Abstract
Background:
Long-term survival for children diagnosed with cancer exceeds 80%. Notably, premature cardiovascular disease has become the leading non-cancer cause of late mortality among these survivors.
Methods/design:
This randomized controlled trial (RCT; NCT03104543) focuses on adult participants in the Childhood Cancer Survivor Study identified as high risk for ischemic heart disease or heart failure due to their cancer treatment. Participants undergo a home-based evaluation of blood pressure and laboratory tests to determine the prevalence of undiagnosed and/or undertreated hypertension, dyslipidemia, and diabetes. Those with abnormal values are then enrolled in an RCT to test the efficacy of a 12-month personalized, remotely delivered survivorship care plan (SCP) intervention designed to reduce undertreatment of these three target conditions. The intervention approximates a clinical encounter and is based on chronic disease self-management strategies.
Results:
With a goal of 750, currently 342 out of 742 eligible participants approached have enrolled (46.1%). Initially, we randomized participants to different recruitment strategies, including shorter approach packets and a tiered consent, but did not find significant differences in participation rates (40.7% to 42.9%; p = .95). Subsequently, slightly greater participation was seen with larger upfront unconditional incentive checks ($50 vs. $25: 50.7% vs. 44.1%; p = .10). Overall, the financial impact of the $50 upfront incentive was cost neutral, and possibly cost-saving, vs. a $25 upfront incentive.
Conclusion:
The overall study will determine if a National Academy of Medicine-recommended SCP intervention can improve cardiovascular outcomes among long-term survivors of childhood cancer. Modifications to the recruitment strategy may improve participation rates over time.
Keywords: Cancer survivor, Childhood cancer, Cardiovascular disease, Randomized clinical trial, Survivorship care plan
1. Introduction
Long-term survival for childhood cancer now exceeds 85% and there are an estimated half-million survivors of childhood cancer now living in the United States [1,2]. As a result, cardiovascular disease is now a leading non-cancer cause of premature mortality in childhood cancer survivors [3,4]. Due to cancer treatment-related exposures, survivors have a greater than five-fold increased risk of serious cardiovascular disease and death, and a greater burden of modifiable cardiovascular risk factors such as hypertension, dyslipidemia, and diabetes compared with the general population [3,4]. In conjunction with cardiotoxic cancer treatment exposures, these modifiable risk factors further increase the risk of serious cardiovascular diseases like ischemic heart disease and heart failure, more than they do in the general population [4]. However, recognition of this excess risk and recommended screening practices among survivors and their healthcare providers is limited [5-8].
In response, we proposed to determine the prevalence of hypertension, dyslipidemia, and diabetes underdiagnosis (i.e., people with these conditions but unaware) and undertreatment (i.e., previously diagnosed but not achieving standard treatment goals) among a national sample of childhood cancer survivors. Among survivors with hypertension, dyslipidemia, or diabetes, we are also currently testing, in a randomized controlled trial (RCT), the efficacy of a personalized survivorship care plan (SCP) intervention further tailored to improve control (i.e., reduce undertreatment) of these three cardiovascular risk factors.
SCPs contain a summary of a survivor's past cancer treatment and a follow-up plan from evidence-based guidelines for survivorship care. They are devised to bridge the knowledge gap regarding cancer treatment late effects among both survivors and primary care providers. However, despite widespread endorsement of SCPs by national and international organizations, including the National Academy of Medicine, few studies have examined the efficacy of SCPs in modifying clinical endpoints, and the overall literature is mixed on the efficacy of SCPs on health outcomes [9,10]. Therefore, our study aims to advance understanding about barriers to cardiovascular risk factor treatment among both survivors and healthcare providers, and to test whether a potentially sustainable, readily disseminated (and widely endorsed) SCP-based intervention can actually improve treatment outcomes – both understudied areas in cancer survivorship care.
This ongoing study leverages one of the largest childhood cancer survivor cohorts in the world, the Childhood Cancer Survivor Study (CCSS; n = 25,664), for recruitment of a subset of adult-aged participants for direct, in-person assessments [11]. Validated CCSS-derived prediction models for ischemic heart disease and heart failure, based on original cancer treatment exposures, are being used to select high risk study participants, provide personalized risk information to participants and their primary care providers, and aid providers' clinical decision-making [12,13]. Herein we describe the detailed methodology for the Communicating Health Information and Improving Coordination with Primary care (CHIIP) study (Clinicaltrials.gov: NCT03104543), as well as the results of various participant recruitment strategies, which may be of interest to other researchers engaged in behavioral intervention studies relevant to improved adherence to clinical care.
2. Methods
2.1. Overall design
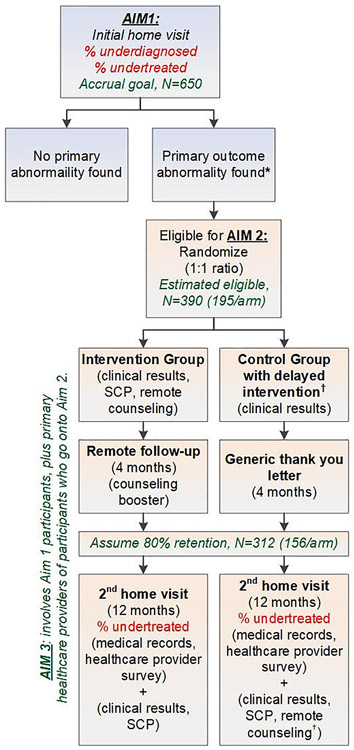
The first aim of the study is to determine the prevalence of (1) underdiagnosis and (2) undertreatment of three conditions (hypertension, dyslipidemia, and diabetes) considered to be risk factors for the development of cardiovascular disease (hereafter, we refer to these three conditions as cardiovascular risk factors). To accomplish this aim, we are recruiting a cross-sectional sample of CCSS participants predicted to be at high cardiovascular risk based upon their original cancer treatment exposures. Those found to have one or more cardiovascular risk factors (see eligibility below) are randomized to an intervention arm or a delayed-intervention control arm to answer the study's second and third aims (Fig. 1). Aim 2 is to determine the efficacy of the RCT and Aim 3 seeks to determine the barriers among survivors and their primary care providers that contribute to undertreatment of hypertension, dyslipidemia, and diabetes in this high-risk population.
Fig. 1.
Overall schema. Aim 1 seeks to determine the overall prevalence of underdiagnosis and undertreatment of target cardiovascular conditions; 750 participants will be enrolled, accounting for drop-out, so that 650 will remain with useable home visit (i.e., clinical) results. Aim 2 features a randomized clinical trial to determine the efficacy of a mailed survivorship care plan (SCP) that contains an explanation of the home visit's clinical results, followed by a telehealth counseling intervention delivered by an advanced practice provider. The control group only receives upfront a letter explaining the clinical results. Aim 3 determines barriers among survivors and their primary care providers with respect to knowledge and self-efficacy towards the care of childhood cancer survivors. (*) Undertreatment or underdiagnosis and eligible for randomization if ≥1 of the following clinical results are found: average blood pressure ≥ 130/80 mmHg, LDL ≥160 mg/dL, triglyceride ≥150 mg/dL, glucose ≥100 mg/dL or HbA1c ≥5.7% (if not previously diabetic), HbA1c ≥7% (if known diabetic). (†) Controls can receive the intervention after the 2nd home visit & survey completion.
2.2. Population
Participants are drawn from members of the CCSS cohort who currently reside in the United States. Given insurance differences, non-US residents are not eligible. Based on CCSS risk prediction models for ischemic heart disease and heart failure [12,13], the study is targeting recruitment of those who are predicted to be at moderate or high cardiovascular risk, and who are able to read, write, and speak English. Participants with known ischemic heart disease or heart failure from prior CCSS surveys are excluded. Other upfront exclusion criteria include current active cancer treatment and pregnancy. However, individuals who become pregnant after enrollment, or are diagnosed with a new cancer after enrollment, remain eligible. Given the logistics of completing hundreds of home visits using an external contractor, Examination Management Services Inc. (EMSI), recruitment has focused on CCSS participants with current addresses within 50 miles of a designated EMSI branch office in each targeted metropolitan area. Geocoding of CCSS participants' last address and identification of major US metropolitan regions with high concentrations of participants have facilitated this effort. Study procedures are then deployed sequentially across each selected site. Details of our recruitment strategy are further described below.
2.3. Measurements
Participants are asked to complete a < 20-min survey that includes items related to past medical history (cancer diagnosis and treatment), current cardiovascular health and medication adherence (if applicable), lifestyle habits (tobacco use, physical activity, fruit/vegetable intake), attitudes towards healthcare, and current mood (PROMIS anxiety and depression short forms [14]). Specifically, the Medication Adherence Scale assesses medication adherence to any blood pressure, lipid-lowering, and diabetes medications [15]. Attitudes towards healthcare are assessed by examining health-related self-efficacy [16] and the Multidimensional Health Locus of Control (MHLC) scale [17].
After consent and completion of the baseline questionnaire, participants' contact information is provided to EMSI, whose staff then schedules a home visit. At this visit, EMSI staff measure participants' standing height, weight, resting blood pressure (three measurements), and waist circumference. Blood is drawn for lipid profile, glucose, insulin, and hemoglobin A1c. To improve ease of scheduling and minimize barriers to participation, we do not require participants to fast prior to the blood draw, but we record the duration of any fasting if it occurred. The elimination of routine fasting is consistent with current clinical practice and national guidelines provide guidance on how to interpret non-fasting laboratory results [18-22]. Blood is then shipped overnight to a CLIA-certified laboratory (St. Jude Children's Research Hospital) and results provided within one business day of receipt. Additional blood is banked if the participant provided additional optional consent. Individuals eligible to participate in the RCT portion of the study (further described below) are scheduled for a second home visit approximately one year after the baseline home visit. At this visit, an EMSI staff member collects the same measurements, including lipid profile, glucose, insulin, and hemoglobin A1c.
2.4. Eligibility for randomized controlled trial (RCT)
Participants who have at least one of the following findings from their baseline measurement are eligible to participate in the RCT: 1) average of the lowest two blood pressure measurements ≥130/ 80 mmHg; 2) low density lipoprotein (LDL) ≥160 mg/dL; 3) triglyceride ≥150 mg/dL (if ≥10 h fast) or ≥200 mg/dL (if <10 h fast); 4) glucose ≥100 mg/dL (if ≥8 h fast and not known to be diabetic) or ≥140 mg/dL (if<8 h fast and not known to be diabetic; or 5) hemoglobin A1c ≥5.7% (if not known to be diabetic) or hemoglobin A1c ≥7% (if known to be diabetic). Individuals who report ischemic heart disease or heart failure/cardiomyopathy on the CHIIP study's baseline questionnaire can have a home visit but are not eligible to continue onto the RCT even if they have an otherwise eligible cardiometabolic abnormality identified (Fig. 1).
2.5. Randomization and Intervention
After RCT eligibility is confirmed by the study team, a software algorithm featuring a random number generator is used to perform a block randomization of RCT-eligible participants with blocks of four, assigning them 1:1 to intervention or delayed-intervention control arm with a random permutation of two interventions and two controls within each block, stratified by site. The intervention is a manualized, telehealth-delivered, personalized, clinician-led, SCP-focused self-management counseling session that is generalizable to a real clinical setting. This assures the methods are sustainable in practice, broadly accessible without depending on survivorship care resources in a local community, and cost-conserving. After randomization, participants in the intervention arm receive a printed, mailed SCP with personalized health history, recommendations, and clinically meaningful results from the baseline home visit (i.e., average blood pressure, lipid profile, diabetes screening, body mass index) (see Supplement for a template). The personalized health information includes both absolute and relative risk estimates of ischemic heart disease and cardiomyopathy/heart failure compared with the general population, based on each participant's prior childhood cancer treatment exposures [12,13]. Study staff then schedule participants for a remote counseling session via telephone or HIPAA-compliant web video, depending on participant choice.
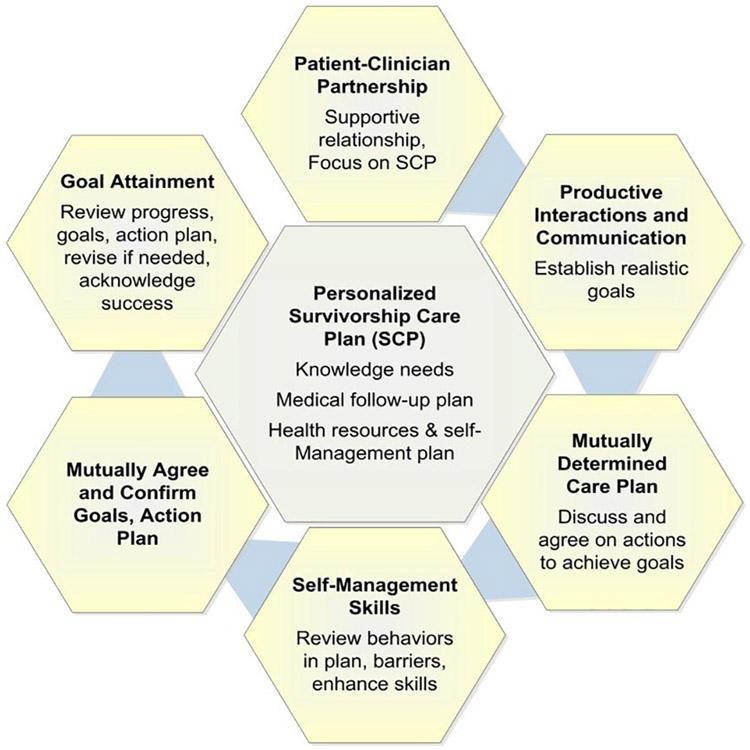
The first session with a study clinician (survivorship-trained advanced practice provider) is designed to be delivered within 30 min. The session includes a review of the SCP and an opportunity for the participant, in discussion with the study clinician, to make mutually agreed upon goals and an action plan to address the cardiovascular risk factors identified. The principle of goal-setting and emphasis on achievable actions that can be implemented in the near-term to meet those goals (plus discussion of potential barriers and solutions) are based on well-accepted chronic disease self-management models (Fig. 2) [23-25]. To facilitate and standardize possible goals, study clinicians follow a standard of care strategy document for hypertension, high LDL/triglyceride, and hyperglycemia management that emphasizes close interval follow-up with primary care, adherence to existing medications, and lifestyle factors, if relevant. Scripted questions are also used to ensure the participants understand the SCP, to elicit participants' intentions to act on the SCP information, and to identify potential barriers and solutions related to any planned actions. After the session, the personalized action plan is mailed to the participant.
Fig. 2.
Self-management model for cancer survivorship care, based on chronic disease management models. Adapted from Schuman-Green et al. (reference 25).
Approximately four months after the initial counseling session, participants are asked to schedule a follow-up session by telephone or web video. This session is designed to be completed within 15 min and provides an opportunity for the study clinician to follow up with the participant on the action plan, address any barriers to plan completion and mutually agree on a revised plan if needed. The clinician also rates the survivor's completion of the action plan on a numeric scale. Both the baseline and four-month follow-up sessions are audiotaped to facilitate process evaluation of content and fidelity of the clinician adherence to the intervention.
2.6. Delayed-intervention control
Participants assigned to the control arm immediately receive a copy of clinically meaningful baseline home visit results, with a general recommendation to seek medical follow-up in instances where any of those values are abnormal. A copy is also sent to their designated healthcare provider. After four months, participants receive a generic thank you letter by mail for participating, which also includes a reminder of the second home visit. With the goal of enhancing control group retention, control participants are reminded that they can receive the study intervention (minus the four-month booster session) after completing the second home visit and any associated surveys. After completing the second home visit, control participants are mailed their personalized SCP and offered the opportunity to receive a self-management telehealth call with the study clinician.
2.7. Interaction with primary care providers
At the time of consent, all participants are asked to list their current and past (within the last two years) primary care provider(s). Current designated providers receive a copy of all materials sent to study participants: SCP with home visit results and an action plan if the participant is in the intervention arm; home visit results only if in the control arm. Following the second home visit, healthcare providers of participants in both study arms complete a survey on their self-efficacy and knowledge about providing care for childhood cancer survivors. Questions are adapted from the Survey of Physicians' Attitudes Regarding the Care of Cancer Survivors (SPARCCS) [26]. At that time study staff also request participant medical records from the past three years. To enhance response rates, the survey has been limited to four pages (less than five minutes) and we apply Dillman's tailored design survey protocol [27]. This includes personalizing the survey to the provider and referencing the study participant, as well as an upfront $20 cash incentive. Non-responding providers are sent a second survey packet approximately a month later with an alternative cover letter, followed by a third and final survey packet three months later with a small token incentive (e.g., $5 coffee card).
2.8. Overall study planned analysis
Due to the nature of the intervention, participants and study staff cannot be blinded. However, EMSI staff who conduct the home visits and the statisticians are blinded to the RCT arm assignment. For Aim 1, we will estimate the prevalence of underdiagnosed and undertreated (as defined earlier under study eligibility) hypertension, dyslipidemia, and diabetes among enrolled participants, assessing potential modifiers of the prevalence. For Aim 2, we will determine if the intervention arm is associated with a lower probability of having an undertreated cardiovascular risk factor, compared with the control arm, at one-year follow-up. As some survivors may contribute up to three outcomes (persistent hypertension / pre-hypertension, dyslipidemia, and diabetes/pre-diabetes), we will use generalized estimating equations, accounting for intra-individual correlation of the three outcomes, to estimate the overall intervention effect as a single parameter (three-element vector). Analyses will be conducted per intent-to-treat and will include all randomized participants. For those participants with missing/unknown outcomes at the one-year follow-up, we will assume they have persistent undertreated risk factors. Finally, for Aim 3, we will examine if participants who are initially underdiagnosed or undertreated have lower knowledge of their prior cancer history and lower health-related self-efficacy compared with those not affected. We also will examine if different health-related behavioral attitudes as defined by the MHLC scale (“worried”, “collaborative”, and “self-controlling”) are associated with differential rates of underdiagnosis or undertreatment [28]. A priori, we hypothesize that the proportion undertreated will be greatest among “self-controlling” survivors vs. “collaborative” and “worried” (least undertreated) survivors.
2.9. Detailed overview of recruitment strategies
For the first study site, research staff mailed potentially eligible participants an introductory packet via US Postal Service containing a one-page cover letter, one-page trifold study brochure, consent with medical release form (9 pages), a baseline questionnaire (14 pages), and a $25 upfront unconditional incentive in the form of a bank check. An additional $25 incentive was promised upon completion of the baseline home visit (further described below). After two weeks, research staff began contacting potential participants by telephone and email to assess interest and to answer any questions regarding the study. After 6–8 weeks, a second packet (without further incentive) was mailed to non-responders. Finally, approximately 3–4 months after the initial approach, study staff mailed a third packet (minus the baseline questionnaire) plus a small token incentive [i.e., $5 coffee gift card]. Research staff continued periodic telephone and email outreach regarding study participation during this time, up to 6 months from the initial packet mailing date. Throughout this time, besides responding by mail, interested participants could also provide consent and complete the study questionnaire online or by telephone.
To try to improve response rates, the study subsequently tested alternative recruitment strategies. We randomized (1:1:1) the content and size of materials sent: 1) standard packet described above; 2) a shorter introductory packet with the standard consent and medical release form but without the baseline questionnaire; and 3) an even shorter introductory packet with a simplified consent and medical release form (7 instead of 9 pages). Potential participants randomized to the second and third strategies were then sent the questionnaires by mail after enrollment, and also with options to complete the questionnaires by telephone or online. With the shortened and simplified consent, only the baseline home visit was described; participants who consented would then be offered a second consent form that described the intervention trial after the home visit was completed. All three strategies contained the upfront unconditional $25 bank check as incentive, with the promise of an additional $25 upon completion of the baseline home visit.
Another strategy to improve recruitment rates tested the timing for delivery of the $50 incentive. For some participants, the study switched entirely to an upfront unconditional $50 bank check incentive to accompany the initial introductory packet. Participants who consented under these conditions do not receive any additional incentive upon completing the baseline home visit. Differences in characteristics among participants vs. non-participants were then compared using t-test (continuous variables) or chi-square test (categorical variables), while participation rates in response to the different recruitment strategies were compared using the chi-square test. All procedures were approved by the human subjects review board at the Fred Hutchinson Cancer Research Center and St. Jude Children's Research Hospital.
3. Results
Participant recruitment began in August 2017, and as of July 2019, active recruitment has been completed at 6 geographically disparate metropolitan regions across the United States with 342 participants enrolled as part of Aim 1 (participation rate 46.1%, with site-specific rates ranging from 40.5% to 56.3%; Table 1). Recruitment is ongoing at several other metropolitan regions with an overall accrual goal of approximately 750 participants enrolled for Aim 1 by Spring 2021, which after accounting for an expected drop-out rate of around 10–15%, will allow the study to have at least 650 participants with useable home visit results. Below, we describe the initial results from our various different recruitment strategies and preliminary differences between participants and non-participants. The results corresponding to Aim 1 (prevalence of underdiagnosed and undertreated cardiovascular risk factors) will be presented separately after enrollment has been completed, and the results corresponding to Aims 2 (randomized clinical trial) and 3 (barriers contributing to undertreatment) will be presented after all participants have completed the intervention period and follow-up testing.
Table 1.
Aim 1 participation rates among first 6 enrolling sites.
| Site | Recruitment period | No. approached | No. consented | No. ineligible | Participation ratea |
|---|---|---|---|---|---|
| 1 | 8/2017–3/2018 | 112 | 50 | 5 | 46.7% |
| 2 | 1/2018–7/2018 | 155 | 60 | 7 | 40.5% |
| 3 | 3/2018–9/2018 | 118 | 49 | 7 | 44.1% |
| 4 | 5/2018–10/2018 | 160 | 71 | 5 | 45.8% |
| 5 | 7/2018–12/2018 | 94 | 49 | 7 | 56.3% |
| 6 | 9/2018–3/2019 | 137 | 63 | 3 | 47.0% |
| Overall | 776 | 342 | 34 | 46.1% |
Excluding those subsequently found to be ineligible (unable to provide informed consent; newly discovered to have coronary artery disease or cardiomyopathy; lives beyond the EMSI service area; deceased).
A randomized trial of recruitment strategies was undertaken in the study's second and third sites (n = 259 approached), but we did not find that a slightly shorter introductory packet (42.9%) nor an even shorter packet with a simplified consent (42.7%) were associated with a markedly improved participation rate compared with the original introductory packet (40.7%; overall p = .95; Table 2). Therefore, in subsequent sites, the study reverted to the original introductory packet given that it was logistically easier for study staff to distribute and track.
Table 2.
Aim 1 participation rates with variable recruitment strategies.
| Recruitment strategy | No. approacheda | No. consented | Participation rate | P-value |
|---|---|---|---|---|
| Randomizedb | 0.95 | |||
| Standard packet | 86 | 35 | 40.7% | |
| Short packet | 84 | 36 | 42.9% | |
| Short packet with simplified consent | 89 | 38 | 42.7% | |
| Nonrandomizedc | 0.10 | |||
| $25 upfront incentive | 521 | 230 | 44.1% | |
| $50 upfront incentive | 221 | 112 | 50.7% | |
Excludes those subsequently found to be ineligible.
Randomized 1:1:1 among sites 2 and 3 (stratified by site).
Initial four sites provided $25 bank check with introductory packet with additional $25 upon completion of home visit; subsequent two sites provided $50 bank check upfront with introductory packet and no additional incentive after completion of home visit.
The participation rate for $25 vs. $50 upfront bank checks appeared to slightly favor the larger amount (44.1% vs. 50.7%; p = .10). Among study participants given a $25 bank check (n = 521), the deposit rate within 6 months after they were mailed was 87.4% among consented participants vs. 34.7% among non-participants (p < .001). When the incentive was increased to $50 (n = 221), the 6-month deposit rate was 74.1% vs. 32.1% among consented participants vs. non-participants as of July 2019 (p < .001). Notably the overall cost per consented enrollee using either strategy was comparable: $52.68 for upfront $50 incentive vs. $54.58 for upfront $25 incentive (which includes an assumption that a similar proportion [87%] of enrolled participants will deposit the second $25 conditional incentive distributed after completing the home visit). The per enrollee cost for the $25 incentive strategy increases to $57.83 if 100% of enrolled participants deposit their second $25 incentive. As a result, the study has adopted the $50 upfront incentive as its standard recruitment strategy.
To date, an examination of demographic and clinical characteristics among participants vs. non-participants indicates that participants are more likely to be female, have had prior chest radiation, and have self-reported hypertension (Table 3). However, we do not see differences in other demographic and treatment characteristics. As of July 2019, 291 out of 342 participants (85.1%) have completed their baseline home visits, and 161 participants have been recruited to and randomized in the RCT. Notably, five participants who initially received the simplified consent and completed their baseline home visit, had results that met RCT eligibility but refused subsequent randomization when offered the second study consent.
Table 3.
Distribution of baseline demographic and clinical characteristics by Aim 1 participation status.
| Characteristic | Participant N = 342 (%) |
Non-participant N = 400 (%) |
P-value |
|---|---|---|---|
| Female sex | 208 (60.8) | 201 (50.3) | 0.004 |
| White non-Hispanic race/ | 294 (86.0) | 335 (83.8) | 0.40 |
| ethnicity | |||
| Cancer diagnosis | 0.87 | ||
| Bone cancer | 17 (5.0) | 22 (5.5) | |
| Central nervous system tumor | 49 (14.3) | 61 (15.3) | |
| Hodgkin lymphoma | 73 (21.3) | 70 (17.5) | |
| Kidney (Wilms) | 43 (12.6) | 48 (12.0) | |
| Leukemia | 99 (28.9) | 118 (29.5) | |
| Non-Hodgkin lymphoma | 21 (6.1) | 23 (5.8) | |
| Neuroblastoma | 29 (8.5) | 39 (9.8) | |
| Soft tissue sarcoma | 11 (3.2) | 19 (4.8) | |
| Median age at diagnosis (range) | 6.4 (0.1–20.9) | 5.3 (0.0–20.7) | 0.30 |
| Median current age (range) | 37.0 (20.2–63.1) | 36.5 (19.0–65.1) | 0.56 |
| Anthracycline exposure | 219 (64.4) | 260 (65.7) | 0.72 |
| Chest radiation exposure | 230 (68.3) | 242 (61.3) | 0.049 |
| Prior self-reported hypertension | 63 (18.4) | 51 (12.8) | 0.03 |
| Prior self-reported dyslipidemia | 51 (14.9) | 55 (13.8) | 0.65 |
| Prior self-reported diabetes | 19 (5.6) | 26 (6.5) | 0.59 |
4. Discussion
Premature cardiovascular disease is a leading contributor to late morbidity and mortality in long-term survivors of childhood cancer [3,4]. Cohort studies from North America and Europe, including the CCSS, have consistently shown that survivors have a greater than five-fold increased risk of serious cardiovascular morbidity or mortality vs. the general population, corresponding to an approximate 5% cumulative incidence by age 45 years [29-32]. Among survivors that have been exposed to cardiotoxic cancer treatments (i.e., anthracyclines and chest radiotherapy), this risk can be markedly greater [12,13]. Demographic characteristics such as age at treatment and gender, and off-target and indirect effects of both radiotherapy and chemotherapy may also affect cardiovascular health [3,33,34].
Multiple studies in childhood and adult cancer survivors also have shown that even after considering treatment exposures, conventional cardiovascular risk factors such as hypertension, diabetes, and dyslipidemia remain important, and further increase the risk of serious cardiovascular disease in more than additive fashion [4,35,36]. Among childhood cancer survivors, these conditions also tend to present at younger ages compared with siblings or the general population [37,38]. Given the relatively young age of onset of these conditions that occur more typically in older adults, and the limited knowledge of cancer survivor-specific guidelines among general practitioners [5,6], most high risk survivors likely do not receive recommended cardiovascular screening studies [8,39,40]. For these reasons, we believe there is a compelling rationale to develop an intervention for this high-risk population designed to target these modifiable cardiovascular risk factors. Our study's first aim will specifically determine the prevalence of underdiagnosed and undertreated cardiovascular risk factors in this population. Specifically, studies that have attempted to examine cardiovascular risk factor undertreatment have been rare among survivors of adult cancers [41-44], and have not been done, to our knowledge, among survivors of childhood cancers. Our study's intervention features a National Academy of Medicine-recommended SCP, which is designed to promote knowledge/awareness of personal health risks among survivors and to help disseminate that information to primary care providers [9]. Prior research has shown that>80% of adult survivors of childhood cancer are followed by primary-care providers [40,45], and that while the majority of internists and family practitioners report caring for childhood cancer survivors, receipt of an SCP remains uncommon and most providers are not familiar with long-term follow-up guidelines for childhood cancer survivors [5,6]. Although the SCP is, by itself, a tool to foster self-management, we are supplementing it with well-established, focused chronic disease self-management strategies (Fig. 2) now being applied to improve coordination of cancer survivorship care [23,25]. Collection of directly measured data also offers an opportunity to further refine risk prediction in the future, beyond using self-report alone.
To date, with a little over half of the target accrual being met, we have learned several lessons from the conduct of this study. We have achieved nearly a 50% participation rate, which is favorable when compared with participation rates for contemporary epidemiologic studies and specifically for many cancer-related or cardiovascular disease prevention trials with young adults [46-48]. Our choice of a larger upfront unconditional incentive may improve our final response rate. In general, the use of upfront unconditional incentives has been associated with an improved participation rate in similar settings [49-51]. In our experience thus far, the greater upfront cost of the larger unconditional incentive has been off-set by higher participation rates and<100% deposit rates of the bank checks. Furthermore, our current cost estimates do not account for the extra research staff time required to process and track the multiple checks associated with the $25 incentive strategy compared with single $50 check, further favoring the latter strategy.
It is possible that participation rates could be further improved by use of a direct cash or gift card incentive, which participants may find more convenient (and thus more attractive) than a bank check. However, the cost associated with cash and most gift card-based incentives cannot typically be recouped by the study should subjects lose their incentive or not respond. While a proportion of non-participants deposited their checks, the deposit rate was significantly lower compared with participants.
In summary, our study is systematically assessing the magnitude of underdiagnosis and undertreatment of potentially modifiable cardiovascular risk factors and contributing survivor- and provider-specific barriers among adult-aged childhood cancer survivors predicted to be at high risk of future cardiovascular disease. Results from this study may significantly advance our understanding of cardiovascular disease risk and its modification among childhood cancer survivors of all ages, given that many of the cancer treatments CCSS participants received remain in common use today [52,53]. If successful, our personalized intervention will increase the proportion of survivors and healthcare providers who are aware of current screening guidelines, who receive/deliver more appropriate cardiovascular treatment, and who adhere to these guidelines and treatments. The cumulative effect will be a mitigation of these high-risk survivors' long-term cardiovascular risk.
Supplementary Material
Acknowledgements
This work was supported by the National Cancer Institute at the U.S. National Institutes of Health (grant numbers CA15704, CA21765, CA55727, CA204378) and the American Lebanese Syrian Associated Charities. None of the funders had any role in the design, conduct, data collection, analysis, interpretation, manuscript preparation, or submission of this study. The authors report no conflicts of interest related to this work.
Abbreviations
- CCSS
Childhood Cancer Survivor Study
- EMSI
Examination Management Services Inc.
- LDL
low density lipoprotein
- MHLC
Multidimensional Health Locus of Control
- RCT
randomized clinical trial
- SCP
survivorship care plan
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2019.105915.
References
- [1].SEER Cancer Statistics Review, https://seer.cancer.gov/csr/1975_2016/, (1975–2016) 2019. (accessed 1 December 2019).
- [2].Robison LL, Hudson MM, Survivors of childhood and adolescent cancer: life-long risks and responsibilities, Nat. Rev. Cancer 14 (1) (2014) 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, Hudson MM, Kremer LC, Landy DC, Miller TL, Oeffinger KC, Rosenthal DN, Sable CA, Sallan SE, Singh GK, Steinberger J, Cochran TR, Wilkinson JD, Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association, Circulation 128 (17) (2013) 1927–1995. [DOI] [PubMed] [Google Scholar]
- [4].Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, Stovall M, Chow EJ, Sklar CA, Mulrooney DA, Mertens AC, Border W, Durand JB, Robison LL, Meacham LR, Modifiable risk factors and major cardiac events among adult survivors of childhood cancer, J. Clin. Oncol 31 (29) (2013) 3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nathan PC, Daugherty CK, Wroblewski KE, Kigin ML, Stewart TV, Hlubocky FJ, Grunfeld E, Del Giudice ME, Ward LA, Galliher JM, Oeffinger KC, Henderson TO, Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer, J. Cancer Surviv 7 (3) (2013) 275–282. [DOI] [PubMed] [Google Scholar]
- [6].Suh E, Daugherty CK, Wroblewski K, Lee H, Kigin ML, Rasinski KA, Ford JS, Tonorezos ES, Nathan PC, Oeffinger KC, Henderson TO, General internists' preferences and knowledge about the care of adult survivors of childhood cancer: a cross-sectional survey, Ann. Intern. Med 160 (1) (2014) 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cox CL, Hudson MM, Mertens A, Oeffinger K, Whitton J, Montgomery M, Robison LL, Medical screening participation in the childhood cancer survivor study, Arch. Intern. Med 169 (5) (2009) 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, Bhatia S, Meeske K, Chen MH, Kinahan KE, Steinberger J, Rosenthal D, Monitoring for cardiovascular disease in survivors of childhood cancer: report from the cardiovascular disease task force of the Children's Oncology Group, Pediatrics 121 (2) (2008) e387–e396. [DOI] [PubMed] [Google Scholar]
- [9].N.C.P.B. Committee on Cancer Survivorship, Improving care and quality of life, in: Hewitt M, Greenfield S, Stovall E (Eds.), From Cancer Patient to Cancer Survivor: Lost in Transition, Institute of Medicine and National Research Council, National Academies Press, Washington, D.C., 2006. [Google Scholar]
- [10].Jacobsen PB, DeRosa AP, Henderson TO, Mayer DK, Moskowitz CS, Paskett ED, Rowland JH, Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery, J. Clin. Oncol 36 (20) (2018) 2088–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, Green DM, Hammond S, Meadows AT, Mertens AC, Mulvihill JJ, Nathan PC, Neglia JP, Packer RJ, Rajaraman P, Sklar CA, Stovall M, Strong LC, Yasui Y, Zeltzer LK, The childhood cancer survivor study: a National Cancer Institute-supported resource for outcome and intervention research, J. Clin. Oncol 27 (14) (2009) 2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chow EJ, Chen Y, Kremer LC, Breslow NE, Hudson MM, Armstrong GT, Border WL, Feijen EA, Green DM, Meacham LR, Meeske KA, Mulrooney DA, Ness KK, Oeffinger KC, Sklar CA, Stovall M, van der Pal HJ, Weathers RE, Robison LL, Yasui Y, Individual prediction of heart failure among childhood cancer survivors, J. Clin. Oncol 33 (5) (2015) 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chow EJ, Chen Y, Hudson MM, Feijen EAM, Kremer LC, Border WL, Green DM, Meacham LR, Mulrooney DA, Ness KK, Oeffinger KC, Ronckers CM, Sklar CA, Stovall M, van der Pal HJ, van Dijk I, van Leeuwen FE, Weathers RE, Robison LL, Armstrong GT, Yasui Y, Prediction of ischemic heart disease and stroke in survivors of childhood cancer, J. Clin. Oncol 36 (1) (2018) 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].HealthMeasures, PROMIS - Patient-Reported Outcomes Measureement Information System, http://www.healthmeasures.net/explore-measurement-systems/promis, (2019) (accessed 1 December 2019).
- [15].Morisky DE, Green LW, Levine DM, Concurrent and predictive validity of a self-reported measure of medication adherence, Med. Care 24 (1) (1986) 67–74. [DOI] [PubMed] [Google Scholar]
- [16].Schwarzer R, Jerusalem M, Generalized self-efficacy scale, in: Weinman J, Wright S, Johnston M (Eds.), Measures in Health Psychology: A User's Portfolio. Casual and Control Beliefs, NFER-NELSON, Windsor, UK, 1995, pp. 35–37. [Google Scholar]
- [17].Wallston KA, Wallston BS, DeVellis R, Development of the multidimensional health locus of control (MHLC) scales, Health Educ. Monogr 6 (2) (1978) 160–170. [DOI] [PubMed] [Google Scholar]
- [18].Nordestgaard BG, A test in context: lipid profile, fasting versus nonfasting, J. Am. Coll. Cardiol 70 (13) (2017) 1637–1646. [DOI] [PubMed] [Google Scholar]
- [19].Professional Practice Committee, Standards of medical care in diabetes-2018, Diabetes Care 41 (Suppl. 1) (2018) S3. [DOI] [PubMed] [Google Scholar]
- [20].Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J, Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics, Circulation 114 (24) (2006) 2710–2738. [DOI] [PubMed] [Google Scholar]
- [21].Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report, Pediatrics 128 (Suppl. 5) (2011) S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Third Report of the National Cholesterol Education Program (NCEP), Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report, Circulation 106 (25) (2002) 3143–3421. [PubMed] [Google Scholar]
- [23].Glasgow RE, Hampson SE, Strycker LA, Ruggiero L, Personal-model beliefs and social-environmental barriers related to diabetes self-management, Diabetes Care 20 (4) (1997) 556–561. [DOI] [PubMed] [Google Scholar]
- [24].Lorig K, Ritter PL, Laurent DD, Plant K, Green M, Jernigan VB, Case S, Online diabetes self-management program: a randomized study, Diabetes Care 33 (6) (2010) 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schulman-Green D, Bradley EH, Knobf MT, Prigerson H, DiGiovanna MP, McCorkle R, Self-management and transitions in women with advanced breast cancer, J. Pain Symptom Manag. 42 (4) (2011) 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blanch-Hartigan D, Forsythe LP, Alfano CM, Smith T, Nekhlyudov L, Ganz PA, Rowland JH, Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians, J. Clin. Oncol 32 (15) (2014) 1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dillman D, Mail and Internet Surveys: The Tailored Design Method: 2007 Update with New Internet, Visual, and Mixed-Mode Guide, John Wiley & Sons, Inc., Hoboken, NJ, 2007. [Google Scholar]
- [28].Cox CL, Zhu L, Hudson MM, Steen BD, Robison LL, Oeffinger KC, Survivor typologies predict medical surveillance participation: the childhood cancer survivor study, Psychooncology 22 (7) (2013) 1534–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobble W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL, Chronic health conditions in adult survivors of childhood cancer, N. Engl. J. Med 355 (15) (2006) 1572–1582. [DOI] [PubMed] [Google Scholar]
- [30].Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, Skinner R, Stevens MC, Hawkins MM, Long-term cause-specific mortality among survivors of childhood cancer, JAMA 304 (2) (2010) 172–179. [DOI] [PubMed] [Google Scholar]
- [31].van der Pal HJ, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van den Bos C, Oldenburger F, Koning CC, van Leeuwen FE, Kremer LC, Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study, Arch. Intern. Med 170 (14) (2010) 1247–1255. [DOI] [PubMed] [Google Scholar]
- [32].Kero AE, Jarvela LS, Arola M, Malila N, Madanat-Harjuoja LM, Matomaki J, Lahteenmaki PM, Cardiovascular morbidity in long-term survivors of early-onset cancer: a population-based study, Int. J. Cancer 134 (3) (2014) 664–673. [DOI] [PubMed] [Google Scholar]
- [33].Lipshultz SE, Landy DC, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, French CA, Rovitelli AM, Proukou C, Adams MJ, Miller TL, Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy, J. Clin. Oncol 30 (10) (2012) 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brouwer CA, Postma A, Hooimeijer HL, Smit AJ, Vonk JM, van Roon AM, van den Berg MP, Dolsma WV, Lefrandt JD, Bink-Boelkens MT, Zwart N, de Vries DG, Tissing WJ, Gietema JA, Endothelial damage in long-term survivors of childhood cancer, J. Clin. Oncol 31 (31) (2013) 3906–3913. [DOI] [PubMed] [Google Scholar]
- [35].Armenian SH, Sun CL, Vase T, Ness KK, Blum E, Francisco L, Venkataraman K, Samoa R, Wong FL, Forman SJ, Bhatia S, Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease, Blood 120 (23) (2012) 4505–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chow EJ, Baker KS, Lee SJ, Flowers ME, Cushing-Haugen KL, Inamoto Y, Khera N, Leisenring WM, Syrjala KL, Martin PJ, Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation, J. Clin. Oncol 32 (3) (2014) 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dengel DR, Kelly AS, Zhang L, Hodges JS, Baker KS, Steinberger J, Signs of early sub-clinical atherosclerosis in childhood cancer survivors, Pediatr. Blood Cancer 61 (3) (2014) 532–537. [DOI] [PubMed] [Google Scholar]
- [38].Smith WA, Li C, Nottage KA, Mulrooney DA, Armstrong GT, Lanctot JQ, Chemaitilly W, Laver JH, Srivastava DK, Robison LL, Hudson MM, Ness KK, Lifestyle and metabolic syndrome in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study, Cancer 120 (17) (2014) 2742–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D, Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline, J. Clin. Oncol 35 (8) (2017) 893–911. [DOI] [PubMed] [Google Scholar]
- [40].Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC, Gurney JG, Donaldson SS, Leisenring WM, Robison LL, Oeffinger KC, Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study, J. Clin. Oncol 26 (27) (2008) 4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baldwin LM, Dobie SA, Cai Y, Saver BG, Green PK, Wang CY, Receipt of general medical care by colorectal cancer patients: a longitudinal study, J. Am. Board Fam. Med 24 (1) (2011) 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Blaser BW, Kim HT, Alyea EP III, Ho VT, Cutler C, Armand P, Koreth J, Antin JH, Plutzky J, Soiffer RJ, Hyperlipidemia and statin use after allogeneic hematopoietic stem cell transplantation, Biol. Blood Marrow. Transplant 18 (4) (2012) 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shin DW, Kim SY, Cho J, Yang HK, Cho B, Nam HS, Kim H, Park JH, Comparison of hypertension management between cancer survivors and the general public, Hypertens. Res 35 (9) (2012) 935–939. [DOI] [PubMed] [Google Scholar]
- [44].Calip GS, Hubbard RA, Stergachis A, Malone KE, Gralow JR, Boudreau DM, Adherence to oral diabetes medications and glycemic control during and following breast cancer treatment, Pharmacoepidemiol. Drug Saf 24 (1) (2015) 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Casillas J, Oeffinger KC, Hudson MM, Greenberg ML, Yeazel MW, Ness KK, Henderson TO, Robison LL, Armstrong GT, Liu Q, Leisenring W, Yasui Y, Nathan PC, Identifying predictors of longitudinal decline in the level of medical care received by adult survivors of childhood cancer: a report from the childhood Cancer survivor study, Health Serv. Res 50 (4) (2015) 1021–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME, Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation, J. Natl. Cancer Inst 111 (3) (2019) 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bayley A, Stahl D, Ashworth M, Cook DG, Whincup PH, Treasure J, Greenough A, Ridge K, Winkley K, Ismail K, Response bias to a randomised controlled trial of a lifestyle intervention in people at high risk of cardiovascular disease: a cross-sectional analysis, BMC Public Health 18 (1) (2018) 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Galea S, Tracy M, Participation rates in epidemiologic studies, Ann. Epidemiol 17 (9) (2007) 643–653. [DOI] [PubMed] [Google Scholar]
- [49].Alexander GL, Divine GW, Couper MP, McClure JB, Stopponi MA, Fortman KK, Tolsma DD, Strecher VJ, Johnson CC, Effect of incentives and mailing features on online health program enrollment, Am. J. Prev. Med 34 (5) (2008) 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rosoff PM, Werner C, Clipp EC, Guill AB, Bonner M, Demark-Wahnefried W, Response rates to a mailed survey targeting childhood cancer survivors: a comparison of conditional versus unconditional incentives, Cancer Epidemiol. Biomark. Prev 14 (5) (2005) 1330–1332. [DOI] [PubMed] [Google Scholar]
- [51].Young JM, O'Halloran A, McAulay C, Pirotta M, Forsdike K, Stacey I, Currow D, Unconditional and conditional incentives differentially improved general practitioners' participation in an online survey: randomized controlled trial, J. Clin. Epidemiol 68 (6) (2015) 693–697. [DOI] [PubMed] [Google Scholar]
- [52].Hudson MM, Neglia JP, Woods WG, Sandlund JT, Pui CH, Kun LE. Robison LL, Green DM, Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies, Pediatr. Blood Cancer 58 (3) (2012) 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Green DM, Run LE, Matthay RR, Meadows AT, Meyer WH, Meyers PA, Spunt SL, Robison LL, Hudson MM, Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors, Pediatr. Blood Cancer 60 (7) (2013) 1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.