Abstract
Aims
Myocardial fibrosis (MF) might represent a key player in pathophysiology of heart failure in aortic stenosis (AS). We aimed to assess its impact on left ventricular (LV) remodelling, recovery, and mortality after transcatheter aortic valve implantation (TAVI) in different AS subtypes.
Methods and results
One hundred patients with severe AS were prospectively characterized clinically and echocardiographically at baseline (BL), 6 months, 1 year, and 2 years following TAVI. Left ventricular biopsies were harvested after valve deployment. Myocardial fibrosis was assessed after Masson’s trichrome staining, and fibrotic area was calculated as percentage of total tissue area. Patients were stratified according to MF above (MF+) or below (MF−) median percentage MF (≥11% or <11%). Myocardial fibrosis burden differed significantly between AS subtypes, with highest levels in low ejection fraction (EF), low-gradient AS and lowest levels in normal EF, high-gradient AS (29.5 ± 26.4% vs. 13.5 ± 16.1%, P = 0.003). In the entire cohort, MF+ was significantly associated with poorer LV function, higher extent of pathological LV remodelling, and more pronounced clinical heart failure at BL. After TAVI, MF+ was associated with a delay in normalization of LV geometry and function but not per se with absence of reverse remodelling and clinical improvement. However, 22 patients died during follow-up (mean, 11 months), and 14 deaths were classified as cardiovascular (CV) (n = 9 arrhythmia-associated). Importantly, 13 of 14 CV deaths occurred in MF+ patients (CV mortality 26.5% in MF+ vs. 2% in MF− patients, P = 0.0003). Multivariate analysis identified MF+ as independent predictor of CV mortality [hazard ratio (HR) 27.4 (2.0–369), P = 0.01].
Conclusion
Histological MF is associated with AS-related pathological LV remodelling and independently predicts CV mortality after TAVI.
Keywords: Aortic stenosis, Myocardial fibrosis, Endomyocardial biopsy, Transcatheter aortic valve implantation
See page 1915 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa151)
Introduction
It is increasingly acknowledged that aortic stenosis (AS) is not only a disease of the aortic valve but also of the left ventricle. Current guidelines strongly recommend aortic valve replacement (AVR) for patients with severe AS who are either symptomatic or have developed left ventricular (LV) systolic dysfunction.1 Thus, the decision to intervene is ultimately driven by the condition of the myocardium, and not by the valve.
Various phenotypes of LV remodelling in AS exist that are characterized by differing LV geometry and varying degrees of LV dysfunction. Histological studies suggested that myocardial fibrosis (MF) plays a significant role in the transition from adaptive LV hypertrophy with preserved EF to maladaptive LV remodelling with reduced EF in patients with severe AS.2 , 3 Furthermore, histological MF was associated with impaired clinical recovery and reduced long-term survival in patients undergoing surgical AVR (SAVR).3–5
Currently, the era of transcatheter aortic valve implantation (TAVI) has dramatically changed demographic and clinical characteristics of AS patients towards increasingly older and sicker individuals. However, no data evaluating the impact of histological MF on LV reverse remodelling and clinical outcome after TAVI is available yet. Moreover, MF burden and its relevance have not yet been investigated in patients with different guideline-defined1 entities of severe AS.
Thus, the purposes of our clinical study were (i) to characterize the different patterns of LV remodelling in AS subtypes, (ii) to link these to MF in endomyocardial LV biopsies, and (iii) to assess the impact of MF on recovery (defined by LV reverse remodelling and clinical benefit) and on cardiovascular (CV) mortality after TAVI.
Methods
Between January 2017 and October 2018, we prospectively enrolled 100 consecutive patients scheduled for transfemoral TAVI. Indication for TAVI was based on heart team consensus according to current guidelines.1 Transfemoral implantation was performed using standard techniques. In the vast majority of cases, the Sapien 3 valve (Edwards Lifesciences Inc., Irvine, CA, USA) was implanted.
At baseline (BL), transthoracic echocardiography (TTE) and transoesophageal echocardiography, 6-min walking test (6mwt), Minnesota Living with Heart failure Quality of life questionnaire (MLHFQ), New York Heart Association (NYHA) status, and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were recorded. Structured follow-up visits at 6 months, 1 year, and 2 years included TTE, 6mwt, MLHFQ, and NT-proBNP.
Cardiovascular mortality during follow-up (VARC-2 definition)6 was defined as the primary clinical endpoint, all-cause mortality as secondary endpoint.
The local ethics committee approved the study and written informed consent was obtained from all patients.
Echocardiography
All echocardiograms were performed using either a Philips ie33 or a Philips Epiq7 system, routinely recorded in a Picture Archiving and Communication System and retrospectively re-evaluated by a single observer using Q Station 3.8.5 (Philips healthcare). Echocardiographic measurements were obtained as recommended.7 For further details, see Supplementary material online, Appendix. Following current guidelines,1 four subtypes of severe AS were defined:
Normal/preserved ejection fraction, high-gradient AS (NEF-HG AS): LVEF ≥50%, v max ≥4 m/s or P mean ≥40 mmHg, and aortic valve area (AVA) ≤1.0 cm2.
Low/reduced ejection fraction, high-gradient AS (LEF-HG AS): LVEF<50%, v max ≥4 m/s, or P mean ≥40 mmHg, and AVA ≤1.0 cm2.
Low/reduced ejection fraction, low-gradient AS (‘classic’ low-flow, low-gradient AS) (LEF-LG AS): LVEF <50%, v max <4 m/s and P mean <40 mmHg, AVA ≤1.0 cm2, and stroke volume index (SVI) ≤35 mL/m2.
Paradoxical low-flow, low-gradient AS (PLF-LG AS): LVEF ≥50%, v max <4 m/s and P mean <40 mmHg, AVA ≤1.0 cm2 and indexed AVA ≤0.6 cm2/m2, and SVI ≤35 mL/m2.
Assessment of myocardial fibrosis in endomyocardial biopsies
After deployment of the transcatheter valve, LV biopsies were harvested from the basal anteroseptum by using a biopsy forceps (Proflex-Bioptom 7F, Medical Imaging Systems), fixed in 10% paraformaldehyde and embedded in paraffin. MF was assessed blinded to clinical and imaging data using quantitative morphometry (Olympus Software cell-Sens 1.6) and defined as blue area in Masson’s trichrome stained biopsy sections (section with positive staining for collagen) in relation to total tissue area.
Cardiovascular magnetic resonance imaging
Cardiovascular magnetic resonance (CMR) imaging was performed on a 3 Tesla MR scanner (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany). Late gadolinium enhancement (LGE) was quantified in grams using a 3 SD threshold (adapted from Ref.8) Extracellular volume fraction was defined as ECV = (1 − Hct) × (DeltaR1myocardium)/(DeltaR1blood). For further details, see Supplementary material online, Appendix.
Statistical analysis
Statistical analysis was performed with graph pad prism version 8.0 and with the Statistical Computing Software R (version 3.4.4; http://www.r-project.org). Continuous variables are presented as mean ± standard deviation and were compared using Student’s t-test or Mann–Whitney U test for two-group comparison (as appropriate). Categorical variables are presented as absolute numbers and percentage and were compared by Fisher’s exact test for two-group comparison and by Pearson’s χ 2 test for multigroup comparison. A P-value of <0.05 was considered statistically significant.
Survival analysis was performed considering procedure (TAVI) to event time for mortality and CV mortality using the R package survival, visualized by Kaplan–Meier plots, and significance was calculated by the log-rank test. For multivariate models, the Cox Proportional Hazards Model was used. Survival analyses were performed for known outcome predictors according to literature or to previous publications of our institution (BL characteristics, echocardiographic measures, AS subtypes, and MF). Only risk stratifiers that were found to be significant in univariate analyses were included in multivariate analyses.
Results
Among the 100 study participants, 40 suffered from NEF-HG AS, 14 from LEF-HG AS, 26 from LEF-LG AS, and 16 from PLF-LG AS. Four patients were retrospectively classified as ‘moderate-to-severe AS’ and excluded from subtype analyses.
Baseline demographic characteristics
The total cohort (35 women and 65 men) was characterized by advanced age (mean 78 ± 7 years), and a high prevalence of coronary artery disease (CAD, 70%), atrial fibrillation (43%), diabetes (45%), and chronic kidney disease (54%). Seventy-four percentage of patients pertained to NYHA Classes III and IV (Table 1).
Table 1.
Baseline clinical characteristics
| Total cohort (n = 100) | NEF-HG AS (n = 40) | LEF-HG AS (n = 14) | LEF-LG AS (n = 26) | PLF-LG AS (n = 16) | P (comparison of all groups) | |
|---|---|---|---|---|---|---|
| Age (years) | 78 ± 7 | 78 ± 7 | 78 ± 9 | 79 ± 6 | 81 ± 5 | 0.37 |
| Sex, female, n (%) | 35 | 15 (38) | 5 (36) | 5 (19)d | 9 (56)c | 0.11 |
| Coronary artery disease, n (%) | 70 | 26 (65) | 9 (64) | 20 (77) | 11 (69) | 0.75 |
| Prior MI, n (%) | 22 | 3 (8)c | 2 (14)c | 12 (46)a , b | 3 (19) | 0.002 |
| Prior PCI, n (%) | 36 | 11 (28)c | 2 (14)c , d | 14 (54)a , b | 8 (50)b | 0.03 |
| Prior CABG, n (%) | 11 | 4 (10) | 1 (7) | 4 (15) | 1 (6) | 0.76 |
| Ischaemic cardiomyopathy, n (%) | 15 | 0b , c | 3 (21)a | 12 (46)a , d | 0c | <0.001 |
| Dilative cardiomyopathy, n (%) | 2 | 0 | 0 | 2 (8) | 0 | 0.14 |
| Atrial fibrillation, n (%) | 43 | 16 (40) | 5 (36) | 10 (38) | 11 (69) | 0.18 |
| Peripheral vascular disease | 27 | 11 (28) | 3 (21) | 9 (35) | 2 (13) | 0.44 |
| Prior cerebral ischaemia event, n (%) | 19 | 8 (20) | 1 (7) | 6 (23) | 4 (25) | 0.60 |
| Chronic pulmonary disease, n (%) | 21 | 6 (15) | 1 (7) | 9 (35) | 5 (31) | 0.10 |
| Diabetes, n (%) | 45 | 18 (45) | 3 (21)a | 14 (54)b | 8 (50) | 0.48 |
| CKD (GFR <60 mL/min), n (%) | 54 | 19 (48) | 6 (43) | 15 (58) | 12 (75) | 0.22 |
| Creatinine (mg/dL) | 1.23 ± 0.7 | 1.26 ± 1.0 | 1.09 ± 0.3 | 1.27 ± 0.4 | 1.27 ± 0.9 | 0.90 |
| NT-proBNP (pg/mL) | 4901 ± 9444 | 2206 ± 3411b , c | 10 061 ± 11 082a , d | 8228 ± 14 712a | 2117 ± 1185b | <0.0001 |
| MLHFQ (points) | 38 ± 18 | 34 ± 19d | 42 ± 17 | 40 ± 14 | 46 ± 15a | 0.09 |
| 6mwt distance (m) | 213 ± 121 | 252 ± 95b , c , d | 169 ± 147a | 186 ± 117a | 168 ± 125a | 0.03 |
Two group comparisons: t-test for continuous variables and the Fisher’s exact test for categorical variables. Comparison of all four groups: one-way analysis of variance for continuous variables and the χ2 test for categorical variables.
6mwt, 6-min walking test; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; MI, myocardial infarction; MLHFQ, Minnesota Living with Heart failure Quality of Life Questionnaire; PCI, percutaneous coronary intervention.
P-value of <0.05 vs. NEF-HG AS.
P-value of <0.05 vs. LEF-HG AS.
P-value of <0.05 vs. LEF-LG AS.
P-value of <0.05 vs. PLF-LG AS.
Among all haemodynamic subgroups, LEF-LG patients represented the sickest cohort with the highest prevalence of relevant CAD [prior myocardial infarction (MI) in 46% and prior coronary artery bypass grafting (CABG) in 54%]. Importantly, we could prove for 54% of LEF-LG patients that reduced LVEF preceded the diagnosis of severe AS, whereas pre-existing systolic dysfunction had been documented in only 21% of LEF-HG patients. In the PLF-LG cohort, the proportion of women (56%) and the prevalence of atrial fibrillation (69%) were highest among all subgroups.
Quantitative measures of heart failure
Six-min walking test distance and NT-proBNP levels consistently identified LEF-HG patients as those with most advanced and NEF-HG patients as those with least advanced clinical heart failure (Table 1). PLF-LG patients exhibited the lowest NT-proBNP levels and also the lowest 6mwt distances of all subtypes.
Patterns of echocardiographic left ventricular remodelling and function
Echocardiographic BL measures are displayed in Supplementary material online, Table S1. NEF-HG patients exhibited a normal LV cavity size with normal indexed left-ventricular end-diastolic volume [LVEDVi, 40 ± 15 mL/m2 body surface area (BSA)], a markedly elevated LV mass index (LVMI, 146 ± 38 g/m2 BSA) and a very high relative wall thickness (RWT, 0.67 ± 0.13), thus indicating significant concentric LV hypertrophy. In contrast, LV geometry of LEF-HG patients was characterized by the combination of LV dilatation and hypertrophy (eccentric hypertrophy), with the highest LVEDVi (64 ± 20 mL/m2 BSA), highest LVMI (181 ± 41 g/m2 BSA), and lowest RWT (0.50 ± 0.10) of all subgroups. This progressive LV remodelling suggests that LEF-HG AS represents a more advanced stage of NEF-HG AS. Echocardiographic LV morphology of LEF-LG patients did not differ significantly from LEF-HG patients despite of significantly lower transaortic gradients (P mean 24 ± 7 vs. 45 ± 11 mmHg, P < 0.001). PLF-LG patients had the smallest LV cavity size (LVEDVi 34 ± 14 mL/m2 BSA) and the lowest LVMI among all subgroups (128 ± 31 g/m2 BSA). However, RWT (0.64 ± 0.07) and LV mass adjusted for end-diastolic volume (4.04 ± 1.32 g/mL) were as high as in NEF-HG AS indicating significant (apparently maladaptive) concentric remodelling/hypertrophy. Despite normal EF, longitudinal myocardial function suggested subclinical LV systolic dysfunction (GLS −16 ± 2%). The phenotype PLF-LG was overrepresented in women. Other new gender-specific findings could not be detected.
Relationship between myocardial fibrosis in endomyocardial biopsies and baseline parameters
Amount of MF differed significantly between the four haemodynamic AS subtypes (Figure 1). LEF-HG (25.6 ± 23.3%) and LEF-LG patients (29.5 ± 26.4%) exhibited a significantly higher MF burden than NEF-HG patients (13.5 ± 16%; P = 0.03 vs. LEF-HG; P = 0.003 vs. LEF-LG). Fibrosis in PLF-LG (15.6 ± 14.0) was numerically higher than in NEF-HG AS and lower than in LEF-HG and LEF-LG AS.
Figure 1.
Burden of myocardial fibrosis in different haemodynamic subtypes of severe aortic stenosis (box plots indicating minimum, maximum, median, and 25th + 75th percentile). Fibrosis was assessed in Masson’s trichrome stained biopsy sections as blue area in relation to total tissue area. NEF-HG, normal EF, high gradient; LEF-HG, reduced EF, high gradient; LEF-LG, reduced EF, low gradient (classic low-flow, low-gradient); PLF-LG, paradoxical low-flow, low-gradient.
Regarding the influence of CAD, we found similar MF levels in study patients with (n = 70) and without CAD (n = 30) (19.3 ± 21.3 vs. 19.8 ± 19.9%, P = 0.92). Furthermore, we stratified into individuals with (n = 27) and without severe CAD (n = 73; defined by the presence or absence of prior MI and/or CABG) and detected no significant difference (25.2 ± 24.0 vs. 17.3 ± 19.2%, P = 0.09) (Supplementary material online, Figure S1). Then, we applied the latter stratification to each AS subtype (Supplementary material online, Figure S2). Thereby, a significant difference by presence of severe CAD could only be detected in NEF-HG AS (P = 0.03) but not in LEF-HG (P = 0.31), LEF-LG (P = 0.84) or PLF-LG AS (P = 0.36). Furthermore, analysis of fibrosis levels by AS subtype (Figure 1) remained qualitatively unchanged after exclusion of patients with severe CAD (Supplementary material online, Figure S2).
For subsequent analyses, we stratified study participants according to their MF burden into patients with myocardial fibrosis above (MF+) and below (MF−) the median of the entire cohort (11%) (Table 2). MF+ patients were younger and more likely to suffer from diabetes (61% vs. 27%; P = 0.0001). In addition, they exhibited a significantly lower 6mwt distance (179 ± 122 vs. 245 ± 112 m, P = 0.01). Among them were only 25% of all NYHA I patients, but 64% of all NYHA IV patients. Concerning echocardiographic measurements, MF+ patients were characterized by lower EF (44 ± 17 vs. 55 ± 11%, P = 0.0002), poorer systolic longitudinal myocardial function (GLS −13.1 ± 4.7 vs. −16.3 ± 4.2%, P = 0.0001), larger left ventricles [left ventricular end-diastolic volume (LVEDV) 107 ± 45 vs. 79 ± 33 mL/m2 BSA; P = 0.0006], higher LVMI (161 ± 43 vs. 142 ± 39 g/m2 BSA, P = 0.03), and consecutively a higher prevalence of eccentric hypertrophy (24% vs. 4%, P = 0.004). In conclusion, MF+ patients exhibited a higher extent of pathological LV remodelling and clinical heart failure.
Table 2.
Comparison of baseline characteristics in patients with myocardial fibrosis below (MF−) and above (MF+) the median
| MF− (MF <11%; n = 51) | MF+ (MF ≥ 11%; n = 49) | P-value | |
|---|---|---|---|
| Age (years) | 80 ± 6 | 77 ± 7 | 0.01 |
| Female gender, n (%) | 20 (39) | 15 (31) | 0.19 |
| Coronary artery disease, n (%) | 38 (75) | 32 (65) | 0.28 |
| Prior myocardial infarction, n (%) | 11 (22) | 11 (22) | 1.0 |
| Atrial fibrillation, n (%) | 20 (39) | 23 (47) | 0.54 |
| Peripheral vascular disease | 9 (18) | 18 (37) | 0.04 |
| Diabetes, n (%) | 14 (27) | 30 (61) | 0.001 |
| CKD (GFR < 60 mL/min), n (%) | 27 (53) | 27 (55) | 1.0 |
| NT-proBNP (pg/mL) | 3142 ± 3824 | 6620 ± 12 569 | 0.09 |
| 6mwt (m) | 245 ± 112 | 179 ± 122 | 0.01 |
| NYHA III + IV, n (%) | 34 (67) | 40 (82) | 0.11 |
| AS subtype, n (%) | 0.03 | ||
| NEF-HG AS | 27/40 (68) | 13/40 (33) | 0.002 |
| LEF-HG AS | 5/14 (36) | 9/14 (64) | 0.13 |
| LEF-LG AS | 9/26 (35) | 17/26 (65) | 0.03 |
| PLF-LG AS | 7/16 (44) | 9/16 (56) | 0.48 |
| LVEF (%) | 55 ± 11 | 44 ± 17 | 0.0002 |
| Global longitudinal strain (GLS) (%) | −16.3 ± 4.2 | −13.1 ± 4.7 | 0.001 |
| Stroke volume index (mL/m²) | 38 ± 11 | 34 ± 8 | 0.04 |
| LVEDV (mL) | 79 ± 33 | 107 ± 45 | 0.0006 |
| LVMI (g/m² BSA) | 142 ± 39 | 161 ± 43 | 0.03 |
| Eccentric hypertrophy, n (%) | 2 (4) | 12 (24) | 0.004 |
| Mean transaortic gradient (mmHg ) | 39 ± 15 | 36 ± 16 | 0.44 |
| Aortic valve area (cm²) | 0.76 ± 0.17 | 0.72 ± 0.18 | 0.29 |
Two group comparisons: t-test for continuous variables and the Fisher’s exact test for categorical variables.
6mwt, 6-min walking test distance; BSA, body surface area; CKD, chronic kidney disease; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; NYHA, New York Heart Association.
Patterns of myocardial fibrosis
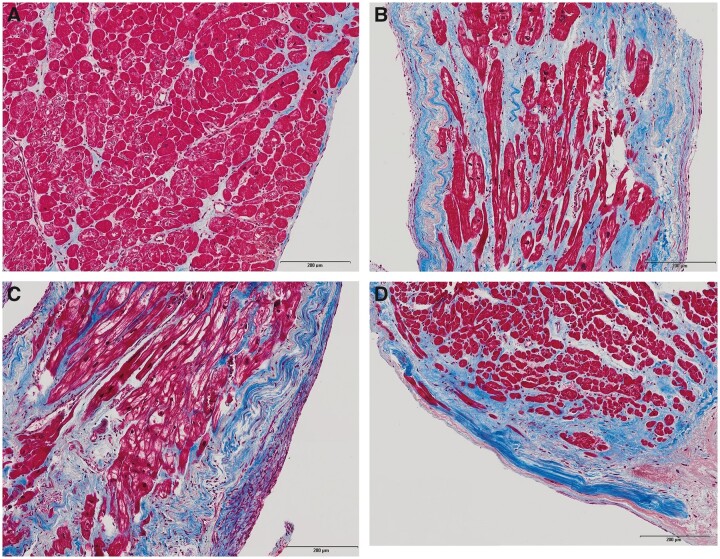
Almost all LV biopsies (90/100) contained endocardium. Regarding the distribution of MF, we predominantly found mid-wall interstitial fibrosis (72%) followed by subendocardial fibrosis (19%) and replacement fibrosis (9%). Histological examples are displayed in Figure 2.
Figure 2.
Masson's trichrome stained endomyocardial biopsies of four patients with different AS subtypes. (A) A 73-year-old man with normal EF, high-gradient AS, coronary artery disease (CAD) excluded, no diabetes, baseline EF 60%, LVEDV 91 mL, and LVMI 124 g/m2; low MF burden (5%), predominantly interstitial (including perivascular) localization; uneventful follow-up with favourable outcome. (B) A 67-year-old man with reduced EF, high-gradient AS, CAD excluded, no diabetes, baseline EF18%, LVEDV 175 mL, and LVMI 224 g/m2; high MF burden (42%) with subendocardial and interstitial localization; uneventful follow-up with very good clinical and echocardiographic recovery (EF at 6 months 52%). (C) An 89-year-old woman with reduced EF, low-gradient AS, CAD with CTO LAD, no diabetes, baseline EF 17%, LVEDV 222 mL, and LVMI 235 mL/m2; high MF burden (40%), predominantly subendocardial localization with massive fibroblast infiltration of the endocardium, and the subendocardial layer; focal replacement fibrosis; direct post-interventional course uneventful, but patient died 5 days after TAVI due to incessant VT and unsuccessful CPR. (D) A 76-year-old man with paradoxical low-flow, low-gradient aortic stenosis, CAD without prior infarction, diabetes, baseline EF 54%, LVEDV 82 mL, LVMI 96 g/m2; high MF burden (45%) with predominantly subendocardial and to a lesser extent interstitial localization; focal replacement fibrosis; patient did not benefit clinically and died 125 days after TAVI (after subsequent cardiac surgery for severe tricuspid regurgitation).
Reverse left ventricular remodelling at 6-month follow-up
At 6 months, 14 of 100 TAVI patients had died, and 19 of 100 were unable or refused to perform the clinical visit. Thus, a complete 6-month follow-up could be obtained in 67 patients (29 MF+ and 38 MF−). The following analyses will rely exclusively on paired observations in these patients. However, a positive selection bias due to death or immobility of the sickest patients has to be postulated.
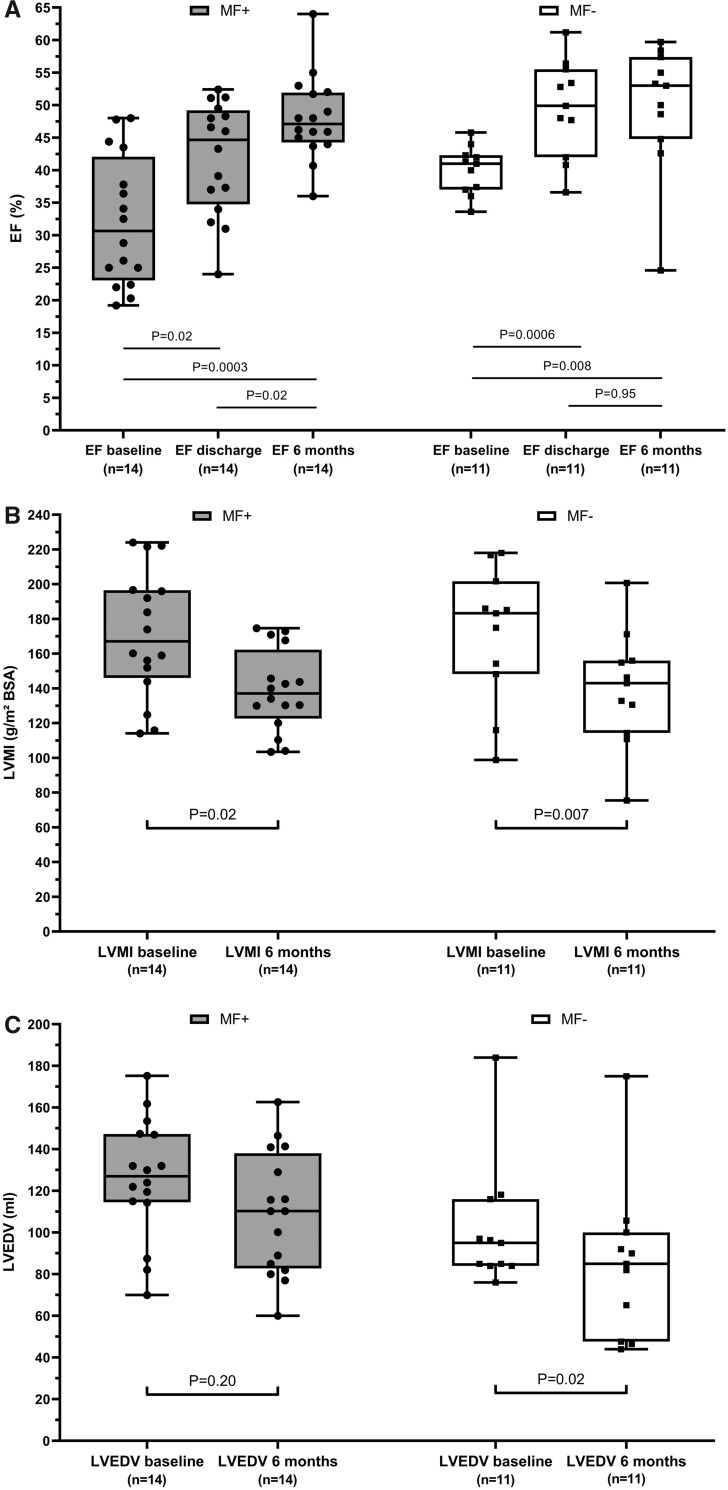
Regarding the entire cohort, a significant GLS improvement (MF+: −13.3 ± 4.5 vs. −16.1 ± 3.0, P = 0.006; MF−: −16.2 ± 4.4 vs. −17.5 ± 2.9, P = 0.04) and LVMI reduction (MF+: 156.4 ± 134.1 vs. 134.1 ± 26.4, P = 0.0001; MF−: 141.2 ± 39.8 vs. 124.2 ± 32.6, P = 0.002) was present in both MF+ and MF− patients, whereas changes in LVEDV were insignificant. We then closer examined both AS subgroups with reduced BL EF and consecutively most advanced pathological BL remodelling (LEF-HG + LEF-LG AS patients, n = 25). In the 14 MF+ patients, BL EF was significantly lower (P = 0.046) and EF recovery at time of discharge poorer (MF+: 33 ± 10 at BL vs. 41 ± 10% at discharge, P = 0.02; MF−: 40 ± 4 at BL vs. 50 ± 7% at discharge, P = 0.0006). However, EF values equalized at 6 months (49 ± 7% in MF+ vs. 50 ± 10% in MF−, P = 0.77) (Figure 3A). Compared to BL, both MF+ and MF− patients exhibited a significant LVMI reduction at 6 months (MF+: 166 ± 41 vs. 141 ± 24 g/m2, P = 0.02; MF−: 171 ± 39 vs. 137 ± 34 g/m2, P = 0.007), but LVMI reduction was more pronounced in the MF− group. Similarly, a significant reduction of LVEDV could only be observed in MF− patients (MF+: 125 ± 31 at BL vs. 109 ± 31 mL at 6 m, P = 0.20; MF−: 102 ± 30 at BL vs. 85 ± 38 mL at 6 m, P = 0.02) (Figure 3B and C).
Figure 3.
Reverse left ventricular remodelling at 6-month follow-up in patients with reduced baseline ejection fraction (reduced ejection fraction, high-gradient and reduced ejection fraction, low-gradient aortic stenosis) in dependence on myocardial fibrosis burden (box plots indicating minimum, maximum, median, and 25th + 75th percentile); only matched observations included. Development of (A) ejection fraction, (B) left ventricular end-diastolic volume, and (C) left ventricular mass index. BL, baseline; EF, ejection fraction; LVEDV, left ventricular end-diastolic volume; LVMI, left ventricular mass index; MF+, myocardial fibrosis ≥11% (=median total cohort); MF−, myocardial fibrosis <11%.
We also performed mixed-effects analyses for the complete cohort (Supplementary material online, Figure S3) and for the AS subtypes with reduced BL EF. Importantly, no significant interactions between change over time and MF status could be detected. In conclusion, MF+ was associated with a delay in (but not per se with absence of) improvement of LV geometry and function.
Clinical measures of heart failure at 6-month follow-up
In the entire cohort, significant improvements in both MF+ and MF− patients could be observed regarding 6mwt distance (MF+: 193 ± 117 vs. 252 ± 137 m, P = 0.006; MF−: 254 ± 115 vs. 276 ± 104 m, P = 0.01), MLHFQ points (MF+: 37 ± 17 vs. 24 ± 19 points, P < 0.0001; MF−: 33 ± 17 vs. 26 ± 19 points, P = 0.02), and NT-proBNP levels (MF+ 3442 ± 4076 vs. 1884 ± 2877 pg/mL, P = 0.02; MF−: 2305 ± 2747 vs. 952 ± 1162 pg/mL, P = 0.005).
In conclusion, both MF+ and MF− patients had gained clinical benefit from TAVI, but measures of heart failure had not yet equalized between groups at 6 months.
All-cause and cardiovascular mortality during follow-up
Procedural mortality was 0%. Follow-up period after TAVI ranged between 6 months and 2 years for each individual patient (mean, 11 months). During follow-up, 22 deaths occurred: 6 of 40 NEF-HG patients (15%), 3 of 14 LEF-HG patients (21%), 8 of 26 LEF-LG patients (31%), and 4 of 16 PLF-LG patients (25%) died. Among these, 14 causes of death were classified as CV: 1 of 40 (2.5%) in NEF-HG AS (endocarditis), 2 of 14 (14%) in LEF-HG AS (two unexplained sudden deaths), 7 of 26 (27%) in LEF-LG AS (heart failure in two cases, documented arrhythmia in four patients, and one unexplained sudden death), and 4 of 16 (25%) in PLF-LG AS (two unexplained sudden deaths, one death after cardiac surgery for tricuspid regurgitation, and one case of endocarditis). Non-cardiac deaths occurred due to malignant diseases (n = 4; three NEF-HG and one LEF-LG), sepsis after surgery for gastric cancer (n = 1, NEF-HG), aspiration (n = 1, NEF-HG), renal failure with refusal of renal replacement therapy (n = 1, NEF-HG), and pneumonia with refusal of intensive care therapy (n = 1, LEF-HG).
Predictors of cardiovascular mortality during follow-up
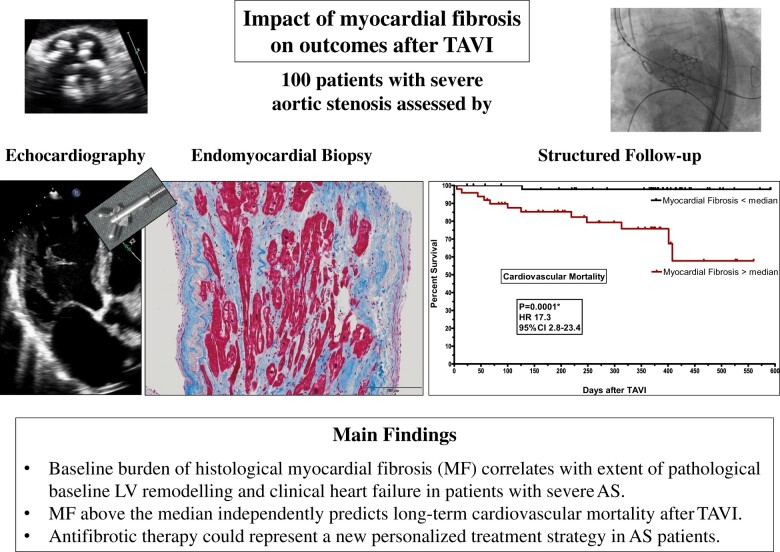
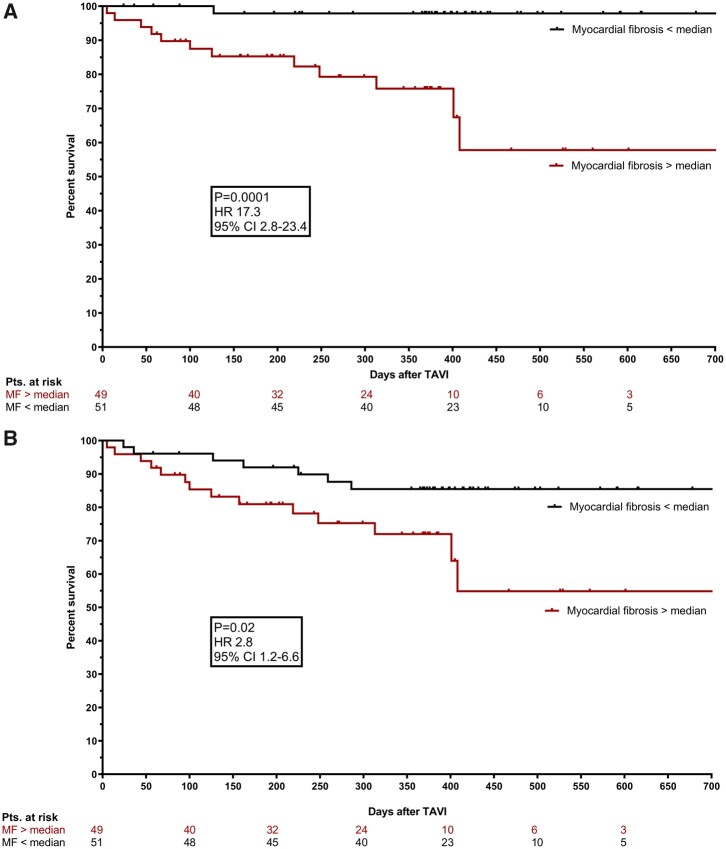
Taking NEF-HG AS as a reference, CV mortality was significantly higher in LEF-LG AS [hazard ratio (HR) 3.28 (1.2–9.4); P = 0.006] and in PLF-LG AS [HR 2.12 (1.0–4.4); P = 0.01] in univariate analysis. Also, a strong trend towards higher cardiac mortality was present in LEF-HG AS [HR 6.04 (0.6–66.7); P = 0.09]. Myocardial fibrosis above the median predicted CV mortality with the highest HR of all significant parameters [HR 17.3 (2.8–23.4), P = 0.0001]. Kaplan–Meier curves for CV mortality in patients with MF below and above the median are displayed in Figure 4A.
Figure 4.
Cardiovascular and all-cause mortality in dependence of fibrotic burden. Kaplan–Meier curves displaying cardiovascular (A) and all-cause (B) mortality in patients with myocardial fibrosis below (black) and above (red) the median.
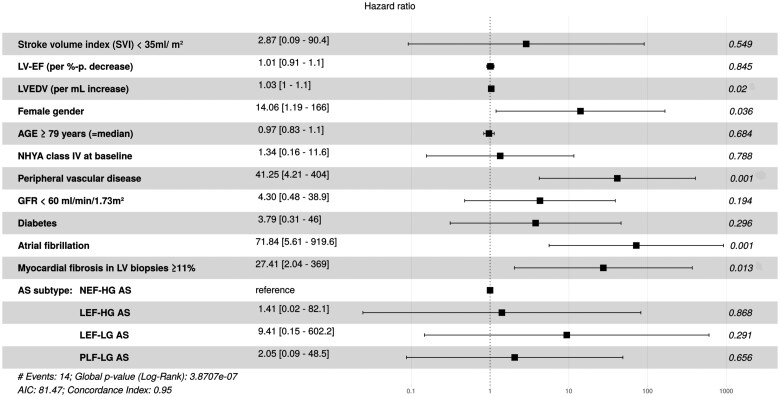
All univariate significant BL parameters, as well as age and gender, were inserted into a Cox Proportional Hazards Model. Thereby, five independent predictors of CV mortality during follow-up could be identified (see also forest plot in Figure 5): female gender [HR 14.1 (1.2–166); P = 0.04], BL LVEDV per mL increase [HR 1.03 (1.0–1.1); P = 0.02], peripheral vascular disease [HR 41.3 (4.2–404); P = 0.001], atrial fibrillation [HR 71.8 (5.6–920); P = 0.001], and MF ≥11% [HR 27.4 (2.0–369); P = 0.01]. Diabetes and AS subtype lost their significance in the presence of MF in the model.
Figure 5.
Multivariate Cox regression analysis for prediction of cardiovascular mortality during follow-up after transcatheter aortic valve implantation (hazard ratios with 95% confidence intervals, displayed as forest plot).
Take home figure.
This figure summarizes flow and main findings of the study which was designed to link myocardial fibrosis to LV remodelling, recovery and clinical outcome after TAVI.
For more accurate depiction of the relationship between MF levels and outcome, analyses by tertiles (lowest tertile: MF ≤7%, n = 35; medium tertile: MF >7–20%, n = 32; and highest tertile: MF > 20%, n = 33) are displayed in the Supplementary material online, Appendix. Cardiovascular mortality was 2.9% in the lowest, 9.4% in the medium, and 30.3% in the highest tertile (Kaplan–Meier curves in Supplementary material online, Figure S4). Multivariate analysis (forest plot in Supplementary material online, Figure S5) identified MF in the highest tertile as independent predictor of CV mortality [HR 24.91 (1.4–446), P = 0.03].
Predictors of all-cause mortality during follow-up
Kaplan–Meier curves for all-cause mortality in patients with MF below and above the median are displayed in Figure 4B. In univariate analysis, MF significantly predicted all-cause mortality (HR 2.8 (1.2–6.6), P = 0.02). However, only peripheral vascular disease and atrial fibrillation emerged as independent predictors of all-cause mortality in multivariate analysis (forest plot displayed in Supplementary material online, Figure S6).
Correlation of histological myocardial fibrosis with fibrosis-estimation by cardiovascular magnetic resonance
Among our 100 study patients, 40 underwent CMR with complete LGE and extracellular volume fraction (ECV) analyses (BL characteristics in Supplementary material online, Table S2). Mean LGE (using a 3 SD threshold8) was 29.97 ± 23.88 g, and mean total ECV 28.11 ± 4.17% (comparisons of ECV and LGE with histological images displayed in Supplementary material online, Figure S7).
Late gadolinium enhancement quantification significantly correlated with histological MF (r 2 = 0.20, P = 0.004), but ECV quantification did not (r 2 = 0.02; P = 0.41). In addition, LGE significantly correlated with echocardiographic parameters as EF (r 2 = 0.21; P = 0.003), LVEDVi (r 2 = 0.28; P = 0.001), LVMI (r 2 = 0.17; P = 0.0007), and GLS (r 2 = 0.19; P = 0.0007). In contrast, ECV only correlated with EF (r 2 = 0.10; P = 0.04). No associations between LGE/ECV quantification and clinical measures of heart failure could be detected. Since only 3 of 14 patients with CV death underwent magnetic resonance imaging (MRI), the prognostic impact of LGE or ECV quantification cannot be determined in this cohort.
Discussion
The present study was designed to link MF to haemodynamic AS phenotypes and BL LV remodelling patterns on the one hand and to recovery and clinical outcome after TAVI on the other.
The main findings are:
Baseline MF above the median independently predicts long-term CV mortality after TAVI.
The amount of MF correlates with the extent of BL LV remodelling and with clinical heart failure. Myocardial fibrosis above the median is associated with lower EF and global longitudinal strain, with larger LV end-diastolic volume and LV mass, and with higher NYHA class and lower 6mwt distance. Accordingly, MF burden is highest in LEF-HG and LEF-LG patients.
Myocardial fibrosis above the median is associated with delayed improvement of LV geometry and function, but MF+ patients nevertheless exhibit significant LV reverse remodelling and clinical benefit 6 months after TAVI.
Assessment of myocardial fibrosis
As to our knowledge, this is the first study in TAVI patients that evaluates the association of histological MF with BL LV remodelling as well as with post-interventional recovery and survival in different AS subtypes.
Biopsy-based analysis of fibrosis has previously been performed in patients undergoing SAVR.2–5 , 9 However, characteristics of previous SAVR patients are different from current TAVI patients. Moreover, recent data suggests that patients’ outcome may differ after SAVR and TAVI10 and, therefore, analysis of underlying pathophysiological processes may not be transferable between procedures.
An increasing number of AS studies assesses MF by CMR imaging.11–14 Cardiovascular magnetic resonance has the major advantage of being non-invasive, thereby easily allowing sequential investigations.
We can provide correlations of CMR and histological data for 40 of our patients. Consistent with Treibel et al.,8 LGE quantification significantly correlated with histological MF and with measures of pathological LV remodelling. However (and again consistent with Treibel8) ECV did not.
ECV has been investigated as a method for detecting diffuse MF, and some clinical studies found good correlations with histological MF,15 whereas others did not.8 Possible reasons for this discordance include differences in severity of AS phenotypes and underestimation of subendocardial MF due to exclusion of the endo- and epicardium to avoid blood pool contamination. However, the T1 mapping technique is in a relatively early stage of development,16 and robust data assessing its prognostic value in AS are still lacking. On the other hand, LGE quantification is well validated in AS and supported by powerful prognostic data, but is commonly considered as to insensitive for the detection of diffuse interstitial fibrosis.16 Thus, myocardial biopsy and histological analysis are still considered the gold standard assessments of MF.16 Our study was designed as biopsy study, and we were indeed able to demonstrate a very high prognostic impact of biopsy-derived MF assessment in AS. Future studies will have to evaluate the prognostic value of different CMR parameters in large AS patients cohorts.
Transition to failure
At BL, MF above the median (MF+) was associated with reduced systolic LV function (as indicated by EF and/or GLS), with increased LVEDV and increased LVMI. In parallel, MF+ patients exhibited higher NYHA classes and lower 6mwt distances. These results are consistent with previous studies suggesting that fibrosis might represent a major determinant of transition from compensated hypertrophy to failure.2–4 , 9
Considering differences in BL characteristics and history in our patients with reduced EF, LV dysfunction seemed to derive predominantly from untreated AS in the majority of LEF-HG patients. Thus, untreated NEF-HG develops into LEF-HG AS in the first place. Accordingly, LEF-HG patients exhibit a significantly higher MF burden than NEF-HG patients do. Regarding LEF-LG patients, CAD may aggravate this process in some patients. However, MF levels do not differ at all in study patients with and without CAD, and even the presence of prior MI and/or CABG does not influence MF burden to the same extent as the AS subtype.
The development of the phenotype PLF-LG AS (overrepresented in women) characterized by small hypertrophied ventricles, high left atrial volumes, and a high prevalence of atrial fibrillation appears to follow a different antecedent pathway. In comparison with NEF-HG, PLF-LG patients suffered from more advanced heart failure symptoms and were more likely to exhibit MF above the median as pre-described,9 indicating that the PLF-LG remodelling phenotype is also maladaptive.
Since correlation of LVMI with MF is very weak in our cohort (r 2 = 0.05, P = 0.03), LV hypertrophy per se does not seem to be a dominant mechanism of MF in AS but rather one of several cofactors.
Impact of myocardial fibrosis on post-transcatheter aortic valve implantation outcomes
Reverse remodelling and clinical benefit
The concept of reverse myocardial remodelling after AS removal has been proven in previous echocardiographic and MRI studies demonstrating LVMI regression of about 20–30% 6–18 months after AVR.17–20 Importantly, patients without BL MF (measured by LGE) were reported to experience greater LVMI regression than those with fibrosis.18 In our own study, we observed an LVMI decrease of about 15% 6 months after TAVI, which was present in individuals with MF above and below the median. However, analysis of LVEDV indicated a less complete and delayed reverse remodelling in MF+ patients.
Sequential biopsy findings from Krayenbuehl et al.3 suggested that the early LV mass reduction after SAVR is predominantly driven by regression of myocardial cellular hypertrophy, whereas a significant decrease of LV fibrous content (however incomplete) can only be observed 6–7 years later.3 In a recent MRI study by Treibel et al.,12 focal fibrosis measured by MRI did not resolve at 1 year after mainly SAVR, but diffuse fibrosis and myocardial cellular hypertrophy regressed accompanied by structural and functional LV improvements. Authors postulate that diffuse fibrosis may be plastic and, therefore, a therapeutic target.
Regarding the recovery of LV systolic function in patients with decreased BL EF, Weidemann et al.4 reported an EF increase after SAVR exclusively in subjects without BL fibrosis. In contrast, we rather observed a delayed EF recovery in MF+ patients: Whereas the rapid EF improvement at time of discharge (which is most likely a simple consequence of immediate afterload reduction) was much more pronounced in MF− patients, EF values of MF− and MF+ patients equalized at 6 months. Moreover, Weidemann et al.4 saw no clinical improvement in patients with severe BL MF, whereas our MF+ patients showed clinical benefit. These outcome differences could be related to the higher invasiveness of SAVR (Weidemann et al.) in comparison with TAVI (our study).
In conclusion, TAVI patients generally exhibit the potential of reverse remodelling and clinical benefit, but normalization of LV geometry and function is delayed in patients with higher fibrotic burden.
Post-transcatheter aortic valve implantation all-cause and cardiovascular mortality
Studies examining the impact of MF on survival after SAVR or TAVI are scarce.5 , 11 , 13 , 14 Azevedo et al.5 remains the only study linking histological MF and survival to date. Authors demonstrated that MF measured by either histopathology or MRI independently predicted all-cause mortality in 28 AS patients after SAVR.
Two other outcome studies measured MF exclusively by LGE in MRI. Dweck et al.21 found that LGE was an independent predictor of mortality in 143 patients with moderate or severe AS (only 70% underwent AVR). The most recent MRI study by Musa et al.11 followed 674 patients for a minimum of 2 years after SAVR or TAVI. Late gadolinium enhancement in BL MRI was independently associated with late mortality (two-fold elevation).
In our own study, histological BL MF above the median of 11% emerged as univariate significant predictor of all-cause mortality and as independent predictor of CV mortality during post-TAVI follow-up. Due to advanced age and multiple comorbidities, we observed a high incidence of non-CV death in our cohort, and our study was not powered for all-cause mortality. However, 13 of 14 CV deaths (including eight sudden cardiac deaths) occurred in MF+ patients. In fact, mortality in MF+ patients was 26.5%, whereas it was only 2% in MF− patients.
Left ventricular biopsies of two patients with cardiac death after TAVI are displayed in Figure 2C and D. Already 30 years ago, it was described that AS patients remain predisposed to sudden death even after AVR, and this finding has been related to advanced LV hypertrophy.22 Myocardial fibrosis might be the missing link between advanced BL LV remodelling and sudden cardiac death after (T)AVR.
As our study and previous publications underline, valve replacement in severe AS is frequently performed after the occurrence of potentially irreversible LV remodelling associated with MF, which negatively influences LV recovery, clinical benefit, and survival after (T)AVR.
At this stage, we cannot prove a causal relationship between pathological LV remodelling, MF, and CV death. Also, approved pharmacological treatment strategies for prevention or reversal of MF are not available to date. These burning issues need to be addressed in future studies.
Conclusions
Histopathological MF in severe AS correlates with AS subtype, extent of LV remodelling, and clinical heart failure. It is associated with delay but not inhibition of reverse LV remodelling and clinical benefit after TAVI. Most importantly, MF above the median independently predicts CV mortality during post-TAVI follow-up. These findings might be hypothesis-generating for future studies.
Limitations
In order to make study findings applicable to daily clinical practice, we recruited all-comers (20% of all TAVI patients treated at our institution during recruitment period) without excluding CAD or other comorbidities. The given CAD-prevalence of 70% in our cohort suggests that histological alterations might at least partly derive from the combination of AS and CAD. However, the influence of AS subtype on MF emerged as much more meaningful than the influence of CAD. Also, our study cohort represents a typical TAVI collective and, therefore, our histological findings are likely to be applicable to TAVI patients in daily clinical practice.
Supplementary Material
Acknowledgements
The authors gratefully thank the study nurses Swetlana Hartmann and Kristina Schröder for study organization, and the technical assistant Annika Erdmann for processing and staining of LV biopsies.
Funding
The study was supported by a grant from the German Research Foundation (DFG; CRC 1002, TP D01 to G.H., TP D04 to K.T., and TP INF) and from Germany's Excellence Strategy - EXC 3067/1 - 390729940 (GH). Research was supported by the UMG biobank by provision of quality-assured biomaterials.
Conflict of interest: none declared.
Contributor Information
Miriam Puls, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany; German Center for Cardiovascular Research (DZHK), site Göttingen, Robert-Koch-Straße 42a, 37075 Göttingen, Germany.
Bo Eric Beuthner, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany; German Center for Cardiovascular Research (DZHK), site Göttingen, Robert-Koch-Straße 42a, 37075 Göttingen, Germany.
Rodi Topci, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany.
Anja Vogelgesang, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany.
Annalen Bleckmann, Department of Medical Bioinformatics, University Medical Center Göttingen, Robert-Koch-Straße 40, 37099 Göttingen , Germany; Department of Hematology and Oncology, University Medical Center Göttingen, Robert-Koch-Straße 40, 37099 Göttingen, Germany.
Maren Sitte, Department of Medical Bioinformatics, University Medical Center Göttingen, Robert-Koch-Straße 40, 37099 Göttingen , Germany.
Torben Lange, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany.
Sören Jan Backhaus, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany.
Andreas Schuster, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany; German Center for Cardiovascular Research (DZHK), site Göttingen, Robert-Koch-Straße 42a, 37075 Göttingen, Germany.
Tim Seidler, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany; German Center for Cardiovascular Research (DZHK), site Göttingen, Robert-Koch-Straße 42a, 37075 Göttingen, Germany.
Ingo Kutschka, Department of Cardiovascular Surgery, University Medical Center Göttingen, Robert-Koch-Straße 40, 37099 Göttingen, Germany.
Karl Toischer, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany; German Center for Cardiovascular Research (DZHK), site Göttingen, Robert-Koch-Straße 42a, 37075 Göttingen, Germany.
Elisabeth Maria Zeisberg, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany; German Center for Cardiovascular Research (DZHK), site Göttingen, Robert-Koch-Straße 42a, 37075 Göttingen, Germany.
Claudius Jacobshagen, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany; German Center for Cardiovascular Research (DZHK), site Göttingen, Robert-Koch-Straße 42a, 37075 Göttingen, Germany.
Gerd Hasenfuß, Clinic of Cardiology and Pneumology, University Medical Center Göttingen, 37099 Göttingen, Germany; German Center for Cardiovascular Research (DZHK), site Göttingen, Robert-Koch-Straße 42a, 37075 Göttingen, Germany.
References
- 1. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 2. Hein S, Arnon E, Kostin S, SchöNburg M, ElsäSser A, Polyakova V, Bauer EP, KlöVekorn W-P, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 2003;107:984–991. [DOI] [PubMed] [Google Scholar]
- 3. Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation 1989;79:744–755. [DOI] [PubMed] [Google Scholar]
- 4. Weidemann F, Herrmann S, StöRk S, Niemann M, Frantz S, Lange V, Beer M, GattenlöHner S, Voelker W, Ertl G, Strotmann JrgM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009;120:577–584. [DOI] [PubMed] [Google Scholar]
- 5. Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010;56:278–287. [DOI] [PubMed] [Google Scholar]
- 6. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodes-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 8. Treibel TA, Lopez B, Gonzalez A, Menacho K, Schofield RS, Ravassa S, Fontana M, White SK, DiSalvo C, Roberts N, Ashworth MT, Diez J, Moon JC. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J 2018;39:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herrmann S, Stork S, Niemann M, Lange V, Strotmann JM, Frantz S, Beer M, Gattenlohner S, Voelker W, Ertl G, Weidemann F. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol 2011;58:402–412. [DOI] [PubMed] [Google Scholar]
- 10. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 11. Musa TA, Treibel TA, Vassiliou VS, Captur G, Singh A, Chin C, Dobson LE, Pica S, Loudon M, Malley T, Rigolli M, Foley JRJ, Bijsterveld P, Law GR, Dweck MR, Myerson SG, McCann GP, Prasad SK, Moon JC, Greenwood JP. Myocardial scar and mortality in severe aortic stenosis. Circulation 2018;138:1935–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva AN, Sheikh A, Lopez B, Gonzalez A, Manisty C, Lloyd G, Kellman P, Diez J, Moon JC. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol 2018;71:860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen H, Zeng J, Liu D, Yang Q. Prognostic value of late gadolinium enhancement on CMR in patients with severe aortic valve disease: a systematic review and meta-analysis. Clin Radiol 2018;73:983.e7–983.e14. [DOI] [PubMed] [Google Scholar]
- 14. Lee H, Park JB, Yoon YE, Park EA, Kim HK, Lee W, Kim YJ, Cho GY, Sohn DW, Greiser A, Lee SP. Noncontrast myocardial T1 mapping by cardiac magnetic resonance predicts outcome in patients with aortic stenosis. JACC Cardiovasc Imaging 2018;11:974–983. [DOI] [PubMed] [Google Scholar]
- 15. Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, Jenkins W, Koo M, Mirsadraee S, White AC, Japp AG, Prasad SK, Semple S, Newby DE, Dweck MR. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 2017;10:1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bing R, Cavalcante JL, Everett RJ, Clavel MA, Newby DE, Dweck MR. Imaging and impact of myocardial fibrosis in aortic stenosis. JACC Cardiovasc Imaging 2019;12:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monrad ES, Hess OM, Murakami T, Nonogi H, Corin WJ, Krayenbuehl HP. Time course of regression of left ventricular hypertrophy after aortic valve replacement. Circulation 1988;77:1345–1355. [DOI] [PubMed] [Google Scholar]
- 18. Dobson LE, Musa TA, Uddin A, Fairbairn TA, Swoboda PP, Erhayiem B, Foley J, Garg P, Haaf P, Fent GJ, Malkin CJ, Blackman DJ, Plein S, Greenwood JP. Acute reverse remodelling after transcatheter aortic valve implantation: a link between myocardial fibrosis and left ventricular mass regression. Can J Cardiol 2016;32:1411–1418. [DOI] [PubMed] [Google Scholar]
- 19. Lamb HJ, Beyerbacht HP, de Roos A, van der Laarse A, Vliegen HW, Leujes F, Bax JJ, van der Wall EE. Left ventricular remodeling early after aortic valve replacement: differential effects on diastolic function in aortic valve stenosis and aortic regurgitation. J Am Coll Cardiol 2002;40:2182–2188. [DOI] [PubMed] [Google Scholar]
- 20. Rost C, Korder S, Wasmeier G, Wu M, Klinghammer L, Flachskampf FA, Daniel WG, Voigt JU. Sequential changes in myocardial function after valve replacement for aortic stenosis by speckle tracking echocardiography. Eur J Echocardiogr 2010;11:584–589. [DOI] [PubMed] [Google Scholar]
- 21. Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 2011;58:1271–1279. [DOI] [PubMed] [Google Scholar]
- 22. Foppl M, Hoffmann A, Amann FW, Roth J, Stulz P, Hasse J, Gradel E, Burckhardt D. Sudden cardiac death after aortic valve surgery: incidence and concomitant factors. Clin Cardiol 1989;12:202–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.