http://aasldpubs.onlinelibrary.wiley.com/hub/journal/10.1002/(ISSN)2046-2484/video/15-5-interview-lau-ward the interview with the author
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ALT
alanine aminotransferase
- APASL
Asian Pacific Association for the Study of the Liver
- AST
aspartate aminotransferase
- CHB
chronic hepatitis B
- CHC
chronic hepatitis C
- CLD
chronic liver disease
- COVID‐19
coronavirus disease 2019
- EASL
European Association for the Study of the Liver
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HCW
health care worker
- LT
liver transplantation
- NAFLD
nonalcoholic fatty liver disease
- NIH
National Institutes of Health
- PPE
personal protective equipment
- SARS‐CoV‐2
Severe Acute Respiratory Syndrome Coronavirus 2
- TKI
tyrosine kinase inhibitor
- ULD
upper limit of normal
To navigate through the stormy unchartered ocean of SARS‐coV‐2 infections and coronavirus disease 2019 (COVID‐19), all practicing hepatologists and other clinicians caring for patients with liver disease need guidance based on the best documented and rapidly evolving knowledge regarding SARS‐CoV‐2 infection and COVID‐19. Prevention of SARS‐CoV‐2 transmission requires the redesign of patient workflow and other measures ensuring delivery of elective and emergency hepatology services without compromising the safety of patients and medical personnel. 1 , 2 Moreover, prevention of severe COVID‐19 and related mortality requires updating management of persons with chronic liver disease (CLD) to diagnose COVID‐19 and being vigilant for drug‐drug interactions and other potential complications of COVID‐19 in persons with CLD. 3 To respond to an urgent need for such information, the Asian Pacific Association for the Study of the Liver (APASL) recently published recommendations of an expert committee to guide infection control and clinical management of patients with CLD during the COVID‐19 pandemic. 4 Previously, two other regional liver associations, American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL), convened expert panels with the same objectives. 5 , 6 This review summarizes the recommendations of the three liver associations for clinical practices to prevent SARS‐CoV‐2 transmission and protect persons with CLD from health risks posed by the emerging COVID‐19 pandemic (Table 1).
Table 1.
Selected AASLD, APASL, and EASL Recommendations for Liver Disease Management During the COVID‐19 Pandemic
| Recommendations | AASLD | APASL | EASL |
|---|---|---|---|
| Limit nosocomial transmission |
|
|
|
| Evaluate and care for patients with COVID‐19 for liver disease |
|
|
|
| Manage hepatitis B; hepatitis C |
|
|
|
| Manage patients with HCC |
|
|
|
| Manage pretransplant and posttransplant patients |
|
|
|
How to Protect Medical Personnel and Patients With Liver Diseases From SARS‐CoV‐2 Infection?
To this end, all three associations recommend physical distancing by limiting face‐to‐face consultations to urgent situations, routine patient contact via telemedicine and phone visits, and use of local laboratories and pharmacies to reduce clinic and hospital visits and patient travel. AASLD guidance is most stringent by discouraging clinic entry of anyone with fever or other COVID‐19 symptoms and SARS‐CoV‐2 testing of these patients. All recommend COVID‐19 testing of liver transplant donors and recipients and patients with encephalopathy or acute decompensation. Recent data showed that many SARS‐CoV‐2–infected patients are asymptomatic, yet capable of transmitting the disease. 7 Ideally, all patients with a history of close contact with cases of possible or confirmed COVID‐19 or from high‐prevalence regions should be tested. APASL recommends SARS‐CoV‐2 testing based on clinical and epidemiological factors. EASL recommends following an institution’s practice. AASLD and APASL describe the personal protective equipment (PPE) requirements for endoscopy and other procedures. Like SARS‐CoV‐2 testing, the limiting factor is the availability of PPE. Without adequate supply, many elective procedures will need to be canceled.
Modifications of the practice of liver transplantation (LT) are recommended by all three associations. With the potential risks of SARS‐CoV‐2 transmission, aggravated by the limited supply of PPE and other resources, many governments have restricted elective medical procedures, including LT. However, in the United States, LTs are considered “high‐acuity surgery” and can proceed as medically warranted. 8 All associations recommend limiting LT to patients with high Model for End‐Stage Liver Disease scores, risk for decompensation, or hepatocellular carcinoma (HCC) progression.
Should LT be Performed in COVID‐19 Recipients, and Should One Use Organs Procured From COVID‐19 Donors?
AASLD recommends against LT in patients with COVID‐19. LT can proceed 21 days after symptom resolution and negative diagnostic tests in recipients. APASL suggests balancing risks of delaying LT against risks of transmission to health care workers (HCWs). EASL does not specifically address this issue. To minimize the risk to HCWs, APASL recommends LT be performed only in patients with COVID‐19 with at least two consecutive negative SARS‐CoV‐2 nucleic acid results and the presence of antibodies. Finally, there is debate whether immunosuppression should be reduced during the COVID‐19 pandemic. So far there are no data to suggest that posttransplant immunosuppression is a risk factor for severe COVID‐19. In contrast, reducing immunosuppression may increase the risk for graft rejection. All three associations recommend against reducing immunosuppressive therapy in LT patients with mild COVID‐19. The dose of azathioprine, mycophenolate, and calcineurin inhibitor may be reduced in the setting of severe lymphopenia or worsening pulmonary status.
What Are the Roles of a Hepatologist in the Management of a Patient With COVID‐19?
Elevation of serum transaminase levels is commonly observed in patients with COVID‐19, and a hepatologist might therefore be consulted. All of the guidance suggests that the underlying cause of liver injury may be related to SARS‐CoV‐2 infections, exacerbation of preexisting CLD, or drug‐induced hepatotoxicity. AASLD and APASL provide an algorithm to clinical evaluations (Fig. 1). A key question is whether patients with CLD have a higher risk for severe COVID‐19. AASLD and APASL suggest nonalcoholic fatty liver disease (NAFLD) as an independent prognostic factor, and patients with CLD should be prioritized as candidates for COVID‐19 drug trials. EASL and AASLD mention that patients with NAFLD are more likely than others to have other comorbidity risks for severe COVID‐19. To date, there is no evidence that patients with stable CLD due to chronic hepatitis B (CHB) or chronic hepatitis C (CHC) have increased susceptibility to SARS‐CoV‐2 infection. It is controversial whether there is an increased risk for flare‐up of CHB or CHC during COVID‐19 and whether prophylactic therapy should be started. Both AASLD and APASL recommend continuing treatment for CHB or CHC in patients with COVID‐19. APASL recommends prophylactic hepatitis B therapy for those planned for anti‐IL‐6 or other immunosuppressive therapy. Initiating prophylactic hepatitis C therapy is not recommended. If there is any suggestion of a flare‐up, therapy should be initiated in patients who are not already receiving hepatitis B or hepatitis C treatment.
Fig 1.
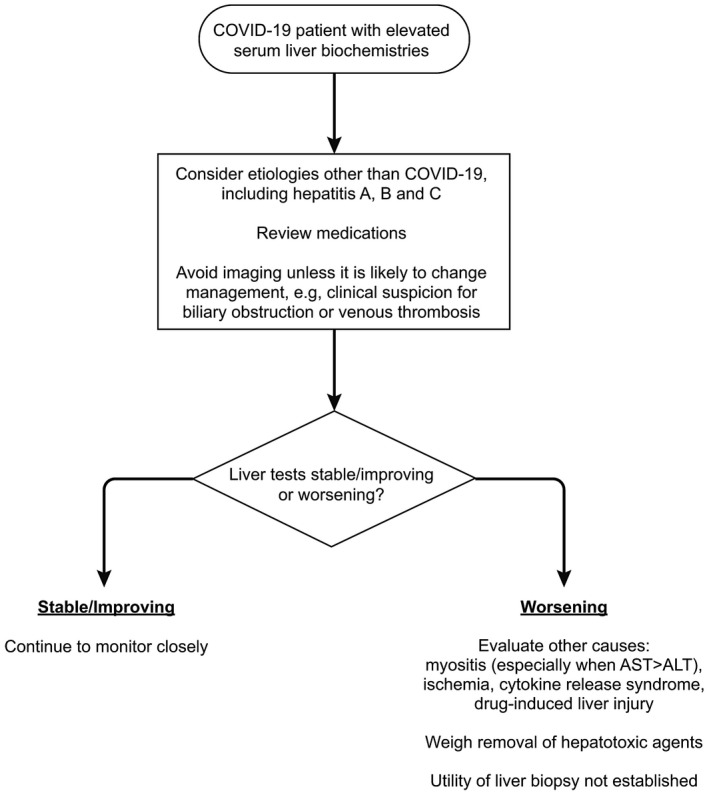
Approach to the patient with COVID‐19 and elevated serum liver biochemistries. Reproduced with permission from Hepatology. 5 Copyright 2020, American Association for the Study of Liver Diseases.
On May 1, 2020, remdesivir, a nucleotide RNA polymerase inhibitor, was authorized by the US Food and Drug Administration under Emergency Use Authorization for treatment of those patients hospitalized with severe COVID‐19. 9 APASL and AASLD recommend close monitoring of liver function in patients, especially those with CLD, who are treated with remdesivir. Patients with decompensated CLD and those with alanine aminotransferase (ALT) >5 times upper limit of normal should not be treated with remdesivir.
How Should We Modify Management of Patients With HCC?
To avoid SARS‐CoV‐2 exposures, all associations recommend reducing patient visits and a delay in HCC ultrasound surveillance. It is uncertain whether HCC treatment should be deferred or started as usual in patients with COVID‐19 with newly diagnosed HCC, and whether tyrosine kinase inhibitors (TKIs) or checkpoint inhibitors should be stopped in patients with COVID‐19 who are already receiving such therapy. Delaying or withdrawing treatment increases the risk for HCC progression with detrimental outcomes, whereas surgical resection may increase risk for transmission to health care personnel, and checkpoint inhibitors might worsen COVID‐19 by exacerbating a cytokine storm. AASLD recommends HCC treatments should proceed. EASL recommends locoregional therapies should be postponed whenever possible and immune‐checkpoint inhibitor therapy be temporarily withdrawn. TKI in nonsevere COVID‐19 should be taken on a case‐by‐case basis. APASL recommends postponing elective transplant/resection surgery, whereas radiofrequency ablation, transcatheter arterial chemoembolization, TKI, or immunotherapy can be initiated with change of immunotherapy schedules to every 4 to 6 weeks.
How to Conduct Clinical Trials?
Both APASL and AASLD recommend using alternative physical distancing processes for study assessments to reduce SARS‐CoV‐2 exposure. APASL specifically recommends seeking local regulators and institutional review board approval of the contingency measures during the COVID‐19 pandemic, obtaining trial participant’s consent, and documentation of all deviations from the contingency measures. These recommendations align with US National Institutes of Health (NIH) revised guidance for NIH‐supported clinical research. 10
Summary
APASL, AASLD, and EASL strongly recommend changes in patient workflow and clinical procedures to protect HCWs and patients from SARS‐CoV‐2 infection. Similarly, the associations generally agree on approaches to evaluation and treatment of patients with COVID‐19 for liver disease, and management of patients with HCC and post–liver transplant patients with slight differences in the populations targeted for SARS‐CoV‐2 testing. These recommendations will evolve with further clinical experience and data from randomized controlled trials. For now, the liver associations provide the best available advice for the management of CLD during the COVID‐19 pandemic.
Potential conflict of interest: Nothing to report.
Contributor Information
George Lau, Email: gkklau@hnhmgl.com.
John W. Ward, Email: jward@taskforce.org.
References
- 1. World Health Organization . Guidance for health workers. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/health-workers. Accessed May 4, 2020.
- 2. Centers for Disease Control and Prevention . Infection control guidance for healthcare professionals about coronavirus (COVID‐19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Updated April 24, 2020. Accessed May 4, 2020.
- 3. World Health Organization . Coronavirus disease (COVID‐19) technical guidance: patient management. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/patient-management. Accessed May 4, 2020.
- 4. APASL Covid‐19 Task Force , Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID‐19 pandemic: APASL expert panel consensus recommendations. Hepatol Int 2020. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fix OK, Hameed B, Fontana RJ. Insights for hepatology and liver transplant providers during the COVID‐19 pandemic. Hepatology. Available at: 10.1002/hep.31281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID Position Paper. JHEP Rep 2020;2:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS‐CoV‐2 infections in residents of a long‐term care skilled nursing facility—King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020;69:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Medicaid and Medicare Services . CMS Adult elective surgery and procedures recommendations: limit all non‐essential planned surgeries and procedures, including dental, until further notice. Available at: https://www.cms.gov/files/document/covid-elective-surgery-recommendations.pdf. Published April 7, 2020. Accessed May 4, 2020.
- 9. US Food and Drug Administration . Fact sheet for health care providers: emergency use authorization (EUA) of Remdesivir (GS‐5734™). Available at: https://www.fda.gov/media/137566/download. Accessed May 4, 2020.
- 10. National Institutes of Health . Guidance for NIH‐funded clinical trials and humansubjectsstudiesaffectedbyCOVID‐19. Notice Number NOT‐OD‐20‐087. Available at: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-20-087.html. Published March 16, 2020. Accessed May 4, 2020.


