Abstract
Background:
This study examined the 6-month follow-up outcomes of the Transdiagnostic Sleep and Circadian Intervention (TranS-C), compared to Psychoeducation about sleep and health (PE).
Methods:
Adolescents (mean [SD] = 14.77 [1.84] years) with eveningness chronotype and “at-risk” in at least one of five health domains were randomized to receive TranS-C (n = 89) or PE (n = 87) at a university-based clinic. Primary outcomes were average weeknight total sleep time and bedtime calculated from sleep diary, a questionnaire measure of circadian preference, and composite risks in five health domains. Secondary outcomes were selected sleep diary indices, sleepiness, and self- and parent-reported sleep, parent-reported risks in five health domains.
Results:
Relative to PE, TranS-C showed treatment effects through 6-month follow-up on only one primary outcome; namely eveningness circadian preference. TranS-C also showed treatment effects on two sleep and circadian secondary outcomes, including the Pittsburgh Sleep Quality Index and sleep-diary measured weeknight-weekend discrepancy in wakeup time. TranS-C did not show treatment effects on self-report or parent-report composite risks in five health domains. PE showed benefit, relative to TranS-C, from posttreatment to 6-month follow-up for reducing parent-reported behavioral health risk (secondary outcome).
Conclusions:
In at-risk adolescents, the evidence supports the TranS-C treatment effects over six months on improving sleep and circadian functioning on selected outcomes but not on reducing risk in five health domains.
Keywords: Adolescents, transdiagnostic, psychopathology, eveningness, sleep, circadian
Introduction
The delayed sleep-wake pattern emerges during adolescence and remains stable until early adulthood (Roenneberg et al., 2004). Adolescents with eveningness chronotype, compared to those with morningness chronotype, follow a delayed sleep schedule, with later bedtime and wakeup time and increased activities later in the day. Eveningness chronotype is associated with greater sleep problems in adolescents, most notably sleep deprivation and greater weekday-weekend discrepancy in sleep (Crowley, Acebo, & Carskadon, 2007; Gradisar et al., 2013), and a wide range of psychological and physical problems, including depression, anxiety, substance use, and physical inactivity (Adan et al., 2012; Schaal, Peter, & Randler, 2010). Indeed, mounting evidence suggests that sleep deprivation and weekday-weekend discrepancy in sleep are prevalent during adolescence and confer more risk for psychopathology and other health problems (Owens, 2014). In light of this evidence, the Transdiagnostic Sleep and Circadian Intervention (TranS-C) for youth was developed to target modifiable psychosocial, cognitive and behavioral factors thought to contribute to eveningness (Harvey, 2016).
In the randomized controlled trial, for which the 6-month follow-up data are reported in this study, we tested whether TranS-C modifies eveningness, improves sleep, and reduces risk in health-related domains from pretreatment to posttreatment. From pretreatment to posttreatment, relative to Psychoeducation (PE), TranS-C was associated with shifting away from eveningness, an earlier endogenous circadian phase, a less weeknight-weekend discrepancy in total sleep time and wakeup time, less daytime sleepiness, and better self- and parent-reported sleep. TranS-C did not directly affect primary and secondary outcomes in health-related domains relative to PE, except for parent-reported risk in the cognitive domain (Harvey et al., 2018). However, a mediation analysis suggested indirect effects of TranS-C (vs. PE) on reducing risk in multiple mental and physical health domains via improving sleep and circadian problems from pretreatment to posttreatment (Dong, Gumport, Martinez, & Harvey, 2019). Although these pretreatment to posttreatment effects of TranS-C are promising, longer-term effects have not been examined.
The goal of the current study is to investigate the longer-term effects of TranS-C relative to PE. The first aim was to examine the effects of TranS-C (vs. PE) on sleep and circadian outcomes at 6-month follow-up, and whether they were maintained from posttreatment to 6-month follow-up. The second aim was to examine the effects of TranS-C (vs. PE) on risks in five health domains at 6-month follow-up, and whether they were maintained from posttreatment to 6-month follow-up. We also conducted exploratory analysis for specific measures comprising the composite risk scores to understand the TranS-C effects further and be consistent with the previous report on the pre-post effects (Harvey et al., 2018). We hypothesized that TranS-C would demonstrate greater improvements than PE in both sleep and circadian outcomes and risks across health domains at 6-month follow-up and that TranS-C treatment effects would be maintained from posttreatment to 6-month follow-up.
Methods
Participants and procedures
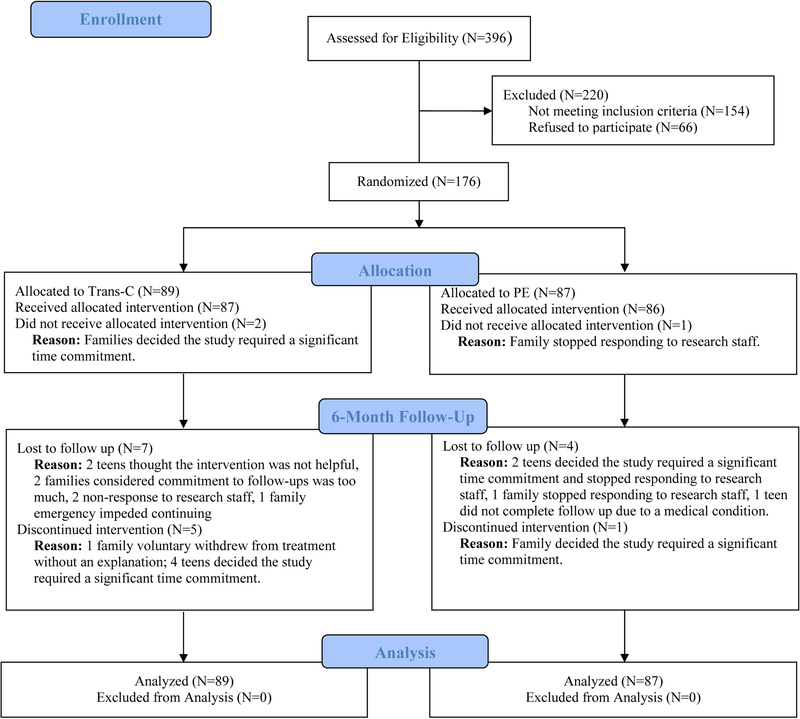
A parallel RCT design was used and CONSORT RCT requirements for nonpharmacological trials were followed (see Appendix S1 in the Supporting Information for CONSORT checklist). Participants were recruited from January 2013 to February 2016 through advertisements and clinician referrals. Participants and their parents or guardians were first screened via phone for eligibility, with potentially eligible individuals completing a subsequent in-person assessment to ascertain eligibility. Eligible participants were randomized to receive TranS-C or PE. Randomization was conducted using a computerized random number generator and was stratified by sex and age. Only the project coordinators in charge of randomization and therapists knew the treatment allocation of each participant. The Committee for Protection of Human Subjects at University of California Berkeley approved the study protocol. Parents or guardians of all participants provided informed consent and participants provided informed assent. A detailed study procedure was reported elsewhere (Harvey et al., 2018). Participant flow was illustrated in Figure 1.
Figure 1.
CONSORT Diagram Illustrating the Flow of Participants Through the Study
Note. 6-month follow-up assessment was completed 6 months after randomization.
Primary outcomes of the trial are weeknight TST, weeknight BT, CMEP, and youth self-report risk composites in the five health domains. Secondary outcomes are weeknight-weekend discrepancy in TST, BT, and wakeup time, sleepiness, PSQI, CBCL Sleep Composite, and parent-report risk composites in the five health domains. These outcomes were pre-registered on clinicaltrials.gov and tested in the previous report on the pre-post effects (Harvey et al., 2018).
Inclusion criteria were: (a) 10- to 18-years old, living with a parent or guardian, and attending a class/job by 9 a.m. at least 3 days per week; (b) fluent in English; (c) able and willing to give informed assent; (d) reported eveningness as demonstrated by scoring within the lowest quartile of the CMEP (27 or lower; Dagys et al., 2012) and had a 7-day sleep diary showing a sleep onset time of 10:40 p.m. or later for 10–13-year-olds, 11 p.m. or later for 14–16-year-olds, and 11:20 p.m. or later for 17–18-year-olds for at least 3 nights per week in the last 3 months1; and (e) participants must fall into an ‘at risk’ range on measures in at least one of the five health domains (see Appendix S2).
Exclusion criteria were: (a) an active, progressive physical illness or neurological degenerative disease directly related to the sleep disturbance; (b) evidence of obstructive sleep apnea, restless legs syndrome, or periodic limb movement disorder via the Duke Structured Interview for Sleep Problems; (c) significantly impairing pervasive developmental disorder; (d) bipolar disorder, schizophrenia, or another current Axis I disorder if there was a risk of harm if treatment was delayed (assessed via the K-SADS-PL); (e) history of substance dependence in the past six months or current suicide risk (assessed via the K-SADS-PL) sufficient to preclude treatment on an outpatient basis; and (f) Participants ceased taking medications that alter sleep (e.g., hypnotics) 4 weeks prior to the assessment (2 weeks for melatonin) or were excluded.
Treatment conditions
Both treatments consisted of a total of six 50-minute sessions delivered over six weeks during the school year by doctoral or masters-level therapists. A key difference between treatment conditions is that TranS-C promotes behavior change whereas PE only provides information without facilitating behavior change, and this treatment differentiation has been confirmed by coding. Full details about the treatment conditions are reported elsewhere (Harvey et al., 2018).
Transdiagnostic Sleep and Circadian Intervention for Youth (TranS-C).
As documented elsewhere (Harvey, 2016; Harvey & Buysse, 2017), TranS-C was developed based on sleep and circadian principles and the transdiagnostic approach. There are four sources for TranS-C: Cognitive Behavior Therapy for Insomnia (Morin et al., 2006; Perlis, Aloia, & Kuhn, 2011), Interpersonal and Social Rhythm Therapy (Frank et al., 2005), Chronotherapy (Wirz-Justice, Benedetti, & Terman, 2009), and Motivational Interviewing (Miller & Rollnick, 2002). TranS-C targets psychosocial, behavioral, and cognitive processes that maintain sleep and circadian problems in youth. TranS-C includes 4 cross-cutting modules (functional analysis, goal setting, motivational interviewing, education), 4 core modules (behavioral components, daytime impairment, unhelpful beliefs, relapse prevention), and 7 optional modules (e.g., bedtime worry).
Psychoeducation (PE).
PE is an active control associated with short- and long-term sleep improvement (Harvey et al., 2015). PE sessions focus on providing information about the interplay between sleep, stress, diet, health, exercise, accidents, and mood. Participants sample through meditation, yoga, and/or outdoor appreciation activities.
Measures
All measures described below were collected at pretreatment, posttreatment, and 6-month follow-up assessment and assessors were blind to treatment allocations.
Sleep and Circadian Outcomes
Sleep diary.
7-day sleep diary was collected every morning via phone by trained research assistants during the week leading up to treatment, the week after treatment, and the week leading up to the 6-month follow-up assessment. A standardized sleep diary was used (Carney et al., 2012). The following variables were derived using the sleep diary: 1) weeknight total sleep time (TST), calculated as time in bed - sleep onset latency - wake after sleep onset - terminal wakefulness; 2) weeknight bedtime (BT), using response to “time getting into bed”; 3) weeknight-weekend discrepancy in TST; 4) weeknight-weekend discrepancy in bedtime; and 5) weeknight-weekend discrepancy in wakeup time (time of final awakening).
Children’s Morningness-Eveningness Preference Scale (CMEP)
(Carskadon, Vieira, & Acebo, 1993). CMEP is a self-report measure of the degree of eveningness. The scores range from 10 (Extreme Evening Preference) to 43 (Extreme Morning Preference). Morningness-eveningness is subject to change in adults (Vedaa, Bjorvatn, Magerøy, Thun, & Pallesen, 2013) and CMEP scores decrease as children age (Díaz-Morales, 2015).
Sleepiness scale.
The Sleepiness Scale consists of ten items from the School Sleep Habits Survey asking about sleepiness in the past two weeks (Wolfson & Carskadon, 1998). Items were rated on a 4-point scale (0 = No, 1 = Struggled to Stay Awake, 2 = Fallen Asleep, 3 = Both Struggled to Stay Awake and Fallen Asleep).
Pittsburgh Sleep Quality Index (PSQI).
The PSQI consists of 19 self-reported questionnaire generating a global score (Buysse et al., 1989). The score ranges from 0 to 21, with higher scores indicating greater sleep problems in the past month.
Child Behavior Checklist (CBCL) sleep composite.
A CBCL sleep composite was derived based on seven items on the parent-report CBCL that measure sleep functioning (Becker, Ramsey, & Byars, 2015). Items were rated on a 3-point scale (0 = Not True, 1 = Somewhat/Sometimes True, 2 = Very True/Often True). Higher scores indicate more sleep problems.
Functioning in five health domains.
Two sets of composite scores were used to indicate functioning or risk in five health domains, namely, youth self-reported composite risk scores and parent-reported composite risk scores. Both sets of composite scores were composed of measures of emotional, cognitive, behavioral, social, and physical health. The Youth Self-Report Composite Risk Scores were derived from psychometrically validated questionnaires representing the five health domains (see Appendix S3). The Parent-Reported Composite Risk Scores are derived using parent responses to the CBCL. Further details on specific measures for each domain are in Appendix S3.
Data analysis
A priori power analysis based on two independent groups t-test yielded 69 participants per condition to achieve at least 80% power, assuming two-sided significance of 0.05 and an expected effect size d = 0.48, which was derived by averaging across estimates of the treatment effects on sleep duration and outcomes in the five health domains (Cappuccio et al., 2008; Dewald, Meijer, Oort, Kerkhof, & Bögels, 2010; Talbot, McGlinchey, Kaplan, Dahl, & Harvey, 2010). The recruitment allowed for at least 20% more for potential attrition. The final sample size for the analysis was 176.
Data analysis was conducted in Stata 15. All analyses were adjusted by age and sex, which were the stratification factors used during the randomization. Intent-to-treat method was used. Multilevel modeling with maximum likelihood estimation with the assumption of missing at random was used to examine all continuous variables. We used multilevel modeling because it ensures that the analysis includes all randomized participants and ensures an unbiased approach to deal with attrition through maximum likelihood estimation with the assumption of missing at random (Enders & Bandalos, 2001). The fixed component of the model included stratification factors (age and sex), indicator variables for time (pretreatment = 0, posttreatment = 1, 6-month follow-up = 2) and treatment (TranS-C = 1, PE = 0), and time by treatment interaction terms. The random part of the model included a subject-specific random intercept and a subject- and occasion-specific error term. Benjamini-Hochberg procedure (Benjamini & Hochberg, 1995) was used to correct for multiple comparisons assuming 15% false discovery rate for four subgroups of analyses: effects of TranS-C vs. PE on change from pre to 6 months follow-up for primary outcomes (1) and secondary outcomes (2), and effects of TranS-C vs. PE on change from post to 6 months follow-up for primary (3) and secondary outcomes (4). The Benjamini-Hochberg critical values are report in Table 2.
Table 2.
Results from Multilevel Modeling for All Outcome Variables.
| Treatment effect on outcome from pretreatment to 6-month follow-up (treatment by time interaction term) | Treatment effect on outcome from posttreatment to 6-month follow-up (treatment by time interaction term) | |||||||
|---|---|---|---|---|---|---|---|---|
| Coef. | SE | p | B-H critical value | Coef. | SE | p | B-H critical value | |
| Sleep and Circadian Outcomes | ||||||||
| TST weeknights* | −0.75 | 11.62 | 0.95 | 0.15 | −13.73 | 11.83 | 0.25 | 0.08 |
| BT weeknights* | 0.20 | 0.16 | 0.21 | 0.06 | 0.24 | 0.16 | 0.14 | 0.04 |
| CMEP* | 1.84 | 0.67 | 0.006 | 0.02 | −0.16 | 0.69 | 0.83 | 0.13 |
| TST weeknight-weekend discrepancy | 18.30 | 23.47 | 0.44 | 0.10 | −29.73 | 24.00 | 0.22 | 0.05 |
| BT weeknight-weekend discrepancy | 0.38 | 0.29 | 0.19 | 0.07 | 0.34 | 0.29 | 0.25 | 0.07 |
| WUP weeknight-weekend discrepancy | 0.72 | 0.29 | 0.013 | 0.014 | 0.02 | 0.29 | 0.95 | 0.15 |
| Sleepiness | −1.08 | 0.73 | 0.14 | 0.05 | 0.61 | 0.76 | 0.43 | 0.10 |
| Pittsburgh Sleep Quality Index | −1.09 | 0.48 | 0.02 | 0.03 | −0.10 | 0.49 | 0.84 | 0.14 |
| CBCL Sleep Composite | −0.06 | 0.30 | 0.85 | 0.15 | 0.70 | 0.30 | 0.019 | 0.01 |
| Youth Self-Report Composite Risk Score* | ||||||||
| Emotional health: | ||||||||
| Children’s Depression Rating Scale | −1.22 | 1.36 | 0.37 | −0.68 | 1.38 | 0.64 | ||
| Multidimensional Anxiety Scale for Children | 1.41 | 2.18 | 0.52 | 2.29 | 2.20 | 0.30 | ||
| Composite* | −0.02 | 0.10 | 0.82 | 0.09 | −0.01 | 0.10 | 0.92 | 0.15 |
| Cognitive health: | ||||||||
| Attention Control Scale | −1.31 | 1.17 | 0.26 | −2.59 | 1.20 | 0.03 | ||
| YSAS (school/cognitive) | −0.27 | 0.60 | 0.65 | 0.28 | 0.62 | 0.67 | ||
| Composite* | 0.01 | 0.12 | 0.92 | 0.13 | 0.17 | 0.13 | 0.16 | 0.06 |
| Behavioral health: | ||||||||
| Sensation Seeking Scale | −0.26 | 0.70 | 0.71 | 0.38 | 0.72 | 0.61 | ||
| Alcohol and Substance Use | 0.50 | 0.64 | 0.43 | 0.53 | 0.65 | 0.42 | ||
| Composite* | 0.01 | 0.07 | 0.83 | 0.11 | 0.07 | 0.07 | 0.32 | 0.11 |
| Social health: | ||||||||
| YSAS: Friends | 0.39 | 0.69 | 0.57 | 1.19 | 0.70 | 0.09 | ||
| YSAS: Family | 0.85 | 0.56 | 0.13 | 0.79 | 0.57 | 0.17 | ||
| YSAS: Romantic | 0.12 | 0.40 | 0.77 | −0.02 | 0.40 | 0.96 | ||
| Composite* | 0.16 | 0.11 | 0.15 | 0.04 | 0.17 | 0.11 | 0.11 | 0.02 |
| Physical health: | ||||||||
| Modifiable Activity Questionnaire | 4.97 | 5.85 | 0.40 | 5.19 | 5.99 | 0.39 | ||
| Physical Health Questionnaire | −0.09 | 0.69 | 0.89 | −0.26 | 0.68 | 0.72 | ||
| Composite* | −0.13 | 0.11 | 0.24 | 0.08 | −0.13 | 0.11 | 0.26 | 0.09 |
| Parent-Reported Composite Risk Score | ||||||||
| Emotional Health | ||||||||
| Anxious/Depressed | 0.47 | 0.44 | 0.29 | 0.49 | 0.45 | 0.27 | ||
| Withdrawn/Depressed | −0.04 | 0.37 | 0.92 | 0.04 | 0.37 | 0.92 | ||
| Composite | 0.06 | 0.11 | 0.57 | 0.11 | 0.08 | 0.12 | 0.50 | 0.11 |
| Cognitive Health | ||||||||
| Thought problems | −0.23 | 0.35 | 0.51 | 0.79 | 0.35 | 0.02 | ||
| Attention problems | 0.01 | 0.44 | 0.98 | 0.37 | 0.44 | 0.40 | ||
| Composite | −0.04 | 0.10 | 0.67 | 0.14 | 0.19 | 0.10 | 0.053 | 0.04 |
| Behavioral Health | ||||||||
| Rule-Breaking Behavior | 0.30 | 0.32 | 0.34 | 1.01 | 0.32 | 0.00 | ||
| Aggressive Behavior | 0.93 | 0.49 | 0.06 | 0.35 | 0.49 | 0.49 | ||
| Composite | 0.18 | 0.11 | 0.10 | 0.04 | 0.26 | 0.11 | 0.023 | 0.03 |
| Social Health | ||||||||
| Social Problems | 0.15 | 0.28 | 0.58 | 0.32 | 0.28 | 0.26 | ||
| Composite | 0.08 | 0.14 | 0.58 | 0.12 | 0.16 | 0.14 | 0.26 | 0.08 |
| Physical Health | ||||||||
| Somatic Complaints | −0.34 | 0.38 | 0.37 | −0.10 | 0.38 | 0.81 | ||
| Composite | −0.13 | 0.14 | 0.37 | 0.08 | −0.04 | 0.14 | 0.81 | 0.12 |
Note.
and bold indicate primary outcomes of the trial. B-H = Benjamin-Hochberg procedure. The p-values in bold remain significant after the Benjamini-Hochberg procedure (< B-H critical value). All models adjusted for age and sex. TST = total sleep time; BT = bedtime. WUP = wakeup time. Sleepiness = Sleepiness subscale from School Sleep Habits Survey. CBCL = Child Behavior Checklist (parent-report). CMEP = Children’s Morningness–Eveningness Preferences. YSAS = Youth Social Adjustment Scale.
Results
Demographic and descriptive statistics
Table S1 presents demographic variables. Table 1 presents the descriptive statistics of all outcome variables. Additional summary statistics (N for each variable, median and range for skewed outcomes) are presented in Table S2. Attrition was not significantly different across treatment groups at post treatment (TranS-C: 6 [6.7%], PE: 4 [4.6%], z = .60, p = .54) or at 6-month follow-up (TranS-C: 9 [10.0%], PE: 5 [5.7%], z = 1.10, p = .28). The two treatment groups did not differ with respect to season in which treatment was provided (X2 = 0.75, p = 0.69) or at 6-month follow-up (X2 = 0.81, p = 0.67).
Table 1.
Descriptive Statistics of Outcome Variables
| TranS-C | PE | |||||
|---|---|---|---|---|---|---|
| Pretreatment | Posttreatment | 6-month follow-up | Pretreatment | Posttreatment | 6-month follow-up | |
| Outcome | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Sleep and Circadian Outcomes | ||||||
| TST weeknights (min)* | 459.06 (64.92) | 482.76 (82.55) | 436.63 (58.15) | 454.96 (61.51) | 464.81 (76.01) | 430.57 (60.63) |
| BT weeknights (hr:min)* | 22:52 (1:04) | 22.51 (0:59) | 22:59 (1:07) | 22:59 (1:03) | 23:02 (1:07) | 22:56 (0:56) |
| CMEP* | 21.09 (3.76) | 25.08 (4.86) | 25.33 (4.77) | 21.52 (3.86) | 23.44 (4.82) | 23.93 (4.32) |
| TST weeknight-weekend discrepancy | −70.39 (113.10) | −31.16 (115.19) | −74.21 (70.71) | −48.91 (89.28) | −56.46 (106.25) | −70.98 (133.91) |
| BT weeknight-weekend discrepancy | −0.79 (1.23) | −0.68 (1.41) | −0.39 (0.98) | −0.58 (1.13) | −0.51 (1.09) | −0.55 (1.89) |
| WUP weeknight-weekend discrepancy | −1.91 (1.36) | −1.13 (1.29) | −1.55 (1.19) | −1.42 (1.28) | −1.32 (1.55) | −1.78 (1.38) |
| Sleepiness | 6.20 (4.52) | 4.67 (3.98) | 3.56 (4.02) | 6.15 (4.01) | 6.37 (4.71) | 4.80 (4.84) |
| Pittsburgh Sleep Quality Index | 7.58 (2.99) | 5.85 (2.56) | 4.88 (3.03) | 7.58 (3.03) | 6.75 (3.48) | 6.00 (3.97) |
| CBCL Sleep Composite | 3.32 (2.03) | 1.84 (1.86) | 2.10 (2.14) | 3.24 (2.13) | 2.54 (1.92) | 2.11 (1.95) |
| Youth Self-Report Composite Risk Score* | ||||||
| Emotional health | ||||||
| Children’s Depression Rating Scale | 33.90 (9.34) | 27.01 (8.72) | 25.87 (7.68) | 33.08 (9.90) | 27.00 (8.16) | 26.63 (10.08) |
| Multidimensional Anxiety Scale for Children | 46.51 (17.73) | 45.45 (17.10) | 42.28 (20.28) | 45.98 (15.99) | 44.74 (18.03) | 40.63 (18.18) |
| Composite* | 0.36 (0.90) | −0.07 (0.79) | −0.24 (0.82) | 0.28 (0.81) | −0.11 (0.79) | −0.25 (0.89) |
| Cognitive health | ||||||
| Attention Control Scale | 50.56 (8.23) | 52.18 (8.09) | 51.25 (8.67) | 51.24 (7.22) | 51.29 (7.77) | 52.70 (10.10) |
| YSAS (school/cognitive) | 11.68 (2.95) | 11.69 (3.14) | 10.21 (3.65) | 11.90 (2.83) | 12.49 (2.94) | 10.68 (3.72) |
| Composite* | 0.10 (0.79) | −0.001 (0.82) | −0.18 (0.75) | 0.07 (0.65) | 0.17 (0.80) | −0.19 (1.00) |
| Behavioral health | ||||||
| Sensation Seeking Scale | 27.28 (5.97) | 27.35 (6.61) | 26.50 (6.45) | 26.36 (6.22) | 27.51 (7.04) | 25.96 (6.65) |
| Alcohol and Substance Use | 5.76 (8.24) | 5.51 (8.10) | 5.60 (7.93) | 5.67 (6.62) | 6.26 (8.37) | 5.46 (7.68) |
| Composite* | 0.03 (0.80) | 0.02 (0.83) | −0.03 (0.79) | −0.04 (0.77) | 0.09 (0.92) | −0.08 (0.85) |
| Social health | ||||||
| YSAS: Friends | 18.53 (4.58) | 17.73 (3.69) | 17.22 (4.21) | 18.81 (4.98) | 18.68 (4.82) | 17.12 (4.94) |
| YSAS: Family | 11.92 (3.50) | 11.33 (3.56) | 10.97 (3.29) | 12.34 (3.67) | 11.68 (4.17) | 10.74 (3.70) |
| YSAS: Romantic | 7.34 (2.03) | 7.62 (1.78) | 6.79 (2.41) | 7.59 (1.69) | 7.62 (1.85) | 6.80 (2.36) |
| Composite* | 0.06 (0.59) | −0.0001 (0.60) | −0.20 (0.62) | 0.18 (0.70) | 0.11 (0.68) | −0.22 (0.72) |
| Physical health | ||||||
| Modifiable Activity Questionnaire | 3.36 (5.35) | 4.20 (8.22) | 5.31 (8.78) | 2.83 (4.31) | 3.40 (5.11) | 3.58 (3.97) |
| Physical Health Questionnaire | 9.30 (5.37) | 7.97 (5.01) | 6.42 (4.88) | 8.58 (4.40) | 7.01 (4.33) | 6.00 (5.09) |
| Composite* | 0.20 (0.68) | 0.01 (0.87) | −0.25 (0.93) | 0.18 (0.58) | −0.02 (0.57) | −0.15 (0.63) |
| Parent-Reported Composite Risk Score | ||||||
| Emotional Health | ||||||
| Anxious/Depressed | 3.13 (3.48) | 2.61 (2.97) | 2.79 (3.22) | 4.11 (3.78) | 3.61 (3.56) | 3.36 (2.88) |
| Withdrawn/Depressed | 2.83 (2.84) | 2.49 (2.54) | 2.57 (2.88) | 3.14 (2.77) | 2.99 (2.72) | 3.09 (2.68) |
| Composite | −0.03 (0.94) | −0.17 (0.80) | −0.13 (0.92) | 0.17 (0.93) | 0.07 (0.89) | 0.05 (0.83) |
| Cognitive Health | ||||||
| Thought problems | 3.56 (2.59) | 2.38 (2.31) | 2.57 (2.78) | 3.75 (2.73) | 3.60 (2.90) | 2.92 (2.63) |
| Attention problems | 4.23 (3.61) | 4.01 (3.85) | 4.03 (3.86) | 4.17 (4.13) | 4.33 (4.30) | 4.07 (4.26) |
| Composite | 0.08 (0.80) | −0.16 (0.81) | −0.12 (0.90) | 0.11 (0.89) | 0.10 (0.96) | −0.05 (0.91) |
| Behavioral Health | ||||||
| Rule-Breaking Behavior | 1.91 (2.31) | 1.39 (1.87) | 1.97 (2.31) | 1.98 (2.16) | 2.31 (2.61) | 1.85 (2.33) |
| Aggressive Behavior | 3.84 (4.02) | 3.62 (4.22) | 3.84 (4.32) | 4.54 (4.52) | 3.76 (3.73) | 3.69 (3.85) |
| Composite | −0.005 (0.91) | −0.14 (0.86) | 0.01 (0.96) | 0.09 (0.88) | 0.07 (0.92) | −0.03 (0.87) |
| Social Health | ||||||
| Social Problems | 1.36 (1.52) | 1.24 (1.81) | 1.25 (1.72) | 1.86 (2.15) | 1.83 (2.49) | 1.44 (1.91) |
| Composite | −0.07 (0.77) | −0.13 (0.92) | −0.13 (0.88) | 0.18 (1.09) | 0.16 (1.27) | −0.03 (0.97) |
| Physical Health | ||||||
| Somatic Complaints | 2.89 (3.11) | 2.14 (2.75) | 1.94 (2.45) | 2.49 (2.74) | 2.01 (2.43) | 1.93 (2.12) |
| Composite | 0.24 (1.17) | −0.04 (1.04) | −0.12 (0.93) | 0.09 (1.04) | −0.09 (0.92) | −0.12 (0.80) |
Notes.
and bold indicate primary outcomes of the trial. Additional summary statistics (N for each variable, median and range for skewed and count outcomes) are presented in Table S2. TST = total sleep time; BT = bedtime. WUP = wakeup time. Sleepiness = Sleepiness subscale from School Sleep Habits Survey. CBCL = Child Behavior Checklist (parent-report). CMEP = Children’s Morningness–Eveningness Preferences. YSAS = Youth Social Adjustment Scale.
Sleep and circadian outcomes (aim 1)
There were three primary sleep and circadian outcomes: weeknight TST, weeknight BT, and CMEP. As shown in Table 2, relative to PE, TranS-C was associated with greater reduction in eveningness as indexed by greater increase in CMEP score from pretreatment through 6-month follow-up (b = 1.84, p = 0.006), and there was no significant difference between treatment conditions on CMEP from posttreatment to 6-month follow-up (b = −0.16, p = 0.83). There was no significant difference between treatment conditions on weeknight TST or BT from pretreatment or posttreatment to 6-month follow-up.
There were six secondary sleep and circadian outcomes: weeknight-weekend discrepancy in TST, BT and wakeup time, sleepiness, PSQI, and CBCL Sleep Composite. Previously we reported that wakeup time weeknight-weekend discrepancy, sleepiness, PSQI, and CBCL Sleep Composite all showed significant improvement for TranS-C relative to PE from pretreatment to posttreatment. As shown in Table 2, among these outcomes, the additional treatment gains associated with TranS-C relative to PE were maintained through 6-month follow-up for wakeup time weeknight-weekend (b = 0.72, p = 0.013) and PSQI (b = −1.09, p = 0.02), and there was no significant difference between treatment conditions from posttreatment to 6-month follow-up on these two measures. For sleepiness, both TranS-C and PE exhibited significant decrease from posttreatment to 6-month follow-up, so there was no advantage of TranS-C over PE by 6-month follow-up. For the CBCL Sleep Composite, there was an increase for TranS-C and a decrease for PE from post to 6-month follow-up, thus at 6-month follow-up TranS-C no longer had an advantage over PE.
Five health risk domains (aim 2)
There were five primary health outcomes: youth self-report composite risk scores in the five health domains. As shown in Table 2, none of the five youth self-report composite risk scores were significantly different between treatment conditions in terms of their changes from pretreatment to posttreatment and to 6-month follow-up. We also conducted exploratory analyses using the specific measures within each health domain. TranS-C did not produce additional changes above and beyond PE on these measures. For the Attention Control Scale (ACS), there was a treatment by time interaction from posttreatment to 6-month follow-up (b = −2.59, p = 0.03) in favor of PE, such that PE had greater improvement in attention control (as indicated by an increase in ACS score) than TranS-C during this period.
There were five secondary health outcomes: parent-reported composite risk scores in the five health domains. None of the five parent-report composite risk scores were significantly different between treatment conditions from pretreatment to 6-month follow-up. As shown in Table 2, from posttreatment to 6-month follow-up, there was a significant treatment by time interaction in the behavioral health domain follow-up in favor of PE (b = 0.26, p = 0.023), such that there was significantly more reduction in the parent-reported composite scores in the behavioral domain for PE over TranS-C during that period. We again conducted exploratory analyses using the specific measures within each health domain. For the CBCL thought problem subscale, while we have previously reported a significant advantage of TranS-C relative to PE from pretreatment to posttreatment, this advantage has diminished by 6-month follow-up (PE had greater reduction of thought problems relative to TranS-C during this period, b = 0.79, p = 0.02).
Discussion
The current study examined TranS-C treatment effects on sleep and health, compared to PE, at 6-month follow-up and whether treatment effects were maintained from posttreatment to 6-month follow-up. Among the three primary outcomes of sleep and circadian functioning, TranS-C treatment effects were maintained through 6-month follow-up only for eveningness (Aim 1). None of the primary outcomes of health-related domains were significant at 6-month follow-up (Aim 2). Among the secondary outcomes, TranS-C treatment effects were maintained through 6-month follow-up for weeknight-weekend discrepancy in wakeup time and PSQI, but not for weeknight-weekend discrepancy in TST, sleepiness, and CBCL sleep composite (Aim 1).
These findings add to the growing literature on cognitive and behavioral sleep interventions in adolescents that shows benefits for improving sleep and reducing risk or symptoms of psychopathology (e.g., Blake et al., 2016; de Bruin, Bögels, Oort, & Meijer, 2018). A previous mediation analysis has shown the indirect effects of TranS-C on the five health domains via improving sleep and circadian outcomes from pretreatment to posttreatment (Dong, Gumport, Martinez, & Harvey, 2019). As such, clinicians working with adolescents should consider targeting eveningness to improve adolescent sleep and health outcomes.
It is noteworthy that previous studies have used wait-list control or a control condition that does not involve sleep-related content. In contrast, the current study used an active control–Psychoeducation–which provides sleep education and produces real benefits (Clarke et al., 2015; Harvey et al., 2015). This may have contributed to the relatively smaller effect sizes associated with TranS-C. PE was associated with improving reducing parent-reported behavioral risk, as well as self- and parent-reported attention control/thought problems in exploratory analysis, from posttreatment to 6-month follow-up. It is possible that the PE-specific activities (e.g., meditation, yoga, outdoor activities) may have produced these effects given prior evidence that they may improve attention (e.g., Semple, 2010; Sheard & Golby, 2006) and self-control in adolescents (Oberle, Schonert-Reichl, Lawlor, & Thomson, 2012). Nevertheless, future research needs to replicate and understand these effects.
Several limitations are important to note. The current paper only included subjective sleep measures. Also, we did not measure the endogenous circadian phase at 6-month follow-up; however, changes in eveningness measured by the CMEP was aligned with changes in Dim Light Melatonin Onset from pretreatment to posttreatment. Generalizability of the current findings to samples with fewer exclusion criteria and to lower-income families should be assessed. Additionally, while in the current study we used a structural interview to screen for sleep disorders, objective sleep recordings are needed for the diagnosis of several sleep disorders (e.g., obstructive sleep apnea).
In sum, adolescence is associated with high a prevalence of eveningness and risk in multiple health domains. TranS-C is a short modular, transdiagnostic intervention that addresses modifiable psychosocial contributors to eveningness and real-world sleep and circadian problems that often co-occur. As such, it has great potential for dissemination. This study provides evidence for the maintenance of TranS-C treatment gains through 6-month follow-up in terms of reducing eveningness and a few other sleep and circadian outcomes, compared to PE. TranS-C did not significantly increase sleep duration or advance bedtime, or reduce overall risk in the five health domains.
Supplementary Material
Appendix S1. CONSORT 2010 checklist.
Appendix S2. Inclusion criteria operationalizing 'at risk' for the five health domains.
Appendix S3.Construction of composite risk scores in five health domains.
Table S1. Demographic variables and sample characteristics.
Table S2. Complete summary statistics for all study variables.
Key points.
The current study reports the effects of a transdiagnostic sleep and circadian intervention (TranS-C), relative to psychoeducation, on sleep, circadian, and health outcomes at 6-month follow-up in a sample of at-risk youth who are “night-owls.”
We found that the durability of TranS-C treatment effects was strongest for selected sleep and circadian outcomes, particularly self-reported eveningness preference.
The trial is consistent with a small number of randomized controlled trials supporting that treating sleep problems in adolescents can benefit not only sleep but also other mental health outcomes.
Acknowledgements
The authors are grateful to NICHD (R01HD071065) for funding this research and to the team for their assistance with project set-up, project co-ordination, conducting assessments, and providing treatment. Clinical Trial Registration: NCT01828320. The authors are also grateful to the families who participated in this study. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
age-group cutoffs based on Giannotti et al. (2002) and Maslowsky & Ozer (2014)
References
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, & Randler C (2012). Circadian Typology: A Comprehensive Review. Chronobiology International, 29, 1153–1175. [DOI] [PubMed] [Google Scholar]
- Becker SP, Ramsey RR, & Byars KC (2015). Convergent validity of the Child Behavior Checklist sleep items with validated sleep measures and sleep disorder diagnoses in children and adolescents referred to a sleep disorders center. Sleep Medicine, 16, 79–86. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. Source Journal of the Royal Statistical Society. Series B, 57, 289–300. [Google Scholar]
- Blake MJ, Waloszek JM, Schwartz O, Raniti M, Simmons JG, Blake L, … Allen NB (2016). The SENSE study: Post intervention effects of a randomized controlled trial of a cognitive–behavioral and mindfulness-based group sleep improvement intervention among at-risk adolescents. Journal of Consulting and Clinical Psychology, 84, 1039–1051. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, III CFR, … Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, & Miller MA (2008). Meta-analysis of short sleep duration and obesity in children and adults. Sleep, 31, 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, & Morin CM (2012). The Consensus Sleep Diary: Standardizing Prospective Sleep Self-Monitoring. Sleep, 35, 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, & Acebo C (1993). Association between puberty and delayed phase preference. Sleep, Vol. 16, pp. 258–262. [DOI] [PubMed] [Google Scholar]
- Clarke G, McGlinchey EL, Hein K, Gullion CM, Dickerson JF, Leo MC, & Harvey AG (2015). Cognitive-behavioral treatment of insomnia and depression in adolescents: A pilot randomized trial. Behaviour Research and Therapy, 69, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, & Carskadon MA (2007). Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Medicine, 8, 602–612. [DOI] [PubMed] [Google Scholar]
- Dagys N, McGlinchey EL, Talbot LS, Kaplan KA, Dahl RE, & Harvey AG (2012). Double trouble? The effects of sleep deprivation and chronotype on adolescent affect. Journal of Child Psychology and Psychiatry and Allied Disciplines, 53, 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin EJ, Bögels SM, Oort FJ, & Meijer AM (2018). Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. Journal of Child Psychology and Psychiatry, 59, 509–522. [DOI] [PubMed] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, & Bögels SM (2010). The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews, 14, 179–189. [DOI] [PubMed] [Google Scholar]
- Díaz-Morales JF (2015). Morningness-Eveningness Scale for Children (MESC): Spanish normative data and factorial invariance according to sex and age. Personality and Individual Differences, 87, 116–120. [Google Scholar]
- Dong L, Gumport NB, Martinez AJ, & Harvey AG (2019). Is improving sleep and circadian problems in adolescence a pathway to improved health? A mediation analysis. Journal of Consulting and Clinical Psychology, 87, 757–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling: A Multidisciplinary Journal, 8, 430–457. [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, … Monk T (2005). Two-Year Outcomes for Interpersonal and Social Rhythm Therapy in Individuals With Bipolar I Disorder. Archives of General Psychiatry, 62, 996. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, & Ottaviano S (2002). Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research, 11, 191–199. [DOI] [PubMed] [Google Scholar]
- Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, & Czeisler CA (2013). The sleep and technology use of Americans: Findings from the National Sleep Foundation’s 2011 sleep in America poll. Journal of Clinical Sleep Medicine, 9, 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG (2016). A Transdiagnostic Intervention for Youth Sleep and Circadian Problems. Cognitive and Behavioral Practice, 23, 341–355. [Google Scholar]
- Harvey AG, & Buysse DJ (2017). Treating Sleep Problems: A Transdiagnostic Approach. New York, NY: The Guilford Press. [Google Scholar]
- Harvey AG, Hein K, Dolsen MR, Dong L, Rabe-Hesketh S, Gumport NB, … Blum DJ (2018). Modifying the Impact of Eveningness Chronotype (“Night-Owls”) in Youth: A Randomized Controlled Trial. Journal of the American Academy of Child & Adolescent Psychiatry, 57, 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Soehner AM, Kaplan KA, Hein K, Lee J, Kanady J, … Buysse DJ (2015). Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: A pilot randomized controlled trial. Journal of Consulting and Clinical Psychology, 83, 564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowsky J, & Ozer EJ (2014). Developmental trends in sleep duration in adolescence and young adulthood: Evidence from a national United States sample. Journal of Adolescent Health, 54, 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, & Rollnick S (2002). Motivational interviewing: Preparing people for change. New York: Guilford Press. [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, & Lichstein KL (2006). Psychological And Behavioral Treatment Of Insomnia: Update Of The Recent Evidence (1998–2004). Sleep, 29, 1398–1414. [DOI] [PubMed] [Google Scholar]
- Oberle E, Schonert-Reichl KA, Lawlor MS, & Thomson KC (2012). Mindfulness and Inhibitory Control in Early Adolescence. The Journal of Early Adolescence, 32, 565–588. [Google Scholar]
- Owens J (2014). Insufficient Sleep in Adolescents and Young Adults: An Update on Causes and Consequences. PEDIATRICS, 134, e921–e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Aloia MS, & Kuhn B (2011). Behavioral Treatment for Sleep Disorders: A Comprehensive Primer of Behavioral Sleep Medicine. Amsterdam: Elsevier. [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, & Merrow M (2004). A marker for the end of adolescence. Current Biology, 14, R1038–R1039. [DOI] [PubMed] [Google Scholar]
- Schaal S, Peter M, & Randler C (2010). Morningness‐eveningness and physical activity in adolescents. International Journal of Sport and Exercise Psychology, 8, 147–159. [Google Scholar]
- Semple RJ (2010). Does Mindfulness Meditation Enhance Attention? A Randomized Controlled Trial. Mindfulness, 1, 121–130. [Google Scholar]
- Sheard M, & Golby J (2006). The Efficacy of an Outdoor Adventure Education Curriculum on Selected … Journal of Experiential Education, 29, 187–209. [Google Scholar]
- Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, & Harvey AG (2010). Sleep Deprivation in Adolescents and Adults: Changes in Affect. Emotion, 10, 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedaa Ø, Bjorvatn B, Magerøy N, Thun E, & Pallesen S (2013). Longitudinal predictors of changes in the morningness-eveningness personality among Norwegian nurses. Personality and Individual Differences, 55, 152–156. [Google Scholar]
- Wirz-Justice A, Benedetti F, & Terman M (2009). Chronotherapeutics for Affective Disorders: A Clinician’s Manual for Light & Wake Therapy. Basel: Karger. [Google Scholar]
- Wolfson AR, & Carskadon MA (1998). Sleep Schedules and Daytime Functioning in Adolescents. Child Development, 69, 875–887. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. CONSORT 2010 checklist.
Appendix S2. Inclusion criteria operationalizing 'at risk' for the five health domains.
Appendix S3.Construction of composite risk scores in five health domains.
Table S1. Demographic variables and sample characteristics.
Table S2. Complete summary statistics for all study variables.