Abstract
Purpose
Although topical agents are often provided during radiation therapy, there is limited consensus and evidence for their use prophylactically to prevent or reduce radiation dermatitis.
Methods
This was a multi-site, randomized, placebo-controlled, blinded study of 191 breast cancer patients to compare the prophylactic effectiveness of three topical agents (Curcumin, HPR Plus™, and Placebo) for reducing radiation dermatitis and associated pain. Patients applied the topical agent to their skin in the radiation area site three times daily starting the first day of radiation therapy (RT) until 1 week after RT completion.
Results
Of the 191 randomized patients, 171 patients were included in the final analyses (87.5% white females, mean age = 58 (range = 36–88)). Mean radiation dermatitis severity (RDS) scores did not significantly differ between study arms (Curcumin = 2.68 [2.49, 2.86]; HPR Plus™ = 2.64 [2.45, 2.82]; Placebo = 2.63 [2.44, 2.83];p = 0.929). Logistic regression analyses showed that increased breast field separation positively correlated with increased radiation dermatitis severity (p = 0.018). In patients with high breast field separation (≥ 25 cm), RDS scores (Curcumin = 2.70 [2.21, 3.19]; HPR Plus™ = 3.57 [3.16, 4.00]; Placebo = 2.95 [2.60, 3.30];p = 0.024) and pain scores (Curcumin = 0.52 [− 0.28, 1.33]; HPR Plus™ = 0.55 [− 0.19, 1.30]; Placebo = 1.73 [0.97, 2.50]; p = 0.046) significantly differed at the end of RT.
Conclusions
Although there were no significant effects of the treatment groups on the overall population, our exploratory subgroup analysis suggests that prophylactic treatment with topical curcumin may be effective for minimizing skin reactions and pain for patients with high breast separation (≥ 25 cm) who may have the worst skin reactions.
Keywords: Radiation dermatitis, Radiation therapy, Breast cancer, Pain
Introduction
During radiation therapy (RT) for cancer, the skin is the first organ exposed to and damaged by radiation. Radiation dermatitis occurs in 90% of patients undergoing RT, most notably in patients with head and neck cancer or breast cancer, with severe reactions occurring in 10–25% of patients [1, 2, 7, 23]. This skin injury is characterized by erythema, dry or moist desquamation, and ulceration. Despite the high prevalence of radiation dermatitis, evidence-based treatments do not exist [7, 14, 26].
Although management guidelines for radiation dermatitis have been published, disagreement regarding optimal management remains. Consensus across institutions is difficult to obtain due to the limited clinical evidence of effective topical agents [7, 9, 14, 23, 26]. A wide variety of topical treatments used in clinical trials, such as aloe vera gel, hyaluronate creams, and dressings, have shown conflicting results in reducing skin symptoms [6, 7, 14, 19, 26–28, 37, 40]. Recent publications [14, 19], along with the Multinational Association of Supportive Care in Cancer (MASCC) guidelines [40], recommend the use topical corticosteroids for radiation dermatitis. Despite these recommendations, Lucey et al. reported only 29% of providers use of topical steroids for radiation dermatitis [26]. More recently, Cleary et al. showed that application of an andrenergic vasoconstrictor to skin before radiation reduced radiation dermatitis severity [3]. Overall, most institutions provide topical agents, such as aquaphor, aloe vera, and/or silver sulfadiazine, after onset of visible changes in the skin [26]. Little evidence is available regarding whether prophylactic treatment with a moisturizing agent can prevent radiation dermatitis.
Curcumin, the active component of turmeric, has been used to treat a variety of skin ailments, including acne and eczema [38, 39]. Curcumin is one of the most widely studied nutraceuticals with supportive evidence for a broad spectrum of biological activity, including antioxidant, anti-inflammatory, anti-cancer, and anti-microbial properties [10, 12, 20, 21, 24, 34, 36]. In 2013, our group showed that prophylactic oral curcumin reduced radiation dermatitis severity in 30 breast cancer patients [31]. Unfortunately, oral curcumin’s effectiveness was not demonstrated in the subsequently published large confirmatory trial [32]. Because of oral curcumin’s low bio-availability, topical administration may be more effective at reducing radiation dermatitis. Curcumin gel, also known as Psoria-Gold® Curcumin, is an approved cosmetic that contains 4% curcumin and has been used in multiple clinical trials with demonstrated benefits for psoriasis and burns [15–18]. HPR Plus™ is an FDA-approved medical device recommended for atopic dermatitis and radiation dermatitis. This study was a preventive, multi-site, randomized, placebo-controlled, blinded study of 191 breast cancer patients to compare the prophylactic effectiveness of Curcumin gel, HPR Plus™, or Placebo for reducing radiation dermatitis and associated pain.
Methods
Patients and study design
Eligible patients were adult females, able to speak and understand English, with a diagnosis of unilateral non-inflammatory breast cancer or carcinoma in situ and scheduled to receive “conventional fractionated” RT. Eligible RT regimens included 1.8 to 2.0 Gy fractions for 22 to 36 sessions (total radiation dose of 44 to 66 Gy) with or without boost. Eligibility required previous chemotherapy that ended at least 2 weeks prior to enrollment. Participants were required to refrain from using any topical treatments in the RT area other than the study topical agent or “standard of care” provided by their treating oncologist. Exclusion criteria included pregnancy; concurrent chemotherapy; bilateral breast cancer; short-course fractionation RT (i.e., 16 sessions or 20 sessions at 2.4 to 2.6 Gy fractions per session); previous radiation to the breast or chest; breast reconstruction prior to RT; previous diagnosis of radiosensitivity disorder, collagen vascular disorder, or vasculitis; and unhealed surgical wounds or dermatological issues in the breast or chest region.
This study was a preventive phase 2, three-arm, parallel group, randomized, semi-blind, placebo-controlled trial conducted via the National Community Oncology Research Program (NCORP) Research Base at five community oncology practices nationwide. The study was conducted under FDA IND 123,414 for Curcumin Gel approved by the University of Rochester Institutional Review Board and NCI Division of Cancer Prevention Office, and registered on ClinicalTrials.gov (Identifier: NCT02536632). Written informed consent was obtained from each patient. Within each site, a computer generated random number table with random block sizes of 3 or 6 was used to assign patients to Curcumin gel, HPR Plus™, or Placebo gel in the ratio of 1:1: 1. The primary objective was to investigate the effectiveness of Curcumin gel or HPR Plus™ at reducing radiation dermatitis severity in breast cancer patients during RT compared to placebo. We also examined the effects of Curcumin gel and HPR Plus™ on radiation-associated pain and the acceptability of these prophylactic topical agents during RT.
Study intervention
Three topical agents were included in this study: (1) HPR Plus™ (PruGen, Scottsdale, AZ), (2) Psoria-Gold® Curcumin gel (Psoria-Gold, Federal Way, WA), and (3) Placebo gel (Psoria-Gold, Federal Way, WA). The Placebo gel was the same color (yellow) and consistency as the Curcumin gel, whereas the HPR Plus™ was a white lotion. The topical agents were packaged in white, opaque, 1.69 ounce air pump bottles. Patients applied three pumps of the study topical to the entire RT area (from the base of the neck to the fold underneath the breast, including the sides of the breast and the underarm area), three times daily starting on the first day of treatment and ending 1 week after completion of RT. Compliance was assessed using a self-report log in which patients checked boxes daily to indicate utilization of the topical agent. “Standard care” was allowed in all study arms if the patient’s skin had a Radiation Dermatitis Severity (RDS) score of 2.0 or higher Each NCORP site submitted their “standard care” regimens prior to the start of the study. The “standard care” regimens varied by NCORP site and included sixteen different topical modalities. All “standard care” topical agents used during the study were reported for each subject.
Study procedures and measures
Eligibility screening and informed consent were performed prior to the start of RT. Patients were assessed at baseline, weekly after every fifth RT session starting week 3 of RT, at the end of RT (EndRT), 1 week post-RT, and 2 weeks post-RT. Study assessments involved clinical skin rating, using the Radiation Dermatitis Severity (RDS) scale and NIH Common Terminology Criteria-Adverse Events (CTCAE), as well as three self-report questionnaires. EndRT assessments also involved digital photographs of radiation-induced skin changes. The RDS scale was developed by dermatologists to evaluate radiation-induced color and texture changes in skin (Table 1) [30–32]. The NIH CTCAE was used as a secondary clinical rating for radiation dermatitis severity [3, 4]. The primary outcome measure was the RDS score performed by the treating oncologist or trained study personnel at EndRT. Secondary outcome measures included pain at RT site (Self-report Pain Diary and Skin-Pain Inventory). Additional exploratory measures included the presence of moist desquamation, time to implementation of standard care, and acceptability and blinding (Feedback Questionnaire). Furthermore, this study investigated the validity of RDS ratings performed by central blinded reviewers on digital photographs of skin.
Table 1.
Radiation dermatitis severity score (RDS)
| Score | Criteria/characteristics |
|---|---|
| 0.0 | None |
| 0.5 | Patchy faint follicular erythema; faint hyperpigmentation. |
| 1.0 | Faint diffuse erythema; diffuse hyperpigmentation. |
| 1.5 | Definite erythema; definite darkening/hyperpigmentation. |
| 2.0 | Definite erythema (or hyperpigmentation) with fine desquamation and pruritus (itchiness). |
| 2.5 | Definite erythema (or hyperpigmentation) with branny desquamation. |
| 3.0 | Deep red erythema (or extreme darkening) with diffuse desquamation, some desquamation in sheets. |
| 3.5 | Violaceous erythema (or extreme darkening) with diffuse desquamation in sheets, patchy crusting. |
| 4.0 | Violaceous erythema (or extreme darkening) with diffuse desquamation in sheets, patchy crusting, and superficial ulceration. |
Throughout the course of the study, patients reported their pain intensity at the RT site on a scale of 0 to 10 in a self-report daily Pain Diary. The Skin-Pain Inventory was a 16-item questionnaire that evaluated the severity of skin problems and pain at the RT site, as well as 9 specific pain symptoms (i.e., itchy, achy, tender, hot-burning, tight, redness, flaking, bumpy) on a 6-point severity scale anchored by 0 (“Not Present”) and 5 (“Very Severe”) [11]. A Feedback Questionnaire consisted of 12 questions regarding likes and dislikes of the topical agent, perceived benefit for skin reactions or pain, use of standard care topical treatments, and their perceived assigned study arm.
Blinded RDS scores of digital images
Coordinators at each site took digital images (i.e., photos) of the worst radiation dermatitis at the EndRT assessment using a Canon Powershot SD1300 IS Digital ELPH camera. The images were uploaded onto a secure, study-specific server. Three blinded reviewers (two Dermatologists and one Radiation Oncologist) rated radiation dermatitis severity (RDS scale). All reviewers were blinded to the treatment arms and site-reported RDS scores. Correlative and measurement error analyses were used to assess the reliability of blinded reviewer RDS scoring of digital skin images. For correlative analyses, Derm 1 (i.e., senior Dermatologist) was used as the standard for RDS ratings.
Statistical analyses
Assuming a standard deviation of 0.9 and significance level of 0.05, 50 participants per group would provide 82% power to detect a 0.5 point difference in the RDS score [31]. Assuming 20% rate of withdrawal, we aimed to recruit a total of 180 participants. Descriptive statistics, using ANOVA or contingency table analyses, were used to identify any baseline differences in participant clinical and demographic characteristics between the three study arms. The primary and secondary analyses were based on subjects with available data (Fig. 1). A general linear model was used to identify differences in mean RDS score at EndRT between study arms using study arm and site as covariates. Similar analyses were performed for mean pain at EndRT using the Pain Diary and Skin-Pain Inventory. Fisher’s exact tests were used to analyze the proportions of patients with moist desquamation. Linear mixed models (LMM) with cubic splines and random quadratic polynomial within subjects evaluated RDS scores and Pain scores over time. Nonparametric correlative analyses, using Spearman’s p, were performed to examine relationships of RDS scores with self-reported pain and skin problems. Sensitivity to missing data for the primary aim was assessed by comparing the available case results with those obtained using multiple imputation (an Intent to treat analysis), assuming the MAR mechanism [25]. The results were very comparable with available cases, which are presented below. A measurement error model was fit to the ratings from three assessors of digital photographs via maximum likelihood using the R package lavaan. Because all assessors judged the same photograph, we were able to estimate the variance of the “true” RDS score (VRDS), the measurement variances of the three assessors (Vj,j = 1,2,3), and biases of two assessors relative to one we considered to be the reference, Dermatologist 1. Test-retest reliabilities were estimated for each assessor using the intraclass correlation, ICC = VRDS/(VRDS + Vj).
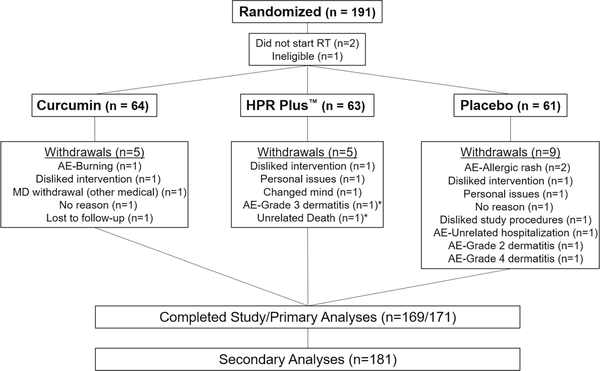
Fig. 1.
CONSORT diagram. This figure presents the patient flow of randomized patients in the study, as well as the number of patients used in primary and secondary analyses
Results
Patient characteristics
Between October 2015 and April 2016, 191 patients with breast cancer were enrolled and randomized into one of three arms (Fig. 1). Of the 191 patients, three patients did not start study procedures due to not starting RT or ineligibility. Of the 188 patients who began the study, 19 (10.1%) patients withdrew and 169 patients completed the study. However, 171 patients were used in the primary analyses because two patients completed the EndRT assessment prior to withdrawal. Reasons for withdrawal included disliked intervention/study procedures (4; 21.1%); radiation dermatitis grade 2–4 (3; 15.8%); allergic reaction (2; 10.5%); and personal issues (2; 10.5%). Baseline characteristics did not differ between study arms (Table 2) nor did compliance (curcumin = 97%; HPR Plus™ = 95%; and placebo = 97%).
Table 2.
Patient demographics
| All N = 191 | Curcumin Gel N = 64 (33.5%) | HPR Plus™ N = 65 (34.0%) | Placebo N = 62 (32.5%) | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean [95% CI] | 59.8 [58.3, 61.3] | 59.0 [56.2, 61.8] | 60.7 [58.0, 63.3] | 59.8 [57.4, 62.2] |
| Race | ||||
| White/Caucasian | 167 (87.4%) | 55 (85.9%) | 55 (84.6%) | 57 (91.9%) |
| Black/African American | 21 (11.0%) | 8 (12.5%) | 9 (13.8%) | 4 (6.5%) |
| Asian | 2 (1.%) | 0 (0.0%) | 1 (1.5%) | 1 (1.6%) |
| Unknown | 1 (0.5%) | 1 (1.6%) | 0 (0.0%) | 0 (0.0%) |
| Ethnicity | ||||
| Hispanic | 3 (1.4%) | 2 (3.1%) | 0 (0.0%) | 1 (1.6%) |
| Non-Hispanic | 186 (97.4%) | 62 (96.9%) | 63 (96.9%) | 61 (98.4%) |
| Unknown | 2 (1.1%) | 0 (0.0%) | 2 (3.1%) | 0 (0.0%) |
| Marital status | ||||
| Married | 119 (62.3%) | 45 (70.3%) | 37 (56.9%) | 37 (59.7%) |
| Domestic partner | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) |
| Divorced | 30 (15.7%) | 5 (7.8%) | 9 (13.8%) | 16 (25.8%) |
| Separated | 2 (1.1%) | 1 (1.6%) | 0 (0.0%) | 1 (1.1%) |
| Single | 18 (9.4%) | 8 (12.5%) | 8 (12.3%) | 2 (3.2%) |
| Widowed | 19 (10%) | 5 (7.8%) | 10 (15.4%) | 4 (6.5%) |
| Tumor location | ||||
| Left | 86 (45%) | 29 (45.3%) | 27 (41.5%) | 30 (48.4%) |
| Right | 103 (53.9%) | 35 (54.7%) | 37 (56.9%) | 31 (50%) |
| Tumor stage | ||||
| DCIS | 28 (14.7%) | 5 (7.8%) | 12 (18.5%) | 11 (17.7%) |
| I | 85 (44.5%) | 34 (53.1%) | 29 (44.6%) | 22 (35.5%) |
| II | 54 (28.3%) | 16 (25%) | 18 (27.7%) | 20 (32.3%) |
| III | 20 (10.5%) | 7 (10.9%) | 5 (7.7%) | 8 (12.9%) |
| IV | 2 (1.1%) | 2 (3.1%) | 0 (0.0%) | 0 (0.0%) |
| Radiation type | ||||
| IMRT | 5 (2.6%) | 1 (1.6%) | 2 (3.1%) | 2 (3.2%) |
| 3D conformai whole breast | 184 (96.3%) | 63 (98.4%) | 62 (95.4%) | 59 (95.2%) |
| Radiation boost | ||||
| Yes | 180 (94.2%) | 60 (93.8%) | 61 (93.8%) | 59 (95.2%) |
| No | 9 (4.7%) | 4 (6.3%) | 3 (4.6%) | 2 (3.2%) |
| Prescribed radiation dose (Gy) | ||||
| Mean [95% CI] | 59 [58.3, 61.3] | 58.4 [57.0, 59.8] | 59.5 [58.4, 60.7] | 59.1 [57.8, 60.4] |
| Breast field separation (cm) | ||||
| Mean [95% CI] | 23.1 [22.4, 23.7] | 22.6 [21.6, 23.6] | 23.8 [22.8, 24.9] | 22.8 [21.4, 24.2] |
| Prior chemotherapy | ||||
| Yes | 90 (47.1%) | 34 (53.1%) | 29 (44.6%) | 27 (43.5%) |
| No | 99 (51.8%) | 30 (46.9%) | 35 (53.8%) | 34 (54.8%) |
| Hormone treatment | ||||
| Yes | 41 (21.5%) | 12 (18.8%) | 16 (24.6%) | 13 (21%) |
| No | 148 (77.5%) | 52 (81.2%) | 48 (73.8%) | 48 (77.4%) |
| Herceptin treatment | ||||
| Yes | 19 (10.0%) | 8 (12.5%) | 8 (12.3%) | 3 (4.8%) |
| No | 170 (89.0%) | 56 (87.5%) | 56 (86.2%) | 58 (93.5%) |
Adverse events
Adverse events were reported during the study, but not all resulted in withdrawal from the study. The most common adverse events included: radiation dermatitis—grades 1–4 (12; 6.3%); pain of skin—grade 1–2 (7; 3.7%); burning of skin—grade 1–2 (3; 1.6%); allergic reaction—grade 2 (2; 1.1%); rash—grade 2 (2; 1.1%); and pruritus—grade 2 (2; 1.1%). One unrelated death and one unrelated hospitalization were reported.
Radiation dermatitis and pain severity
Mean study arm differences in RDS scores and the presence of moist desquamation at EndRT were not statistically significant (Table 3). Longitudinal trends showed RDS peaked at Week 6 with no significant difference between study arms (Fig. 2a). Mean self-reported skin problem severity at EndRT, as captured by the Skin-Pain Inventory, was not significantly different between study arms (Table 3). Longitudinal analyses of self-reported skin problem severity showed an earlier peak at week 5 compared to the RDS scores (Fig. 2b). Similarly, the NIH CTCAE ratings showed mean grade of 2.2 across arms with 77% patients having grade 1 or 2 radiation dermatitis. Although the presence of moist desquamation at EndRT did not differ between arms, it did differ across the five NCORP sites (36%, 17%, 8%, 21%, and 26%; p = 0.047). Standard care was initiated on 57% of patients during the study (Curcumin = 63%; HPR Plus = 53%; Placebo = 54%). The most commonly prescribed modalities in all arms were Aquaphor (20–32%), Silver Sulfadiazine Cream (26–27%), and topical steroid (8–12%). The mean session number for initiation of standard care did not differ between arms. Standard care was most commonly initiated at session 30 (35%), session 25 (30%), and session 20 (25%).
Table 3.
Radiation dermatitis severity and pain scores at EndRT
| Curcumin | HPR Plus | Placebo | |
|---|---|---|---|
| RDS score EndRT | |||
| Mean | 2.68 | 2.64 | 2.63 |
| 95% CI | [2.49, 2.86] | [2.45, 2.82] | [2.44, 2.83] |
| p = 0.929 | |||
| Moist desquamation EndRT | |||
| %(N) | 25.42% (15) | 20.34% (12) | 22.64% (12) |
| p = 0.805 | |||
| Skin severity EndRT (Skin-Pain Inventory) | |||
| Mean | 1.93 | 1.76 | 2.08 |
| 95% CI | [1.63, 2.23] | [1.47, 2.06] | [1.77, 2.39] |
| p = 0.139 | |||
| Pain Diary score EndRT | |||
| Mean | 1.96 | 2.28 | 2.44 |
| 95% CI | [1.31, 2.62] | [1.63, 2.92] | [1.74, 3.14] |
| p = 0.840 | |||
| Pain severity EndRT (Skin-Pain Inventory) | |||
| Mean | 1.47 | 1.44 | 1.64 |
| 95% CI | [1.12, 1.81] | [1.10, 1.78] | [1.28, 2.00] |
| p = 0.685 | |||
Fig. 2.
Radiation dermatitis severity and pain over time. a, b Longitudinal analyses of RDS scores (a) and self-reported skin problems (b) did not differ between treatment arms. c, d Longitudinal analyses of pain scores from Pain Diary (c) and Skin-Pain Inventory (d). Pain Diary scores (c) showed significant difference in pain over time with curcumin arm lower at week 6 compared to the other two arms (*p <0.001)
There were no significant differences between arms in mean Pain Diary scores or self-reported pain severity at EndRT (Table 3). Longitudinal analyses revealed a highly significant arm by time interaction for mean pain diary scores (p < 0.001). Additionally, more patients using Curcumin or HPR Plus™ reported none or very mild itchiness (73% and 76%) or redness (37% and 39%) compared to placebo (51%, p = 0.009 and 19%, p = 0.044, respectively).
Subgroup analyses: high breast field separation
Logistic regression analyses showed that increased breast field separation positively correlated with increased radiation dermatitis severity (p = 0.018). Breast field separation can be used to estimate breast size [13, 29, 33]. We performed a subgroup analysis on patients with a breast field separation of ≥ 25 cm who were more likely to have a severe radiation-induced skin reaction (n = 22). ANOVA showed significant differences in RDS scores and pain scores by study arm at EndRT (Fig. 3a, b). The Curcumin arm had lower RDS scores over time compared to HPR Plus™ and Placebo (Fig. 3c). Curcumin and HPR Plus™ arms had lower pain scores over time compared to Placebo (Fig. 3d). These subgroup analyses suggest that topical curcumin may be beneficial for radiation dermatitis and pain in patients with breast field separation of ≥ 25 cm undergoing RT.
Fig. 3.
Curcumin gel may benefit patients with breast field separation ≥ 25 cm. Subgroup ANOVA analyses of RDS scores (a) and pain scores (b) showed a significant difference between arms at EndRT. Longitudinal analyses showed significant differences in mean RDS scores (c) and pain scores (d) between arms over time
Correlation of patient-reported measures
Self-reported skin problems and pain showed stronger correlation with each other than with RDS scores at week 4 and week 5 during RT. Non-parametric correlative analyses demonstrated a strong association of self-reported skin problems and pain at Week 4 (ρ = 0.697, p < 0.0001) and week 5 (ρ = 0.766, p < 0.0001). In contrast, RDS scores at week 4 and week 5 weakly correlated with self-reported skin problems (ρ = 0.510 and ρ = 0.454, p < 0.0001) and pain scores (ρ = 0.368 and ρ = 0.347, p < 0.0001).
Radiation dermatitis severity from digital images
Two dermatologists and one radiation oncologist performed RDS ratings on digital images of the radiation-induced skin reactions taken at EndRT for 173 patients. ANOVA did not reveal any significant differences between arms in RDS ratings from blinded reviewers. For correlative analyses, Derm 1 (i.e., senior Dermatologist) was used as the standard for RDS ratings. Although RadOnc (r =1.08) and site (r = 0.98) ratings correlated with Derm 1 (r =1.00), a structural equation-based measurement error analysis revealed substantial intra-rater variance with RDS ratings. The intraclass coefficient (ICC) showed substantial measurement error in RDS ratings (Derm1 ICC = 0.71; Derm 2 = 0.57; RadOnc = 0.73). Although RDS ratings from digital images by a senior dermatologist or radiation oncologist had similar reliability as the in-person RDS ratings, a more reliable measure for radiation dermatitis severity is imperative.
Blinding and acceptability
Assessment of blinding was critical in this study due to the different appearance of HPR Plus™ compared to the Curcumin and Placebo gels. A multiple-choice question on the Feedback Questionnaire asked subjects to select their assigned study arm. Despite the color difference of HPR Plus™ compared to the Curcumin and Placebo gels, patients appeared blinded to their assigned topical agent. Only 30% of subjects correctly chose their assigned study arm (Curcumin arm: 45% chose Curcumin; HPR Plus™ arm: 25% chose HPR Plus™; and Placebo arm: 18% chose Placebo). In HPR Plus™ and Placebo arms, majority of subjects selected Curcumin as their assigned study arm (HPR Plus™ arm: 39% chose Curcumin; Placebo arm: 67% chose Curcumin). These results suggest that blindness was intact. Furthermore, over 50% of patients stated that they would use their assigned topical agent in the future, suggesting acceptability of the three topical agents.
Discussion
The primary analysis comparing the RDS score at EndRT did not demonstrate a difference between treatment groups. Additionally, no differences were detected in self-reported skin problems or pain ratings between the treatment groups in the total study sample. The allowance of standard care in the study was a limitation and may have resulted in lower overall radiation dermatitis severity. Additionally, the severity of both skin problems and pain peaked prior to EndRT. Future studies should evaluate peak severity score during radiation therapy as the primary outcome. Despite the limitations, this study provides important data to inform the design of future trials on radiation dermatitis. The low mean severity of skin problems and pain in our sample may have precluded our ability to detect a treatment effect due to a so-called floor effect [8, 35]. In order to test this hypothesis, we looked for demographic variables that were associated with more severe radiation dermatitis. Because demographic variables are known prior to randomization they can be incorporated into entry criteria for future studies to provide a reasonable basis for exploratory subgroup analyses. We found that higher breast separation (i.e., larger breast size) predicted more severe radiation dermatitis. Subgroup analyses in patients with breast separation of 25 cm or larger showed a benefit of Curcumin over Placebo and HPR Plus™ (p values < 0.05). These data suggest that future studies of preventive treatments for RT should target patients who are more likely to develop moderate to severe radiation dermatitis for better detection of treatment response. Our data provides further support that larger breast separation could be one specified entry criterion in a radiation dermatitis trial. Additionally, evaluation of preventive treatments for radiation dermatitis in head and neck cancer patients should be considered because of the higher radiation dose and dermatitis severity. An alternative approach is to evaluate topical interventions on established radiation dermatitis rather than prophylactic response. However, this approach tests a different hypothesis and would only be advantageous for treatments that reverse established radiation-induced skin damage.
The primary outcome measure in this trial was the RDS score, a clinician-reported scale for the severity of radiation dermatitis. It has been validated for use by experienced dermatologists, but its reliability in multicenter trials, where a dermatologist is not available, is largely unknown. In order to address this question, skin reactions were documented using digital photography to allow for blinded ratings by two external dermatologists and one radiation oncologist. We found similar correlations among the site ratings, the senior dermatologist ratings, and the radiation oncologist. Considering the higher correlation of patient-reported skin problems and pain, as well as the variability of RDS scores, self-report outcome measures for radiation dermatitis and pain may be more useful in future studies. Recently, NIH developed the patient-reported outcome version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) to be used in conjunction with the NCI CTCAE. The PRO-CTCAE enables standard assessment of systematic adverse events from the patient perspective, which could provide valuable information on the safety and tolerability of treatments and medications [5, 22]. The utility of the PRO-CTCAE in radiation dermatitis and pain trials has yet to be determined, but could be informative in future trials.
Conclusions
Overall, our primary analyses did not show a significant difference in radiation dermatitis severity at the EndRT between treatment groups. Our exploratory subgroup analysis suggests that prophylactic treatment with topical curcumin may be effective for minimizing skin reactions and pain for patients with high breast separation (≥ 25 cm) who may have the worst skin reactions. These conclusions are limited because this analysis was not pre-planned. However, we performed only one subgroup analysis, which lowers the chances that these findings are random. Additionally, utilization of patient-reported measures for radiation dermatitis severity and pain are critical for future trials.
Acknowledgments
The authors thank all patients that participated in this clinical study.
Funding information This study was supported by the Food & Drug Administration (FDA) Investigational New Drug (IND) approval 123,414, National Cancer Institute (NCI) 1R21CA178648-01A1, and National Cancer Institute (NCI) National Community Oncology Research Program (NCORP) UG1CA189961.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Compliance with ethical standards
Conflict of interest Dr. Ryan Wolf has full control of the primary data and agree to allow the journal to review these data if requested. Dr. Ryan Wolf discloses support from NCI/NIH grant to conduct this study. Dr. Gewandter discloses support from NCI/NIH grant to conduct this study, as well as personal fees from Disarm, MundiPharma, Asahi, Regenacy, and SK LifeSciences for work unrelated to submitted study. All other authors do not have any conflicts of interest to disclose.
References
- 1.Arron S (2016) Anatomy of the skin and pathophysiology of radiation dermatitis In: Fowble B, Yom S, Yuen F, Arron S (eds) Skin care in radiation oncology: a practical guide. Spinger International Publishing, Switzerland, pp 9–14 [Google Scholar]
- 2.Bray FN, Simmons BJ, Wolfson AH, Nouri K (2016) Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther 6:185–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleary JF, Anderson BM, Eickhoff JC, Khuntia D, Fahl WE (2017) Significant suppression of radiation dermatitis in breast cancer patients using a topically applied adrenergic vasoconstrictor. Radiat Oncol 12:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Common terminology criteria-adverse events (CTCAE) Version 4.03, http://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5×7.pdf
- 5.Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, Atkinson TM, Bennett AV, Denicoff AM, O’Mara AM, Li Y, Clauser SB, Bryant DM, Bearden JD 3rd, Gillis TA, Harness JK, Siegel RD, Paul DB, Cleeland CS, Schrag D, Sloan JA, Abernethy AP, Bruner DW, Minasian LM, Basch E, National Cancer Institute PROCSG (2015) Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol 1:1051–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falkowski S, Trouillas P, Duroux JL, Bonnetblanc JM, Clavere P (2011) Radiodermatitis prevention with sucralfate in breast cancer: fundamental and clinical studies. Support Care Cancer 19:57–65 [DOI] [PubMed] [Google Scholar]
- 7.Ferreira EB, Vasques CI, Gadia R, Chan RJ, Guerra EN, Mezzomo LA, De Luca CG, Dos Reis PE (2017) Topical interventions to prevent acute radiation dermatitis in head and neck cancer patients: a systematic review. Support Care Cancer 25:1001–1011 [DOI] [PubMed] [Google Scholar]
- 8.Fogg L, Gross D (2000) Threats to validity in randomized clinical trials. Res Nurs Health 23:79–87 [DOI] [PubMed] [Google Scholar]
- 9.Fowble B, Park C, Yuen F (2016) Breast Cancer In: Fowblle B, Yom S, Yuen F, Arron S (eds) Skin Care in radiation oncology: a practical guide. Springer International Publishing, Switzerland, pp 93–122 [Google Scholar]
- 10.Gallardo M, Calaf GM (2016) Curcumin and epithelial-mesenchymal transition in breast cancer cells transformed by low doses of radiation and estrogen. Int J Oncol 48:2534–2542 [DOI] [PubMed] [Google Scholar]
- 11.Gewandter JS, Walker J, Heckler CE, Morrow GR, Ryan JL (2013) Characterization of skin reactions and pain reported by patients receiving radiation therapy for cancer at different sites. J Support Oncol 11:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta SC, Patchva S, Aggarwal BB (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15:195–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gursel B, Meydan D, Ozbek N, Ofluoglu T (2011) Dosimetric comparison of three different external beam whole breast irradiation techniques. Adv Ther 28:1114–1125 [DOI] [PubMed] [Google Scholar]
- 14.Haruna F, Lipsett A, Marignol L (2017) Topical management of acute radiation dermatitis in breast cancer patients: a systematic review and meta-analysis. Anticancer Res 37:5343–5353 [DOI] [PubMed] [Google Scholar]
- 15.Heng MC (2010) Curcumin targeted signaling pathways: basis for anti-photoaging and anti-carcinogenic therapy. Int J Dermatol 49: 608–622 [DOI] [PubMed] [Google Scholar]
- 16.Heng MC (2011) Wound healing in adult skin: aiming for perfect regeneration. Int J Dermatol 50:1058–1066 [DOI] [PubMed] [Google Scholar]
- 17.Heng MC (2013) Signaling pathways targeted by curcumin in acute and chronic injury: burns and photo-damaged skin. Int J Dermatol 52:531–543 [DOI] [PubMed] [Google Scholar]
- 18.Heng MC, Song MK, Harker J, Heng MK (2000) Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol 143:937–949 [DOI] [PubMed] [Google Scholar]
- 19.Ho AY, Olm-Shipman M, Zhang Z, Siu CT, Wilgucki M, Phung A, Arnold BB, Porinchak M, Lacouture M, McCormick B, Powell SN, Gelblum DY (2018) A randomized trial of mometasone furoate 0.1% to reduce high-grade acute radiation dermatitis in breast cancer patients receiving postmastectomy radiation. Int J Radiat Oncol BiolPhys 101:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayakumar S, Patwardhan RS, Pal D, Sharma D, Sandur SK (2016) Dimethoxycurcumin, a metabolically stable analogue of curcumin enhances the radiosensitivity of cancer cells: possible involvement of ROS and thioredoxin reductase. Biochem Biophys Res Commun 478:446–454 [DOI] [PubMed] [Google Scholar]
- 21.Khafif A, Lev-Ari S, Vexler A, BarneaI, Starr A, Karaush V, Haif S, Ben-Yosef R (2009) Curcumin: a potential radio-enhancer in head and neck cancer. Laryngoscope 119:2019–2026 [DOI] [PubMed] [Google Scholar]
- 22.Kluetz PG, Chingos DT, Basch EM, Mitchell SA (2016) Patient-reported outcomes in cancer clinical trials: measuring symptomatic adverse events with the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ Book 35:67–73 [DOI] [PubMed] [Google Scholar]
- 23.Kole AJ, Kole L, Moran MS (2017) Acute radiation dermatitis in breast cancer patients: challenges and solutions. Breast Cancer (Dove Med Press) 9:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar B, Yadav A, Hideg K, Kuppusamy P, Teknos TN, Kumar P (2014) A novel curcumin analog (H-4073) enhances the therapeutic efficacy of cisplatin treatment in head and neck cancer. PLoS One 9: e93208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little RJA, Rubin DB (2002) Statistical analysis with missing data. Wiley, Hoboken, NJ [Google Scholar]
- 26.Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN (2017) Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer 25:2857–2862 [DOI] [PubMed] [Google Scholar]
- 27.Meghrajani CF, Co HS, Arcillas JG, Maano CC, Cupino NA (2016) A randomized, double-blind trial on the use of 1% hydrocortisone cream for the prevention of acute radiation dermatitis. Expert Rev Clin Pharmacol 9:483–491 [DOI] [PubMed] [Google Scholar]
- 28.Rao S, Hegde SK, Baliga-Rao MP, Palatty PL, George T, Baliga MS (2017) An aloe vera-based cosmeceutical cream delays and mitigates ionizing radiation-induced dermatitis in head and neck cancer patients undergoing curative radiotherapy: a clinical study. Medicines (Basel, Witzerland) 4, 44:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell NS, Krul IM, van Eggermond AM, Aleman BMP, Cooke R, Kuiper S, Allen SD, Wallis MG, Llanas D, Diallo I, de Vathaire F, Smith SA, Hauptmann M, Broeks A, Swerdlow AJ, Van Leeuwen FE (2017) Retrospective methods to estimate radiation dose at the site of breast cancer development after Hodgkin lymphoma radiotherapyClin. Transl Radiat Oncol 7:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan JL (2012) Ionizing radiation: the good, the bad, and the ugly. J Investig Dermatol 132:985–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP, Morrow GR (2013) Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res 180:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan Wolf J, Heckler CE, Guido JJ, Peoples AR, Gewandter JS, Ling M, Vinciguerra VP, Anderson T, Evans L, Wade J, Pentland AP, Morrow GR (2018) Oral curcumin for radiation dermatitis: a URCC NCORP study of 686 breast cancer patients. Support Care Cancer 26:1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaitelman SF, Schlembach PJ, Arzu I, Ballo M, Bloom ES, Buchholz D, Chronowski GM, Dvorak T, Grade E, Hoffman KE, Kelly P, Ludwig M, Perkins GH, Reed V, Shah S, Stauder MC, Strom EA, Tereffe W, Woodward WA, Ensor J, Baumann D, Thompson AM, Amaya D, Davis T, Guerra W, Hamblin L, Hortobagyi G, Hunt KK, Buchholz TA, Smith BD (2015) Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: a randomized clinical trial. JAMA Oncol 1:931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shehzad A, Qureshi M, Anwar MN, Lee YS (2017) Multifunctional curcumin mediate multitherapeutic effects. J Food Sci 82:2006–2015 [DOI] [PubMed] [Google Scholar]
- 35.Spriensma AS, Eelchout I, de Boer MR, Luime JJ, de Jong PH, Bahcecitapar MK, Heymans MW, Twisk JW (2018) Analysing outcome variables with floor effects due to censoring: a simulation study with longitudinal trial data. Epidemiology, Biostatistics, and Public Health 15(2):1–9 [Google Scholar]
- 36.Tajbakhsh A, Hasanzadeh M, Rezaee M, Khedri M, Khazaei M, ShahidSales S, Ferns GA, Hassanian SM, Avan A (2018) Therapeutic potential of novel formulated forms of curcumin in the treatment of breast cancer by the targeting of cellular and physiological dysregulated pathways. J Cell Physiol 233:2183–2192 [DOI] [PubMed] [Google Scholar]
- 37.Ulff E, Maroti M, Serup J, Nilsson M, Falkmer U (2017) Prophylactic treatment with a potent corticosteroid cream ameliorates radiodermatitis, independent of radiation schedule: a randomized double blinded study. Radiother Oncol 122:50–53 [DOI] [PubMed] [Google Scholar]
- 38.Vaughn AR, Branum A, Sivamani RK (2016) Effects of turmeric (Curcuma longa) on skin health: a systematic review of the clinical evidence. Phytother Res 30:1243–1264 [DOI] [PubMed] [Google Scholar]
- 39.Verma V (2016) Relationship and interactions of curcumin with radiation therapy World. J Clin Oncol 7:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong RK, Bensadoun RJ, Boers-Doets CB, Bryce J, Chan A, Epstein JB, Eaby-Sandy B, Lacouture ME (2013) Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer 21:2933–2948 [DOI] [PubMed] [Google Scholar]