Abstract
Background
Low socioeconomic status (SES) is associated with higher risk of certain gastrointestinal (e.g. colorectal, pancreatic, and liver) cancers in Western populations. Evidence is very limited in China where correlates and determinants of SES differ from those in the West.
Methods
The prospective China Kadoorie Biobank recruited 512,715 adults (59% women, mean age 51 years) from 10 (5 urban, 5 rural) regions. During 10 years of follow-up, 27,940 incident cancers (including 3061 colorectal, 805 pancreatic, and 2904 liver) were recorded among 510,131 participants without prior cancer at baseline. Cox regression was used to estimate adjusted hazard ratios (HRs) for specific cancers associated with area-level (e.g. per capita gross domestic product, disposable income) and individual-level (e.g. education, household income) SES.
Results
Area-level SES and household income showed positive associations with incident colorectal and pancreatic cancer and inverse associations with liver cancer (p for trend <0.05). Education showed no association with colorectal cancer but inverse associations with pancreatic and liver cancer, with adjusted HRs comparing university to no formal schooling being 1.05 (95% CI 0.85-1.29), 0.49 (0.28-0.85), and 0.61 (0.47-0.81), respectively. Potential risk factors (e.g. smoking, alcohol) explained partly the inverse associations of education with pancreatic and liver cancer (17.6% and 60.4%, respectively), respectively.
Conclusions
Among Chinese adults, the associations of SES with gastrointestinal cancers differed by cancer type and SES indicator. Potential risk factors partially explained the inverse associations of education with pancreatic and liver cancer.
Impact
The different associations between SES with gastrointestinal cancers may inform cancer prevention strategies.
Keywords: socioeconomic status, colorectal cancer, pancreatic cancer, liver cancer, Chinese
Introduction
Socioeconomic status (SES) is one of the most important contributors to health disparities.1 In epidemiological studies, SES is often measured at group level, e.g. by neighbourhood income and deprivation, or at individual level, e.g. by family income, education, and social class indicators.1 Studies in North America and Europe have shown that low SES is a risk factor for a range of non-communicable diseases including several types of cancer (e.g. gastrointestinal and lung cancers), with a similar pattern for cancer incidence and mortality.2–9 There is also evidence that the excess risk in low-SES populations could be partially explained by the higher prevalence of lifestyle risk factors assessed at adulthood including smoking, alcohol, unhealthy diet, physical inactivity, and obesity.2–5
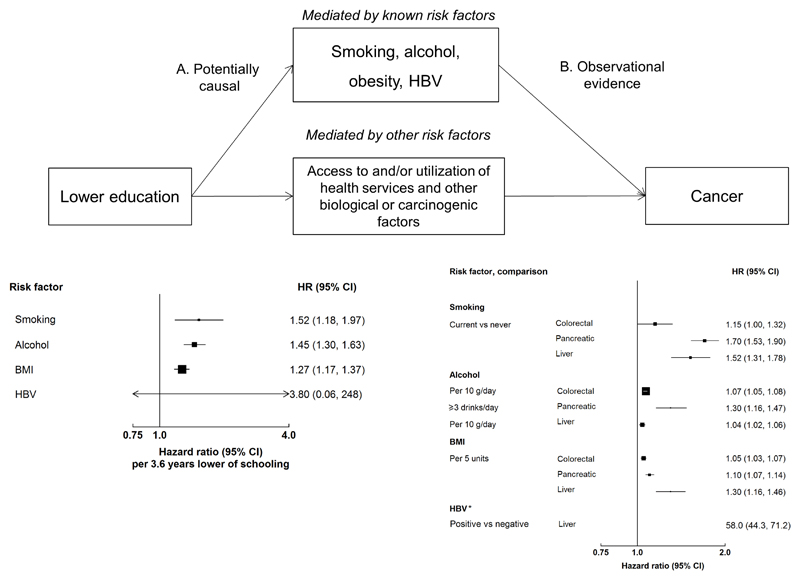
In China, the rates and patterns of cancer, particularly of major gastrointestinal cancers, and the associations of lifestyle factors with SES differ importantly from those in the West.10,11 The age-standardised incidence rate of liver cancer is much higher in China than in Western populations,12 whereas the converse is true for colorectal and pancreatic cancer.10,11 In contrast to the patterns in Western countries, the prevalence of obesity, physical inactivity, and intake frequency of animal-origin foods in China are higher among individuals with high SES.13–15 However, there is limited evidence on the associations of SES with cancer risk in the Chinese population. Furthermore, it is not clear to what extent the difference in prevalence of lifestyle risk factors could explain the SES-cancer associations. While affluence assessed either at group or individual level is a major driver of cancer disparities, education has been shown to be associated with several potential risk factors for cancer in Western populations (Figure 1).2,4–7
Figure 1. The associations of education, potential risk factors, and GI cancer risk.
Conceptual model of the associations of education, potential risk factors, and GI cancer risk. It is hypothesized that the indirect effect of the association of education with GI cancer is mediated through possible risk factors and access to and/or use of healthcare services, while the direct effect is the potential biological or carcinogenic factor.37 However, no data is currently available to examine the direct effect between SES and cancer or the effect of access to and/or utilisation of health services on the SES-cancer associations. To assess the causal effects of education on possible risk factors for cancer, independent summary statistics from genome wide association studies (GWAS) were obtained for education single nucleotide polymorphisms (SNPs) from the Social Science Genetic Association Consortium (PMID: 27225129), for HBV from a Korean study (PMID: 23760081), and for smoking the TAG consortium (PMID: 20418890). The SNPs for alcohol (the UK Biobank) and BMI (the GIANT consortium) were obtained through MR-Base (www.mrbase.org). We used a conventional inverse-variance weighted (IVW) Mendelian randomisation analysis in which the SNP to education estimate is regressed on the SNP to each risk factor, with the y-axis intercept forced through the origin. The pooled relative risks (RRs) between risk factors and cancer are extracted from the largest meta-analysis of prospective studies (PMID: 18193270, 19816941, 19720726, 19142968; World Cancer Research Fund Continuous Update Project), except for HBV (meta-analysis of cross-sectional, case-control, and prospective studies; PMID: 28230038). * For HBV the HR is not plotted due to its very high value.
Therefore, we examined the associations of SES with the incidence and mortality of three major gastrointestinal (GI) cancers which have been shown to be associated with metabolic risk factors, i.e. colorectal, pancreatic, and liver cancer, in the China Kadoorie Biobank (CKB).16 We also assessed whether risk factors (i.e. smoking, alcohol, physical inactivity, diet, adiposity, and hepatitis B virus [HBV] for liver cancer), could explain the associations of education with risks of incident cancers.
Materials and methods
Study design
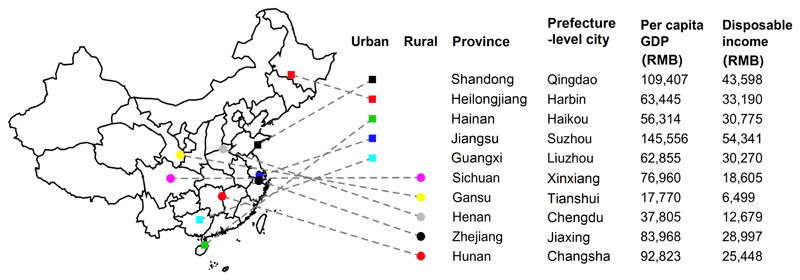
The CKB study recruited 512,715 participants from 10 geographically defined localities (5 urban and 5 rural) in China between 2004 and 2008.17 These 10 regions were selected across China to provide a diverse range of patterns of major chronic diseases and risk exposures, levels of socioeconomic development, population stability, and local infrastructure. Each of the 10 regions (i.e. administrative district) was selected from a prefecture-level city within a different province (Figure 2 and Supplementary Table 1). In each region, 100-150 administrative units, either rural villages or urban residential committees, were randomly selected. We will use the term ‘area’ to describe prefecture-level cities and the term ‘region’ to refer to the 10 regions from which participants were recruited in CKB. Details of the study design, survey methods, and population characteristics have been described in the study protocol.17 All participants eligible for this study had completed a written informed consent form. All methods were performed in accordance with relevant guidelines and regulations. The CKB study was approved by the Ethical Review Committee of the Chinese Center for Disease Control and Prevention and the Oxford Tropical Research Ethics Committee, University of Oxford. In 10 regions, all men and women aged 35 to 74 years who were permanently resident and without major disability were eligible for the study. At local study assessment clinics, participants completed an interviewer-administered laptop-based questionnaire (sociodemographic characteristics, lifestyle factors, personal and family medical history) and underwent a physical examination (height, weight, hip and waist circumference, bioimpedance, lung function, blood pressure and heart rate). In addition, blood spot tests were used to measure random plasma glucose and hepatitis B surface antigen (HBsAg).
Figure 2. The locations and area-level SES of the 10 regions in CKB.
The geographical locations, per capita GDP, and disposable income of the 10 CKB regions are shown separately for urban and rural areas. A prefecture-level city is an administrative unit comprising a main central urban area and its surrounding rural area consisting of smaller cities, towns, and villages. A prefecture-level city ranks below a province and above a county in China's administrative structure.
Assessment of socioeconomic status
SES was assessed at both area and individual levels. Area-level SES included prefecture-level per capita gross domestic product (GDP) and disposable income, obtained from the 2011 Statistical Yearbook (median year of follow-up in CKB).18 Per capita GDP was used as a general measure of area-level economic development, while disposable income was used to approximate the average household income in an area. Previous nationwide reports in China have suggested the inclusion of per capita GDP to inform the implementation of cancer prevention strategies.19 Individual-level SES included self-reported highest education, household income, and an asset-based indicator. Household income was defined as annual income of all members living in a household received from work, investments, or pension. All participants were asked whether they owned a house or an apartment, toilet for private use, motor vehicle (e.g. car or motorbike), telephone or mobile phone, and refrigerator. An asset-based indicator was calculated as the total number of these five items in the household. All 10 regions were divided into three categories by per capita GDP (<60,000, 60,000-99,999, and ≥100,000 RMB/year) and disposable income (<20,000, 20,000-39,999, and ≥40,000 RMB/year) (Figure 2 and Supplementary Table 1). Information on education covered six categories: no formal school, primary school, middle school, high school, technical school/college, and university. Similarly, household income had six categories: <2500, 2500-4999, 5000-9999, 10,000-19,999, 20,000-34,999, ≥35,000 RMB/year.
Assessment of lifestyle risk factors
For smoking, the questionnaire covered frequency, duration, amount, and type of tobacco, as well as the ages at which participants began smoking regularly and stopped smoking, and the main reason for stopping.1 Smoking status was categorised as (1) never (not smoking at baseline and had smoked <100 cigarettes in lifetime), (2) occasional (neither never nor former smokers and had not stopped smoking completely for at least the 6 months before baseline), (3) former regular (had smoked ≥100 cigarettes but had quit smoking by choice for ≥6 months before baseline), or (4) current regular smoker (ever smoked ≥1 cigarettes daily for ≥6 months). For alcohol drinking, the questionnaire covered frequency, the type (beer, wine or spirits) and amount of each type consumed in a typical drinking week, as well as the age at which participants started drinking.2 Drinking status was classified into five categories as (1) abstainers (had never drunk alcohol in the past year and had not drunk weekly in the past), (2) occasional drinkers (had drunk alcohol occasionally, monthly but less than weekly, or during certain seasons, and had not drunk weekly in the past), (3) reduced-intake drinkers (had drunk alcohol occasionally, monthly but less than weekly, or during certain seasons, but had drunk weekly in the past), (4) ex-weekly drinkers (had never drunk alcohol in the past year but had drunk weekly in the past), or (5) weekly drinkers (often drank at least weekly during the past year).
All anthropometric measurements were taken to the nearest 0.1 cm or 0.1 kg by trained staff. Standing and sitting height were measured with a stadiometer. Sitting height was measured as the length of the body from the crown of the head to buttocks. Weight was measured with a body composition analyser (TANITA-TBF-300GS, Tanita Corporation, Tokyo, Japan), subtracting the weight of clothing according to season (0.5 kg in summer and 2.0-2.5 kg in winter). Body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in metres). BMI was classified into five categories (<20.0, 20.0 to <22.5, 22.5 to <25.0, 25.0 to <27.0, and ≥27.0 kg/m2). BMI at age 25 (BMI25) was derived from the weight recalled at age 25 and the height measured at baseline. BMI25 was missing in 81,880 participants due to difficulty with recalling and was imputed using multiple imputation (‘mice’ package in R). The imputation model included age at baseline, sex, regions, education, household income, smoking, alcohol, self-rated health, family history of cancer (any of father, mother, and siblings), and cumulative hazard of total cancer.
Outcome assessment
The CKB study ascertained the vital status of each participant (1) through periodical reports from China Centre for Disease Control and Prevention’s Disease Surveillance Points system, (2) by regular checks against local residential and health insurance records, and (3) by annual active confirmation by street committees or village administrators.20 Additional information about cancer incidence and hospitalisation was collected through linkages with cancer registries and national health insurance databases (with a coverage rate of 98% in all study regions). All disease events were coded using the 10th Revision of International Classification of Diseases (ICD-10) by trained staff blinded to baseline information. By 1.1.2017, 44,066 (8.6%) participants had died, and 4751 (<1%) were lost to follow-up. Overall, 27,940 (5.4%) had developed cancer, including 3061 colorectal (C18-20), 806 pancreatic (C25), and 2904 liver (C22) cancer cases.
Statistical methods
The present study excluded participants with a history of cancer at baseline (n=2584), leaving 510,131 individuals for the main analyses. Prevalences and mean values of baseline characteristics were calculated for categories of education and household income separately, standardised to the age, sex, and region structure of the CKB population. Cancer incidence and mortality rates were calculated for categories of area- and individual-level SES, standardised by age, sex, and region (for individual-level SES only).
In our analysis, we first examined the association of each area- and individual-level SES variable with risks of colorectal, pancreatic, and liver cancer. Cox proportional hazards models were used to estimate adjusted hazard ratios (HRs) of incidence of a specific cancer associated with SES, stratified by sex and region (10 regions, for individual-level SES only) and adjusted for age at baseline (basic model). Age was used as the underlying time scale, with delayed entry at age at baseline. For household income, the basic model also included household size. For variables with more than two categories, all HRs are presented with ‘floating’ standard errors to facilitate comparisons between groups.21
We then examined the extent to which possible risk factors explained the association of education with specific cancers. Possible risk factors were smoking, alcohol, obesity, and HBsAg (for liver cancer). These factors are selected because they potentially showed causal associations with education and they are associated with GI cancers in prospective studies (Figure 1 and Supplementary Table 2). For obesity, we used BMI25 because BMI assessed at baseline might be affected by reverse causality.22,23 Smoking was modelled as a categorical variable with six categories (never, occasional, former regular, and among current regular, 0-10, 11-20, and ≥20 cigarettes/day). Alcohol drinking was modelled as a categorical variable with seven categories (abstainers, ex-weekly drinkers, reduced-intake drinkers, occasional drinkers, and among weekly drinkers, 0-279, 280-419, and ≥420 g/week). BMI25 was modelled as a continuous variable. HBsAg was modelled as a categorical variable with four categories (negative, positive, unclear, or missing). We included each lifestyle risk factor in the basic model and examined the percent change in the logHRs comparing the highest and lowest categories of education or household income. The proportion of the association of education or household income with a specific cancer that was explained by a lifestyle risk factor was calculated as follows: ((logHRbasic model - logHRadjusted model) / (logHRbasic model)) X 100%. Statistical analysis was done using R version 3.5.1.
Results
Baseline characteristics by education and household income
Among the 510,131 participants included, the mean (SD) baseline age was 51.5 (10.7) years, and 59% were women. Overall, 20.9% of participants had high school or higher education, and 18.0% had annual household income ≥35,000 RMB (conversion rate: 1 RMB = 0.15 USD). Per capita GDP and disposable income of all 10 regions are shown in Figure 2. The correlation was low to moderate between area-level SES, household income, and education (Spearman correlation coefficient: 0.37 between area-level per capita GDP and household income, 0.51 between area-level disposable income and household income, and 0.28 between education and household income). Participants with higher education were less likely to smoke or drink alcohol and reported lower physical activity and higher sedentary leisure time (Table 1). They had lower systolic blood pressure and random plasma glucose, higher adiposity at baseline, and lower BMI at age 25, and were less likely to test positive for HBsAg. The pattern of baseline characteristics by household income was generally similar to that by education except that no pattern was identified for HBsAg (Supplementary Table 3).
Table 1. Baseline characteristics by level of education.
| Variable* | Highest education |
|||||
|---|---|---|---|---|---|---|
| No formal school (n=95,221) | Primary school (n=165,216) | Middle school (n=144,913) | High school (n=77,527) | Technical/college (n=18,294) | University (n=11,720) | |
| Age (SD), year | 52.3 (10.2) | 51.8 (4.4) | 51.1 (3.6) | 51.2 (5.0) | 51.0 (8.1) | 50.9 (8.1) |
| Female, % | 83.3 | 60.4 | 47.1 | 43.6 | 33.2 | 27.1 |
| Socioeconomic and lifestyle factors | ||||||
| Urban region, % | 20.5 | 26.5 | 56.1 | 74.6 | 89.9 | 93.9 |
| Household income ≥35 000 RMB/year, % | 9.6 | 12.3 | 17.8 | 27.3 | 43.6 | 54.8 |
| Ever regular smoking, % | ||||||
| Male | 69.6 | 71.5 | 68.6 | 61.8 | 51.5 | 43.2 |
| Female | 5.3 | 4.0 | 2.4 | 1.5 | 1.2 | 0.2 |
| Weekly drinking, % | ||||||
| Male | 31.9 | 33.7 | 33.7 | 31.2 | 30.3 | 29.5 |
| Female | 2.3 | 1.8 | 2.0 | 1.8 | 2.0 | 2.0 |
| Total physical activity (SD), MET-h/day | 20.4 (14.3) | 21.1 (14.3) | 20.7 (14.3) | 19.3 (12.7) | 17.3 (9.0) | 17.3 (8.1) |
| Sedentary leisure time (SD), h/day | 2.8 (1.7) | 3.0 (1.5) | 3.2 (1.5) | 3.3 (1.5) | 3.4 (1.5) | 3.3 (1.5) |
| Daily intake, % | ||||||
| Fresh fruits | 11.7 | 14.6 | 21.0 | 28.2 | 40.0 | 41.7 |
| Fresh vegetables | 93.1 | 94.3 | 95.3 | 95.9 | 97.1 | 98.3 |
| Red meat | 23.8 | 26.8 | 30.6 | 35.4 | 43.7 | 46.0 |
| Blood pressure and anthropometry | ||||||
| SBP (SD), mmHg | 133.3 (23.3) | 131.6 (21.4) | 130.4 (19.4) | 129.3 (19.2) | 127.6 (19.2) | 126.1 (19.3) |
| RPG (SD), mmol/L | 6.2 (2.5) | 6.1 (2.4) | 6.1 (2.3) | 6.0 (2.1) | 6.1 (2.2) | 6.0 (2.2) |
| BMI (SD), kg/m2 | 23.7 (3.5) | 23.7 (3.4) | 23.7 (3.3) | 23.7 (3.3) | 23.8 (3.2) | 23.9 (3.2) |
| Waist circumference (SD), cm | 80.3 (10.0) | 80.6 (9.7) | 80.5 (9.6) | 80.4 (9.7) | 80.7 (9.9) | 81.1 (10.0) |
| Hip circumference (SD), cm | 90.5 (6.8) | 90.9 (6.7) | 91.2 (6.8) | 91.4 (6.6) | 92.0 (6.2) | 92.5 (6.3) |
| Waist to hip ratio (SD) | 0.89 (0.07) | 0.89 (0.07) | 0.88 (0.07) | 0.88 (0.07) | 0.88 (0.08) | 0.88 (0.07) |
| Body fat percentage (SD), % | 28.1 (8.8) | 28.1 (8.9) | 28.1 (8.1) | 27.9 (7.6) | 28.2 (6.9) | 27.8 (6.8) |
| BMI at age 25, kg/m2 | 22.2 (2.8) | 22.2 (2.6) | 21.9 (2.5) | 21.6 (2.5) | 21.3 (2.4) | 21.2 (2.4) |
| Height (SD), cm | 157.3 (7.2) | 158.1 (7.8) | 159.0 (8.0) | 159.8 (8.0) | 160.8 (7.8) | 161.3 (7.9) |
| HBsAg positive, % | 3.3 | 3.3 | 3.0 | 2.8 | 2.6 | 1.9 |
Abbreviations: BMI=body mass index, MET=metabolic equivalent of task, RPG=random plasma glucose, SBP=systolic blood pressure.
Results were standardised by age, sex, and region (where appropriate). Values are means unless otherwise stated.
P-values for trend: all <0.001 except for weekly drinking in females (0.38).
Area- and individual-level SES and specific GI cancers
For area-level SES, per capita GDP and disposable income showed positive associations with colorectal and pancreatic cancer and inverse associations with liver cancer (Table 2). Similar to area-level SES, household income showed positive associations with colorectal and pancreatic cancer and an inverse association with liver cancer (Table 3). There were also positive associations of the number of assets (apartment/house, private toilet, motor vehicle, refrigerator, and phone) with colorectal and pancreatic cancer and an inverse association with liver cancer (Table 3 and Supplementary Table 4). By contrast, education showed inverse associations with pancreatic and liver cancer and no association with colorectal cancer (Table 3).
Table 2. Standardised incidence rates (per 100,000) and HRs of colorectal, pancreatic, and liver cancer by area-level SES.
| Colorectal |
Pancreatic |
Liver |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. cases | Incidence rate (per 100,000) | HR (95% CI) | No. cases | Incidence rate (per 100,000) | HR (95% CI) | No. cases | Incidence rate (per 100,000) | HR (95% CI) | |
| Urbanity | |||||||||
| Rural | 1309 | 46.6 | Reference | 396 | 14.1 | Reference | 1659 | 59.0 | Reference |
| Urban | 1752 | 80.4 | 1.58 (1.47, 1.70) | 409 | 18.7 | 1.19 (1.04, 1.37) | 1245 | 56.9 | 0.91 (0.84, 0.98) |
| Per capita GDP (RMB) | |||||||||
| <60,000 | 489 | 41.9 | 1.00 (0.92, 1.09) | 126 | 8.8 | 1.00 (0.84, 1.19) | 838 | 63.7 | 1.00 (0.93, 1.07) |
| 60,000-99,999 | 1487 | 65.3 | 1.73 (1.64, 1.82) | 321 | 14.1 | 1.42 (1.27, 1.58) | 1273 | 57.6 | 0.88 (0.83, 0.93) |
| ≥100,000 | 1085 | 72.1 | 1.95 (1.84, 2.07) | 358 | 24.8 | 2.48 (2.23, 2.75) | 793 | 53.8 | 0.84 (0.78, 0.90) |
| Disposable income (RMB) | |||||||||
| <20,000 | 649 | 41.4 | 1.00 (0.93, 1.08) | 200 | 12.8 | 1.00 (0.87, 1.15) | 1019 | 64.2 | 1.00 (0.94, 1.06) |
| 20,000-39,999 | 1241 | 62.1 | 1.41 (1.33, 1.49) | 264 | 12.5 | 0.94 (0.83, 1.06) | 1101 | 55.3 | 0.81 (0.76, 0.86) |
| ≥40,000 | 1171 | 84.9 | 1.87 (1.77, 1.98) | 341 | 24.8 | 1.72 (1.55, 1.91) | 784 | 55.8 | 0.80 (0.75, 0.86) |
Model was stratified by sex and region and adjusted for age at baseline. Conversion rate: 1 RMB = 0.15 USD.
P-value for trend by per capita GDP and disposable income: all <0.01.
Table 3. Standardised incidence rates (per 100,000) and HRs of colorectal, pancreatic, and liver cancer by individual-level SES.
| Colorectal |
Pancreatic |
Liver |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. cases | Incidence rate (per 100,000) | HR (95% CI) | No. cases | Incidence rate (per 100,000) | HR (95% CI) | No. cases | Incidence rate (per 100,000) | HR (95% CI) | |
| Education | |||||||||
| No formal school | 653 | 64.7 | 1.00 (0.92, 1.09) | 228 | 20.9 | 1.00 (0.86, 1.16) | 622 | 68.0 | 1.00 (0.91, 1.09) |
| Primary school | 1048 | 61.2 | 1.07 (1.00, 1.14) | 283 | 16.0 | 0.84 (0.75, 0.95) | 1141 | 62.8 | 1.00 (0.94, 1.06) |
| Middle school | 764 | 62.1 | 1.18 (1.09, 1.27) | 161 | 13.5 | 0.83 (0.70, 0.98) | 697 | 52.8 | 0.92 (0.85, 1.00) |
| High school | 409 | 61.6 | 1.09 (0.99, 1.21) | 102 | 16.8 | 0.94 (0.76, 1.15) | 320 | 47.5 | 0.79 (0.70, 0.88) |
| Technical school | 97 | 42.1 | 0.95 (0.78, 1.16) | 18 | 11.9 | 0.59 (0.37, 0.95) | 71 | 32.7 | 0.68 (0.54, 0.86) |
| University | 90 | 103.4 | 1.05 (0.85, 1.29) | 13 | 4.3 | 0.49 (0.28, 0.85) | 53 | 25.0 | 0.61 (0.47, 0.81) |
| Household income (RMB) | |||||||||
| <2,500 | 98 | 51.1 | 1.00 (0.81, 1.24) | 34 | 8.8 | 1.00 (0.69, 1.44) | 168 | 88.0 | 1.00 (0.85, 1.18) |
| 2,500 to 4,999 | 189 | 49.6 | 1.16 (1.00, 1.35) | 62 | 16.8 | 1.19 (0.92, 1.55) | 265 | 79.4 | 0.84 (0.74, 0.96) |
| 5,000 to 9,999 | 446 | 54.3 | 1.31 (1.19, 1.44) | 123 | 14.0 | 1.22 (1.01, 1.46) | 606 | 68.1 | 0.85 (0.78, 0.92) |
| 10,000 to 19,999 | 906 | 61.9 | 1.59 (1.49, 1.69) | 218 | 15.1 | 1.36 (1.19, 1.55) | 841 | 58.2 | 0.71 (0.67, 0.76) |
| 20,000 to 34,999 | 824 | 66.6 | 1.79 (1.67, 1.92) | 203 | 17.8 | 1.62 (1.40, 1.86) | 608 | 50.6 | 0.62 (0.57, 0.67) |
| ≥35,000 | 598 | 69.9 | 1.86 (1.70, 2.02) | 165 | 20.2 | 1.88 (1.59, 2.21) | 416 | 47.6 | 0.57 (0.52, 0.63) |
| Assets | |||||||||
| None | 149 | 46.2 | 1.00 (0.85, 1.18) | 50 | 13.8 | 1.00 (0.75, 1.33) | 263 | 83.2 | 1.00 (0.88, 1.13) |
| One | 273 | 55.5 | 1.22 (1.08, 1.38) | 85 | 14.5 | 1.22 (0.98, 1.52) | 398 | 74.3 | 0.98 (0.89, 1.09) |
| Two | 500 | 59.5 | 1.38 (1.26, 1.50) | 127 | 15.1 | 1.23 (1.04, 1.47) | 581 | 63.6 | 0.95 (0.87, 1.03) |
| Three | 960 | 66.0 | 1.55 (1.45, 1.66) | 227 | 15.6 | 1.35 (1.18, 1.55) | 793 | 58.2 | 0.86 (0.80, 0.92) |
| Four | 737 | 64.2 | 1.53 (1.43, 1.65) | 181 | 16.4 | 1.39 (1.20, 1.61) | 567 | 51.5 | 0.75 (0.69, 0.82) |
| Five | 442 | 69.3 | 1.66 (1.51, 1.82) | 135 | 21.8 | 1.89 (1.59, 2.24) | 302 | 44.7 | 0.67 (0.60, 0.75) |
Model was stratified by sex and region (for individual-SES only) and adjusted for age at baseline, household size and education (for household income and assets), and household income (for education).
P-value for trend: all <0.001 except for education and colorectal cancer (0.45).
Effects of potential risk factors on education with risks of cancers
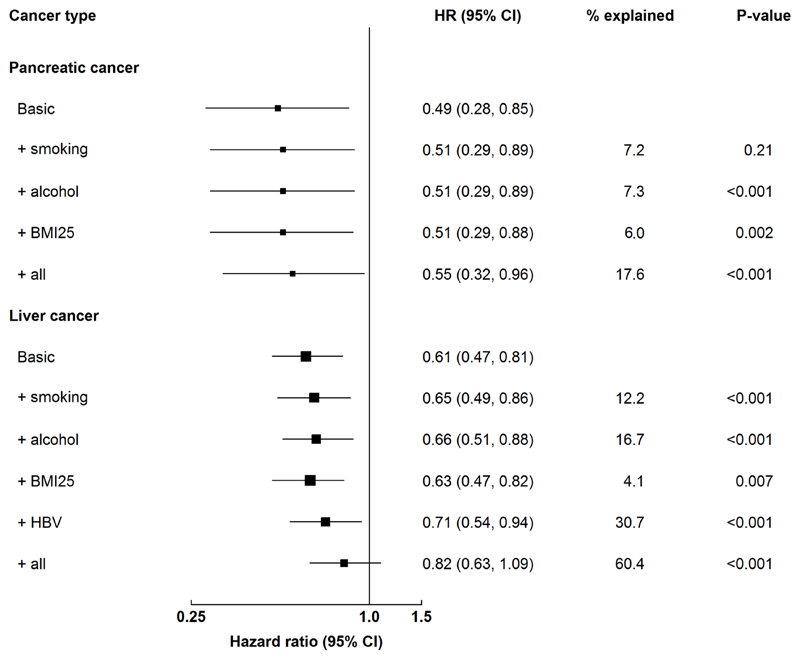
For pancreatic cancer (Figure 3), differences in the prevalence of smoking, alcohol, and young adulthood obesity explained between 6.0% (BMI25) and 7.3% (alcohol) of the inverse association for education, with the three factors together explaining 17.6% of the inverse association. For liver cancer (Figure 3), differences in the prevalence of smoking, alcohol, young adulthood obesity, and HBsAg explained between 4.1% (BMI25) and 30.7% (HBsAg) of the inverse association for education with the four factors together explaining 60.4% of the inverse association.
Figure 3. Associations of education and pancreatic and liver cancer with additional adjustment for potential risk factors.
The basic model was stratified by sex and region and adjusted for age at baseline. The adjusted model included variables in the basic model plus each risk factor. The likelihood ratio test was used to compare the basic model and the adjusted model. P-values from the comparison were reported for each risk factor. Boxes represent HRs associated with central adiposity by number of metabolic risk factors. The sizes of the boxes are proportional to the inverse of the ‘floated’ variance of the log hazard ratios.
Sensitivity analyses
The associations of area- and individual-level SES with risks of the three cancers did not differ by age at baseline or region (Supplementary Figures 1-2). The associations of area-level SES with colorectal cancer and the associations of individual-level SES with the three cancers did not differ by sex (Supplementary Figure 3). For area-level SES, the positive associations with pancreatic cancer were stronger in men, while the inverse associations with liver cancer were stronger in women (Supplementary Figure 3). For colorectal cancer, the positive associations of SES were stronger for colon than rectal cancer (p for heterogeneity <0.001-0.02, Supplementary Table 5), except for per capita GDP (p for heterogeneity 0.56). For cancer mortality (Supplementary Table 6), the positive associations for household income and assets attenuated towards the null for colorectal cancer but changed little for other SES variables. For pancreatic and liver cancer, the associations with mortality were similar to those with incidence. The associations of education with the three GI cancers did not differ by household income (Supplementary Figure 4). The associations of household income with pancreatic and liver cancer did not differ by education, whereas the positive association for colorectal was weaker among participants with higher level of education (Supplementary Figure 4).
Discussion
In this adult population from 10 diverse urban and rural regions of China, the associations of SES with specific GI cancers differed by cancer type and SES indicator. For area-level SES, there were positive associations of per capita GDP and disposable income with colorectal and pancreatic cancer and inverse associations with liver cancer. For these three cancers, the patterns for household income and numbers of assets were similar to those for area-level SES. By contrast, education showed inverse associations with pancreatic and liver cancer and no association with colorectal cancer. For pancreatic and liver cancer, differences in the prevalence of smoking, alcohol, young adulthood adiposity, and HBsAg (for liver cancer) partially explained the inverse associations for education.
For individual-level SES, the majority of previous studies in North America and Europe have shown inverse associations of education with colorectal, pancreatic, and liver cancer (Supplementary Figure 5),4–6,24–26 In CKB, findings for pancreatic and liver cancer were consistent with previous studies in Western populations. For colorectal cancer, however, there was no association for education, in line with a European cohort.4 For household income, a US cohort reported a null association with colorectal cancer,24 while two Korean cohort studies reported a null association with colorectal cancer and an inverse association with liver cancer (Supplementary Figure 5).27,28 Our finding for liver cancer in CKB was consistent with the Korean studies where HBV is also the major risk factor,27 while we showed positive associations of household income with colorectal and pancreatic cancer. For pancreatic and colorectal cancer, we observed an inconsistent pattern for education and income (i.e. household income and assets). This is possibly because of the opposite associations of SES indicators with potential risk factors for these cancers (Table 1 and Supplementary Table 3). For example, participants with higher education were less likely to be regular drinkers and smokers. While participants with higher household income were more likely to be regular drinkers, there was no clear pattern for smoking status. In addition, the positive associations for income might reflect the higher prevalence of an unhealthful lifestyle (e.g. low physical activity, high sedentary leisure time, high consumption of energy and animal-origin foods) among the wealthy population,14,15,29,30 which may be difficult to quantify in regression models. For area-level SES, previous prospective studies in North America and Europe have shown lower risks of colorectal, liver, and pancreatic cancer in neighbourhoods with higher income or lower deprivation (Supplementary Figure 6).3,7–9,25,31–34 In CKB, the risk of liver cancer was lower in regions with higher per capita GDP and disposable income, consistent with previous studies in Western populations and in China.35,36 In contrast, risks of colorectal and pancreatic cancer in CKB were higher in regions with higher per capita GDP and disposable income, consistent with previous reports from the National Central Cancer Registry that assessed urbanisation rates and per capita GDP.35,36
The associations of SES with cancer are likely to reflect the complex relationships between SES, lifestyle risk factors, healthcare, and cancer risk.2,37 Both CKB and previous studies have shown that potential risk factors (mostly lifestyle-related) could partially explain the inverse associations of education with cancer risk. When additionally adjusting for smoking, alcohol, diet, physical activity, and BMI, the NIH-AARP Diet and Health Study in the US reported that the inverse associations with pancreatic and liver cancer attenuated towards the null (50% reduction for pancreatic and 60% for liver).2,5 In CKB, smoking, alcohol, and young adulthood adiposity explained 17% of the inverse association with pancreatic cancer, while smoking, alcohol, young adulthood adiposity, and HBV explained 60% of the association with liver cancer. Compared with household income, educational attainment is more strongly related to early life SES which is important in the adoption and maintenance of a healthy lifestyle in adulthood.38 Indeed, Mendelian randomisation studies have shown that lower education is causally associated with smoking, alcohol, obesity, and possibly HBV infection, which are associated with cancer risk (Figure 1).39 In contrast, household income reflects adulthood SES which may not capture SES over the life course and may also be subject to reverse causality.40
For area-level SES, the patterns for colorectal and pancreatic cancer were opposite in CKB and Western countries. It is possible that the excess risks in high-income individuals in CKB may be partly explained by the higher prevalence of sedentary lifestyle and a diet rich in energy and animal-origin foods and low in dietary fibre and wholegrains,14,15,29,30 which may be difficult to evaluate by individual-level factors in regression models. The positive associations of area-level and household income with colorectal cancer incidence may also reflect the lack of population-based screening in China.43 Randomised controlled trials in high-income countries have shown that colorectal cancer screening is associated with a lower risk of developing colorectal cancer.44 In the US, there was a positive association between area-level household income with colorectal cancer incidence between 1973-1998 and the positive association became inverse after 1998 when colorectal cancer screening was introduced.45 It is possible that individuals with high SES in China have better access to health care services and therefore are more likely to be diagnosed with colorectal cancer.46–49
The strengths of the CKB include its prospective design, the large and diverse study population with complete follow-up, and the ability to assess area- and individual-level SES and a range of risk factors for cancer. This study also has limitations. First, the CKB cohort included 5 urban and 5 rural areas and may not have covered substantial SES disparities across the country. Second, factors related to access to, and use of, health care services were not collected, which may explain the SES-cancer associations. Representative national surveys showed that individuals with higher education and income were more likely to utilise preventive health services including immunisation and cancer screening (Supplementary Table 7). However, only 1% of individuals reported cancer screening in the past year, which is unlikely to explain the SES-cancer associations in the current study. Third, other unmeasured or unknown variables might partly explain the associations of SES with cancer risk (e.g. comorbidities, medications, occupational exposures, environmental chemicals, built environment, access and use of health care services).50 However, a sensitivity analysis showed that an unaccounted for variable would have to be associated with SES and cancer with a relative risk of ~3 to explain away the observed HR (Supplementary Table 8).51
In conclusion, both area- and individual-level income showed positive associations with risks of colorectal and pancreatic cancer and inverse associations with liver cancer. For education, there were inverse associations with pancreatic and liver cancer and no association with colorectal cancer. The inverse associations of education with pancreatic and liver cancer may be explained, at least to a certain extent, by potential risk factors particularly smoking, alcohol, young adulthood adiposity, and HBV (for liver cancer). More studies are warranted in the Chinese population to understand the SES-cancer associations to inform targeted interventions and track cancer disparities.
Supplementary Material
Acknowledgments
The chief acknowledgment is to the participants, the project staff, and the China National Centre for Disease Control and Prevention and its regional offices for access to death and disease registries. The Chinese National Health Insurance scheme provided electronic linkage to all hospital admission data.
Funding
Baseline survey: Kadoorie Charitable Foundation, Hong Kong. Long-term continuation: UK Wellcome Trust (088158/Z/09/Z, 104085/Z/14/Z), Chinese Ministry of Science and Technology (2011BAI09B01, 2012-14), Chinese National Natural Science Foundation (81390541). The British Heart Foundation, UK Medical Research Council and Cancer Research UK provide core funding to the Oxford CTSU. F. Bragg acknowledges support from the BHF Centre of Research Excellence, Oxford. M.V. Holmes is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/23/33512) and the National Institute for Health Research Oxford Biomedical Research Centre. The funders had no role in the collection, analysis or interpretation of data; in the writing of the report or in the decision to submit the article for publication.
Footnotes
Conflicts of interest
We declare that we have no conflict of interest.
References
- 1.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99(9):1013–23. [PMC free article] [PubMed] [Google Scholar]
- 2.Doubeni CA, Major JM, Laiyemo AO, Schootman M, Zauber AG, Hollenbeck AR, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. 2012;104(18):1353–62. doi: 10.1093/jnci/djs346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D, Masyn KE, Kawachi I, Laden F, Colditz GA. Neighborhood socioeconomic status and behavioral pathways to risks of colon and rectal cancer in women. Cancer. 2010;116(17):4187–96. doi: 10.1002/cncr.25195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leufkens AM, Van Duijnhoven FJ, Boshuizen HC, Siersema PD, Kunst AE, Mouw T, et al. Educational level and risk of colorectal cancer in EPIC with specific reference to tumor location. Int J Cancer. 2012;130(3):622–30. doi: 10.1002/ijc.26030. [DOI] [PubMed] [Google Scholar]
- 5.Mouw T, Koster A, Wright ME, Blank MM, Moore SC, Hollenbeck A, et al. Education and risk of cancer in a large cohort of men and women in the United States. PLoS One. 2008;3(11):e3639. doi: 10.1371/journal.pone.0003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99(18):1384–94. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 7.Anyiwe K, Qiao Y, De P, Yoshida EM, Earle CC, Thein HH. Effect of socioeconomic status on hepatocellular carcinoma incidence and stage at diagnosis, a population-based cohort study. Liver Int. 2016;36(6):902–10. doi: 10.1111/liv.12982. [DOI] [PubMed] [Google Scholar]
- 8.Boscoe FP, Johnson CJ, Sherman RL, Stinchcomb DG, Lin G, Henry KA. The relationship between area poverty rate and site-specific cancer incidence in the United States. Cancer. 2014;120(14):2191–8. doi: 10.1002/cncr.28632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackillop WJ, Zhang-Salomons J, Boyd CJ, Groome PA. Associations between community income and cancer incidence in Canada and the United States. Cancer. 2000;89(4):901–12. doi: 10.1002/1097-0142(20000815)89:4<901::aid-cncr25>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 11.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 12.Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–72. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 13.Jaacks LM, Gordon-Larsen P, Mayer-Davis EJ, Adair LS, Popkin B. Age, period and cohort effects on adult body mass index and overweight from 1991 to 2009 in China: the China Health and Nutrition Survey. Int J Epidemiol. 2013;42(3):828–37. doi: 10.1093/ije/dyt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao J, Wu X. Urbanization, socioeconomic status and health disparity in China. Health Place. 2016;42:87–95. doi: 10.1016/j.healthplace.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Ng SW, Howard AG, Wang HJ, Su C, Zhang B. The physical activity transition among adults in China: 1991-2011. Obes Rev. 2014;15(Suppl 1):27–36. doi: 10.1111/obr.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: a Global Perspecrtive. Continuous Update Project Expert Report 2018. 2018 Available at dietandcancerreport.org.
- 17.Chen Z, Chen J, Collins R, Guo Y, Peto R, Wu F, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40(6):1652–66. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.China Statistics Yearbook. 2012.
- 19.Yang Z, Zheng R, Zhang S, Zeng H, Xia C, Li H, et al. Comparison of cancer incidence and mortality in three GDP per capita levels in China, 2013. Chin J Cancer Res. 2017;29(5):385–94. doi: 10.21147/j.issn.1000-9604.2017.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G, Rao C, Ma J, Wang L, Wan X, Dubrovsky G, et al. Validation of verbal autopsy procedures for adult deaths in China. Int J Epidemiol. 2006;35(3):741–8. doi: 10.1093/ije/dyi181. [DOI] [PubMed] [Google Scholar]
- 21.Asadzadeh Vostakolaei F, Karim-Kos HE, Janssen-Heijnen ML, Visser O, Verbeek AL, Kiemeney LA. The validity of the mortality to incidence ratio as a proxy for site-specific cancer survival. Eur J Public Health. 2011;21(5):573–7. doi: 10.1093/eurpub/ckq120. [DOI] [PubMed] [Google Scholar]
- 22.Sunkara V, Hebert JR. The colorectal cancer mortality-to-incidence ratio as an indicator of global cancer screening and care. Cancer. 2015;121(10):1563–9. doi: 10.1002/cncr.29228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10(7):1025–35. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 24.Pang Y, Holmes MV, Kartsonaki C, Guo Y, Yang L, Bian Z, et al. Young adulthood and adulthood adiposity in relation to incidence of pancreatic cancer: a prospective study of 0.5 million Chinese adults and a meta-analysis. J Epidemiol Community Health. 2017;71(11):1059–67. doi: 10.1136/jech-2017-208895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang Y, Kartsonaki C, Turnbull I, Guo Y, Chen Y, Clarke R, et al. Adiposity in relation to risks of fatty liver, cirrhosis and liver cancer: a prospective study of 0.5 million Chinese adults. Sci Rep. 2019;9(1):785. doi: 10.1038/s41598-018-36460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–35. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doubeni CA, Laiyemo AO, Major JM, Schootman M, Lian M, Park Y, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer. 2012;118(14):3636–44. doi: 10.1002/cncr.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jemal A, Simard EP, Xu J, Ma J, Anderson RN. Selected cancers with increasing mortality rates by educational attainment in 26 states in the United States, 1993-2007. Cancer Causes Control. 2013;24(3):559–65. doi: 10.1007/s10552-012-9993-y. [DOI] [PubMed] [Google Scholar]
- 29.Kim JM, Kim HM, Jung BY, Park EC, Cho WH, Lee SG. The association between cancer incidence and family income: analysis of Korean National Health Insurance cancer registration data. Asian Pac J Cancer Prev. 2012;13(4):1371–6. doi: 10.7314/apjcp.2012.13.4.1371. [DOI] [PubMed] [Google Scholar]
- 30.Yun EH, Lim MK, Oh JK, Park JH, Shin A, Sung J, et al. Combined effect of socioeconomic status, viral hepatitis, and lifestyles on hepatocelluar carcinoma risk in Korea. Br J Cancer. 2010;103(5):741–6. doi: 10.1038/sj.bjc.6605803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladabaum U, Clarke CA, Press DJ, Mannalithara A, Myer PA, Cheng I, et al. Colorectal cancer incidence in Asian populations in California: effect of nativity and neighborhood-level factors. Am J Gastroenterol. 2014;109(4):579–88. doi: 10.1038/ajg.2013.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcella S, Miller JE. Racial differences in colorectal cancer mortality. The importance of stage and socioeconomic status. J Clin Epidemiol. 2001;54(4):359–66. doi: 10.1016/s0895-4356(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 33.Shebl FM, Capo-Ramos DE, Graubard BI, McGlynn KA, Altekruse SF. Socioeconomic status and hepatocellular carcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1330–5. doi: 10.1158/1055-9965.EPI-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Major JM, Sargent JD, Graubard BI, Carlos HA, Hollenbeck AR, Altekruse SF, et al. Local geographic variation in chronic liver disease and hepatocellular carcinoma: contributions of socioeconomic deprivation, alcohol retail outlets, and lifestyle. Ann Epidemiol. 2014;24(2):104–10. doi: 10.1016/j.annepidem.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Zheng R, Zhang H, Zuo T, Xia C, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29(1):1–10. doi: 10.21147/j.issn.1000-9604.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z, Zheng R, Zhang S, Zeng H, Xia C, Li H, et al. Comparison of cancer incidence and mortality in three GDP per capita levels in China. Chin J Cancer Res. 2017;29(5):385–94. doi: 10.21147/j.issn.1000-9604.2017.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 38.Akinyemiju T, Ogunsina K, Okwali M, Sakhuja S, Braithwaite D. Lifecourse socioeconomic status and cancer-related risk factors: Analysis of the WHO study on global ageing and adult health (SAGE) Int J Cancer. 2017;140(4):777–87. doi: 10.1002/ijc.30499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tillmann T, Vaucher J, Okbay A, Pikhart H, Peasey A, Kubinova R, et al. Education and coronary heart disease: mendelian randomisation study. BMJ. 2017;358 doi: 10.1136/bmj.j3542. j3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett JA, Brown P, Cameron L, Whitehead LC, Porter D, McPherson KM. Changes in employment and household income during the 24 months following a cancer diagnosis. Support Care Cancer. 2009;17(8):1057–64. doi: 10.1007/s00520-008-0540-z. [DOI] [PubMed] [Google Scholar]
- 41.Du H, Li L, Whitlock G, Bennett D, Guo Y, Bian Z, et al. Patterns and socio-demographic correlates of domain-specific physical activities and their associations with adiposity in the China Kadoorie Biobank study. BMC Public Health. 2014;14:826. doi: 10.1186/1471-2458-14-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu C, Shi Z, Lv J, Du H, Qi L, Guo Y, et al. Major Dietary Patterns in Relation to General and Central Obesity among Chinese Adults. Nutrients. 2015;7(7):5834–49. doi: 10.3390/nu7075253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang LN, Q RL. Cancer screening and prevention in China. Cancer Control. 2014:131–3. [Google Scholar]
- 44.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 45.Liang PS, Mayer JD, Wakefield J, Ko CW. Temporal trends in geographic and sociodemographic disparities in colorectal cancer among medicare patients, 1973-2010. J Rural Health. 2017;33(4):361–70. doi: 10.1111/jrh.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aarts MJ, Lemmens VE, Louwman MW, Kunst AE, Coebergh JW. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer. 2010;46(15):2681–95. doi: 10.1016/j.ejca.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 47.Doubeni CA, Laiyemo AO, Reed G, Field TS, Fletcher RH. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2170–5. doi: 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Shi J, Yu S, Wang L, Liu J, Ren J, et al. Effect of socioeconomic status on stage at diagnosis of lung cancer in a hospital-based multicenter retrospective clinical epidemiological study in China, 2005-2014. Cancer Med. 2017;6(10):2440–52. doi: 10.1002/cam4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q, Li J, Zheng S, Li JY, Pang Y, Huang R, et al. Breast cancer stage at diagnosis and area-based socioeconomic status: a multicenter 10-year retrospective clinical epidemiological study in China. BMC Cancer. 2012;12:122. doi: 10.1186/1471-2407-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez SL, Shariff-Marco S, DeRouen M, Keegan TH, Yen IH, Mujahid M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer. 2015;121(14):2314–30. doi: 10.1002/cncr.29345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–72. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.