http://aasldpubs.onlinelibrary.wiley.com/hub/journal/10.1002/(ISSN)2046-2484/video/15-5-interview-reddy the interview with the author
Abbreviations
- ART
antiretroviral therapy
- COVID‐19
coronavirus disease 2019
- DDI
drug‐drug interaction
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- ICU
intensive care unit
- PWID
person who injects drugs
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Globally, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus has variably affected the various parts of the world and has been a major cause for significant morbidity and mortality. Coronavirus disease 2019 (COVID‐19), the disease caused by SARS‐CoV‐2, has been reported to have multiple clinical manifestations, although primarily they have been pulmonary manifestations. Hepatic manifestations have variably been present in up to 50% of infected individuals. 1 , 2 The spectrum ranges from asymptomatic abnormalities in hepatic biochemical tests to the rare case of acute liver failure. The cause for hepatic manifestations is unclear at this stage and may be caused by a variety of reasons, such as a manifestation of a systemic illness, ischemic liver injury, immune‐mediated liver injury, drug‐induced liver injury, or a direct cytopathic effect of the virus. 3 , 4 , 5 , 6 Not uncommonly, patients have concomitant infections, such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) infection, either alone or as co‐infections, and the impact of the pandemic and SARS‐CoV‐2 on these infections and associated liver diseases is unknown. Further, the implications in people who inject drugs (PWIDs) may be unique. Observations continue to evolve regarding hepatic manifestations and challenges with COVID‐19 and the liver, and as such, expectations and guidance on issues relevant to the multiple viral infections are important.
A meta‐analysis, primarily involving reports from China, noted a 3% prevalence rate of underlying chronic liver disease in those with COVID‐19, although it does not provide specific data on the prevalence of HBV and HCV infections. 7 HBV and HCV are chronic infections that are frequently encountered worldwide, and the former is particularly common in China, where the first cases of COVID‐19 were reported. Thus, there has been concern about the impact of SARS‐CoV‐2 infection on the course of HCV and HBV. Thus far, fortunately, COVID‐19 has been reported infrequently in those with HBV and HCV infections in the United States. In a large series of 5700 hospitalized patients with COVID‐19 in the northeastern United States, HBV and HCV infections were encountered in 0.1% and <0.1% of patients, respectively 8 (Table 1). In contrast, a large hospitalized patient series from Wuhan, China, observed that 2.1% (23/1099) of patients were HBV infected and represented 2.4% of nonsevere cases and 0.6% of severe cases. 9 A single‐center retrospective study from China noted that 12.2% (15/123) of patients with COVID‐19 had HBV infection, and a higher percentage with comorbid HBV, relative to HBV‐negative patients, had higher total bilirubin levels, developed a more severe course (46.7% versus 24.1%), and had a higher mortality rate (13.3% versus 2.8%). 10 Zha et al. 11 noted a background HBV prevalence rate of 6.5% (2/31) while reporting on their experience with the use of corticosteroids in COVID‐19; further, they observed delayed SARS‐CoV‐2 clearance in those with HBV infection.
Table 1.
SARS‐CoV‐2/COVID‐19 and Hepatitis B and C
| Authors | Infection | Study Characteristics | Observations | Unique Considerations |
|---|---|---|---|---|
| Chen et al. 10 | HBV | Retrospective analysis of hospitalized patients with COVID‐19 in a single center in Wuhan, China |
|
|
| Zha et al. 11 | Observational study investigating the efficacy of corticosteroid treatment in hospitalized patients with COVID‐19 in China |
|
||
| Richardson et al. 8 | Case series of hospitalized patients with COVID‐19 in 12 hospitals in the New York City metro area |
|
||
| Guan et al. 9 | Retrospective multicenter analysis of hospitalized patients with COVID‐19 in China |
|
||
| Richardson et al. 8 | HCV | Case series of hospitalized patients with COVID‐19 in 12 hospitals in the New York City metro area |
|
|
| Blanco et al. 12 | HIV | Clinical case series of 5 hospitalized COVID‐19 patients in a single center in Spain |
|
|
| Zhu et al. 13 | Case study in China in a HIV/SARS‐CoV‐2 co‐infected patient |
|
||
| Chen et al. 15 | Case study in China in a patient co‐infected with HIV/SARS‐CoV‐2 |
|
||
| Aydin et al. 14 | Case series of 4 patients co‐infected with HIV/SARS‐CoV‐2 in Turkey |
|
||
| Zhao et al. 16 | HCV+HIV | Case study in China of HIV/HCV co‐infected patient with COVID‐19 |
|
There have been only sparse reports, involving case series, on the impact of COVID‐19 in patients with HIV infection. 12 , 13 , 14 , 15 A Barcelona experience with hospitalized patients with COVID‐19 noted that HIV‐infected individuals accounted for close to only 1% of these patients. Those with HIV infection were younger than 50 years, they self‐identified as men who have sex with men, their clinical picture was similar to those who were HIV negative/COVID‐19 positive, and there was no mortality reported. 12 There have been only sporadic reports of COVID‐19 in those co‐infected with HIV and HCV, and unique considerations in either HIV mono‐infected or HIV/HCV co‐infected patients would be of adjustments to antiretroviral therapy (ART) based on potential drug‐drug interactions (DDIs); further, antibody response to SARS‐CoV‐2 may be impaired or delayed in this population. 16 Thus far, studies have not been presented on the frequency and impact of COVID‐19 in PWIDs, a population vulnerable to the consequences of SARS‐CoV‐2 due to several comorbidities, such as other viral infections, heart disease, and renal disease. 17
Unique considerations in those with HBV and HCV infections involve possible precautions for HBV and HCV therapies in those with or without SARS‐CoV‐2 infection and COVID‐19 manifestations of abnormalities in hepatic biochemical tests (Fig. 1). As per the American Association for the Study of Liver Diseases guidance document, it would seem reasonable to initiate HCV therapy, in newly diagnosed cases of HCV, in those without a SARS‐CoV‐2 infection if adequate resources are available and if those resources have not been deployed for COVID‐19 activities (e.g., pharmacy services, personnel for approval of therapy, blood testing service, follow‐up facilities through telemedicine or face‐to‐face). 18 In those with COVID‐19 in the background of a recently diagnosed HCV infection, it seems reasonable to defer HCV therapy until a time when COVID‐19 has cleared, whereas already initiated therapy can be continued while monitoring for DDIs. In those patients with HBV, it is important to be aware of the risk for HBV reactivation related to medications, such as tocilizumab and corticosteroids, used in the context of COVID‐19. Reactivation of HBV following the use of tocilizumab and prednisone has been described, and thus prophylaxis against HBV reactivation should be a consideration. 19 , 20 In addition, chronic HBV therapy where indicated as per guidelines 21 can be initiated in those with newly diagnosed HBV and continued if receiving therapy, regardless of COVID‐19. Last but not least, caution needs to be exercised in initiating COVID‐19‐related therapy in those with advanced liver disease; thus, established guidelines on such use need to be followed to minimize the risk for hepatic decompensation, although the risk/benefit of an intervention is likely to weigh in heavily in dealing with the highly lethal condition of COVID‐19.
Fig 1.
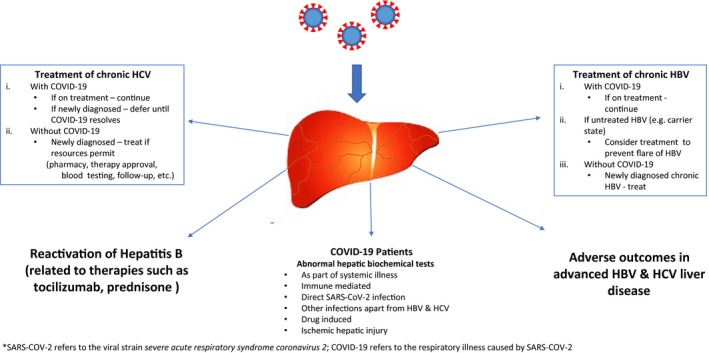
SARS‐CoV‐2 and COVID‐19: considerations in HBV and HCV. COVID‐19 refers to the respiratory illness caused by SARS‐CoV‐2.
Potential conflict of interest: K.R.R. consults for Mallinckrodt, Merck, and Gilead, and his institution receives grants from Mallinckrodt, Gilead, Merck, HepQuant, Exact Sciences, BMS, Sequana, and Intercept.
References
- 1. Bangash MN, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. Available at: 10.1016/S2468-1253(20)30084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang C, Shi L, Wang F‐S. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun J, Aghemo A, Forner A, et al. COVID‐19 and liver disease. Liver Int. Available at: 10.1111/liv.14470 [DOI] [PubMed] [Google Scholar]
- 4. Xu Z, Shi L, Wang Y, al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. eBiomedicine 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. bioRxiv. Available at: 10.1101/2020.02.03.931766 [DOI] [Google Scholar]
- 7. Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: a meta‐analysis. Liver Int. Available at: 10.1111/liv.14465 [DOI] [PubMed] [Google Scholar]
- 8. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. Available at: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen X, Jiang Q, Ma Z, et al. Clinical characteristics of hospitalized patients with SARS‐CoV‐2 and hepatitis B virus co‐infection. medRxiv Available at: 10.1101/2020.03.23.20040733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zha L, Li S, Pan L, et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID‐19). Med J Australia. Available at: 10.5694/mja2.50577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanco JL, Ambrosioni J, Garcia F, et al. COVID‐19 in patients with HIV: clinical case series. Lancet HIV 2020;7:e314‐e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu F, Cao Y, Xu S, et al. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol 2020;92:529‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Altuntas Aydin O, Kumbasar Karaosmanoglu H, Kart Yasar K. HIV/SARS‐CoV‐2 co‐infected patients in Istanbul, Turkey. J Med Virol. Available at: 10.1002/jmv.25955 [DOI] [PubMed] [Google Scholar]
- 15. Chen J, Cheng X, Wang R, et al. Computed tomography imaging of an HIV‐infected patient with coronavirus disease 2019 (COVID‐19). J Med Virol. Available at: 10.1002/jmv.25879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao J, Liao X, Wang H, et al. Early virus clearance and delayed antibody response in a case of COVID‐19 with a history of co‐infection with HIV‐1 and HCV. Clin Infect Dis. Available at: 10.1093/cid/ciaa408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farhoudian A, Baldacchino AM, Clark N, et al. COVID‐19 and substance use disorders: recommendations to a comprehensive healthcare response. An International Society of Addiction Medicine (ISAM) Practice and Policy Interest Group position paper. Basic Clin Neurosci. Available at: 10.32598/bcn.11.covid19.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD expert panel consensus statement. Hepatology. Available at: 10.1002/hep.31281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reddy KR, Beavers KL, Hammond SP, et al. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:215‐219. [DOI] [PubMed] [Google Scholar]
- 20. Chen L‐F, Mo Y‐Q, Jing J, et al. Short‐course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis 2017;20:859‐869. [DOI] [PubMed] [Google Scholar]
- 21. Terrault NA, Lok AS, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]


