Abstract
Despite the burdens costs can place on adults with diabetes, few evidence-based, scalable interventions have been identified that address prevalent health-related financial burdens and unmet social risk factors that serve as major obstacles to effective diabetes management. In this study, we will test the effectiveness of CareAvenue – an automated e-health tool that screens for unmet social risk factors and informs and activates individuals to take steps to connect to resources and engage in self-care. We will determine the effectiveness of CareAvenue relative to standard care with respect to improving glycemic control and patient-centered outcomes such as cost-related non-adherence (CRN) behaviors and perceived financial burden. We will also examine the role of patient risk factors (moderators) and behavioral factors (mediators) on the effectiveness of CareAvenue in improving outcomes. We will recruit 720 patients in a large health system with uncontrolled Type 1 diabetes mellitus (T1DM) or Type 2 diabetes mellitus (T2DM) who engage in CRN or perceive financial burden. Participants will be randomized to one of two arms: 1) receipt of a 15–20 minute web-based program with routine follow-up (CareAvenue); or 2) receipt of contact information for existing health system assistance services. Outcomes will be assessed at baseline and 6- and 12-month follow-up.
Keywords: social determinants of health, diabetes, self-management, cost-related non-adherence
Introduction
Increases in patient cost-sharing across all insurance types coupled with unmet social risk factors have created significant stressors for individuals with complex and expensive conditions like Type 1 and Type 2 Diabetes Mellitus (T2DM) [1–3]. One in 4 families report financial burdens from managing their out-of-pocket healthcare expenses [1], and 1 in 5 report trouble meeting basic needs (e.g., food insecurity, unstable housing) [4]. Financial stressors are major barriers to treatment adherence [5]. Nearly one-third of chronically ill adults report cost-related non-adherence (CRN) associated with medication, such as taking smaller doses of medication, taking medications less frequently, delaying or not fulfilling prescriptions, or borrowing medicines from others to avoid additional expenses [6]. Eleven percent of people with chronic illnesses (17.5 million Americans) report both CRN and food insecurity [4]. Failure to identify and address unmet social risk factors related to health leads to uncontrolled disease, high rates of avoidable urgent care use (emergency department visits and hospitalizations), high healthcare costs, and preventable morbidity and mortality [7–9]. Because social factors are key determinants of health, delivery systems serving patients with multifaceted needs require efficient and effective approaches to ensure that their patients can access affordable, necessary resources to effectively manage their health.
There is increased recognition of the need to assess and address health-related financial burdens and unmet social risk factors as a core element of medical management of chronic illness. Community and health system partnerships to address broader unmet social risk factors, including health-related financial burdens, have become more common with Affordable Care Act initiatives [10]. Many of these initiatives emphasize the integration of services across different entities (e.g., state, health plan, health system) and social service and community resource organizations [11]. Randomized controlled trials evaluating the benefits of systematic screening for unmet social risk factors have shown increased uptake of social resources and fewer unmet needs among patients [12–14]. However, few rigorous evaluations are available on the most effective approaches that also improve physiologic control, perception of health-related financial burden, and other care outcomes.
In diabetes, little is known about the impact of social risk-targeted interventions on patients [15]. The few existing interventions have focused on screening for social risk factors and using lay health workers and health care team members to connect patients to resources [16]. In most trials to date that have focused on addressing unmet social risk factors only, no changes in HbA1c were observed [17].
Further, existing approaches have relied heavily on personnel linking patients to resources, and no studies have sought to develop the capacity and skillsets of individual patients themselves to engage in addressing their social risk factors. Patient-facing interventions that can both activate individuals to address social risk factors and support their disease management could maximize the time of both patients and providers, and represents a scalable approach for diverse practice settings. To address these needs, we will determine the extent to which activating patients to address social risk factors that impact treatment adherence improves diabetes control, and we will identify the mechanisms through which observed intervention effects occur. Specifically, we will evaluate the impact of CareAvenue – an automated, e-health social-needs screening, feedback and skills training intervention that addresses social risk factors for poor health and disease management.
Conceptual Model
Figure 1 shows the conceptual framework of the proposed causal pathways through which we hypothesize that clinical outcomes will be improved by participants’ use of CareAvenue. CareAvenue is informed by a strong theoretical framework guided by Social Cognitive Theory (SCT) and Self-Determination Theory (SDT). SCT posits that successful performance of a behavior depends on a person’s behavioral capability as well as cognitive and environmental influences on behavior [18]. SCT proposes that if people are to perform a particular behavior, they must know the behavior’s significance and components (knowledge and beliefs), know how to perform the behavior (skills), and have the confidence to do so (self-efficacy) [18].
Figure 1. Conceptual Model.
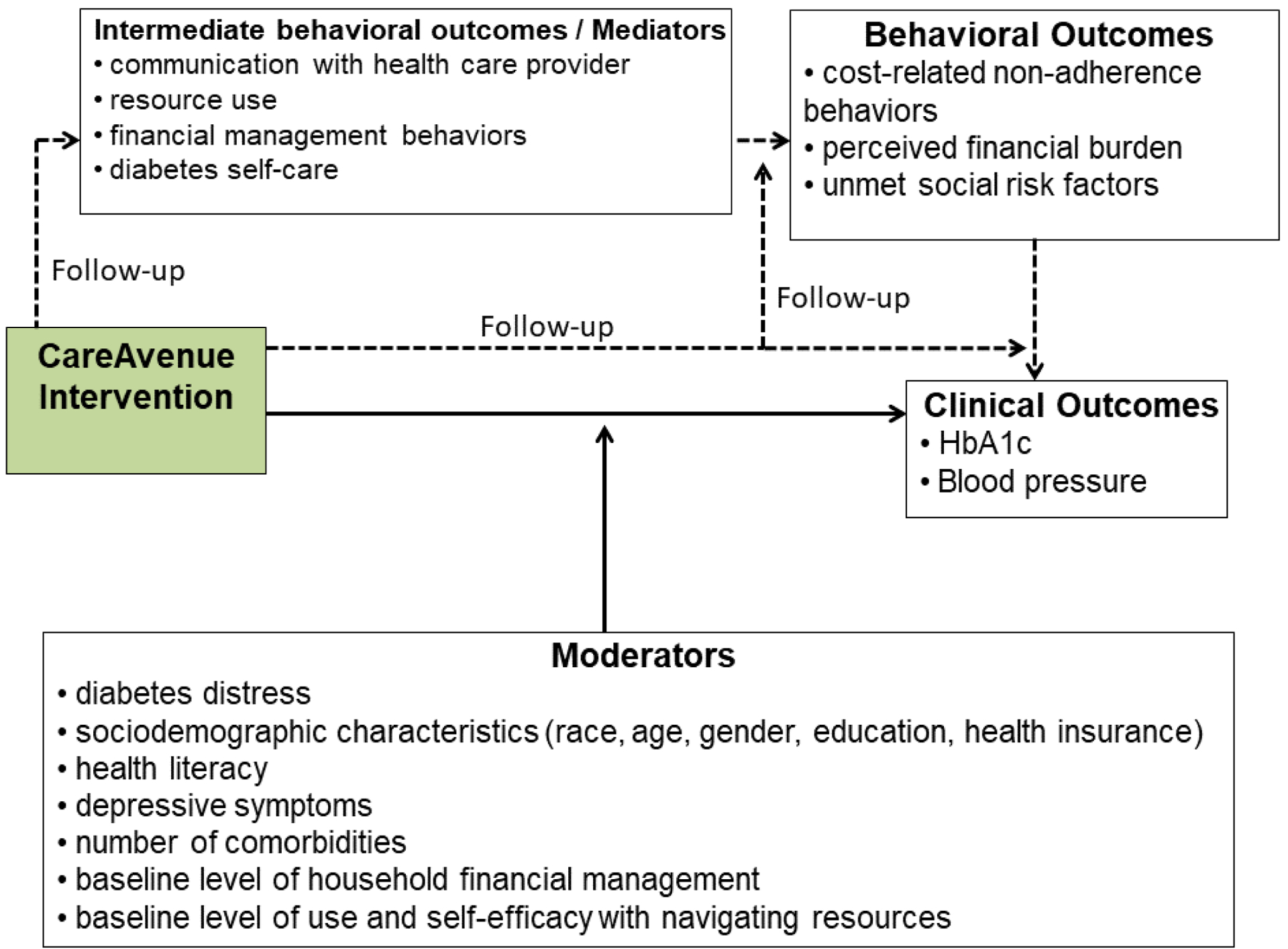
Self-Determination Theory (SDT) posits that three primary needs drive motivation and behavioral engagement. These are an individual’s experience of autonomy, competence, and relatedness [19]. CareAvenue is designed to develop participants’ motivation and behavioral capability to seek out resources in order to reduce perceived financial burden and modify the impact of perceived financial burden and unmet social risk factors on CRN behaviors. It is also designed to change users’ beliefs that there are no viable solutions to address their financial burdens with care and unmet needs. CareAvenue will provide motivation, information and skills training on how to navigate low-cost resources to address social risk factors, how to develop more effective financial management behaviors, and how to improve follow-up with providers related to financial concerns (competence). The program employs active learning strategies, modeling (relatedness), and follow-up via text messaging and interactive voice response phone calls (IVR) to encourage engagement with online resources, coach participants through incremental steps toward a mastery experience in order to raise self-efficacy, [18] increase uptake of lower-cost options to decrease CRN, and improve glycemic control. Finally, CareAvenue will support participants’ autonomy by providing a menu of options from which participants can select to address their financial burdens or unmet social needs they identify.
Methods
Study Design
This is a parallel 2-arm, randomized clinical trial in a large health system among individuals with uncontrolled diabetes who report CRN or health-related financial burdens. The trial will compare CareAvenue- an automated e-health tool that screens for unmet social risk factors and informs and activates individuals to take steps to connect to resources and engage in self-care, and existing usual care services to address financial burden and unmet social risk factors. Providing contact information for a health system assistance program to the control arm provides an opportunity to assess the additional benefits of an autonomy supportive, multi-component skills-training intervention in addition to information on available resources on outcomes. Outcomes will be assessed via physiologic measurement and surveys at 6 months and 12 months following participants’ first engagement with the interventions. Data collectors and analysts will be blind to the study hypotheses.
Specific Aims
Determine the effectiveness of CareAvenue relative to usual care in improving glycemic control and patient-centered outcomes such as CRN behaviors and perceived financial burdens. We hypothesize that at 12-month follow-up participants randomized to CareAvenue will experience clinically meaningful improvements in their HbA1c (primary outcome) and other patient-centered outcomes (secondary outcomes) compared to standard care.
Examine mediators and moderators of the effectiveness of CareAvenue on primary and secondary outcomes. We hypothesize that the intervention will significantly improve skills and self-efficacy in the use of resources leading to decreased financial burdens, lessen perceived stress of social risk factors, and improve adherence to treatment regimens. Based on the conceptual framework driving the intervention design, we assume that these changes will be the primary mechanisms through which improvements in glycemic control are achieved (mediators). We also hypothesize that CareAvenue will be most effective among patients with greater needs for resources and among those with low self-efficacy in navigating resources at baseline (moderators).
CareAvenue Intervention Arm
CareAvenue is an interactive website that builds participants’ capacity to address financial burden and unmet social risk factors while managing diabetes. The website includes a series of short animated videos, step-by-step guidance on navigating a set of low-cost resources, and general tips for financial management. Table 1 and Figure 2 detail content in the website and the order in which participants navigate through website modules. Participants first watch an introduction video that provides an overview of CareAvenue and introduces characters who they will follow throughout the series as they learn new skills and resources. Participants are then asked to identify aspects of their health that they find financially burdensome and identify areas of their health and general life situation where they would like more information on low-cost options. Participants are guided through seven resource modules with videos. Once a participant completes all of the modules, the website generates an action plan that summarizes the needs the participant identified for themselves, the resources they viewed and general tips, and provides an open space for participants to include questions and/or action items to discuss with their health care provider. CareAvenue is optimized for use on a desktop or laptop computer, smartphone or tablet interface.
Table 1.
CareAvenue Website and Video Content
| Module Content | ||
|---|---|---|
| Introduction | Provides participant overview of what to expect when using Care Avenue | Landing page |
| Participant assessment of their needs for resources | N/A | Survey to identify aspects of participant health that they find financially burdensome, and identify areas of their health and general life situation where they would like more information for low-cost options |
| Meet the Care Team | Provide information on key care team members that may be able to support participants in diabetes management | Description of the roles of different types of health care professionals |
| Medication and supplies | Encourages participants to explore low-cost resources such as GoodRx.com, discuss cost of medications with their doctor, and ask for a referral to a clinical pharmacist who can also be helpful | Walk through of how to use GoodRx.com |
| Nutrition and food assistance | Encourages participants to explore food and nutrition resources, discuss food-related resource needs with their doctor and secure referrals to a nutritionist, who can also support them | Walk through how to find local programs through the Food and Nutrition Service Agency website |
| Diabetes education | Encourages participants to explore diabetes education resources, discuss diabetes education resource needs with their doctor and secure referrals to a diabetes educator, who can also support them | Walk through of how to find local programs through American Diabetes Association and American Association of Diabetes Educators |
| Health insurance | Encourages participants to explore resources to assist them with navigating their health insurance, particularly an enrollment assister | Walk through of how to navigate healthcare.gov ‘local help locator’ |
| Other unmet needs | Encourages participants to explore a comprehensive resource for various unmet social needs, discuss unmet resource needs with their doctor and secure referrals to a social worker, who can also support them | Walk through of how to navigate resources through national phone line 2-1-1 |
| Financial management tips | Video demonstrating financial management tips on four domains:
|
General financial management tips in-depth |
Figure 2. CareAvenue participant flow.
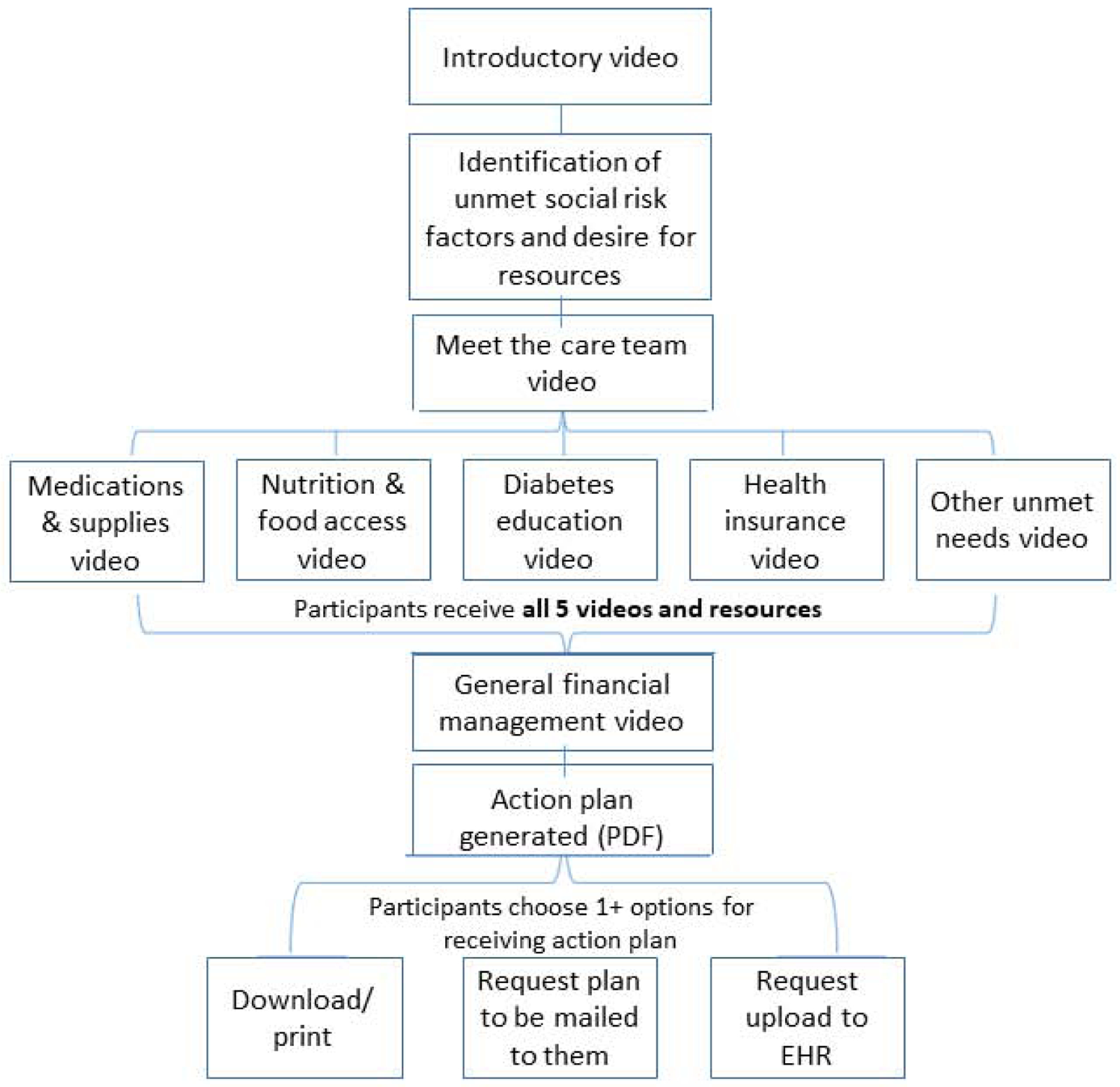
Videos
Table 1 provides a description of each of the videos. The videos model for participants how to ask their doctor to obtain a referral or facilitate connection to available professionals within the care team with expertise addressing specific sources of financial burden or unmet need. Videos also explain the roles of different health professionals and who may be best equipped to assist with specific sources of burden. The videos use storytelling methods to communicate key learning points. Storytelling is an effective patient education method for inspiring behavior change and increasing health literacy by simplifying complex ideas while making them actionable in a short period of time [20–21]. CareAvenue presents content using realistic scenarios, case studies, and characters in an active learning, entertaining format. Words, phrases and concepts are those which are familiar to the user, rather than system-oriented terms. Only relevant information appears in a natural and logical order designed in a manner that minimizes the user’s memory load.
Standard Care Control Arm
We will provide control group participants with contact information for the Guest Assistance Program (GAP) at the University of Michigan Health System. GAP social workers provide assistance with medical and non-medical needs and resources to patients receiving medical care within the health system.
Interactive Voice Response (IVR) and Text Message Follow-Up
Both intervention and control participants receive weekly communication for 52 weeks post-baseline in their study arm. These methods have a proven track record for improving self-management behaviors in chronic disease management broadly [22–23]. Intervention participants receive one IVR call and 4–5 text messages per week. For the intervention group, IVR and text message follow-up will 1) encourage participants randomized to CareAvenue to complete all modules in the website and continue to engage with the content; 2) inquire about needs being met and appropriate follow-up; 3) provide self-management support messages related to adherence of diet, exercise, and medication plans, and 4) build motivation and empathy. Control participants receive 3–4 text messages per week related to assessing their diabetes self-care and supporting their self-management. Text-messages are tailored based on diabetes self-care responses, and for the intervention group, IVR call content is tailored based on the participants’ responses throughout the IVR calls. IVR calls will take less than 5 minutes to complete. The text message system is linked to the web-based platform via an application program interface (API) so that text messages can also be tailored according to which modules the user has and has not completed. Table 2 details IVR and text message follow-up. Both IVR and text messaging are supported by a HIPAA-compliant platform within the University of Michigan Health System.
Table 2.
CareAvenue IVR and SMS content
| Content | Participant Group | Method of Delivery | Post-Baseline Duration |
|---|---|---|---|
| Reminder to complete weekly IVR call | Intervention | SMS | Weekly for 52 weeks |
| Prompts to complete website | Intervention | SMS | Twice weekly until initial completion of all modules in the website |
Prompts to continue to:
|
Intervention | SMS | Weekly for 52 weeks |
Prompts to continue to:
|
Intervention and control | SMS | Weekly for 52 weeks |
| Assess participant general perception of their diabetes self-management | Intervention and control | Two-way SMS | Weekly for 52 weeks |
| Assesses participants’ financial stress each month (compared to previous month) Assesses if any resources would be helpful with addressing their financial burden Prompts study team to follow up with participant if request assistance Focuses on one resource at a time Offers tailored empathy messages if participant is doing financially worse/same |
Intervention | IVR | Every 4 weeks for 52 weeks |
| Assesses if participants have attempted to use any resources they identified needing during monthly call Assesses if participants require assistance using resources from website Prompts study team to follow up with participant if request assistance |
Intervention | IVR | Weekly for 3 weeks each month for 12 months (52 weeks) |
Participants
Participants will include 720 adults with uncontrolled T1DM and T2DM. Study participants will meet the following criteria: 1) 18–75 years of age, 2) diagnosis of T1DM or T2DM with prescribed oral or injectable anti-hyperglycemic medication, 3) most recent (within the past 6 months) recorded hemoglobin HbA1c level of ≥7.5% for individuals ≤70 years and >8.0% for individuals between 70–75 years in age, 4) positive report of financial burden or CRN using screening questions developed and validated from prior work [24–26], and 5) access to a mobile phone that can send and receive text messages. Exclusion criteria include significant cognitive impairment precluding individuals from completing the study as evidenced by inability to complete study intake procedures.
Potential participants will be initially identified via the University of Michigan’s Diabetes Research Registry [27], and other patient registries available to researchers in the health system. The Diabetes Research Registry provides streamlined access to potential subjects through the electronic health records (EHR). Over 6,000 (18–75 years) diabetes patients are included in the registry and receive care at the University of Michigan’s ambulatory care clinics. Registries will provide us with information to generate an initial pool of potential participants based on inclusion criteria of age, diabetes status, prescribed anti-hyperglycemic medication, and HbA1c level.
Enrollment/Randomization
Trained recruitment staff will make initial contact with potential participants via telephone and screen them for the remaining inclusion/exclusion criteria over the phone. Participants who meet inclusion criteria will be consented at their baseline appointment, prior to their baseline assessments. The consent will describe the voluntary and confidential nature of the study and that participants who agree to participate will be randomly assigned to one of two groups who receive resources for better managing diabetes. All participants will be informed that upon completing each baseline, 6-, and 12-month follow-up survey and in-person HbA1c and blood pressure assessment, they will receive a $25 MasterCard gift card. Data will be collected from people who indicate they are not interested in participating in the study (reason for non-interest and socio-demographics) and from people who are determined to be ineligible to participate in the study.
At the beginning of the study, a block randomization schedule was created using SAS to generate blocks of three for random assignment of participants. Randomization was stratified within blocks defined by self-reported income (three levels: very low, low, and middle/high) [28–29]. This randomization ensures that income will be evenly distributed across the two arms of the study, thereby reducing bias due to this potentially confounding factor in the comparative analysis.
Each participant will be scheduled for an in-person appointment to complete their assessments. Trained research staff will measure blood pressure, HbA1c, and collect survey data via a computer-assisted interview through the use of Qualtrics software. Upon completion of baseline assessments, participants will be randomized to one of two study conditions based on the randomization scheme. Control group participants will receive information for GAP services, and intervention participants will receive the domain name for the CareAvenue website to browse on their own in locations convenient to them. Intervention participants will be provided with a unique User ID and password to login to the website. The research team will monitor their use of the website using web analytics data. They will also provide instructions for troubleshooting technical difficulties.
Study Outcomes and Measures
Study outcomes will be measured at baseline, and 6 months, and 12 months post randomization.
Primary Outcome Measure
Disease control: HbA1c
HbA1c is a measure of the average level of glucose in blood over the past 3 months measured as a percentage. We will measure changes in disease control through measurements of HbA1c via a fingerstick blood test using the DCA Vantage Analyzer.
Secondary Outcome Measures
Blood pressure
Blood pressure is measured as systolic blood pressure/diastolic blood pressure in millimeters of mercury (e.g., 120/80 mm Hg). Change in blood pressure will be measured using an automated blood pressure machine. Blood pressure measurements will be taken twice at each appointment, waiting one minute in between.
Cost-Related Non-Adherence Behaviors with Prescribed Treatment Regimens
Cost-Related Non-Adherence (CRN) Behaviors related to diabetes and other conditions will be measured by 4-items adapted from the Medicare Current Beneficiary Survey and 2 items adapted from the National Health Interview Survey that ask about taking smaller doses, skipping doses, delaying prescription refills, deciding not to fill a prescription, delaying seeing a healthcare provider, and not seeing a healthcare provider at all due to cost [30–31]. The items are measured with a 4-point Likert scale. Participants answering “often” or “sometimes” to any of the items are indicated as exhibiting CRN.
Perceived Financial Burden
Perceived Financial Burden will be measured by the 12-item Comprehensive Score for Financial Toxicity (COST) - Functional Assessment of Chronic Illness Therapy (FACIT) [32]. The items are measured with a 5-point Likert scale. Higher scores indicate greater perception of financial well-being.
Unmet Social Risk Factors
Change in Unmet Social Risk Factors will be measured by 20 items adapted from the Accountable Health Communities Health-Related Social Needs Screening Tool, the Health Leads Social Needs Screening Toolkit, the Kaiser Permanente Your Current Life Situation Questionnaire, and the National Health Interview Survey [31, 33–34]. Items assess presence or absence of everyday needs. Number of “yes” responses indicates number of unmet social risk factors.
Mediators and Moderators
To understand intervention facilitators and barriers and specific mechanisms through which changes in outcomes are observed, several mediators and moderators will also be measured. Table 3 describes mediators and moderators and their measurement.
Table 3.
Mediators and Moderators
| Mediators | Measure |
|---|---|
| Diabetes self-care | Summary of Diabetes Self-Care Activities [35] |
| Enactment and self-efficacy with financial management behaviors | Financial Management Behavior Scale [36] |
| Resource use and self-efficacy with navigating resources | Adapted from the Kaiser Permanente’s Your Current Life Situation Questionnaire & Health Leads [33–34] |
| Communication with healthcare providers regarding out-of-pocket costs | Communication with healthcare providers regarding out-of-pocket costs [37–38] |
| Moderators | |
| Diabetes-related distress | Diabetes Distress Scale [39] |
| Depressive symptoms | Patient Health Questionnaire-4 (PHQ-4) [40] |
| Health literacy | [41] |
| Social support | ENRICHD Social Support Inventory [42] |
| Demographics and self-reported clinical history | Survey items |
Sample size and power calculation
We calculated the sample size for detecting differences between study arms at follow-up in our primary outcome of HbA1c. A 0.5 or greater reduction in HbA1c is considered a clinically meaningful improvement in HbA1c control [43]. We designed the study to have 80% power at an alpha level of 0.05 to detect a change difference of 0.56 unit change in hemoglobin HbA1c between study arms, with a standard deviation of 1.5 from prior studies [44]. This requires a total of 508 participants (254 per arm). With this sample size, we will also have sufficient power to detect differences in our other self-reported secondary outcomes. Some cross-over is inevitable because many patients in the Diabetes Research Registry see both a specialist and primary care provider and these patients may be randomized to different arms. We have increased the sample size by 10% (508 to 558) to account for the potential that physicians’ behavior will spill over onto control participants. To account for a potential 20% loss to follow-up, we will recruit an additional 162 participants for a final sample of 720 participants.
Data analysis
Aim 1 Analyses
We will examine baseline clinical differences between study arms. Any differences that are identified will be included in outcome analyses as covariates to prevent confounding. We will use t-tests and analysis of variance (ANOVA) to assess effects of covariates (e.g., gender, age, education, health insurance type) on continuous outcomes (e.g., HbA1c). For categorical variables, we will calculate two-way contingency tables (e.g., percent improved by arm), and use odds ratios and chi-square tests to evaluate differences in proportions by study arm. These results will be used to guide multivariate regression analyses.
For the primary outcome (HbA1c), we will fit a longitudinal mixed-effects model to all repeated measurements (i.e., baseline, 6 months, and 12 months) during the pre-randomization baseline period and the 12-month intervention period. The model will have the intervention (yes/no) as the main independent variable and outcome HbA1c accounted for as a repeated measure within patients. Thus, we will model HbA1c as a function of fixed effects for the treatment arm (the intervention group vs. the control group), the observation period (baseline vs. 6-month and 12-month intervention period), and a treatment-by-time period interaction term, with adjustment for potential confounding covariates (age, gender, race, income, health insurance type, comorbidities, health literacy). Randomization helps greatly alleviate the impact of confounding, so as to improve the power of analysis and interpretability of results. For this model, the coefficient of the treatment-by-time period interaction effect estimates the key parameter of interest: changes in HbA1c levels. Random effects will be included in the model to adjust for HbA1c variation over time within patients. We will take a similar modeling approach to secondary outcome measures (blood pressure, CRN behaviors, perceived financial burden, unmet social risk factors).
Aim 2 Analyses
Using data collected from Aim 1, we will examine moderators and mediators of intervention effectiveness. We will use t-tests for continuous variables and chi-square statistics for categorical variables for a summary of differences on outcomes for each mediator and moderator of interest.
We expect moderators to show an interactive effect with intervention assignment. Moderators of interest are detailed in Table 3. To identify possible moderators, we will use regression models with predictors representing intervention group assignment, the main effect of the moderator, and the multiplicative interaction between the two.
We will also evaluate potential mediators (see Table 3) of intervention effects via structural equation modeling (SEM). Among those randomized to CareAvenue in Aim 1, we will assess the change in magnitude of the direct effect of the intervention before and after the adjustment of mediators of interest. We will examine the Lagrange Multiplier and Wald Tests to consider the deletion or inclusion of paths based on our hypotheses [45]. Once the model is identified, we will test for group differences (i.e., intervention vs. control) in latent constructs and in the paths between these constructs. This method will allow us to estimate the intervention effects on the constructs directly as well as their relationships to one another. We will follow guidelines for adequate reporting of SEM using three goodness-of-fit indices: Bentler-Bonnet’s Normed Fit Index (NFI), Bentler-Bonnet’s Non-Normed Fit Index (NNFI), and the Comparative Fit Index (CFI). We will also verify the root mean-square error of approximation (RMSEA) as an index of misfit. Well-fitting models will have fit indices of .90 or higher and <.06 for RMSEA. In addition, we will assess the causal effect of the intervention through the counterfactual type of analysis in this randomized trial.
Missing data
All primary analyses will be intention-to-treat. We will use multiple imputation on any variables with >5% missingness in linear mixed-effects models. Linear mixed-effects models are robust to missing-at-random types of missing data due to its use of maximum likelihood estimation in the statistical analysis. We will apply the inverse probability weighting method to generalized estimating equation (GEE) models to handle dropout cases to perform complete case analysis as a sensitivity analysis. We will conduct other sensitivity analyses to assess any impact of missing data.
Process Evaluation
On a weekly basis once the interventions are deployed, a research staff member will monitor resources being promoted. We will measure program engagement by completed IVR calls and web analytics data from CareAvenue (e.g., unique log-ins, time on page, click-throughs, etc.). We will also conduct in-depth interviews with a purposive sample of 20 intervention participants selected based on differing levels of engagement with CareAvenue and varying changes in primary and secondary outcome measures.
Discussion
The high prevalence of patients’ health-related financial burdens and unmet social risk factors, the lack of attention these burdens receive in clinical settings, and the serious consequences of CRN on health all underscore the need to test and evaluate practical, scalable, and effective solutions to the spectrum of burdens impacting health. Through the proposed study, we will provide a more in-depth understanding of the extent to which automating screening to identify health-related financial burdens and unmet social risk factors and activating patients to take steps to access resources and engage in self-care improves disease control. We will also identify the mechanisms through which access to these resources affects patients’ health and self-care. The results of this study could have broad translational importance to improve clinical outcomes of complex patients and reduce health disparities.
The proposed work will evaluate the effectiveness of this patient-facing approach in a population of adults with uncontrolled diabetes. It will also provide rich information on how the impact of our intervention on health status and disease control varies across groups defined by patient characteristics. Aim 2 will also provide evidence regarding behavioral mechanisms through which improved outcomes are achieved in Aim 1. The findings from this study will be adaptable to other chronic medical conditions, specialties, and healthcare settings. The ultimate goal is to find innovative ways to decrease healthcare disparities through mechanisms that enhance a patient’s ability to self-manage chronic conditions and improve their health in the face of cost barriers and unmet basic needs. The completion of these aims will yield an automated and scalable method for activating diabetes patients to address unmet social risk factors, including health-related financial burden, as a barrier to treatment adherence.
Acknowledgements
The project described was supported with core resources from P30DK092926 (Michigan Center for Diabetes and Translational Research) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: ClinicalTrials.gov ID NCT03950973, May 2019
Conflicts of interest and funding
The authors have no conflicts of interest to disclose. This work is supported through the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [Patel- R01 DK116715-01A1]. John Piette is a VA Research Career Scientist and also is supported by the Michigan Center for Diabetes Translational Research (P30DK092926)
References
- 1.Richman IB, Brodie M. A National study of burdensome health care costs among non-elderly Americans. BMC Health Serv Res. 2014. September 25;14:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight CK, Probst JC, Liese AD, Sercye E, Jones SJ. Household food insecurity and medication “scrimping” among US adults with diabetes. Prev Med. 2016. February;83:41–5. [DOI] [PubMed] [Google Scholar]
- 3.Vijayaraghavan M, Jacobs EA, Seligman H, et al. The association between housing instability, food insecurity, and diabetes self-efficacy in low-income adults. J Health Care Poor Underserved 2011; 22(4):1279–91. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med 2014; 127(4):303–310.e3. [DOI] [PubMed] [Google Scholar]
- 5.Mathes T, Jaschinski T, Pieper D. Adherence influencing factors - a systematic review of systematic reviews. Arch Public Health. 2014. October 27;72(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009. Nov-Dec;15(9):728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heerman WJ, Wallston KA, Osborn CY, et al. Food insecurity is associated with diabetes self-care behaviours and glycaemic control. Diabet Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heisler M, Choi H, Rosen AB, Vijan S, Kabeto M, Langa KM, et al. Hospitalizations and deaths among adults with cardiovascular disease who underuse medications because of cost: a longitudinal analysis. Med Care 2010. February;48(2):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piette JD, Wagner TH, Potter MB, Schillinger D. Health insurance status, cost-related medication underuse, and outcomes among diabetes patients in three systems of care. Med Care. 2004. February;42(2):102–9. [DOI] [PubMed] [Google Scholar]
- 10.Alley DE, Asomugha CN, Conway PH, Sanghavi DM. Accountable Health Communities--Addressing Social Needs through Medicare and Medicaid. N Engl J Med. 2016. January 7;374(1):8–11. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb LM, Wing H, Adler NE. A Systematic Review of Interventions on Patients’ Social and Economic Needs. Am J Prev Med. 2017. Nov;53(5):719–729. [DOI] [PubMed] [Google Scholar]
- 12.Garg A, Butz AM, Dworkin PH, Lewis RA, Thompson RE, Serwint JR. Improving the management of family psychosocial problems at low-income children’s well-child care visits: the WE CARE Project. Pediatrics. 2007;120(3):547–558. [DOI] [PubMed] [Google Scholar]
- 13.Garg A, Toy S, Tripodis Y, Silverstein M, Freeman E. Addressing social determinants of health at well child care visits. Pediatrics. 2015:peds.2014–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb L, Hessler D, Long D, Amaya A, Adler N. A randomized trial on screening for social determinants of health: the iScreen study. Pediatrics. 2014;134(6):e1611–1618. [DOI] [PubMed] [Google Scholar]
- 15.Hessler D, Bowyer V, Gold R, Shields-Zeeman L, Cottrell E, Gottlieb LM. Bringing Social Context into Diabetes Care: Intervening on Social Risks versus Providing Contextualized Care. Curr Diab Rep. 2019. April 29;19(6):30. [DOI] [PubMed] [Google Scholar]
- 16.Nelson K, Taylor L, Silverman J, Kiefer M, Hebert P, Lessler D, et al. Randomized controlled trial of a community health worker self-management support intervention among low-income adults with diabetes, Seattle, Washington, 2010–2014. Prev Chronic Dis. 2017;14:E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berkowitz SA, Hulberg AC, Standish S, Reznor G, Atlas SJ. Addressing unmet basic resource needs as part of chronic cardiometabolic disease management. JAMA Intern Med. 2017;177(2):244–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelder SH, Hoelscher D, Perry CL. (2015). How individuals, environments, and health behavior interact: Social cognitive theory In Glanz K, Rimer BK, & Viswanath K (Eds.), Health behavior: theory, research, and practice (pp. 159–178) San Francisco, CA: Jossey-Bass. [Google Scholar]
- 19.Patrick H, Williams GC. Self-determination theory: its application to health behavior and complementarity with motivational interviewing. The International Journal of Behavioral Nutrition and Physical Activity. 2012;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter LA. Stories as integrated patterns of knowing in nursing education. Int J Nurs Educ Scholarsh. 2008;5:Article 38. [DOI] [PubMed] [Google Scholar]
- 21.Haigh C, Hardy P. Tell me a story--a conceptual exploration of storytelling in healthcare education. Nurse Educ Today. 2011. May;31(4):408–11. [DOI] [PubMed] [Google Scholar]
- 22.Piette JD, List J, Rana GK, Townsend W, Striplin D, Heisler M. Mobile Health Devices as Tools for Worldwide Cardiovascular Risk Reduction and Disease Management. Circulation. 2015. November 24;132(21):2012–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wald DS, Butt S, Bestwick JP. One-way versus two-way text messaging on improving medication adherence: meta-analysis of randomized trials. Am J Med. 2015. October;128(10):1139.e1–5. [DOI] [PubMed] [Google Scholar]
- 24.Burcu M, Alexander GC, Ng X, et al. Construct validity and factor structure of survey-based assessment of cost-related medication burden. Med Care 2015; 53(2):199–206. [DOI] [PubMed] [Google Scholar]
- 25.Pierre-Jacques M, Safran DG, Zhang F, Ross-Degnan D, Adams AS, Gurwitz J, et al. Reliability of new measures of cost-related medication nonadherence. Med Care 2008. April;46(4):444–448. [DOI] [PubMed] [Google Scholar]
- 26.Piette JD, Heisler M, Wagner TH. Cost-related medication underuse among chronically ill adults: the treatments people forgo, how often, and who is at risk. Am J Public Health 2004. Oct;94(10):1782–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan MH, Bernstein SJ, Gendler S, Hanauer D, Herman WH. Design, development and deployment of a Diabetes Research Registry to facilitate recruitment in clinical research. Contemp Clin Trials. 2016. March;47:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Department of Housing and Urban Development. HUD USER. Office of Policy Development and Research. FY 2019 Income Limits Summary: Statewide Income Limits For Michigan. FY 2019 Income Limits Documentation System. Retrieved from https://www.huduser.gov/portal/datasets/il.html
- 29.Kochhar R The American middle class is stable in size, but losing ground financially to upper-income families PEW Research Center, Washington, D.C: (2018. September 6) Retrieved from https://www.pewresearch.org/fact-tank/2018/09/06/the-american-middle-class-is-stable-in-size-but-losing-ground-financially-to-upper-income-families/ [Google Scholar]
- 30.Centers for Medicare & Medicaid Services. (2019). 2013 Medicare Current Beneficiary Survey Public Use File [Data file and code book]. Retrieved from https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/MCBS-Public-Use-File/index.html
- 31.National Center for Health Statistics. 2012 National Health Interview Survey. Public-use data file and documentation. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm. [Google Scholar]
- 32.de Souza JA, Yap BJ, Wroblewski K, Blinder V, Araújo FS, Hlubocky FJ, Nicholas LH, O’Connor JM, Brockstein B, Ratain MJ, Daugherty CK, Cella D. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017. February 1;123(3):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Billoux AK, Verlander SA, & Alley D. Standardized screening for health-related social needs in clinical settings: The accountable health communities screening tool 2017. Discussion Paper, National Academy of Medicine, Washington, DC. [Google Scholar]
- 34.Your Current Life Situation Survey. Kaiser Permanente Care Management Institute Center for Population Health. 2017www.thepermanentejournal.org/files/2018/18-189-Suppl.pdf Accessed April 18, 2019. [Google Scholar]
- 35.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure. Diabetes Care. 2000. 23: 943–950. [DOI] [PubMed] [Google Scholar]
- 36.Dew J, Xiao JJ. The financial management behavior scale: Development and validation. Journal of Financial Counseling and Planning, 2011; 22(1), 19–35. [Google Scholar]
- 37.Alexander GC, Casalino LP, Tseng CW, McFadden D, Meltzer DO. Barriers to patient-physician communication about out-of-pocket costs. J Gen Intern Med 2004. August;19(8):856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander GC, Casalino LP, Meltzer DO. Patient-physician communication about out-of-pocket costs. Jama. 2003. 290(7): 953–958. [DOI] [PubMed] [Google Scholar]
- 39.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing psychosocial distress in diabetes. Diabetes Care. 2005. March; 28(3): 626–631. [DOI] [PubMed] [Google Scholar]
- 40.Lowe B, Wahl I, Rose M, Spitzer C, Glaesmer H, Wingenfeld K, Schneider A, Brahler E. A 4-item measure of depression and anxiety: Validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. Journal of Affective Disorders. 2010. 122: 86–95. [DOI] [PubMed] [Google Scholar]
- 41.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004. 36(8): 588–594. [PubMed] [Google Scholar]
- 42.Mitchell PH, Powell L, Blumenthal J, Norten J, Ironson G, Pitula CR, Froelicher ES, Czajkowski S, Youngblood M, Huber M, Berkman LF. A short social support measure for patients recovering from myocardia infarction: the enrichd social support inventory. Journal of Cardiopulmonary Rehabilitation. 2003. 23: 398–403. [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes Association. Standards of medical care in diabetes 2013.Diabetes Care. 2013. January;36 Suppl 1:S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elliott DJ, Robinson EJ, Anthony KB, Stillman PL. Patient-centered outcomes of a value-based insurance design program for patients with diabetes. Popul Health Manag 2013. April;16(2):99–106. [DOI] [PubMed] [Google Scholar]
- 45.Kline RB (2011). Principles and practice of structural equation modeling (3rd ed.). New York, NY: Guilford Press. [Google Scholar]


