Abstract
Bacteria can attach to essentially all materials and form multicellular biofilms with high-level tolerance to antimicrobials. Detrimental biofilms are responsible for a variety of problems ranging from food and water contamination, bio-corrosion, to drug resistant infections. Besides the challenges in control, biofilms are also difficult to detect due to the lack of biofilm-specific biomarkers and methods for non-destructive imaging. In this article, we present a concise review of recent advancements in this field, with a focus on medical device-associated infections. We also discuss the technologies that have potential for non-destructive detection of bacterial biofilms.
Bacterial biofilms
As the oldest life form on earth, bacteria have developed remarkable capabilities to survive in harsh environments. One such strategy is to colonize surfaces and form biofilms, which are complex structures of bacterial cells embedded in an extracellular matrix comprised of polysaccharides, proteins, DNA and lipids produced intrinsically by these bacteria [1]. Biofilm cells are up to 1000 times more tolerant to antibiotics than their planktonic counterparts, rendering biofilm-related infections largely unresponsive to antibiotic therapies [2]. Thus, detrimental biofilms are of great concern especially when formed by pathogenic species on implanted medical devices and biomaterials such as indwelling catheters, orthopedic implants, cochlear implants, among others [3–6].
Challenges in biofilm detection
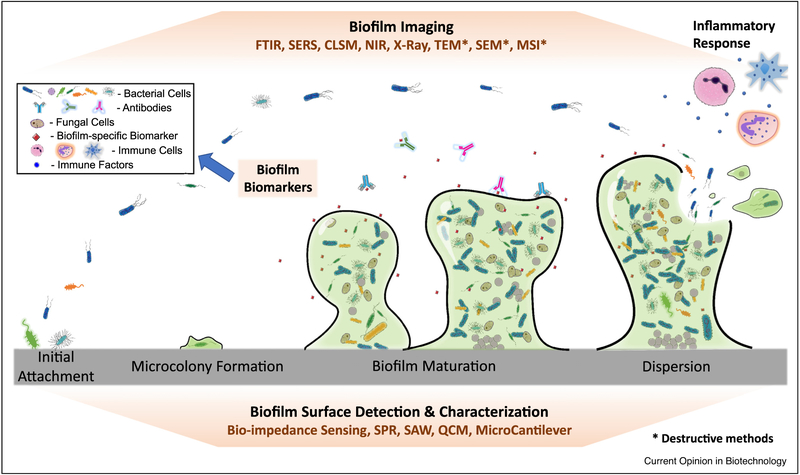
Although a number of methods have become quite routine for biofilm characterization in controlled laboratory settings [7•], biofilm detection in clinical settings has multiple challenges [8–10]. In general, biofilm associated infections (BAI) are chronic and remain local to the infection sites such as an implant. Additionally, clinical evidence suggests that for long periods of time biofilms can produce occult, or subclinical, infections, in which the inflammatory symptoms are less pronounced than acute infections, only revealing themselves when biofilm cells shed off resulting in bacteremia [11]. Usually BAIs involving foreign bodies are not confirmed until the device is explanted and even then often a diagnosis of biofilm is based on anecdotal evidence [9]. When a biofilm is formed on an implanted medical device, it cannot be directly sampled without a surgical procedure. This essentially precludes the detection using standard microbiological methods such as bacterial culturing on agar plates until intra-operative access. Even if the cells can be sampled, slow-growing variants of bacteria and dormant persister cells [12] may not form colonies under routine culturing conditions and thus cause a false negative result [13]. Culturing methods also face challenges with the highly heterogeneous distribution of biofilms and the involvement of fastidious strains and/or mixed-species biofilms that require specific growth factors [9]. A standardized, reliable method for the detection of biofilms in clinical settings is still missing. Besides BAI diagnosis in vivo, microbial detection on explanted medical devices is also challenging if the cells are difficult to sample (if trapped in crevices such as those in endo-scopes) or culture (if dormant or missing growth factors). Sampling may not be effective without removing bacteria using a stronger force such as sonication [14]. In this article, we provide a concise review of recent advancements (focusing on the past three years) in biofilm detection in medical settings and possible future directions (Figure 1).
Figure 1.
Schematic of biofilm formation and potential targets for detection. Biofilm formation causes significant changes in bacterial gene expression and metabolism, triggers host immune response, and alters the chemical/physical properties of the substrate material. These changes are potential targets for biofilm detection.
Molecular methods
Compared to culturing methods, DNA-based analyses are more sensitive in detecting microbes including unculturable cells and samples with mixed species. Conventional polymerase chain reaction (PCR)-based approaches target ribosomal RNA for detection [15]. Inclusion of specific gene targets can reveal additional information such as antibiotic resistance [16••]. Compared to PCR tests that target individual genes, meta-genomic sequencing can reveal more information regarding antibiotic resistance, virulence factors and other important physiological traits. Recent development of whole-genome sequencing (WGS) has drastically improved the throughput and accuracy, and lowered the cost [17•]. DNA-based technologies do have limitations, one being the lack of information about the viability of cells and not being able to distinguish whether the organisms are in a biofilm or planktonic phenotype (if the cells cannot be separated during sampling). Combining DNA detection with messenger RNA analysis for biofilm-specific gene expression may be able to distinguish the phenotype of the organisms. However, contaminating DNA from the clinical environment (including the patient’s skin, surgical instruments, gloves and irritants) are also a concern.
Biofilm associated biomarkers
Although biofilms formed on implanted devices cannot be directly sampled, biofilm growth may produce unique molecules, or stimulate biofilm-specific host responses, that can be detected using standard methods. Antibodies may not be detectable during acute infections, but can be used to help with chronic BAI diagnosis. For example, alpha defensin, an antimicrobial peptide produced by the body to fight infection, has been found in the synovial fluid of infected hip and knee joints and shows good sensitivity and specificity for periprosthetic joint infection (PJI) [18•]. However, like antibody tests, alpha defensin is not biofilm specific.
Besides host factors, identifying biofilm-specific markers on bacterial cells will enable effective detection of biofilms. Since the discovery of biofilm-associated protein (Bap) in Staphylococcus aureus, Bap homologs have been found in many bacterial species. These proteins are present on bacterial surfaces and many are involved in biofilm formation [19] and chronic infection in mammary glands [20]. Recombinant subunits of Acinetobacter baumannii Bap has been shown to stimulate an immune response in mice [21]. However, bap was not detected in S. aureus isolates from patients with urinary tract infections (UTI) [22]. Further studies are needed to identify markers that are unique to bacterial biofilms, especially those present in multiple bacterial species.
In addition to cellular targets, biofilm matrix components provide potential markers of biofilms and may offer species identification. One of such possible biomarkers is cellulose, a major component of the biofilm matrix of uropathogenic Escherichia coli (UPEC). Antypas et al. [23•] developed an assay based on optotracing that can detect cellulose in urine in less than 45 min, which can help determine if biofilm is involved in UTI. Similarly, exopolysaccharide (EPS) is a possible marker for detecting biofilm as has been demonstrated for Histophilus somni biofilms [24]. Chronic lung infection by mucoid Pseudomonas aeruginosa biofilms leads to an IgG antibody response against P. aeruginosa components including alginate, lipopolysaccharides and proteins, which is currently used for diagnosis [25•]. Interestingly, many species of bacteria incorporate their own extracellular DNA (eDNA) into the matrix; and thus, cell-free DNA analysis [26] in combination with polysaccharide analysis [27] might provide evidence of biofilm and identify the species involved. Another possibility is to quantify biomass by measuring proteins in biofilms. For example, Guan et al. [28••] modified the o-phthalaldehyde (OPA) protein assay to achieve extraction-free detection of biofilms.
Quorum sensing (QS) is a well-known system found in numerous microbial species, which enables the cells to regulate cell density-dependent activities by sensing and responding to signaling molecules named autoinducers [29]. For lung infection by P. aeruginosa, QS signal profiling has been shown to be a potential biomarker for biofilm detection [30]. QS signals are also known to alter the level of immune factors and immune cell proliferation [31,32], which may help identify new biomarkers for biofilm detection. The chronic nature of BAIs indicates that host immunity is unable to eradicate biofilm cells. Thus, it is believed that the innate and acquired immune responses coexist [33•]. Deciphering the types and dynamics of immune factors involved in such infections may provide new biomarkers for better diagnosis.
Biofilm imaging
Laboratory methods for biofilm culturing, imaging and analysis have been well summarized in recent reviews [7•]. Confocal laser scanning microscopy (CLSM), fluorescence in situ hybridization (FISH), and later improved peptide nucleic acid (PNA)-FISH [34] and locked nucleic acids (LNA)-FISH [35], can provide spatial information about biofilms and the location of different species. But these advanced imaging techniques require a clear line of sight with the specimen, which are destructive and not applicable for in situ detection/diagnosis. Similar issues exist for scanning electron microscopy (SEM), time-of-flight secondary ion mass spectrometry (TOF-SIMS), transmission electron microscopy (TEM) and Mass Spectrometry Imaging (MSI).
In comparison, Raman spectroscopy and surface enhanced Raman spectroscopy (SERS) offer non-destructive molecular detection with high sensitivity [36•]. By detecting the photons that change energy when scattering off a material (Raman scattering), Raman spectroscopy is highly sensitive in detecting low-abundant biomolecules, e.g. 5 ppb of P. aeruginosa pyocyanin in sputa [37] and quorum sensing signals in P. aeruginosa biofilms in mice [38].
For preoperative and intraoperative guidance, imaging techniques that can ‘see’ through tissues into the body will arguably have the greatest medical utility. A useful tool for non-invasive biofilm detection is Near Infra-Red (NIR) imaging. Systems with such capabilities have been commercialized. For example, the Spectrum In Vivo Imaging System (IVIS) from PerkinElmer combines 2D optical imaging and 3D NIR tomography, achieving 3D tracking of bioluminescence and fluorescence signals. Combining IVIS with biofilm-specific markers may improve biofilm diagnoses.
Hyperspectral imaging is a label-free method that has been used in wound monitoring and to detect biofilms in the natural environment. This technology provides visible and near-IR spectra in a 2D image and may have future potential for detecting color changes associated with biofilms themselves or reaction of the host tissues to biofilms.
While signs of infection can be diagnosed from observing host tissues in X-rays, direct detection of biofilm will likely require a contrast agent. For example, with appropriate contrast agents such as iron sulfate, X-ray tomography can differentiate biofilm from surrounding water and allow 3D quantification of biofilm structures[39••]. Further improvement in biofilm characterization can be achieved using X-ray micro-force computed tomography (μCT), which has been used for non-destructive analysis of biofilm grown in central venous catheters (CVC) [40]. Sellmyer et al. [41] synthesized [18F] fluoropropyl-trimethoprim and showed it can label bacteria but not inflammatory or cancer cells in rodents, allowing direct visualization of infection using positron emission tomography imaging. Future work to improve sensitivity and reduce background signals from bowel and bone uptake may bring exciting opportunities for non-invasive imaging of biofilm infections.
Currently medical imaging modalities do not have the spatial resolution to detect biofilms which can exist as aggregates with a diameter of 10–100 μm diameter. If biofilm specific labels can be found, radiolabeling has the potential to increase sensitivity in signal detection and would also offer theranostic potential, in which localized imaging is combined with a therapeutic benefit [42].
Unconventional methods
Bio-impedance based sensing
Biofilm formation on an implant and associated inflammation can significantly alter the environment near the device surface and device-host tissue interface, which provides an opportunity for biofilm-specific sensing. One approach to characterize such changes is to use electric impedance, which represents the retardation of a circuit under alternating current/voltage applied at specific frequencies [43]. Previous improvements in instrumentation and data analysis [44,45] have made EIS a promising technology for biofilm detection.
Recently, a CMOS on-chip impedance analysis system was engineered using lithography. These devices are highly sensitive, compact, and can be reproduce data large scale by modern fabrication processes [46]. Single-frequency impedance spectroscopy has been developed as a label-free system for monitoring biofilm growth and treatment [43,47]. EIS could become a promising monitoring method in the future since it does not require sample preparation and can be done continuously without physically sampling the biofilm, a major advantage over many other methods.
Recent advances have also made it possible to integrate impedimetric sensors with existing electronic medical devices. The most important addition in such an integration is the electrode/probe, which has been demonstrated in the form of a microfluidic chip [48••]. This device combines real-time monitoring and a threshold activated treatment. It was later adapted by the same group into a wireless monitoring enabled urinary catheter [49]. It is worth noting that synergy between antibiotics and electrochemical treatment has been reported to have significant effect on killing biofilms including persister cells [50–52]. With the capability to combine treatment and monitoring, it is possible to engineer smart medical devices that can deliver on-demand control of biofilms.
Surface acoustic waves
Surface Acoustic Waves (SAW) are referred to a form of vibrational wave propagating through a solid material affected by its elasticity. Combing with Love Waves (LW), which travel across a surface, LW-SAW devices can provide label-free real-time monitoring of high-molecular weight molecules with high sensitivity [53].
LW-SAW sensors have been developed for early-detection of biofilm formation on surfaces with pg level sensitivity and real-time biofilm monitoring [54]. Using gold nanoparticles as a signal enhancer, LW-SAW can also be used to detect antigen at pg/mL level [55]. Such high sensitivity might be useful for detecting biofilm markers in the future.
A new device based on SAW was engineered recently, which can be clamped onto a liquid filled tube and act as an actuator to monitor soft layer deposition on the tube surface. This device is capable of distinguishing between a soft layer such as biofilm or a hard layer such as limescale in a metal tube [56]. This adaptable device has possible applications in piping inspection and medical catheter check-ups, demonstrating an alternative to integrating into devices. Instead of putting SAW sensor onto the surface of interest, it rather turns the said surface into a substrate for analysis. Besides sensing, SAW has also been used in biofilm treatment [57], presenting a future possibility to engineer smart devices.
Other approaches
With advancements in analytic biotechnology and device fabrication, researchers continuously push the boundary towards more sensitive and portable detection systems. For example, a disposable sensor consisting of a small segment of optical fiber and antibody surface coating developed for C-reactive protein sensing has a consistent linear response to the target protein between 0.01–20 μg/mL [58]. Meanwhile, Zhang et al. [59] reported the capability to monitor biofilm formation and treatment in real time using surface plasmon resonance waveguide mode. Cantilever technology can be used to form an array sensor, and achieve simultaneous detection of multiple targets of interest [60]. Although some of these are not directly applicable to biofilm monitoring in vivo, they have potential for biomarker detection and analysis of explanted medical devices. Future development in this field will also benefit from new technologies that combine molecular markers with advanced imaging [61•], and more in-depth understanding of biofilm physiology [62].
Conclusions
With the increasing challenges associated with microbial biofilms, there is an urgent need to develop the capability for non-destructive real-time detection of microbial biofilms. However, the patchy nature, size scale and lack of biofilm specific targets provide obstacles in meeting this goal. Conventional methods for microbial detection are largely based on culturing methods and the detection of immunoglobulin antibodies in the blood or other bodily fluids. Although these methods are effective in diagnosing acute infections, they commonly fail in diagnosing BAIs. Similar challenges exist in detecting microbes from explanted medical devices. Many of the future development will rely on the discovery of new biomarkers and engineering more sensitive detection systems. Most BAIs are culturing negative and involve multiple species, which require universal biomarkers rather than species-specific molecules for detection. It is also important to improve the knowledge of in vivo biofilm formation, which has significant differences than that in in vitro pure-culture systems. Understanding the dynamics and inter-species interactions will be essential for identifying the right bio-markers. Achieving effective biofilm detection also requires low-cost, easy-to-manufacture, portable/wearable/implantable devices that have a long-life span and require minimal maintenance. Addressing these challenges will bring exciting new technologies for safer medical devices and better healthcare.
Acknowledgements
The authors thank the U.S. National Science Foundation for an ERC planning grant (1936926), which facilitates discussions of future research on microbial control and partially led to this review article. PS is funded by the NIH R01GM124436. DR’s current research on catheters is supported by NIH 1R21AI142424-01.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Singh S, Singh SK, Chowdhury I, Singh R: Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol J 2017, 11:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebeaux D, Ghigo JM, Beloin C: Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev 2014, 78:510–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donlan RM: Biofilms: microbial life on surfaces. Emerg Infect Dis 2002, 8:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawlowski KS, Wawro D, Roland PS: Bacterial biofilm formation on a human cochlear implant. Otol Neurotol 2005, 26:972–975. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerli W, Sendi P: Orthopaedic biofilm infections. APMIS 2017, 125:353–364. [DOI] [PubMed] [Google Scholar]
- 6.Veerachamy S, Yarlagadda T, Manivasagam G, Yarlagadda PK: Bacterial adherence and biofilm formation on medical implants: a review. Proc Inst Mech Eng H 2014, 228:1083–1099. [DOI] [PubMed] [Google Scholar]
- 7•. Azeredo J, Azevedo NF, Briandet R, Cerca N, Coenye T, Costa AR, Desvaux M, Di Bonaventura G, Hébraud M, Jaglic Z et al. : Critical review on biofilm methods. Crit Rev Microbiol 2017, 43:313–351 [DOI] [PubMed] [Google Scholar]; Authors reviewed the traditional and cutting-edge methods for studying biofilms, from the measurement of biomass, viability, and composition, to the imaging and monitoring of cellular behaviors, covering a wide range of techniques for biofilm research.
- 8.Høiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, Hall-Stoodley L, Holá V, Imbert C, Kirketerp-Møller K et al. : ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 2015, 21(Suppl. 1):S1–25. [DOI] [PubMed] [Google Scholar]
- 9.Hall-Stoodley L, Stoodley P, Kathju S, Høiby N, Moser C, Costerton JW, Moter A, Bjarnsholt T: Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol Med Microbiol 2012, 65:127–145. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Renz N, Trampuz A: Management of periprosthetic joint infection. Hip Pelvis 2018, 30:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donlan RM, Costerton JW: Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002, 15:167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher RA, Gollan B, Helaine S: Persistent bacterial infections and persister cells. Nat Rev Microbiol 2017, 15:453–464. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Stojicic S, Haapasalo M: Bacterial viability in starved and revitalized biofilms: comparison of viability staining and direct culture. J Endod 2010, 36:1820–1823. [DOI] [PubMed] [Google Scholar]
- 14.Bjerkan G, Witsø E, Bergh K: Sonication is superior to scraping for retrieval of bacteria in biofilm on titanium and steel surfaces in vitro. Acta Orthop 2009, 80:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Zhang J, Ji Y: PCR-based approaches for the detection of clinical methicillin-resistant Staphylococcus aureus. Open Microbiol J 2016, 10:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Tsalik EL, Bonomo RA, Fowler VG: New molecular diagnostic approaches to bacterial infections and antibacterial resistance. Annu Rev Med 2018, 69:379–394 [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reviews new methods for molecular diagnostics, including both host-focused and pathogen-focused approaches. The review covers the technologies in development and those that have been commercialized.
- 17•.Quainoo S, Coolen JPM, van Hijum SAFT, Huynen MA, Melchers WJG, van Schaik W, Wertheim HFL: Whole-genome sequencing of bacterial pathogens: the future of nosocomial outbreak analysis. Clin Microbiol Rev 2017, 30:1015–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a review of conventional molecular approaches for characterizing bacterial strains, and various sequencing technologies that can be integrated with bioinformatic tools to achieve whole-genome sequencing based nosocomial outbreak analysis.
- 18•. Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE: The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res 2015, 473:2229–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports the use of alpha-defensin as a possible marker for diagnosing periprosthetic joint infection by a wide spectrum of microbes.
- 19.Lasa I, Penadés JR: Bap: a family of surface proteins involved in biofilm formation. Res Microbiol 2006, 157:99–107. [DOI] [PubMed] [Google Scholar]
- 20.Cucarella C, Tormo MA, Ubeda C, Trotonda MP, Monzón M, Peris C, Amorena B, Lasa I, Penadés JR: Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect Immun 2004, 72:2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noori E, Rasooli I, Owlia P, Mousavi Gargari SL, Ebrahimizadeh W: A conserved region from biofilm associated protein as a biomarker for detection of Acinetobacter baumannii. Microb Pathog 2014, 77:84–88. [DOI] [PubMed] [Google Scholar]
- 22.Yousefi M, Pourmand MR, Fallah F, Hashemi A, Mashhadi R, Nazari-Alam A: Characterization of Staphylococcus aureus biofilm formation in urinary tract infection. Iran J Public Health 2016, 45:485–493. [PMC free article] [PubMed] [Google Scholar]
- 23•.Antypas H, Choong FX, Libberton B, Brauner A, Richter-Dahlfors A: Rapid diagnostic assay for detection of cellulose in urine as biomarker for biofilm-related urinary tract infections. NPJ Biofilms Microbiomes 2018, 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a new method for rapid detection of uropathogenic Escherichia coli using cellulose as a biomaker of biofilms. The detection was achieved by using optotracing.
- 24.Pan Y, Fisher T, Olk C, Inzana TJ: Detection of antibodies to the biofilm exopolysaccharide of Histophilus somni following infection in cattle by enzyme-linked immunosorbent assay. Clin Vaccine Immunol 2014, 21:1463–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Høiby N, Bjarnsholt T, Moser C, Jensen P, Kolpen M, Qvist T, Aanaes K, Pressler T, Skov M, Ciofu O: Diagnosis of biofilm infections in cystic fibrosis patients. APMIS 2017, 125:339–343 [DOI] [PubMed] [Google Scholar]; This article summarizes the current methods for the diagnosis of bacterial infections in cystic fibrosis patients, with P.aeruginosa infections as a focus.
- 26.Nagler M, Insam H, Pietramellara G, Ascher-Jenull J: Extracellular DNA in natural environments: features, relevance and applications. Appl Microbiol Biotechnol 2018, 102:6343–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo Pedraza MC, Novais TF, Faustoferri RC, Quivey RG, Terekhov A, Hamaker BR, Klein MI: Extracellular DNA and lipoteichoic acids interact with exopolysaccharides in the extracellular matrix of Streptococcus mutans biofilms. Biofouling 2017, 33:722–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Guan A, Wang Y, Phillips KS: An extraction free modified o-phthalaldehyde assay for quantifying residual protein and microbial biofilms on surfaces. Biofouling 2018, 34:925–934 [DOI] [PubMed] [Google Scholar]; This article describes a modified o-phthalaldehyde (OPA) protein assay, which can quantify proteins in surface-attached biofilms without extraction. This has potential applications in biofilm detection in hard-to-reach areas such as those in endoscopes.
- 29.Li YH, Tian X: Quorum sensing and bacterial social interactions in biofilms. Sensors (Basel) 2012, 12:2519–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP: Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000, 407:762–764. [DOI] [PubMed] [Google Scholar]
- 31.Skindersoe ME, Zeuthen LH, Brix S, Fink LN, Lazenby J, Whittall C, Williams P, Diggle SP, Froekiaer H, Cooley M et al. : Pseudomonas aeruginosa quorum-sensing signal molecules interfere with dendritic cell-induced T-cell proliferation. FEMS Immunol Med Microbiol 2009, 55:335–345. [DOI] [PubMed] [Google Scholar]
- 32.Jensen P, Givskov M, Bjarnsholt T, Moser C: The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol 2010, 59:292–305. [DOI] [PubMed] [Google Scholar]
- 33•.Moser C, Pedersen HT, Lerche CJ, Kolpen M, Line L, Thomsen K, Høiby N, Jensen P: Biofilms and host response - helpful or harmful. APMIS 2017, 125:320–338 [DOI] [PubMed] [Google Scholar]; The article discusses various types of immune response and their interaction with biofilm infection, both acute and chronic; and the possibility of diagnosing biofilm infections by monitoring acquired immune response.
- 34.Azevedo NF, Vieira MJ, Keevil CW: Establishment of a continuous model system to study Helicobacter pylori survival in potable water biofilms. Water Sci Technol 2003, 47:155–160. [PubMed] [Google Scholar]
- 35.You Y, Moreira BG, Behlke MA, Owczarzy R: Design of LNA probes that improve mismatch discrimination. Nucleic Acids Res 2006, 34:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Nguyen CQ, Thrift WJ, Bhattacharjee A, Ranjbar S, Gallagher T, Darvishzadeh-Varcheie M, Sanderson RN, Capolino F, Whiteson K, Baldi P et al. : Longitudinal monitoring of biofilm formation via robust surface-enhanced Raman scattering quantification of Pseudomonas aeruginosa-produced metabolites. ACS Appl Mater Interfaces 2018, 10:12364–12373 [DOI] [PubMed] [Google Scholar]; This study reports a micorfluidic SERS device for quantitative detection of quorum sensing in P. pseudomonas biofilms, with a capability to detect 1 ng/mL of pyocyanin.
- 37.Wu X, Chen J, Li X, Zhao Y, Zughaier SM: Culture-free diagnostics of Pseudomonas aeruginosa infection by silver nanorod array based SERS from clinical sputum samples. Nanomed: Nanotechnol Biol Med 2014, 10:1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodelón G, Montes-García V, López-Puente V, Hill EH, Hamon C, Sanz-Ortiz MN, Rodal-Cedeira S, Costas C, Celiksoy S, Pérez-Juste I et al. : Detection and imaging of quorum sensing in Pseudomonas aeruginosabiofilmcommunitiesbysurface-enhancedresonance Raman scattering. Nat Mater 2016, 15:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Carrel M, Beltran MA, Morales VL, Derlon N, Morgenroth E, Kaufmann R, Holzner M: Biofilm imaging in porous media by laboratory X-Ray tomography: combining a non-destructive contrast agent with propagation-based phase-contrast imaging tools. PLoS One 2017, 12:e0180374. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors reported a new contrast agent (iron sulfate) for X-ray tomography that is non-reactive compared to conventional contrast agents. This new contrast agent is integrated into the extracellular matrix during biofilm growth, enabling biofilm imaging in opaque porous media.
- 40.Niehaus WL, Howlin RP, Johnston DA, Bull DJ, Jones GL, Calton E, Mavrogordato MN, Clarke SC, Thurner PJ, Faust SN et al. : Development of X-ray micro-focus computed tomography to image and quantify biofilms in central venous catheter models in vitro. Microbiology 2016, 162:1629–1640. [DOI] [PubMed] [Google Scholar]
- 41.Sellmyer MA, Lee I, Hou C, Weng CC, Li S, Lieberman BP, Zeng C, Mankoff DA, Mach RH: Bacterial infection imaging with. Proc Natl Acad Sci U S A 2017, 114:8372–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine R, Krenning EP: Clinical history of the theranostic radionuclide approach to neuroendocrine tumors and other types of cancer: historical review based on an interview of EricP. Krenning by Rachel Levine. J Nucl Med 2017, 58:3S–9S. [DOI] [PubMed] [Google Scholar]
- 43.van Duuren JBJH, Müsken M, Karge B, Tomasch J, Wittmann C, Haüssler S, Brönstrup M: Use of single-frequency impedance spectroscopy to characterize the growth dynamics of biofilm formation in Pseudomonas aeruginosa. Sci Rep 2017, 7:5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L, Xu Y, Cui F, Xia Y, Chen L, Mou X, Lv J: Monitoring of bacteria biofilms forming process by in-situ impedimetric biosensor chip. Biosens Bioelectron 2018, 112:86–92. [DOI] [PubMed] [Google Scholar]
- 45.Furst AL, Francis MB: Impedance-based detection of bacteria. Chem Rev 2019, 119:700–726. [DOI] [PubMed] [Google Scholar]
- 46.Yu H, Yan M, Huang X: CMOS Impedance Sensor. CMOS Integrated Lab-on-a-Chip System for Personalized Biomedical Diagnosis. 2018:60–75. [Google Scholar]
- 47.Gutiérrez D, Hidalgo-Cantabrana C, Rodríguez A, García P, Ruas-Madiedo P: Monitoring in real time the formation and removal of biofilms from clinical related pathogens using an impedance-based technology. PLoS One 2016, 11:e0163966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••. Subramanian S, Tolstaya EI, Winkler TE, Bentley WE, Ghodssi R: An integrated microsystem for real-time detection and threshold-activated treatment of bacterial biofilms. ACS Appl Mater Interfaces 2017, 9:31362–31371 [DOI] [PubMed] [Google Scholar]; This study combined impedance-based detection and bioelectric treatment in one integrated chip targeting E. coli K-12 biofilms.
- 49.Huiszoon RC, Subramanian S, Rajasekaran PR, Beardslee LA, Bentley WE, Ghodssi R: Flexible platform for in situ impedimetric detection and bioelectric effect treatment of Escherichia coli biofilms. IEEE Trans Biomed Eng 2019, 66:1337–1345. [DOI] [PubMed] [Google Scholar]
- 50.Freebairn D, Linton D, Harkin-Jones E, Jones DS, Gilmore BF, Gorman SP: Electrical methods of controlling bacterial adhesion and biofilm on device surfaces. Expert Rev Med Devices 2013, 10:85–103. [DOI] [PubMed] [Google Scholar]
- 51.Niepa TH, Gilbert JL, Ren D: Controlling Pseudomonas aeruginosa persister cells by weak electrochemical currents and synergistic effects with tobramycin. Biomaterials 2012, 33:7356–7365. [DOI] [PubMed] [Google Scholar]
- 52.Sultana ST, Call DR, Beyenal H: Eradication of Pseudomonas aeruginosa biofilms and persister cells using an electrochemical scaffold and enhanced antibiotic susceptibility. NPJ Biofilms Microbiomes 2016, 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puiu M, Gurban A-M, Rotariu L, Brajnicov S, Viespe C, Bala C: Enhanced sensitive love wave surface acoustic wave sensor designed for immunoassay formats. Sensors (Basel, Switzerland) 2015, 15:10511–10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim YW, Meyer MT, Berkovich A, Subramanian S, Iliadis AA, Bentley WE, Ghodssi R: A surface acoustic wave biofilm sensor integrated with a treatment method based on the bioelectric effect. Sens Actuators A: Phys 2016, 238:140–149. [Google Scholar]
- 55.Li S, Wan Y, Su Y, Fan C, Bhethanabotla VR: Gold nanoparticle-based low limit of detection Love wave biosensor for carcinoembryonic antigens. Biosens Bioelectron 2017, 95:48–54. [DOI] [PubMed] [Google Scholar]
- 56.Tietze S, Singer F, Lasota S, Ebert S, Landskron J, Schwuchow K, Drese KS, Lindner G: Monitoring of soft deposition layers in liquid-filled tubes with guided acoustic waves excited by clamp-on transducers. Sensors 2018, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Teng F, Yang X, Guo X, Tu J, Zhang C, Zhang D: Preventing microbial biofilms on catheter tubes using ultrasonic guided waves. Sci Rep 2017, 7:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang W, Mai Z, Chen Y, Wang J, Li L, Su Q, Li X, Hong X: A label-free fiber optic SPR biosensor for specific detection of C-reactive protein. Sci Rep 2017, 7:16904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang P, Guo J-S, Yan P, Chen Y-P, Wang W, Dai Y-Z, Fang F, Wang G-X, Shen Y: Dynamic dispersal of surface layer biofilm induced by nanosized TiO2 based on surface Plasmon resonance and waveguide. Appl Environ Microbiol 2018, 84: e00047–00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng F, Wang P, Du Q, Chen Y, Liu N: Simultaneous and ultrasensitive detection of foodborne bacteria by gold nanoparticles-amplified microcantilever array biosensor. Front Chem 2019, 7:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Akram AR, Avlonitis N, Scholefield E, Vendrell M, McDonald N, Aslam T, Craven TH, Gray C, Collie DS, Fisher AJ et al. : Enhanced avidity from a multivalent fluorescent antimicrobial peptide enables pathogen detection in a human lung model. Sci Rep 2019, 9:8422. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes a new system for high resolution imaging of pathogen detection in explanted human lungs.
- 62.Wan N, Wang H, Ng CK, Mukherjee M, Ren D, Cao B, Tang YJ: Bacterial Metabolism During Biofilm Growth Investigated by 13C Tracing. Front Microbiol 2018, 9:2657. [DOI] [PMC free article] [PubMed] [Google Scholar]