral contrast radiography, but video capsule endoscopy being still more sensitive for mucosal disease.
Although inflammatory disease behaviour [B1] predominated at diagnosis in this paediatric CD cohort, stricturing and/or penetrating disease was more common in those with isolated ileal disease compared with both ileocolonic and colonic Crohn’s disease. This observation provides an explanation for the greater proportion of older children [A1b] with complicated disease, a finding consistent with numerous paediatric studies.40,41 Unlike the EUROkids registry,6 we did not find an association with B2/B3 disease and the occurrence of perianal disease. There was an increase in the prevalence of perianal fistulising disease with age at diagnosis, in keeping with observations made by the Improve Care Now Network, a multicentre paediatric IBD quality improvement collaborative.40 Our observation of more frequent mucosal granulomas in children with perianal disease merits further investigation.42 Crohn’s-like colonic IBD with granulomatous inflammation and perianal disease are common in patients with chronic granulomatous disease [CGD], where mutations in NADPH pathway genes cause defective innate immunity and impaired intracellular killing of phagocytosed microorganisms.43 Polymorphisms in other NADPH oxidase pathway genes, distinct from those associated with CGD, have also been identified as IBD susceptibility genes.44
Historically, impairment of linear growth before diagnosis and even before development of overt intestinal symptoms was common in CD, but very rarely observed in UC.45 Height z-scores of Canadian children presenting with UC/IBD-U in our cohort were comparable to healthy peers. In contrast, across all age categories, the mean height z-scores in our CD cohort were below those expected for age- and gender-matched healthy children. It is encouraging, however, that children newly diagnosed with CD in Canada currently are less stunted compared with previously. For example, pre-pubertal CD patients diagnosed in the Greater Toronto Area of Canada during the time period 1980–1986 had a mean height z-score of -1.1 ± 1.3.46 The lesser degree of linear growth delay currently observed nationally in our cohort is likely attributable to greater awareness among primary care physicians and therefore shorter duration of symptoms before referral for diagnostic investigations.29
In this cohort of children with IBD, the percentage with South Asian ethnicity was higher than the 4% in the entire Canadian paediatric population; this over-representation in our cohort suggests increased susceptibility to the development of IBD.47 Indeed, linking Ontario provincial health administrative data with Canadian immigration data, Benchimol et al. found incidence of IBD among Canadian-born children of immigrants from South Asia to reach a level equivalent to that of Caucasian children, whereas Canadian-born children in other immigrant families, such as those of East Asian ethnicity, retained a lower likelihood of developing IBD.47,48 Very early life in the Canadian environment presumably increases the risk of IBD development in genetically susceptible South Asian children.
This inception cohort study portrays the clinical spectrum of IBD across the full paediatric age range in a country with high incidence. The strengths of the study are its prospective and comprehensive data collection at centres where young patients are thoroughly and systematically evaluated. Canadian practice patterns mean that paediatric gastroenterologists affiliated with the Network’s academic institutions are indeed responsible for the breadth of paediatric IBD. Nevertheless, it is a limitation that the cohort cannot be considered truly population-based. Particularly in the older paediatric age range, patients may be referred directly to adult gastroenterologists.
The Canadian Children IBD network was established with the dual goals of facilitating research to explain the increases in what were once rare paediatric disorders and optimising outcomes of all affected children. Monogenic mimickers of complex IBD are being identified in paediatric patients with phenotypes at the extremes either of age of onset and/or disease severity, but leading to confusion as to which children warrant gene sequencing. ‘VEO-IBD’ is often wrongly construed to equate with monogenic IBD. We have demonstrated that CD and UC are clinically heterogeneous across all paediatric ages. Defining clinical heterogeneity and understanding its biological basis are required to advance precision medicine for IBD. To this end, pre-treatment biospecimens, including serum, DNA, stool, and biopsies, have been collected from this carefully characterised inception cohort. Our observations confirm the greater prevalence of colon-only IBD, the relative rarity of ileal CD, and therefore the presence of less stricturing and penetrating CD in younger children, but a similar spectrum of clinical disease severity and less severe endoscopic disease compared with older children.
Funding
This work was supported by grant 297862 from the Canadian Institutes of Health Research [CIHR] in partnership with the Children’s Intestinal and Liver Disease [Ch.I.L.D.] Foundation.
Supplementary Material
This paper was presented in part at the Annual Meeting of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition 2018, Geneva.
Conflict of Interest
The authors report no conflicts directly related to this manuscript. The individual authors disclose the following relationships with industry involved in treatment of IBD. TDWs: consultant: Abbvie, Ferring, Janssen, Merck; speaker: Abbvie, Ferring, Janssen, Merck, Nestle. HQH: advisory board Abbvie, Janssen, Merck; research support: Janssen. KJ: advisory board member: Abbvie, Janssen, Merck; speaker’s bureau: Abbvie Janssen; investigator-initiated research support: Janssen. ARO: advisory board: Abbvie, Janssen, Shire; consultant: Abbvie, Janssen, Lilly; research site: Abbvie, Janssen, Takeda. JD: advisory board: Abbvie, Janssen, Merck. WE-M: advisory board member: Abbvie, Janssen, Merck. CDs: advisory board member: Abbvie, Janssen, Merck. MS: advisory board member: Abbvie, Merck. KB: advisory board: Abbvie, Mead Johnson; pharmaceutical trials: Abbvie, Takeda, Janssen, Allergan, Pfizer; speaker: Mead Johnson; unrestricted educational grant: Abbvie. PJ: consultant: Abbvie, Janssen; advisory board: Ferring. EW: consultant: Abbvie, Janssen. MC: advisory board member: Abbvie, Janssen. SL: advisory board member: Janssen; speaker: Abbvie. JV: consulting, travel, and/or speaker fees and research support: Abbvie, Janssen, Nestlé Health Science, Merck, P&G, GSK, Illumina, Otsuka. PC: consultant: Abbvie, Ferring, Janssen, Merck;speaker: Abbvie; research support: Abbvie. AMG: consultant: Abbvie, Merck, Janssen, Eli Lilly, Pfizer, Gilead, Roche, Takeda; speaker: Abbvie, Janssen, Shire; investigator-initiated research support: Abbvie.
Author Contributions
JD interpreted data, drafted the manuscript and approved final draft submitted. TDW planned and conducted the study, collected and interpreted data, and approved final draft submitted. DRM planned and conducted the study, collected data, and approved final draft submitted. HHQ planned and conducted the study, collected data, and approved final draft submitted. KJ planned and conducted the study, collected data, reviewed manuscript, and approved final draft submitted. ARO planned and conducted the study, collected data, and approved final draft submitted. JD planned and conducted the study, collected data, reviewed manuscript, and approved final draft submitted. WE-M planned and conducted the study, collected data, reviewed manuscript, and approved final draft submitted. CD planned and conducted the study, collected data, and approved final draft submitted. MS collected data and approved final draft submitted. JC planned and conducted the study, collected data, and approved final draft submitted. KB collected data and approved final draft submitted. ES planned and conducted the study, collected data, and approved final draft submitted. PJ collected data and approved final draft submitted. AR collected data, reviewed manuscript, and approved final draft submitted. MR collected data and approved final draft submitted. AM planned and conducted the study and approved final draft submitted. EW planned and conducted the study, collected data, and approved final draft submitted. MC collected data and approved final draft submitted. SL collected data and approved final draft submitted. JVL collected data and approved final draft submitted. EB planned and conducted the study, collected data, reviewed manuscript, and approved final draft submitted. PC collected and interpreted data, reviewed manuscript, and approved final draft submitted. AMG planned and conducted the study, collected and interpreted data, drafted the manuscript, and approved final draft submitted.
References
- 1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 2. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis 2011;17:423–39. [DOI] [PubMed] [Google Scholar]
- 3. Perminow G, Brackmann S, Lyckander LG, et al. ; IBSEN-II Group A characterization in childhood inflammatory bowel disease, a new population-based inception cohort from South-Eastern Norway, 2005-07, showing increased incidence in Crohn’s disease. Scand J Gastroenterol 2009;44:446–56. [DOI] [PubMed] [Google Scholar]
- 4. Benchimol EI, Bernstein CN, Bitton A, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol 2017;112:1120–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 6. de Bie CI, Paerregaard A, Kolacek S, et al. ; EUROKIDS Porto IBD Working Group of ESPGHAN Disease phenotype at diagnosis in pediatric Crohn’s disease: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis 2013;19:378–85. [DOI] [PubMed] [Google Scholar]
- 7. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture [REDCap]–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bousvaros A, Antonioli DA, Colletti RB, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr 2007;44:653–74. [DOI] [PubMed] [Google Scholar]
- 9. Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr 1991;12:439–47. [PubMed] [Google Scholar]
- 10. Turner D, Griffiths AM, Walters TD, et al. Mathematical weighting of the pediatric Crohn’s disease activity index [PCDAI] and comparison with its other short versions. Inflamm Bowel Dis 2012;18:55–62. [DOI] [PubMed] [Google Scholar]
- 11. Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423–32. [DOI] [PubMed] [Google Scholar]
- 12. Mohammed Vashist N, Samaan M, Mosli MH, et al. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev 2018;1:CD011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khanna R, Nelson SA, Feagan BG, et al. Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev 2016;CD010642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention, National Center for Health Statistics. https://www.cdc.gov/growthcharts/zscore.html Accessed August 4, 2009.
- 15. Hyams JS, Davis S, Mack DR, et al. Factors associated with early outcomes following standardised therapy in children with ulcerative colitis [PROTECT]: a multicentre inception cohort study. Lancet Gastroenterol Hepatol 2017;2:855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Batura V, Muise AM. Very early onset IBD: novel genetic aetiologies. Curr Opin Allergy Clin Immunol 2018;18:470–80. [DOI] [PubMed] [Google Scholar]
- 17. Oliva-Hemker M, Hutfless S, Al Kazzi ES, et al. Clinical presentation and five-year therapeutic management of very early-onset inflammatory bowel disease in a large North American Cohort. J Pediatr 2015;167:527–32.e1–3. [DOI] [PubMed] [Google Scholar]
- 18. Ghione S, Sarter H, Fumery M, et al. ; Epimad Group Dramatic increase in incidence of ulcerative colitis and Crohn’s disease [1988-2011]: a population-based study of French adolescents. Am J Gastroenterol 2018;113:265–72. [DOI] [PubMed] [Google Scholar]
- 19. Müller KE, Lakatos PL, Arató A, et al. ; Hungarian IBD Registry Group [HUPIR] Incidence, Paris classification, and follow-up in a nationwide incident cohort of pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2013;57:576–82. [DOI] [PubMed] [Google Scholar]
- 20. Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci 2013;58:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brant SR, Nguyen GC. Is there a gender difference in the prevalence of Crohn’s disease or ulcerative colitis? Inflamm Bowel Dis 2008;14[Suppl 2]:S2–3. [DOI] [PubMed] [Google Scholar]
- 22. Levine A, Koletzko S, Turner D, et al. ; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 2014;58:795–806. [DOI] [PubMed] [Google Scholar]
- 23. Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease [IBD]: analysis of a pediatric IBD consortium registry. J Pediatr 2005;146:35–40. [DOI] [PubMed] [Google Scholar]
- 24. Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22. [DOI] [PubMed] [Google Scholar]
- 25. De Greef E, Mahachie John JM, Hoffman I, et al. ; IBD working group of the Belgian Society of Pediatric Gastroenterology, Hepatology and Nutrition [BeSPGHAN]; Belgian IBD Research and Development Profile of pediatric Crohn’s disease in Belgium. J Crohns Colitis 2013;7:e588–98. [DOI] [PubMed] [Google Scholar]
- 26. Malmborg P, Grahnquist L, Ideström M, et al. Presentation and progression of childhood-onset inflammatory bowel disease in Northern Stockholm County. Inflamm Bowel Dis 2015;21:1098–108. [DOI] [PubMed] [Google Scholar]
- 27. Gower-Rousseau C, Vasseur F, Fumery M, et al. Epidemiology of inflammatory bowel diseases: new insights from a French population-based registry [EPIMAD]. Dig Liver Dis 2013;45:89–94. [DOI] [PubMed] [Google Scholar]
- 28. Ricciuto A, Fish JR, Tomalty DE, et al. Diagnostic delay in Canadian children with inflammatory bowel disease is more common in Crohn’s disease and associated with decreased height. Arch Dis Child 2018;103:319–26. [DOI] [PubMed] [Google Scholar]
- 29. Vavricka SR, Spigaglia SM, Rogler G, et al. ; Swiss IBD Cohort Study Group Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:496–505. [DOI] [PubMed] [Google Scholar]
- 30. Gullberg E, Söderholm JD. Peyer’s patches and M cells as potential sites of the inflammatory onset in Crohn’s disease. Ann N Y Acad Sci 2006;1072:218–32. [DOI] [PubMed] [Google Scholar]
- 31. Van Kruiningen HJ, Ganley LM, Freda BJ. The role of Peyer’s patches in the age-related incidence of Crohn’s disease. J Clin Gastroenterol 1997;25:470–5. [DOI] [PubMed] [Google Scholar]
- 32. Van Kruiningen HJ, West AB, Freda BJ, Holmes KA. Distribution of Peyer’s patches in the distal ileum. Inflamm Bowel Dis 2002;8:180–5. [DOI] [PubMed] [Google Scholar]
- 33. Aloi M, Birimberg-Schwartz L, Buderus S, et al. Treatment options and outcomes of pediatric IBDU compared with other IBD subtypes: a retrospective multicenter study from the IBD Porto group of ESPGHAN. Inflamm Bowel Dis 2016;22:1378–83. [DOI] [PubMed] [Google Scholar]
- 34. Meinzer U, Ideström M, Alberti C, et al. Ileal involvement is age dependent in pediatric Crohn’s disease. Inflamm Bowel Dis 2005;11:639–44. [DOI] [PubMed] [Google Scholar]
- 35. Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis: a pathologic and clinical entity. JAMA 1932;99:1323–9. [PubMed] [Google Scholar]
- 36. Cleynen I, Boucher G, Jostins L, et al. ; International Inflammatory Bowel Disease Genetics Consortium Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet 2016;387:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hugot JP, Zaccaria I, Cavanaugh J, et al. Prevalence of CARD15/NOD2 mutations in Caucasian healthy people. Am J Gastroenterol 2007;102:1259–67. [DOI] [PubMed] [Google Scholar]
- 38. Cleynen I, González JR, Figueroa C, et al. Genetic factors conferring an increased susceptibility to develop Crohn’s disease also influence disease phenotype: results from the IBDchip European Project. Gut 2013;62:1556–65. [DOI] [PubMed] [Google Scholar]
- 39. Cleynen I, Boucher G, Jostins L, et al. ; International Inflammatory Bowel Disease Genetics Consortium Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet 2016;387:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adler J, Dong S, Eder SJ, Dombkowski KJ; ImproveCareNow Pediatric IBD Learning Health System Perianal Crohn disease in a large multicenter pediatric collaborative. J Pediatr Gastroenterol Nutr 2017;64:e117–24. [DOI] [PubMed] [Google Scholar]
- 41. Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology 2008;135:1106–13. [DOI] [PubMed] [Google Scholar]
- 42. Johnson CM, Hartman DJ, Ramos-Rivers C, et al. Epithelioid granulomas associate with increased severity and progression of Crohn’s disease, based on 6-year follow-up. Clin Gastroenterol Hepatol 2018;16:900–7.e1. [DOI] [PubMed] [Google Scholar]
- 43. Marks DJ, Miyagi K, Rahman FZ, Novelli M, Bloom SL, Segal AW. Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn’s disease. Am J Gastroenterol 2009;104:117–24. [DOI] [PubMed] [Google Scholar]
- 44. Hayes P, Dhillon S, O’Neill K, et al. Defects in NADPH oxidase genes NOX1 and DUOX2 in very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 2015;1:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moeeni V, Day AS. Impact of inflammatory bowel disease upon growth in children and adolescents. ISRN Pediatr 2011;2011:365712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Griffiths AM. Growth retardation in early-onset inflammatory bowel disease: should we monitor and treat these patients differently? Dig Dis 2009;27:404–11. [DOI] [PubMed] [Google Scholar]
- 47. Minister responsible for Statistics Canada https://www12.statcan.gc.ca/census-recensement/2016/as-sa/98-200-x/2016016/98-200-x2016016-eng.cfm Accessed April 3, 2019.
- 48. Benchimol EI, Mack DR, Guttmann A, et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol 2015;110:553–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.