Abstract
Objective:
Developing effective therapies to reduce morbidity and mortality requires knowing the responsible pathophysiologies and the therapeutic advances that are likely to be impactful. Our objective was to determine at the individual patient level the important pathophysiological processes and needed therapeutic additions and advances that could prevent or ameliorate morbidities and mortalities.
Design:
Structured chart review by pediatric intensivists of pediatric Intensive care unit (PICU) children discharged with significant new morbidity or mortality to determine the pathophysiologies responsible for poor outcomes and needed therapeutic advances.
Setting:
Multi-center study (eight sites) from the Collaborative Pediatric Critical Care Research Network (CPCCRN) of general and cardiac PICUs conducted from December 2011 to April 2013.
Measurements and Main Results:
292 patients were randomly selected from 681 patients discharged with significant new morbidity or mortality. The median age was 2.4 years, 233 (79.8%) were in medical/surgical ICUs, 59 (20.2%) were in cardiac ICUs. Sixty five (22.3%) were surgical admissions. The outcomes included 117 deaths and 175 significant new morbidities. The most common pathophysiologies contributing to the poor outcomes were impaired substrate delivery (n = 158, 54.1%) and inflammation (n = 104, 35.6%). There were no strong correlations between the pathophysiologies and no remarkable clusters among them. The most common therapeutic needs involved new drugs (n = 149, 51.0%), cell regeneration (n = 115, 39.4%), and immune and inflammatory modulation (n = 79, 27.1%). As with the pathophysiologies, there was a lack of strong correlations or meaningful clusters in the suggested therapeutic needs.
Conclusions:
There was no single dominant pathophysiology or cluster of pathophysiologies responsible for poor pediatric critical care outcomes. Therapeutic needs often involved therapies that are not close to implementation such as cell regeneration, improved organ transplant, improved extra-corporeal support and artificial organs, and improved drugs.
Keywords: Research, research agenda, mortality, morbidity, pediatric critical care, pediatrics
Article Tweet:
The research agenda for PICU patients is very broad because of the diversity of pathophysiologies and needed therapeutic advances.
INTRODUCTION
Effective therapeutic advances to reduce pediatric critical care morbidity and mortality are often directed at the pathophysiological cause, traditionally classified by diagnostic classifications or the primary system of dysfunction.1–5 Yet, traditional classification systems may lack meaningful pathophysiological relationships to adverse outcomes. For example, morbidity and mortality may be associated with secondary symptom complexes such as respiratory distress syndrome that have multiple pathophysiological triggers, each of which might be responsible for the adverse outcome.6 Adverse outcomes may also be secondary to pathophysiologies not captured by the acute diagnosis such as underlying conditions or complications of medications. Conditions such as sepsis involve multiple pathophysiological processes, each potentially responsible for adverse outcomes.7,8
Research agendas are developed to derive a future benefit from an investment of research time and dollars. Both formal and informal processes have been used to develop research agendas, but the agenda-setting process has primarily focused on integrating the knowledge and values of content experts,9,10 especially for critical care.11–13 If the experts have insufficient or inaccurate information or if they are overly committed to a specific issue, the final agenda may not reflect the most productive path.
There has not been an effort in pediatric critical care to identify a research agenda that would maximally reduce morbidity and mortality. To inform such an agenda, the CPCCRN prioritized the identification of pathophysiologies responsible for new morbidities and mortality and the therapeutic advances that might ameliorate or prevent these adverse outcomes. The primary aims of this initiative, Informing the Research Agenda, were to determine the following at the individual patient level which could improve clinical outcomes: a) the important pathophysiological processes resulting in morbidity and mortality, and b) needed therapeutic additions and advances that could prevent or ameliorate morbidity and mortality. Importantly, we investigated this aim at the individual patient level with structured chart reviews rather than using expert opinion or diagnostic lists. Secondary aims included a) the development of classification schemes for important pathophysiological processes and needed therapeutic additions and advances, and b) the development of a generalizable structured chart review methodology appropriate for the primary aim and applicable to other medical issues. The chart review methodology has been published.14 This analysis focuses on the overall assessment of pathophysiologies and needed therapeutic advances. A companion analysis focuses on the specific issues identified at the patient level.15
METHODS
Patients
The patients for this analysis originated in the Trichotomous Outcome Prediction in Critical Care (TOPCC) study conducted by CPCCRN. Data collection methods and institutional characteristics have been previously described.16 There were seven funded sites, one being composed of two institutions. In brief, patients aged from newborn to less than 18 years were randomly selected and stratified by hospital from December 4, 2011 to April 7, 2013. Patients from both general-medical and cardiac-cardiovascular PICUs were included. Only the first PICU admission during a hospitalization was included. The protocol was approved by all Institutional Review Boards.
Patients discharged with a significant new morbidity or who died during their hospitalization were eligible for inclusion. A significant new morbidity was defined as an increase in the Functional Status Scale (FSS) score of 2 or more in a single functional domain from their pre-illness baseline.17,18 The previous definition of a significant new morbidity was an FSS increase of 3 or more. Since 95.4% of those patients had an increase of at least 2 in a single domain, we adopted that simpler and more conservative definition for this analysis.18 New morbidities were classified as moderate (FSS = 9–13), severe (FSS = 14–20), and very severe (FSS = >20).18 Mortalities were included if they were potentially savable on admission as indicated by a mortality risk of < 80%.19 Eligible patients at each clinical site were randomized by the data coordinating center and reviewed in the randomization sequence until 25 or more patients per site were evaluated.
Structured Chart Review
We developed a time-limited, structured chart review method based on methods initially developed for the assessment of safety and quality of health care.20–22 The method, validity, reliability, and reviewer qualifications have been published.14 In brief, reviewers at each site (3rd year critical care fellows or attendings) read the study protocol, attended a small group, web-based session which included the study overview, the structured chart review process, and the electronic data capture system, and conducted 2–4 initial reviews with one of the project co-PI’s (MMP, KLM) who served as central reviewers. The review was intended to take an average of 30 minutes per case. For each subsequent case review, the site reviewer went over their assessments with a central reviewer to maintain consistency in the classifications across sites. During this process, the reviewers confirmed the data collected in the TOPICC project and assessed the classification for the pathophysiologies and therapies (below).
Categorizing Pathophysiological Processes, and Needed Therapeutic Additions or Advances
We anticipated that developing meaningful classification systems for the primary aims might be challenging. First, causes of morbidity and mortality are often conditions or symptom complexes (e.g. respiratory distress syndrome) that have many etiologies.6 Second, symptom complexes often lack specificity because they were sometimes chosen for a high sensitivity and low specificity (e.g. systemic inflammatory response syndrome).23 Third, even when the diagnosis is known, critical care diagnoses may have several potential pathophysiological processes. For example, sepsis has multiple clinical phenotypes and pathophysiological processes that have different prognostic and therapeutic significance.7,8 Fourth, the events and pathophysiological processes most immediately associated with the adverse outcome may not be the underlying cause of the outcome that, if it had been interrupted, would have ameliorated or prevented the outcome. Finally, adverse outcomes may be related to complications of care that may not be captured by the acute diagnosis.24,25
We used free-listing to determine the classification schema for the pathophysiological process, and needed interventions and life support technologies.26–28 Free listing, a qualitative research technique, requires the recognition of the “domains of interest” (pathophysiological processes, therapeutic advances) and the use of an expert group to choose the content, scope, and domain structure. Free-listing was used as an iterative process with CPCCRN Steering Committee over three sessions. The CPCCRN Steering Committee consisted of PIs, co-PIs, and alternate PIs for the investigative sites, the data coordinating center, and representatives of the Eunice Kennedy Shriver National Institute of Child Health and Human Development with expertise relevant to this project. An iterative process with inter-current analytic summaries was used. Briefly, each individual was asked to list all pathophysiologies potentially contributing to morbidity or mortality in PICUs. Each item on each list was typed on a card. Next, cards were sorted into piles representing the same or similar pathophysiologies. The results were presented to the group and each individual was asked to suggest items to add, delete or combine. The suggestions were organized by placing similar suggestions together. MMP and KLM independently revised the list of pathophysiologies based on the group’s suggestions, and then compared their revisions. Through review and discussion, the final list of main pathophysiologic categories was generated. The category “other” was added to capture additional pathophysiologies that might be identified during the medical record review (Table 1). A similar process of free-listing and pile-sorting was used to generate the list of major therapeutic additions and advances (Table 2).
Table 1.
Pathophysiologies. The primary pathophysiology is followed by the categories.
| Impaired Substrate Delivery |
|---|
| Oxygen (hypoxia)/Blood/Other |
| Coagulation Dysfunction |
| Thrombotic disorders - congenital/acquired |
| Thrombotic disorder- clinical (platelet/clotting factors/other) |
| Bleeding disorder - congenital/acquired |
| Bleeding disorder - clinical (platelet/clotting factors/other) |
| Other |
| Inflammation |
| Infection with organism |
| Oxidative injury (acute or chronic) |
| Oxidative injury (molecular mechanism) |
| Other |
| Immune Dysfunction |
| Function increased/decreased/other |
| Toxicities |
| Drugs/endogenous substances/electrolytes/other |
| Tissue Injury (direct) |
| Trauma/burns/other |
| Malnutrition |
| General malnutrition/Other |
| Electrical Signaling Dysfunction |
| Neurological/cardiac/other |
| Abnormal Growth/Abnormal Cell Cycle |
| Malignancy/disorders of apoptosis/disorders of necrosis |
| Capillary/Vascular Dysfunction |
| Capillary leak syndrome/other |
| Mitochondrial Dysfunction |
| Other |
Table 2.
Therapeutic Interventions and Advances
| Mechanical Respiratory Support |
| Inhaled Respiratory Support |
| Renal Replacement and Plasmapheresis |
| Extra-corporeal Support and Artificial Organs |
| Extra-corporeal oxygenation |
| Extra-corporeal circulatory support |
| Other |
| Organ Transplant |
| Blood and Blood Products |
| Drugs |
| Drug Delivery |
| Immune and Inflammatory Modulation |
| Nutritional Support |
| Therapeutic Devices |
| Defibrillator |
| Nerve Stimulator |
| Stents |
| Temperature regulation |
| Vascular access |
| Ventricular drains |
| Other |
| Monitoring Devices |
| Brain oxygenation |
| Cardiac output |
| Cellular metabolism |
| Electro-encephalogram |
| Intra-cranial pressure |
| Regional blood flow (specify region) |
| Substrate utilization (i.e. oxygen, glucose, -other). |
| Gas exchange (i.e. transcutaneous) |
| Other |
| Cell Regeneration |
| Suspended Animation |
| Mitochondrial Support |
| Other |
The reviewers recorded the process(es) and sub-process(es) occurring for each patient to support their conclusion and these was reviewed with the co-PIs. Additionally, the reviewers and co-PIs recorded if a chronic condition29 contributed to the morbidity or mortality.
Data
Descriptive data have been described.16 Morbidity was determined with the FSS and severity of illness was characterized using the Pediatric Risk of Mortality (PRISM) score.19
Each reviewer confirmed the patient data consisting of age, baseline and hospital discharge functional status and the admission and discharge dates. Since the functional status in the TOPICC database was obtained using information from the bedside care givers as well as the medical record, we expected that some of the TOPICC morbidity data would not be confirmed using the medical record. If the morbidity could not be confirmed, the patient was excluded.
Multiple pathophysiologies and needed therapeutic additions or advances could be selected for each patient. Additionally, both the site and central reviewers constructed pathophysiological sequences for the morbidity or mortality. After all reviews were completed, the central reveiwers reviewed all cases together to further ensure consistency in the classifications.
Statistics
Summaries for continuous variables use medians [Q1, Q3] while categorical summaries are presented using counts and percentages. To assess the differences among included and excluded patients, the Wilcoxon rank-sum test was used to compare age, PRISM, and baseline FSS. Categorical variables are compared using Fisher’s exact test. For patients surviving to hospital discharge, the Cochran-Armitage trend test was used to assess any directional tendency in dysfunction severity at hospital discharge. Cluster analysis approaches using the single linkage method as implemented in the R function hclust30 were used to display relationships within and between pathophysiologies, chronic conditions, and therapeutic conditions and advances. The Canberra distance metric31 was used for clustering of pairwise count data, while standard Euclidean distance was used for clustering of Spearman correlations. Dendrograms summarizing relationships between factors were constructed using the Euclidean distance metric with the heat map function. Graphical displays of matrices and dendrograms were generated using the R packages reshape232 and ggplot2.33 The matrices and dendrograms presented should not necessarily be construed as “best” or optimal summaries of associations, but rather as guides to identifying relatively similar and dissimilar factors.
RESULTS
Of 10,078 children in the TOPICC study, 681 had a significant new morbidity or mortality at hospital discharge. Among these patients, 327 were randomly selected for chart review. Thirty-five patients (10.7%) were excluded because data could not be confirmed (Supplemental Table 1). While age, sex, and discharge FSS scores did not differ between the included and excluded samples, the excluded sample had lower mortality rates (2.9% vs. 40.1%, p < 0.001) and PRISM scores (2.0 [0.0, 10] vs.7.0 [0.5, 14.0], p < 0.009). The differences in mortality rates and PRISM scores were expected because the primary exclusion criterion was the inability to confirm the discharge FSS which was only present in survivors.
The sample characteristics of the 292 included patients are shown in Table 3. The median age was 2.4 years, 55.5% were male, 79.8% were in combined medical-surgical ICUs, 20.2% were in cardiac ICUs, 22.3% were surgical admissions, and their median PRISM score was 7.0. The outcomes included 40.1% mortality and 59.9% new morbidity rates. A total of 65.1% of the survivors were discharged with moderate functional disability (change in FSS of 4.0), 25.1% with severe disability (change in FSS of 7.5), and 9.7% with very severe disability (change in FSS of 15.0).
Table 3.
Sample Characteristics
| Overall (n=292) | |
|---|---|
| Age in years at PICU Admission | 2.4 [0.4, 9.5] |
| Sex | |
| Male | 162 (55.5%) |
| Female | 130 (44.5%) |
| Race | |
| White | 135 (46.2%) |
| Black | 78 (26.7%) |
| Other/Unknown | 79 (27.1%) |
| Ethnicity | |
| Hispanic or Latino | 54 (18.5%) |
| Not Hispanic or Latino | 198 (67.8%) |
| Unknown or Not Reported | 40 (13.7%) |
| Elective/Emergency status | |
| Elective | 59 (20.2%) |
| Emergency | 233 (79.8%) |
| Admission category1 | |
| Post-intervention - Cardiac | 35 (12.0%) |
| Post-intervention - Non-Cardiac | 30 (10.3%) |
| Medical Admission (non-intervention) | 227 (77.7%) |
| Admission source | |
| Operating Room/Post-Anesthesia Recovery Unit | 65 (22.3%) |
| Inpatient Unit from Same Hospital | 57 (19.5%) |
| Direct Admission From Other Hospital | 84 (28.8%) |
| Emergency Department | 86 (29.5%) |
| Payer | |
| Commercial | 99 (33.9%) |
| Government | 169 (57.9%) |
| Other | 24 (8.2%) |
| PICU type | |
| Cardiac | 59 (20.2%) |
| Medical/Surgical/Other | 233 (79.8%) |
| PICU Length of stay (days) | 8.9 [2.8, 22.2] |
| Hospital length of stay (days) | 20.8 [8.2, 45.4] |
| Baseline FSS Score | 6.0 [6.0, 8.0] |
| PRISM Score | 7.0 [0.5, 14.0] |
| Discharge Outcome | |
| Died | 117 (40.1%) |
| Morbidity2 | 175 (59.9%) |
| Moderate dysfunction | 114 (65.1%) |
| Severe dysfunction | 44 (25.1%) |
| Very severe dysfunction | 17 (9.7%) |
Intervention includes operations and interventional catheterizations
FSS categories for dysfunction: Moderate = 9–13; Severe = 14–20 ; Very Severe = >20. Percentages of the morbidity categories refer to survivors.
The pathophysiologies responsible for morbidities and mortalities are shown in Table 4. Overall, there were 2.9 ± 1.4 pathophysiologies/patient. Impaired substrate delivery (n = 158, 54.1%), inflammation (35.6%) and direct tissue injury (21.9%) were the most common with all other pathophysiologies except mitochondrial dysfunction present in >10% of cases. The highest mortality rates were observed in patients with coagulation dysfunction (61.5%), impaired substrate delivery (58.9%), vascular/capillary dysfunction (55.8%) and immune dysfunction (53.1%). The PRISM score hierarchy was similar. The highest morbidity rates were in those with toxicities (72.5%), malnutrition (72.2%), and electrical signaling (69.2%) categories. Chronic conditions contributed to the morbidity or mortality in 156 children (53.4%) and had an associated mortality rate of 45.5%. Impaired substrate delivery, capillary/vascular dysfunction, and coagulation dysfunction were more frequently (p<.05) associated with mortality than morbidity (Supplemental Table 2).
Table 4.
Pathophysiologies and Needed Therapeutic Additions and Advances.
| N (%) | Age (years) (1) | Deaths (N (%)) (2) | PRISM Score (1) | |
|---|---|---|---|---|
| PATHOPHYSIOLOGIES | ||||
| Impaired Substrate Delivery | 158 (54.1%) | 1.5 [0.3, 7.8] | 93 (58.9%) | 11.0 [3.0, 19.0] |
| Inflammation | 104 (35.6%) | 3.5 [0.7, 11.1] | 47 (45.2%) | 5.0 [0.0, 12.0] |
| Tissue injury | 64 (21.9%) | 4.5 [1.0, 10.6] | 25 (39.1%) | 8.0 [3.0, 16.0] |
| Electrical Signaling Dysfunction | 52 (17.8%) | 1.6 [0.4, 8.0] | 16 (30.8%) | 5.0 [0.0, 13.5] |
| Abnormal Growth / Abnormal Cell Cycle | 52 (17.8%) | 2.9 [0.5, 7.5] | 22 (42.3%) | 6.0 [0.0, 12.0] |
| Capillary / Vascular Dysfunction | 52 (17.8%) | 2.3 [0.5, 8.7] | 29 (55.8%) | 7.0 [2.5, 15.0] |
| Toxicities | 51 (17.5%) | 3.1 [0.5, 10.3] | 14 (27.5%) | 7.0 [0.0, 16.0] |
| Immune Dysfunction | 49 (16.8%) | 10.1 [3.0, 13.7] | 26 (53.1%) | 8.0 [1.0, 13.0] |
| Coagulation Dysfunction | 39 (13.4%) | 2.2 [0.4, 12.6] | 24 (61.5%) | 9.0 [3.0, 19.0] |
| Malnutrition | 36 (12.3%) | 1.1 [0.2, 4.5] | 10 (27.8%) | 3.5 [0.0, 8.0] |
| Mitochondrial Dysfunction | 5 (1.7%) | 0.7 [0.5, 13.7] | 2 (40.0%) | 7.0 [6.0, 12.0] |
| OTHER | 19 (6.5%) | 1.2 (0.2, 12.8) | 6 (31.6%) | 6.0 (3.9, 12.0) |
| NEEDED THERAPEUTIC ADDITIONS/ADVANCES (3) | ||||
| Drugs | 149 (51.0%) | 3.1 [0.5, 9.5] | 57 (38.3%) | 6.0 [0.0, 13.0] |
| Cellular Regeneration | 115 (39.4%) | 3.1 [0.4, 10.4] | 39 (33.9%) | 9.0 [3.0, 18.0] |
| Immune and Inflammatory Modulation | 79 (27.1%) | 7.0 [1.4, 13.4] | 39 (49.4%) | 7.0 [2.0, 14.0] |
| Extra-corporeal Support and Artificial Organs | 47 (16.1%) | 0.6 [0.0, 4.9] | 33 (70.2%) | 10.0 [3.0, 18.0] |
| Organ Transplantation | 47 (16.1%) | 1.9 [0.2, 10.1] | 33 (70.2%) | 10.0 [3.0, 15.0] |
| Mechanical Respiratory Support | 41 (14.0%) | 1.7 [0.7, 9.5] | 16 (39.0%) | 5.0 [0.0, 12.0] |
| Nutritional Support | 39 (13.4%) | 1.1 [0.2, 4.7] | 10 (25.6%) | 4.0 [0.0, 8.0] |
| Therapeutic Devices | 28 (9.6%) | 0.8 [0.2, 5.4] | 15 (53.6%) | 7.5 [3.0, 17.5] |
| Monitoring Devices | 28 (9.6%) | 2.2 [0.3, 7.9] | 13 (46.4%) | 7.5 [0.5, 21.5] |
| Blood and Blood Products | 9 (3.1%) | 4.3 [1.4, 12.6] | 6 (66.7%) | 13.0 [5.0, 20.0] |
| Renal Replacement Therapy and Plasmapheresis | 8 (2.7%) | 6.5 [3.5, 15.8] | 6 (75.0%) | 7.0 [2.5, 17.0] |
| Mitochondrial Support | 6 (2.1%) | 0.6 [0.4, 13.7] | 3 (50.0%) | 6.5 [2.0, 12.0] |
| Inhaled Respiratory Support | 5 (1.7%) | 3.0 [0.6, 11.1] | 0 (0.0%) | 2.0 [1.0, 4.0] |
| Suspended Animation | 2 (0.7%) | 0.6 [0.4, 0.7] | 2 (100.0%) | 14.5 [3.0, 26.0] |
| Other | 92 (31.5%) | 2.3 (0.4, 9.3) | 40 (43.5%) | 7.0 (1.0, 13.0) |
|
| ||||
Median (Quartile 1, Quartile 3).
The reported death rate is out of those with the specific therapy.
Drug Delivery Methods were not selected.
There was a lack of strong pairwise associations between the pathophysiologies. The two most frequent pathophysiology pairs were impaired substrate delivery and inflammation (n = 57), and capillary and vascular dysfunction and impaired substrate delivery (n = 38) (Supplemental Figure 1). Similarly, there were few strong associations between the pathophysiologies (Supplemental Figure 2). The highest positive Spearman correlation coefficients were between immune dysfunction and inflammation (r = .34) with other weak positive correlations between vascular/capillary dysfunction and impaired substrate delivery (r = .18), and coagulation dysfunction and impaired substrate delivery (r = .18). The highest negative correlation occurred between abnormal growth/abnormal cell cycle and direct tissue injury (r=−.18). The dendrogram cluster analysis was consistent with these associations with no pairs or clusters of strong similarity
The proposed therapeutic additions and advances are show in Table 4. Overall, there were 2.3 ± 1.2 therapeutic additions and advances/patient identified. Because this tabulation was unique, we expected that some of the categories might not be frequently selected. The most common suggested advances involved new drugs (n = 149, 51.0%), cell regeneration (n = 115, 39.4%), and immune and inflammatory modulation (n = 79, 27.1%). One category was not selected (drug delivery methods) and seven were selected in less than 10% of the cases. The highest death rates occurred in the categories of renal replacement and plasmapheresis (75.0%); extracorporeal support and artificial organs (70.2%); and organ transplant (70.2%).
Similar to the pathophysiological processes, there was a lack of strong pairwise association between the therapeutic additions and advances. Only two pairs had at least 40 occurrences, new drugs and cell regeneration (n=47), and new drugs and immune and inflammatory modulation (n=46) (Supplemental Figure 4). Similarly, there were few strong associations (Supplemental Figure 5) with the highest positive correlations between extracorporeal support and artificial organs and organ transplant (r = .39), mitochondrial support and renal replacement and plasmapheresis (r = .27), and immune and inflammatory modulation and renal replacement and plasmapheresis (r = .23). The highest negative correlation occurred between organ transplant and cell regeneration (r = −22). The dendrogram cluster analysis (Supplemental Figure 6) indicated that six categories were relatively similar: suspended animation, drug delivery, mitochondrial support, inhaled respiratory support, renal replacement and plasmapheresis, and blood and blood products, primarily because they were very infrequently chosen.
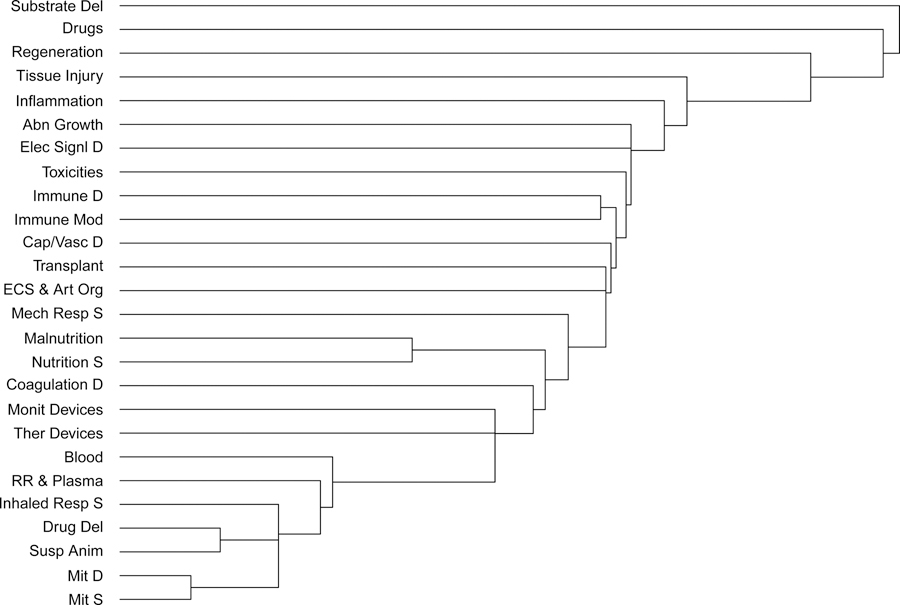
Figure 1 shows the cluster analysis for both the pathophysiological processes and proposed therapeutic additions and advances. Several of the pathophysiologies and therapeutic additions and advances show the expected pairwise similarities including malnutrition and nutritional support, transplantation and extra-corporeal support/artificial organs, and immune dysfunction and immune and inflammatory modulation. The most similar cluster of items included suspended animation, drug delivery, inhaled respiratory support, renal replacement and plasmapheresis, mitochondrial dysfunction and support, and blood products but their similarities were primarily a result of their lack of selection.
Figure 1.
Clustering of Pathophysiologies and Therapeutic Innovations. In this Figure, the algorithm recursively combines the pathophysiologies into clusters. The clustering process is seen from bottom to top, with the height of each “branch” reflecting relative similarities between clusters using the Euclidean distance. Longer “branches” indicate weaker associations.
Abbreviations: Impaired Substrate Delivery = Substrate Del; Electrical Signaling Dysfunction = Elec Sign D; Abnormal Growth / Abnormal Cell Cycle = Abn Growth; Capillary / Vascular Dysfunction = Cap/Vasc D; Immune Dysfunction = Immune D; Coagulation Dysfunction = Coagulation D; Mitochondrial Dysfunction = Mit D. Cell Regeneration = Regeneration; Immune and Inflammatory Modulation = Immune Mod; Extra-corporeal Support and Artificial Organs = ECS & Art Org; Organ Transplant = Transplant; Mechanical Respiratory Support = Mech Resp S; Nutritional Support = Nutrition; Therapeutic Devices = Ther Devices; Monitoring Devices = Monit Devices; Blood and Blood Products = Blood; Renal Replacement and Plasmapheresis = RR & Plasma; Mitochondrial Support = Mit S; Inhaled Respiratory Support = Inhaled Resp S; Suspended Animation = Susp Anim.
DISCUSSION
CPCCRN, first formed in 2005, was funded “to initiate a multi-centered program designed to investigate the safety and efficacy of treatment and management strategies to care for critically ill children, as well as the pathophysiologic basis of critical illness and injury in childhood.”34–36 While the network has placed a high priority on better understanding the pathophysiologies responsible for new morbidities and mortality and the therapeutic advances that might have ameliorated or prevented these adverse outcomes, it had not undertaken a formal assessment of these issues. The primary goal of this initiative was to identify research areas that could have the greatest impact on outcomes.
The major finding of this analysis is the lack of a single dominant pathophysiology or cluster of pathophysiologies responsible for the adverse outcomes. Impaired substrate delivery was the only pathophysiology noted in over half of the individuals (54.1%) and inflammation was the only other pathophysiology noted in over a third of individuals (35.6%). There were no strong pathophysiological associations when assessed with correlation or cluster analyses. Although not a pathophysiology, chronic conditions such as congenital heart disease, neuromuscular conditions, or malignancy were noted as a significant contributor in 53.4% of the cases and were most frequently paired with impaired substrate delivery. Several of the pathophysiological contributors to poor outcomes are potentially approachable without major new advances. Reviewers judged that malnutrition was a significant pathophysiology in 12.3% and toxicities were a significant contributor in 17.5% of cases. These problems can often be approached with emphasis on nutritional support and drug and electrolyte monitoring.
The most common therapeutic advances involved new drugs (51.0%), cell regeneration (39.4%), and inflammatory and immune modulation (27.1%). Proposed therapeutic advances often illustrated the difficulties of caring for patients without effective therapies including cell regeneration (39.4%), improved organ transplant (16.1%), improved extra-corporeal support and artificial organs (16.1%), and improved drugs (51.0%). The reviewers often noted the frustrating situation of not having effective therapies available. While some of these therapies may seem like fantasies, there is sufficient effort to generate optimism that these approaches may improve therapeutic options in the future.37–43
This effort focusing on the assessment of pathophysiologies and therapeutic needs at the individual patient level is unique, especially for identifying a research agenda. Previous efforts at agenda setting have relied on expert opinion without explicit assessments of the magnitude of the need.11–13 Others, predominantly trauma programs, have focused on preventable acute care deaths identified by routine clinical and review criteria.44,45 The Pediatric Emergency Care Applied Research Network developed its research priorities using expert opinion that explicitly included prevalence, seriousness, and practicality.46
There are several important limitations to this study. First, the focus on individual patients required subjective conclusions by experienced content experts conducting the chart reviews and collaboration with central reviewers to insure classification consistency amongst the sites. While we provided an organization for the classifications, the classifications at the individual patient level were a subjective interpretation of the medical record. While previous analysis demonstrated strong inter-rater reliability at two sites,14 this was not done at all sites. Second, the classification schemes for both pathophysiologies and therapeutic advances are unique. It remains to be seen how useful these classifications will be. Third, this manuscript does not focus on prevention or the timing of detection or therapy. The companion analysis includes these issues identified at the patient level.15
CONCLUSIONS
The research agenda for pediatric critical care should be driven in large part by what is needed to reduce or prevent adverse outcomes. Unfortunately, there was a lack of a dominant causative pathophysiology or needed therapy addition or advance. This diversity makes this task harder. A companion paper analyzes the issue at the patient level, describing the specific issues identified for each of the patients in this analysis.15
Supplementary Material
Supplemental Table 1. Comparison of Included and Excluded Patients
Supplemental Figure 1. Pairs of Pathophysiologies. Abbreviations: Impaired Substrate Delivery = Substrate Del; Electrical Signaling Dysfunction = Elec Sign D; Abnormal Growth / Abnormal Cell Cycle = Abn Growth; Capillary / Vascular Dysfunction = Cap/Vasc D; Immune Dysfunction = Immune D; Coagulation Dysfunction = Coagulation D; Mitochondrial Dysfunction = Mit D.
Supplemental Figure 2. Correlations between Pathophysiologies. Abbreviations: Impaired Substrate Delivery = Substrate Del; Electrical Signaling Dysfunction = Elec Sign D; Abnormal Growth / Abnormal Cell Cycle = Abn Growth; Capillary / Vascular Dysfunction = Cap/Vasc D; Immune Dysfunction = Immune D; Coagulation Dysfunction = Coagulation D; Mitochondrial Dysfunction = Mit D.
Supplemental Figure 3. Clustering of Pathophysiologies. In this Figure, the algorithm recursively combines the pathophysiologies into clusters. The clustering process is seen from bottom to top, with the height of each “branch” reflecting relative similarities between clusters using the Euclidean distance. Longer “branches” indicate weaker associations.
Supplemental Figure 4. Pairs of Therapeutic Innovations. Abbreviations: Cell Regeneration = Regeneration; Immune and Inflammatory Modulation = Immune Mod; Extra-corporeal Support and Artificial Organs = ECS & Art Org; Organ Transplant = Transplant; Mechanical Respiratory Support = Mech Resp S; Nutritional Support = Nutrition; Therapeutic Devices = Ther Devices; Monitoring Devices = Monit Devices; Blood and Blood Products = Blood; Renal Replacement and Plasmapheresis = RR & Plasma; Mitochondrial Support = Mit S; Inhaled Respiratory Support = Inhaled Resp S; Suspended Animation = Susp Anim.
Supplemental Figure 5. Correlations Between Therapeutic Innovations. Abbreviations: Cell Regeneration = Regeneration; Immune and Inflammatory Modulation = Immune Mod; Extra-corporeal Support and Artificial Organs = ECS & Art Org; Organ Transplant = Transplant; Mechanical Respiratory Support = Mech Resp S; Nutritional Support = Nutrition; Therapeutic Devices = Ther Devices; Monitoring Devices = Monit Devices; Blood and Blood Products = Blood; Renal Replacement and Plasmapheresis = RR & Plasma; Mitochondrial Support = Mit S; Inhaled Respiratory Support = Inhaled Resp S; Suspended Animation = Susp Anim.
Supplemental Figure 6. Clustering of Therapeutic Innovations. In this Figure, the algorithm recursively combines the pathophysiologies into clusters. The clustering process is seen from bottom to top, with the height of each “branch” reflecting relative similarities between clusters using the Euclidean distance. Longer “branches” indicate weaker associations. Abbreviations: Cell Regeneration = Regeneration; Immune and Inflammatory Modulation = Immune Mod; Extra-corporeal Support and Artificial Organs = ECS & Art Org; Organ Transplant = Transplant; Mechanical Respiratory Support = Mech Resp S; Nutritional Support = Nutrition; Therapeutic Devices = Ther Devices; Monitoring Devices = Monit Devices; Blood and Blood Products = Blood; Renal Replacement and Plasmapheresis = RR & Plasma; Mitochondrial Support = Mit S; Inhaled Respiratory Support = Inhaled Resp S; Suspended Animation = Susp Anim.
Acknowledgments
Funding Source: Supported, in part, by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services: U10HD050096, U10HD049981, U10HD049983, U10HD050012, U10HD063108, U10HD063114 and U01HD049934. UG1HD083171, UG1HD083166, and UG1HD083170.
Authors from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network.
(alphabetical order)
Christian Bauerfeld, MD; Department of Pediatrics, Children's Hospital of Michigan, Wayne State University, Detroit, MI
David Beyda, MD; Critical Care Medicine, Phoenix Children’s Hospital, and University of Arizona College of Medicine-Phoenix, Phoenix, AZ
Robert A. Berg, MD; Department of Anesthesiology and Critical Care, Children’s Hospital of Philadelphia, Philadelphia, PA
Melissa M. Bolton, MBA; Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT
Yonca Bulut, MD; Department of Pediatrics, UCLA Mattel Children's Hospital, University of California, Los Angeles, CA
Randall S. Burd, MD, PhD; Division of Trauma and Burn Surgery, Center for Surgical Care, Children's National Medical Center, Washington, DC
Joseph Carcillo, MD; Department of Critical Care Medicine, Children's Hospital of Pittsburgh, Pittsburgh, PA
J. Michael Dean, MD; Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT
Eleanor Gradidge, MD; Phoenix Children’s Hospital, Phoenix, AZ
Mark W. Hall, MD; Division of Critical Care, Department of Pediatrics, Nationwide Children's Hospital, Columbus, OH and Center for Clinical and Translational Research, The Research Institute at Nationwide Children's Hospital, Columbus, OH.
Peter M. Mourani, MD; Department of Pediatrics, University of Colorado School of Medicine and Children’s Hospital of Colorado, Aurora, CO.
Christopher J. L. Newth, MD, FRCPC; Department of Anesthesiology and Critical Care Medicine, Children's Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, CA
Daniel A. Notterman, MD; Department of Molecular Biology, Princeton University, 219 Lewis Thomas Lab, Princeton, NJ.
Margaret A. Priestley, MD; Department of Anesthesiology and Critical Care , Children’s Hospital of Philadelphia, Philadelphia, PA
Ashley Siems, MD; Department of Pediatrics, Children’s National Health System and the George Washington University School of Medicine and Health Sciences, Washington DC
David L. Wessel, MD; Department of Pediatrics, Children's National Medical Center and George Washington University School of Medicine, Washington DC
Andrew R. Yates, MD; Department of Pediatrics, Nationwide Children's Hospital, The Ohio State University, Columbus, OH
Footnotes
Copyright form disclosure: Drs. Pollack, Banks, Holubkov, Meert, Berg, Bolton, Dean, Mourani, Newth, and Wessel’s institutions received funding from the National Institutes of Health (NIH). Drs. Pollack, Banks, Holubkov, Meert, Berg, Bolton, Carcillo, Dean, Hall, Mourani, Newth, Priestley, Wessel and Yates received support for article research from the NIH. Drs. Banks and Bolton disclosed government work. Dr. Holubkov received funding from Pfizer (DSMB), Medimmune (DSMB), Physicians Committee for Responsible Medicine (biostatistical consulting), Revance (DSMB), Armaron Bio (DSMB), and DURECT Corporation (biostatistical consulting). Drs. Carcillo, Hall, and Yates institutions received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Dr. Hall received funding from LaJolla Pharmaceuticals (DSMB service). Dr. Newth received funding from Philips Research North America and Hamilton Medical AG. Dr. Priestley’s institution received funding from Collaborative Pediatric Critical Care Research Network (CPCCRN) of the NICHD. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Murray M. Pollack, Department of Pediatrics, Children’s National Health System and the George Washington University School of Medicine and Health Sciences, Washington DC.
Russell Banks, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT.
Richard Holubkov, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT.
Kathleen L. Meert, Department of Pediatrics, Children’s Hospital of Michigan, Detroit, MI.
Eunice Kennedy Shriver, National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network.
REFERENCES
- 1.Centers for Disease Control. ICD-10-CM Official Guidelines for Coding and Reporting. FY 2019. (October 1, 2018 - September 30, 2019). 2019; https://www.cdc.gov/nchs/icd/data/10cmguidelines-FY2019-final.pdf.
- 2.Bone MF, Feinglass JM, Goodman DM. Risk factors for acquiring functional and cognitive disabilities during admission to a PICU*. Pediatr Crit Care Med 2014;15(7):640–648. [DOI] [PubMed] [Google Scholar]
- 3.Watson RS, Choong K, Colville G, et al. Life after Critical Illness in Children-Toward an Understanding of Pediatric Post-intensive Care Syndrome. The Journal of pediatrics. 2018;198:16–24. [DOI] [PubMed] [Google Scholar]
- 4.Jones S, Rantell K, Stevens K, et al. Outcome at 6 months after admission for pediatric intensive care: a report of a national study of pediatric intensive care units in the United kingdom. Pediatrics. 2006;118(5):2101–2108. [DOI] [PubMed] [Google Scholar]
- 5.Ong C, Lee JH, Leow MK, Puthucheary ZA. Functional Outcomes and Physical Impairments in Pediatric Critical Care Survivors: A Scoping Review. Pediatr Crit Care Med 2016;17(5):e247–259. [DOI] [PubMed] [Google Scholar]
- 6.El-Haddad H, Jang H, Chen W, Soubani AO. Effect of ARDS Severity and Etiology on Short-Term Outcomes. Respiratory care. 2017;62(9):1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carcillo JA, Podd B, Aneja R, et al. Pathophysiology of Pediatric Multiple Organ Dysfunction Syndrome. Pediatr Crit Care Med 2017;18(3_suppl Suppl 1):S32–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seymour CW, Gomez H, Chang CH, et al. Precision medicine for all? Challenges and opportunities for a precision medicine approach to critical illness. Crit Care. 2017;21(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson E, Goyal NK, Dhepyasuwan N, et al. Prioritizing a research agenda: a Delphi study of the better outcomes through research for newborns (BORN) network. Hosp 2014;4(4):195–202. [DOI] [PubMed] [Google Scholar]
- 10.Collins FS. Research agenda. Opportunities for research and NIH. Science. 2010;327(5961):36–37. [DOI] [PubMed] [Google Scholar]
- 11.Peters MJ, Argent A, Festa M, et al. The intensive care medicine clinical research agenda in paediatrics. Intensive Care Med 2017;43(9):1210–1224. [DOI] [PubMed] [Google Scholar]
- 12.Deutschman CS, Ahrens T, Cairns CB, Sessler CN, Parsons PE, Critical Care Societies Collaborative UTFoCCR. Multisociety Task Force for Critical Care Research: key issues and recommendations. Crit Care Med 2012;40(1):254–260. [DOI] [PubMed] [Google Scholar]
- 13.Weiss CH, Krishnan JA, Au DH, et al. An Official American Thoracic Society Research Statement: Implementation Science in Pulmonary, Critical Care, and Sleep Medicine. Am J Respir Crit Care Med 2016;194(8):1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siems ABR, Holubkov R, et al. Structured Chart Review: Assessment of a Structured Chart Review Methodology Hosp Pediatr 2019;Accepted. [DOI] [PMC free article] [PubMed]
- 15.Meert KLBR, Holubkov R, et al. Morbidity and Mortality in Critically Ill Children. II. Specific Diseases, Conditions and Potential Solutions. Critical Care Medicine. 2019;submitted.
- 16.Pollack MM, Holubkov R, Funai T, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med 2015;43(8):1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124(1):e18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollack MM, Holubkov R, Funai T, et al. Pediatric Intensive Care Outcomes: Development of New Morbidities During Pediatric Critical Care. Pediatr Crit Care Med 2014;15(9):821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollack MM, Holubkov R, Funai T, et al. The Pediatric Risk of Mortality Score: Update 2015. Pediatr Crit Care Med 2016;17(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med 1991;324(6):370–376. [DOI] [PubMed] [Google Scholar]
- 21.Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med 1991;324(6):377–384. [DOI] [PubMed] [Google Scholar]
- 22.Hiatt HH, Barnes BA, Brennan TA, et al. A study of medical injury and medical malpractice. N Engl J Med 1989;321(7):480–484. [DOI] [PubMed] [Google Scholar]
- 23.Vincent J-L, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet 2013;381(9868):774–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howell AM, Panesar SS, Burns EM, Donaldson LJ, Darzi A. Reducing the burden of surgical harm: a systematic review of the interventions used to reduce adverse events in surgery. Ann Surg 2014;259(4):630–641. [DOI] [PubMed] [Google Scholar]
- 25.Devlin JW, Mallow-Corbett S, Riker RR. Adverse drug events associated with the use of analgesics, sedatives, and antipsychotics in the intensive care unit. Crit Care Med 2010;38(6 Suppl):S231–243. [DOI] [PubMed] [Google Scholar]
- 26.Weiss W, Bolton P. Training in Qualitative Resarch Methods for PVOs and NGOs (and counterparts) A Trainers Guide to Strenghten Program Planning and Evaluation. January 2000. ed. Johns Hopkins University: Center for Refugee and Disaster Studies. The Johns Hopkins Univeristy School of Public Health; 2000:1–18. [Google Scholar]
- 27.Rollins N, Chanza H, Chimbwandira F, et al. Prioritizing the PMTCT implementation research agenda in 3 African countries: INtegrating and Scaling up PMTCT through Implementation REsearch (INSPIRE). Journal of acquired immune deficiency syndromes (1999). 2014;67 Suppl 2:S108–113. [DOI] [PubMed] [Google Scholar]
- 28.Regis T, Steiner MJ, Ford CA, Byerley JS. Professionalism expectations seen through the eyes of resident physicians and patient families. Pediatrics. 2011;127(2):317–324. [DOI] [PubMed] [Google Scholar]
- 29.Dosa NP, Boeing NM, Ms N, Kanter RK. Excess risk of severe acute illness in children with chronic health conditions. Pediatrics. 2001;107(3):499–504. [DOI] [PubMed] [Google Scholar]
- 30.R-Core-Team. R: A language and environment for statistical computing. 2018; https://www.R-project.org/. Accessed April 1, 2019.
- 31.Lance GNWW. Mixed-data classificatory programs I.) Agglomerative Systems. Australian Computer Journal. 1967:15–20.
- 32.Wickham H Reshaping data with the reshape package. Journal of Statistical Software. 2007;21(12):1–20. [Google Scholar]
- 33.Wickham H ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 34.National Institute of Child Health and Human Development DoHaHS. RFA-HD-04–004. April 2004
- 35.Willson DF, Dean JM, Meert KL, et al. Collaborative pediatric critical care research network: looking back and moving forward. Pediatr Crit Care Med 2010;11(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willson DF, Dean JM, Newth C, et al. Collaborative Pediatric Critical Care Research Network (CPCCRN). Pediatr Crit Care Med 2006;7(4):301–307. [DOI] [PubMed] [Google Scholar]
- 37.Sueblinvong V, Weiss DJ. Cell therapy approaches for lung diseases: current status. Current opinion in pharmacology. 2009;9(3):268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werner JK, Stevens RD. Traumatic brain injury: recent advances in plasticity and regeneration. Current opinion in neurology. 2015;28(6):565–573. [DOI] [PubMed] [Google Scholar]
- 39.Mobius MA, Thebaud B. Stem Cells and Their Mediators - Next Generation Therapy for Bronchopulmonary Dysplasia. Frontiers in medicine. 2015;2:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang L, Jones S, Jia X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. International journal of molecular sciences. 2017;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorgersen EB, Barratt-Due A, Haugaa H, et al. The role of complement in liver injury, regeneration and transplantation. Hepatology (Baltimore, Md). 2019. [DOI] [PMC free article] [PubMed]
- 42.Ciccocioppo R, Dos Santos CC, Baumgart DC, et al. Proceedings of the signature series event of the international society for cellular therapy: “Advancements in cellular therapies and regenerative medicine in digestive diseases,” London, United Kingdom, May 3, 2017. Cytotherapy. 2018;20(3):461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh DK, Kim YS, Oh YM. Lung Regeneration Therapy for Chronic Obstructive Pulmonary Disease. Tuberculosis and respiratory diseases. 2017;80(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkins DH, Cioffi WG, Cocanour CS, et al. Position statement of the Coalition for National Trauma Research on the National Academies of Sciences, Engineering and Medicine report, A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths After Injury. J Trauma Acute Care Surg 2016;81(5):816–818. [DOI] [PubMed] [Google Scholar]
- 45.Berwick DM, Downey AS, Cornett EA. A National Trauma Care System to Achieve Zero Preventable Deaths After Injury: Recommendations From a National Academies of Sciences, Engineering, and Medicine Report. Jama 2016;316(9):927–928. [DOI] [PubMed] [Google Scholar]
- 46.Tzimenatos L, Kim E, Kuppermann N. The Pediatric Emergency Care Applied Research Network: a history of multicenter collaboration in the United States. Pediatric Emergency Care.31(1):70–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Comparison of Included and Excluded Patients
Supplemental Figure 1. Pairs of Pathophysiologies. Abbreviations: Impaired Substrate Delivery = Substrate Del; Electrical Signaling Dysfunction = Elec Sign D; Abnormal Growth / Abnormal Cell Cycle = Abn Growth; Capillary / Vascular Dysfunction = Cap/Vasc D; Immune Dysfunction = Immune D; Coagulation Dysfunction = Coagulation D; Mitochondrial Dysfunction = Mit D.
Supplemental Figure 2. Correlations between Pathophysiologies. Abbreviations: Impaired Substrate Delivery = Substrate Del; Electrical Signaling Dysfunction = Elec Sign D; Abnormal Growth / Abnormal Cell Cycle = Abn Growth; Capillary / Vascular Dysfunction = Cap/Vasc D; Immune Dysfunction = Immune D; Coagulation Dysfunction = Coagulation D; Mitochondrial Dysfunction = Mit D.
Supplemental Figure 3. Clustering of Pathophysiologies. In this Figure, the algorithm recursively combines the pathophysiologies into clusters. The clustering process is seen from bottom to top, with the height of each “branch” reflecting relative similarities between clusters using the Euclidean distance. Longer “branches” indicate weaker associations.
Supplemental Figure 4. Pairs of Therapeutic Innovations. Abbreviations: Cell Regeneration = Regeneration; Immune and Inflammatory Modulation = Immune Mod; Extra-corporeal Support and Artificial Organs = ECS & Art Org; Organ Transplant = Transplant; Mechanical Respiratory Support = Mech Resp S; Nutritional Support = Nutrition; Therapeutic Devices = Ther Devices; Monitoring Devices = Monit Devices; Blood and Blood Products = Blood; Renal Replacement and Plasmapheresis = RR & Plasma; Mitochondrial Support = Mit S; Inhaled Respiratory Support = Inhaled Resp S; Suspended Animation = Susp Anim.
Supplemental Figure 5. Correlations Between Therapeutic Innovations. Abbreviations: Cell Regeneration = Regeneration; Immune and Inflammatory Modulation = Immune Mod; Extra-corporeal Support and Artificial Organs = ECS & Art Org; Organ Transplant = Transplant; Mechanical Respiratory Support = Mech Resp S; Nutritional Support = Nutrition; Therapeutic Devices = Ther Devices; Monitoring Devices = Monit Devices; Blood and Blood Products = Blood; Renal Replacement and Plasmapheresis = RR & Plasma; Mitochondrial Support = Mit S; Inhaled Respiratory Support = Inhaled Resp S; Suspended Animation = Susp Anim.
Supplemental Figure 6. Clustering of Therapeutic Innovations. In this Figure, the algorithm recursively combines the pathophysiologies into clusters. The clustering process is seen from bottom to top, with the height of each “branch” reflecting relative similarities between clusters using the Euclidean distance. Longer “branches” indicate weaker associations. Abbreviations: Cell Regeneration = Regeneration; Immune and Inflammatory Modulation = Immune Mod; Extra-corporeal Support and Artificial Organs = ECS & Art Org; Organ Transplant = Transplant; Mechanical Respiratory Support = Mech Resp S; Nutritional Support = Nutrition; Therapeutic Devices = Ther Devices; Monitoring Devices = Monit Devices; Blood and Blood Products = Blood; Renal Replacement and Plasmapheresis = RR & Plasma; Mitochondrial Support = Mit S; Inhaled Respiratory Support = Inhaled Resp S; Suspended Animation = Susp Anim.