Abstract
Objective:
To characterize acute alterations of circadian and ultradian rest-activity rhythms in critically ill patients and their association with brain dysfunction, systemic multiorgan dysfunction, and melatonin rhythms.
Design:
Prospective study observing a cohort for 48 hours beginning within the first day of intensive care unit admission.
Setting:
Intensive care units within an academic medical center.
Patients:
Patients presenting from the community with acute onset of either intracerebral hemorrhage (ICH) or sepsis as representative neurologic and systemic critical illnesses. Healthy control patients were studied in the community, during hospital bedrest, and during sleep deprivation.
Measurements and Main Results:
Circadian and ultradian characteristics of rest-activity patterns were measured by wrist actigraphy, severity of neurologic and systemic illness by Glasgow Coma Scale (GCS) and Sequential Organ Failure Assessment (SOFA), and central circadian rhythm by melatonin profile. We studied 112 critically ill patients, including 53 with sepsis and 59 with ICH, along with 53 control participants. Total daily activity was markedly reduced and rest-activity rhythmicity was undetectable, neither of which was replicated by hospital bedrest in healthy controls. Circadian rest-activity rhythm fragmentation and attenuation and ultradian disorganization was associated with GCS and SOFA in adjusted models. Rest-activity rhythms showed no detectable phase coherence with melatonin rhythms.
Conclusions:
Critically ill patients rapidly enter a state of behavioral quiescence proportionate to their illness severity with concomitant disturbance of circadian and ultradian rest-activity rhythms and loss of phase coherence with the melatonin rhythm. Quiescence characteristics in rest-activity rhythms were not different in patients with and without delirium, suggesting them to be distinct phenomena. Animal models of severe physiologic stress have shown that specific neural pathway separate from the sleep-wake regulatory pathway induce behavioral quiescence and rest-activity arrhythmia, and facilitate recovery of cellular homeostasis. Whether quiescence is a conserved protective response pathway in humans is not yet understood.
Keywords: circadian, critical illness, intensive care, quiescence, encephalopathy, actigraphy
Introduction
As more patients survive critical illness and experience chronic disability, common Post-Intensive Care Syndrome impairments like sleep disturbance are being recognized as therapeutic targets.(1) Circadian misalignment and sleep deprivation are pervasive during and after critical illness and may contribute broadly to critical illness morbidity through their effects on brain health, autonomic stability, cardiovascular function, immune function and metabolic homeostasis.(2, 3) Recent work in animal models has shown that severe physiologic stressors induce a sleep-like state of behavioral quiescence, rest-activity arrhythmia and increased arousal threshold that may have an important role in facilitating homeostasis recovery.(4–6) Whether that distinct pathway and brain state are conserved in humans is unknown.
During critical illness, electrographic sleep macrostructure markers are replaced or obscured by findings which resemble neither wake nor sleep.(7–9) Analogously, actigraphy estimation of sleep onset and offset become imprecise.(10, 11) If humans experience a stress-induced brain state that is distinct from sleep and wake rather than a derangement of those states, then methods based on inference to a sleep-wake paradigm may be invalid. Alternative methods to analyze circadian and ultradian rhythmicity (variability, phase, amplitude, architectural organization) have been informative in animal models as well as in non-critically ill human populations, and measure the brain state transitions in these patients more directly.(10, 12–15) The objective of this study was to characterize acute alterations of circadian and ultradian rest-activity rhythms in critically ill patients and their association with brain dysfunction, systemic multiorgan dysfunction, and melatonin rhythms.
Methods
Critically Ill Patients
Patients presenting to intensive care units (ICUs) at Northwestern Memorial Hospital between April 2014 and December 2018 were prospectively enrolled in an observational cohort study. This paper reports cross-sectional data from the first 48 hours of assessment. The study was approved by the Institutional Review Board (IRB). Written informed consent was obtained from patients or their legally authorized representative. We enrolled patients ≥18 years old with either spontaneous intracerebral hemorrhage (ICH) as a representative neurologic critical illness or acute sepsis as a representative systemic critical illness. Intracerebral hemorrhage was diagnosed by a board certified neurologist, excluding hemorrhages attributed to trauma, hemorrhagic conversion of ischemic stroke, structural lesions, or vascular malformations, as we have described elsewhere in detail.(16) Sepsis was diagnosed by a board certified intensivist as organ dysfunction caused by an acute infection evidenced by an increase in the Sequential Organ Failure Assessment (SOFA) score of 2 points or more, consistent with the current consensus definition.(17) We restricted inclusion to patients presenting emergently from the community with demonstrably acute onset of symptoms and excluded patients unlikely to survive for at least 24 hours or unlikely to require at least 48 hours of ICU care, those with baseline need for renal replacement therapy or with hemoglobin concentrations less than 7 g/dL.
We characterized rest-activity rhythms with actigraphy. Actigraphy utilizes wearable devices with accelerometers to detect and record body movement over time.(10) In this study we used the common research implementation consisting of a watch-like wrist actigraphy device that quantified movement recorded in 30-second epochs. An activity-light monitor (Actiwatch-L; Philips/Respironics, Bend, OR, USA) was affixed to the hospital bed at the patients’ eye level to record illuminescence and another to the patients’ dominant wrist (or non-dominant wrist in the context of dominant extremity hemiparesis) to record physical activity, as we have previously described.(18) Study measurements were initiated within 24 hours of emergency department presentation and within two hours of enrollment and continued until death, ICU discharge or for 48 hours, whichever occurred first. Measurements included Glasgow Coma Scale hourly for encephalopathy severity, Richmond Agitation Sedation Scale (RASS) and Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) at least twice daily, continuous light and activity monitoring, blood melatonin sampling every two hours, disease-specific prognostic scores (ICH Score for ICH, Sequential Organ Failure Assessment [SOFA] for sepsis), and other common clinical variables as detailed in Supplemental Table 1 and described elsewhere.(16, 19–21)
Control Groups
Age-matched community dwelling volunteers underwent wrist actigraphy during usual life routines. Hospital bedrest volunteers and sleep deprivation volunteers underwent actigraphy monitoring in our hospital clinical research unit for 24 hours while confined to bedrest in dim light conditions with an intravenous catheter in place or maintained continuously awake, reinforced by research staff members being physically present to provide alertness stimulation as needed. Finally, we studied a group of critically ill patients receiving a continuous infusion of cisatracurium to induce complete neuromuscular paralysis concomitant with intravenous sedation for management of severe hypoxemic respiratory failure in order to characterize the contribution of high intensity care activities on the actigraphy record.
Actigraphy Analyses
We used standardized, previously published methods to calculate nonparametric wrist actigraphy variables.(22) Activity counts reflect the peak acceleration detected over each 30-second epoch. The activity index is the percentage of 30-second measurement epochs within the hour in which movement was detected, M10 and L5 are the average activity over the most active 10 hours and least active 5 hours of the day, respectively, and relative amplitude is the ratio of the difference between M10 and L5 and the sum of M10 and L5. Intradaily variability quantified the fragmentation of rest-activity, converging to zero for a sine wave pattern and two for Gaussian noise, conventionally calculated based on a 60 minute sampling interval alone (IV-60), although we also calculated intradaily variability across multiple sampling intervals (IVm) given recent research indicating that the IVm approach is superior to IV-60.(22, 23) We also characterized fragmentation using the coefficient of variation (relative standard deviation), a general statistic not specific to circadian analysis techniques that is defined as the ratio of the sample standard deviation to the mean. Fractal analysis was performed on actigraphy records using detrended fluctuation analysis over intervals from four minutes to eight hours and power law exponents were obtained for each record to characterize the integrity of ultradian rhythms. Lower power law exponents indicate degradation of the normal positive temporal autocorrelation in activity rhythms toward irregular disruption and randomness.(13) We selected the analysis interval based on prior research showing that scale-invariant correlations within the temporal interval of minutes to eight hours diminish with worsening cognitive impairment in ambulatory human patients, a finding linked to suprachiasmatic nucleus (SCN) pathology in humans and characteristic of experimental animals with central circadian rhythm disruption due to SCN lesions.(14, 15)
Statistical Analyses
The sample size for this study was determined based on calculations for two other study aims, not for the analyses presented here. We used Spearman’s rank order correlation and Wilcoxon rank sum tests for univariate comparisons with continuous or ordinal variables. We selected variables for inclusion into multivariable models by a priori selection based on published associations, and assessed inclusion of other covariates based on univariate associations and covariate removal for parsimony based on model fit using Akaike Information Criteria to yield the reported final models. Activity amplitude was log-transformed for use as a dependent variable in linear regression. Change over time effects were assessed using repeated measures ANOVA in order to avoid assumptions about patterns of change. Rest onset timing was assessed using Rayleigh z and Rao spacing tests to evaluate for unimodal versus uniform distribution and were compared to healthy patient groups using Watson’s U2 and Rao tests, consistent with recommendations for complementary tests for circular data analyses.(24) These circular statistical tests evaluate the hypothesis that the distribution of melatonin acrophase timing is distributed randomly and uniformly throughout the 24 hour day, or when comparing two groups, that the distribution of the acrophase timing over the day is the same in both groups with respect to mean time and the dispersion of individual acrophase times around that mean. We assessed phase coherence between M10, L5 and melatonin acrophase using a significance test of the correlation coefficient for angular variables. Statistical analyses were performed in R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
We studied 112 critically ill patients, including 53 with sepsis and 59 with ICH. The demographic and clinical characteristics of the cohort are shown in the Table with further details provided in the Online Supplemental Table 2. Most patients had no baseline functional impairment (65%) and preexisting dementia was rare (6%). At the time of study enrollment, median GCS was 13 [interquartile range 10, 15], the median ICH Score for patients with ICH was 2 [1,3], and the median SOFA was 5 [2.75, 9] for patients with sepsis, of which 89% had septic shock at the time of initial presentation. By the time the circadian rhythm characterization began, after initial stabilization but within the first 24 hours of hospitalization, 31.3% of patients remained vasopressor dependent. In addition to the 112 critically ill patients described above, actigraphy was obtained in 101 control patients including 53 age-matched community-dwelling control participants, 31 hospital bedrest control participants, 13 sleep deprivation control participants, and 4 patients under pharmacologic paralysis.
Table:
Patient Characteristics
| Baseline Characteristics on Admission | N, Mean or Median |
|---|---|
| Number of critically ill patients | 112 |
| Sepsis (%) | 53 (47.3) |
| Intracerebral hemorrhage (%) | 59 (52.7) |
| Age (mean (SD)) | 63.74 (16.66) |
| Sex = male (%) | 65 (58.6) |
| Race (%) | |
| Asian | 4 (3.6) |
| Black | 35 (31.2) |
| White | 73 (65.2) |
| Ethnicity (%) | |
| Hispanic or Latino | 13 (11.7) |
| Not Hispanic or Latino | 97 (87.4) |
| Unknown or Not Reported | 1 (0.9) |
| Pre-existing dementia | 7 (6.3) |
| Baseline disability by modified Ranking Scale (median [IQR]) | 0 [0, 2] |
| Glasgow Coma Scale at initial presentation (median [IQR]) | 13.00 [10.00, 15.00] |
| ICH Score (median [IQR], only reported for patients with ICH) | 1.00 [1.00, 2.00] |
| Sepsis patients in shock at initial presentation (%) | 46 (88.5) |
| Exposures During Circadian Characterization Interval | |
| Median Glasgow Coma Scale score over 24 hours (median [IQR]) | 14.00 [10.00, 15.00] |
| Glasgow Coma Scale score nadir (median [IQR]) | 14.00 [8.00, 15.00] |
| Median Richmond Agitation Sedation Scale score over 24 hours (median [IQR]) | 0.00 [−2.00, 0.00] |
| Richmond Agitation Sedation Scale score nadir (median [IQR]) | −1.00 [−3.25, 0.00] |
| Delirium by CAM-ICU (%) | 10 (8.9) |
| Sequential Organ Failure Assessment score (SOFA; median [IQR]) | 5 [2.75, 9] |
| SOFA for sepsis patients only | 7 [3, 11] |
| Mechanical ventilation (%) | 43 (38.4) |
| Intravenous sedatives (%) | 46 (41.1) |
| SOFA blood pressure categories (%) | |
| 0: No hypotension | 57 (50.9) |
| 1: MAP <70 mmHg | 20 (17.9) |
| 3: Dopamine >5, epinephrine ≤0.1, or norepinephrine ≤0.1 mcg/kg/min | 12 (10.7) |
| 4: Dopamine >15, epinephrine >0.1, or norepinephrine >0.1 mcg/kg/min | 23 (20.5) |
| Acute kidney injury by KDIGO category (%) | |
| No AKI | 79 (71.2) |
| Stage 1 | 15 (13.5) |
| Stage 2 | 11 (9.9) |
| Stage 3 | 6 (5.4) |
| Renal replacement therapy (%) | 2 (1.8) |
Activity Intensity, Variability and Rest-Activity Rhythm Amplitudes
Rest-activity patterns of the critically ill patients are shown in Figure 1, divided by level of neurologic impairment per GCS score into categories of unimpaired (GCS 15; alert, oriented and following commands), intermediate (GCS 9–14), and comatose (GCS 3–8), alongside actigraphy records from the reference control groups. Tactile stimuli and arousals, estimated using the actigraphy records of the pharmacologically paralyzed control patients, showed that those care-related stimuli were spread uniformly throughout the day and contributed trivially to the overall actigraphy record. Rest-activity rhythms in the age-matched community control participants showed a typical circadian rhythm with daytime activity and quiescence at night. Patients in the sleep deprivation protocol exhibited no rest-activity rhythmicity (p=0.26) and activity levels just mildly lower than the daytime activity measured in community dwelling participants. Healthy controls confined to hospital bedrest exhibited a decrease in absolute activity levels but preserved rest-activity rhythmicity (p<0.001) and a more modest decrease in the activity index compared to community dwelling.
Figure 1. Activity levels in Critically III Patients Compared to Control Groups.
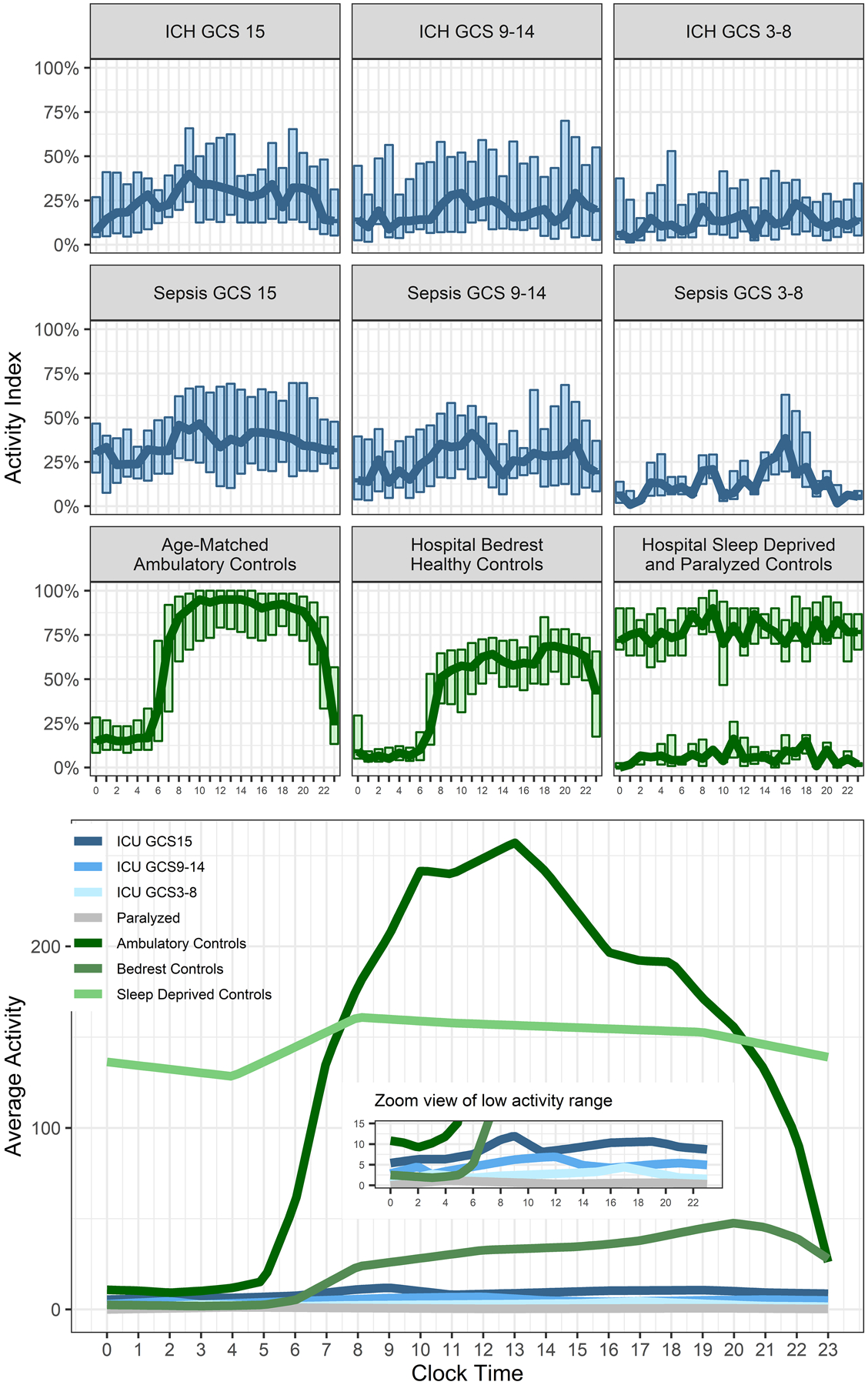
Figure 1 shows activity levels in critically ill patients (blue) and control groups (green) across the day. Critically ill patients are separated into groups by Glasgow Coma Scale scores (GCS). The upper panels show the activity index as boxplots with the hourly median values superimposed as a trendline. The lower panel shows the average activity with an inset panel that uses a smaller y-axis scale to better display difference between the different groups of critically ill patients.
Activity levels were uniformly lower in critically ill patients compared to controls and diminished by neurologic status (activity index amplitudes: ambulatory controls, 91%; bedrest controls, 66%; GCS unimpaired, 36%; intermediate encephalopathy, 39%; comatose, 18%; and M10: ambulatory controls, 326; bedrest controls, 189; unimpaired, 20.7; intermediate encephalopathy, 16.2; comatose, 3.9). In the critically ill participants, total daily activity, M10 and relative amplitude were associated with GCS. Better GCS was also correlated with lower coefficient of variation (rho −0.45, p<0.0001) but not intradaily variability, either measured at usual hourly intervals (IV-60, p=0.56) or averaged across sampling intervals (IVm, p=0.61). In adjusted models, median GCS (β 0.14, 95% confidence interval [0.075, 0.21], p<0.001), SOFA score (β 0.079 [0.016, 0.14], p=0.015), and premorbid disability by modified Rankin Scale (β −0.20 [−0.37, −0.036], p=0.018) were independently associated with activity amplitudes and M10 (median GCS β 0.17 [0.099, 0.25], p<0.001; SOFA β 0.11 [0.037, 0.17], p=0.0029; mRS β −0.20 [−0.38, −0.034], p=0.020). There were no associations between illness type (sepsis or ICH) and any measure of rest-activity rhythmicity by univariate tests or after adjustment for median GCS. Moreover, use of mechanical ventilation and use of sedation were not associated with rest-activity rhythmicity after adjustment for GCS.
Rest-Activity Rhythm Timing
Figure 2 shows rest-activity timing for the ambulatory and hospital bedrest control participants and critically ill patients based on the time of rest onset (the start time of L5). Ambulatory and hospital bedrest control participants demonstrated a unimodal circadian pattern (p<0.001) with clustering of L5 onset at mean time 00:23, and no significant between-group difference in timing of distribution. In contrast, rest onset in the critically ill groups appeared to be randomly distributed throughout the day (all p>0.05 testing against a null hypothesis of uniform circular distribution) as a group and for each arousal subgroup (unimpaired, intermediate encephalopathy or comatose).
Figure 2. Timing of Maximum Rest Interval (L5) Onset.
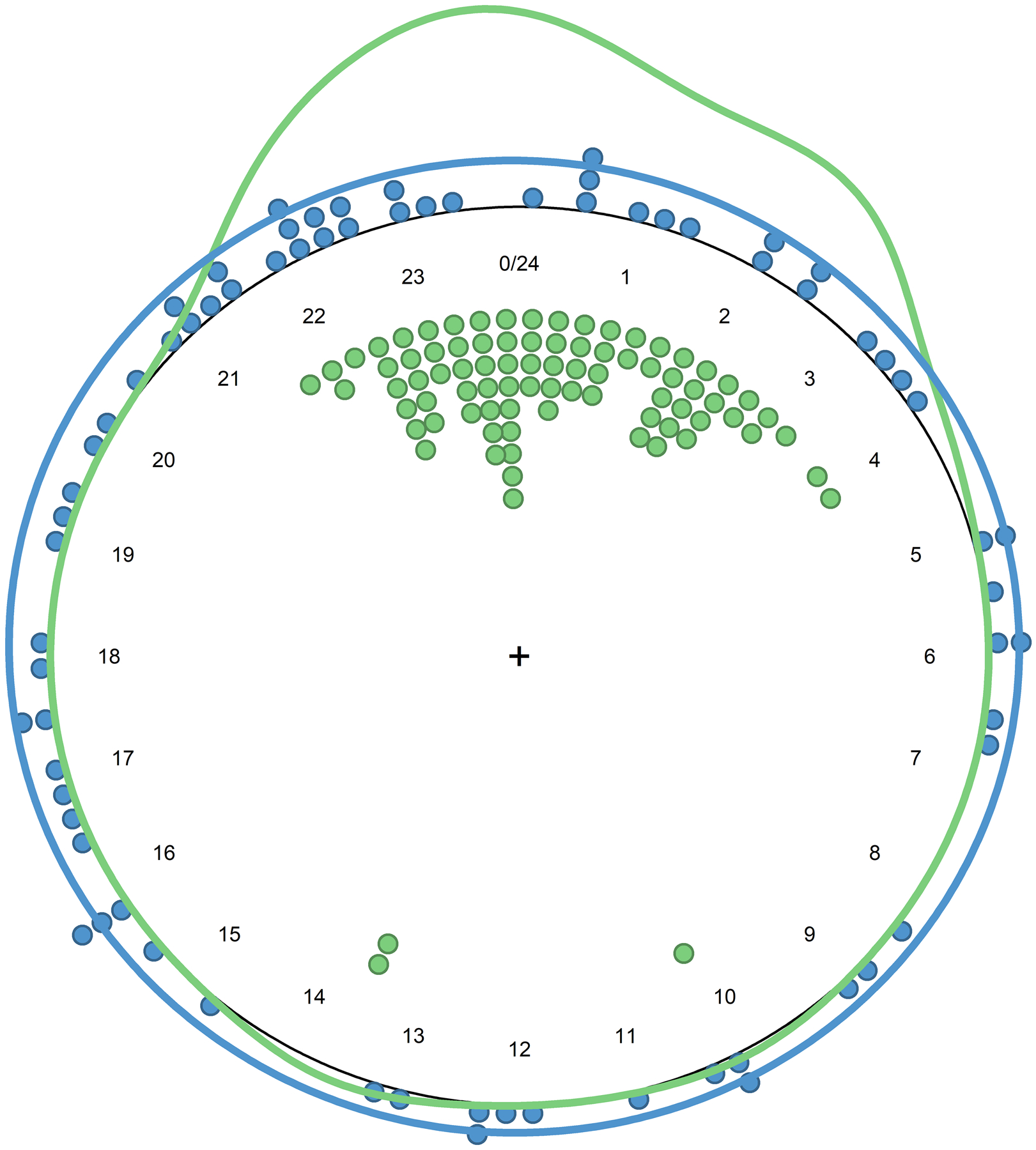
Figure 2 shows the timing of the onset of the maximum five hour rest interval for critically ill patients (blue) and healthy control participants (green). Points indicate individual measurements and the solid line is the circular kernel density estimation plot.
Ultradian Rest-Activity Architecture
Abnormal circadian activity characteristics were associated with derangements in ultradian activity rhythms. In a model adjusting for age (β −0.00014 [−0.0015, 0.77]) and premorbid dementia (β −0.036 [−0.15, 0.079]), the power law exponent from detrended fluctuation analysis was associated with GCS (β 0.0099 [0.0011, 0.019], p=0.028) but not SOFA (β 0.0048 [−0.0016, 0.011], p=0.14). Figure 3 illustrates the correlation between power law exponent and activity amplitude (rho 0.78, p<0.0001) and intradaily variability (rho −0.78, p<0.0001), which illustrates that disturbance of ultradian rhythms is closely linked to disrupted circadian rhythms. Illness type (sepsis or ICH) was not associated with power law exponent.
Figure 3. Power Law Characteristics and Activity.
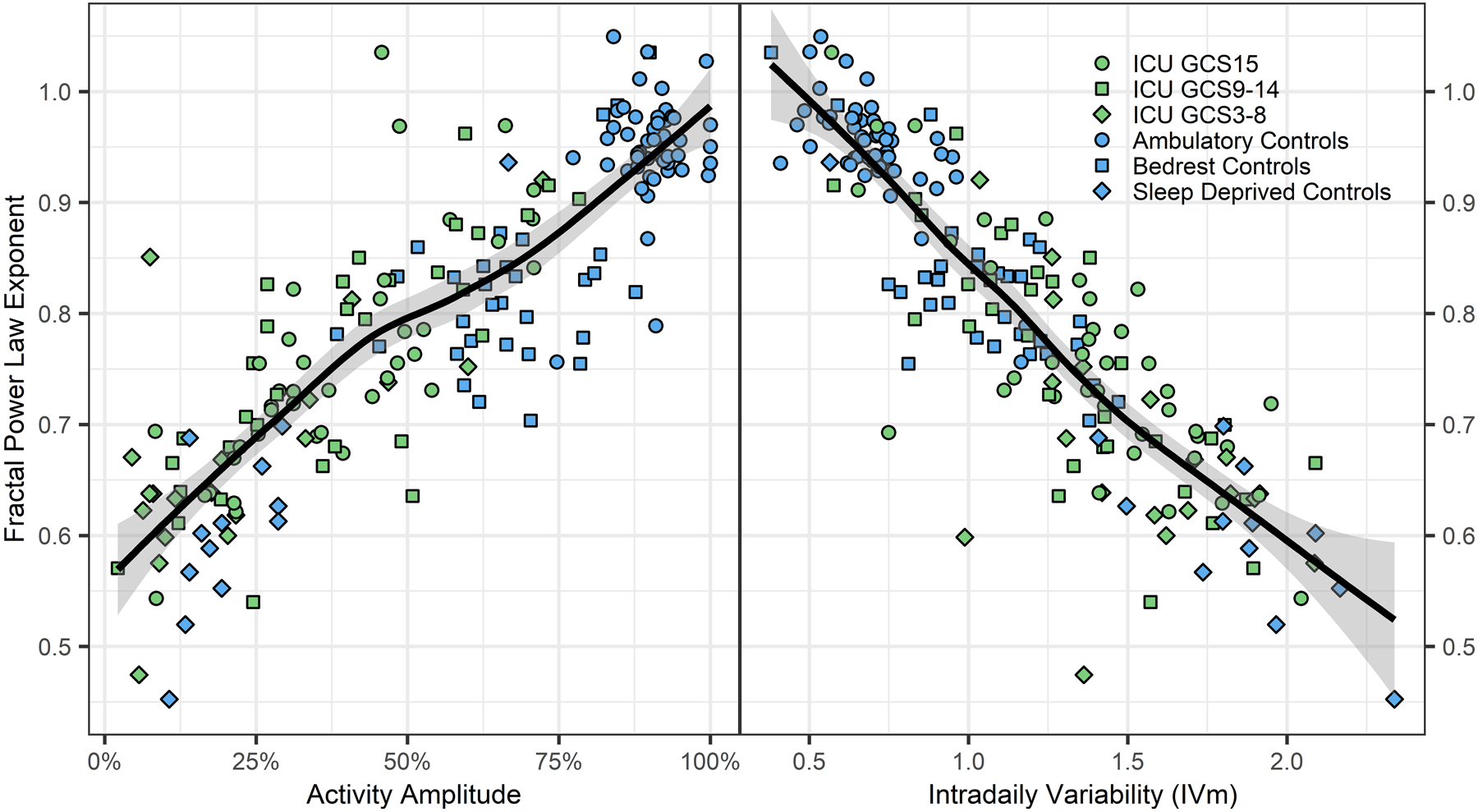
Figure 3 shows the power law exponent derived from detrended fluctuation analysis of actigraphy records plotted against activity amplitude (left panel) and sampling interval averaged intradaily variability (IVm; right panel). The power law exponent is a measure of ultradian rhythm integrity (near 1 is normal for healthy humans and 0.5 is randomness suggestive of poor biological control), whereas activity amplitude and IVm are measures of circadian rhythm integrity. The solid line and shaded area show the moving average trendline and its 95% confidence interval, respectively. These data show that deterioration in circadian rhythms, as measured by activity amplitude (rhythm strength) and intradaily variability (rhythm fragmentation) is strongly correlated with ultradian rhythm deterioration (lower fractal power law exponent).
Rest-Activity and Delirium
Delirium was detected by CAM-ICU assessment in 10 (8.9%; 1 hyperactive [RASS >0] and 6 hypoactive [RASS <0]) patients within the initial 24 hour circadian characterization interval, and another 21 (18.8%) were unassessable at all evaluation timepoints. Unassessable patients had lower arousal, as measured by GCS, and correspondingly lower activity levels, compared to assessable patients. Compared to patients without delirium, patients with delirium had higher total daily activity (median 32335 vs 14941, p=0.015) but no difference in circadian or ultradian measures of activity (activity index, IV-60, IVm, RA, and power law exponent; all p>0.1). Inclusion of delirium as a covariate in the adjusted models of activity amplitude and M10 showed that delirium was not independently associated with either outcome, and the significant relationships with GCS, SOFA and premorbid disability persisted.
Rest-Activity Rhythms and Melatonin Rhythms
There were no correlations between the 24 hour melatonin rhythm amplitude and any summary measure of activity (total daily activity: correlation coefficient rho 0.11, p=0.32; activity variability: rho 0.11, p=0.35), nonparametric circadian measures (absolute activity amplitude: rho 0.17, p=0.14; activity index: rho 0.17, p=0.13; relative amplitude: rho 0.026, p=0.83; IV-60: rho −0.12, p=0.30; IVm: −0.17, p=0.14), or power law exponent of ultradian activity (rho 0.13, p=0.25). There was no temporal correlation between melatonin acrophase and either M10 onset (angular correlation coefficient rho 0.02, p=0.84) or L5 onset (rho −0.17, p=0.09).
Discussion
We observed that patients with both neurologic and systemic critical illness rapidly entered a state of profound behavioral quiescence with the onset of illness. Comparison to healthy patients experimentally confined to hospital bedrest confirmed that the physical constraints of care did not account for the loss of circadian rhythmicity and only partially explained the reduction in total activity. Moreover, experimental continuous sleep deprivation for a similar time interval did not cause a similar reduction in spontaneous activity. Beyond quiescence, we observed a severe temporal disorganization of rest-activity rhythm in both circadian and ultradian scales along with dissociation from melatonin rhythms. Delirium was less common than quiescence and not independently associated with quiescence characteristics in circadian or ultradian rest-activity rhythms, suggesting that delirium and quiescence are distinct phenomena.
The suprachiasmatic nucleus functions as the pacemaker of the central circadian rhythm, relaying signals to various brain regions and indirectly to peripheral tissues through regulated secretion of melatonin from the pineal gland and autonomic signals.(25) Sleep is regulated by input from the circadian clock and a sleep homeostat, with some interaction between those parallel processes.(26) Sleep duration and fragmentation are well established as important health determinants in ambulatory patients, yet their significance during critical illness is unknown. Sleep is a complex phenomenon comprising multiple parallel processes, and it is not known how each individual component may persist or cease in the context of altered mental states due to sedation or encephalopathy. For example, glymphatic clearance and memory consolidation are both increased by sleep, however, anesthetic medications, which are widely used in critically ill patients, replicate the glymphatic flow augmentation of sleep but impede memory consolidation.(27, 28) Traditional EEG scoring methods for sleep are unusable due to encephalopathy changes and proposed alternative methods are descriptive rather than based on biological sleep function, so it is not possible to infer which underlying sleep-associated neurophysiologic process may remain functional during rest intervals.(29–32) Conceptualizing isoelectric brain activity as “atypical sleep” is perhaps like considering shock stabilization with multiple concurrent vasopressors “atypical normotension”: superficially similar but physiologically misrepresentative.(32)
Stress-induced behavioral quiescence may be an altogether distinct brain state triggered by adaptive mechanisms rather than a blurred state on the spectrum between wake and sleep, and potentially health promoting. Animal models used to explore the basic neurobiology of sleep rhythms have found that various strong physiologic stressors induce a state that is superficially indistinguishable from normal, timed sleep but is effectuated by a distinct neuronal process.(4–6) Importantly, stress-induced sleep in animals does not modify all body functions identically to timed sleep, and occurs in tandem with mechanisms that act to restore cellular homeostasis, indicating that it is a protective, adaptive process.(6) Actigraphy has been used in critically ill patients in prior studies where analyses focused on quantifying total activity and average differences between daytime and nighttime activity, most with the objective of assessing sedation, delirium and mobilization.(11) Although actigraphy does not measure sleep onset and offset precisely compared to EEG, these data show that nonparametric circadian and power law exponent measurements of the actigraphy record revealed a loss of rhythmicity and activity autocorrelation that was correlated with the severity of the underlying physiologic stressor, which parallels the characteristics of stress-induced sleep in animals.(11) Given the noninvasiveness of actigraphy, rhythmicity parameters may be useful as severity biomarkers trended over the course of illness, and could additionally provide insights into the interaction between sleep rhythms, circadian normalization, and health outcomes after critical illness. In particular, power law analysis is correlated with changes in cognitive status in ambulatory patient populations, and a growing body of literature supports its utility in characterizing sleep-wake architecture.(13–15, 33)
The neurologic symptoms of severe illness, described as “phrenitis” by ancient Greeks and subject of speculation through the pre-modern era, are so common to the human experience that they received little attention as a significant medical problem until recently when the cognitive symptoms of critical illness encephalopathy, especially its well-known manifestation in the form of delirium, have been studied.(34, 35) Other than categorizing delirium as hyperactive and hypoactive, the behavioral manifestations of encephalopathy have received comparatively less attention than cognitive deficits, and the physiologic implications of the most prominent features- behavioral quiescence and rest-activity fragmentation- are not well understood in humans. Thorough exploration of the physiology of behavioral changes during critical illness may be important for guiding interventions, whether targeted at restoring circadian rhythms and normal sleep after illness, or early mobilization. In these data, delirium did not contribute as an explanatory variable to circadian or ultradian characteristics of rest-activity independent of the contributions of overall encephalopathy, as measured by the GCS, overall multiorgan dysfunction severity (SOFA) and premorbid vulnerability (mRS). There has been substantial attention to early mobilization of critically ill patients as a means to improve functional outcomes, but a systematic review of the recent literature shows limited evidence of efficacy.(36) The cellular mechanisms of skeletal muscle growth and repair are circadian clock regulated, with 17% of skeletal muscle genes exhibiting circadian transcriptome oscillation, and loss of circadian regulation mechanisms leads to severe sarcopenia.(37) Without concomitant normal peripheral clock function in muscle, physical exertion may be futile for attenuating muscle wasting. Likewise, sleep consolidation may be incompatible with quiescence.
Circadian molecular rhythms are ubiquitous in human cells. Approximately 43% of mammalian protein-encoding genes demonstrate circadian transcription rhythms, mostly in an organ-specific pattern.(38) The ability to respond to changing time patterns in the environment is an important adaptive mechanism for health preservation, and abrupt changes like sleep deprivation in otherwise stable individuals can have immediate, deleterious effects like impaired metabolic stability, endocrine functions and immune responsiveness.(39, 40) In contrast to an experimental context with healthy test subjects for whom abrupt system perturbations have no adaptive benefit, in the context of critical physiologic instability, collateral pathways overwhelming circadian control mechanisms may be beneficial to stabilize stressors with greater immediacy.(41) For example, usual circadian fluctuations in core body temperature give way to intermittent fevers in the context of sepsis. Attempts to alter illness response physiology back to normal circadian patterns prematurely may be harmful.
Expression of many genes directly affecting function and growth in muscle tissue show circadian patterns.(42) Muscle function measurably fluctuations in humans on a diurnal cycle, and various animal models have shown that loss of circadian inputs result in impaired muscle function and growth, even independent of physical activity.(37, 42, 43) Human data describing early mobilization after critical illness are mixed and inconclusive, summarized in a recent Cochrane review as showing insufficient evidence for affecting “physical function or performance, adverse events, muscle strength and health-related quality of life.”(36) Circadian research on muscle physiology indicates that intact circadian rhythmicity and phase-congruent timing of activity is required for anabolic responses to physical activity.(44, 45) If behavioral quiescence is associated with cellular stabilizing functions in humans akin to those seen in animal models, early mobilization during that window, if misapplied, could be a harmful disruption. Monitoring for reestablishment of circadian activity rhythms and normalization of ultradian rhythm integrity as biomarkers of the transition from protective quiescence to regular behavior patterns merits exploration as a personalized medicine mobilization strategy.(46) Moreover, augmenting the relatively crude ordinal activity scales or customary actigraphy measurements with rhythmicity measurements, as we have done in this sample, may improve the characterization of sleep and activity recovery after illness.(47)
There are limitations to this approach. The most important, which we have discussed above, is that we could not measure the status of various basic physiologic processes that comprise healthy sleep, like glymphatic clearance. This prevents us from comparing actigraphy rest-activity measurements with physiologic changes to develop additional insights into how rest intervals during stress quiescence resemble and differ from the physiology of sleep. We measured and compared rest-activity rhythms with melatonin rhythms to analyze circadian rhythmicity, and while melatonin is the most commonly used and robust marker of circadian phase in physiologically stable humans, melatonin alterations during critical illness may diminish its reliability as a circadian biomarker.(48) Using ICH and sepsis as representative populations was helpful in contrasting neurologic and systemic critical illness, but those conditions do not represent all forms of critical illness, so generalizability will require further studies. Use of physical restraints was not measured and may have influenced activity measurements. Finally, our own data show that environmental stimuli and physical constraints alter actigraphy records, changing both total activity and rhythmicity patterns, which means extrinsic abnormalities confound the effects of intrinsic abnormalities. This confounding was partially mitigated by increasing the uniformity of extrinsic factors by studying all patients within the early phase of illness onset to reduce cumulative effects, selecting two model diseases that are managed with relatively uniform care practices, by the uniformity of the environment and nursing practices within the single institution, and by confirming that we could still observe a graded effect of illness severity on our measurement endpoints consistent with intrinsic disease effects.
Conclusions
These data show that critically ill patients rapidly enter a state of behavioral quiescence proportionate to their illness severity with concomitant disturbance of rest-activity rhythms within the circadian and ultradian time scales and loss of phase coherence with the melatonin rhythm. Comparative animal models of stress induced behavioral quiescence and activity dysrhythmia suggest the phenomena are activated by a neural pathway distinct from normal sleep-wake regulation and may be protective in those organisms. Further investigation is needed to understand the underlying mechanisms and physiologic consequences of stress-induced quiescence in humans.
Supplementary Material
Funding
Dr. Maas received support from National Institutes of Health grants K23NS092975 and L30NS080176, and a Dixon Translational Research Grant from the Northwestern Memorial Foundation. Drs. Abbott and Reid received support through the Northwestern Center for Circadian and Sleep Medicine. Drs. Reid and Zee receive support from National Institutes of Health grants UM1HL112856, R01HL140580 and P01AG011412. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences grant UL1TR000150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Copyright form disclosure: Drs. Maas, Reid, and Zee’s institution received funding from the National Institutes of Health (NIH). Dr. Maas’ institution received funding from Northwestern Memorial Foundation. Drs. Maas, Reid, and Zee received support for article research from the NIH. Dr. Abbott’s institution received funding from American Sleep Medicine Foundation Grant, and she received funding from American Board of Internal Medicine (Sleep Medicine Exam Writing Committee Member) and UptoDate. Dr. Reid received funding from the Sleep Research Society Foundation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012;40(2):502–509. [DOI] [PubMed] [Google Scholar]
- 2.Chan MC, Spieth PM, Quinn K, et al. Circadian rhythms: from basic mechanisms to the intensive care unit. Crit Care Med 2012;40(1):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman MT, Knauert MP, Pisani MA. Sleep Disturbance after Hospitalization and Critical Illness: A Systematic Review. Ann Am Thorac Soc 2017;14(9):1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iannacone MJ, Raizen DM. Sleep: How Many Switches Does It Take To Turn Off the Lights? Curr Biol 2016;26(18):R847–R849. [DOI] [PubMed] [Google Scholar]
- 5.Trojanowski NF, Nelson MD, Flavell SW, et al. Distinct Mechanisms Underlie Quiescence during Two Caenorhabditis elegans Sleep-Like States. J Neurosci 2015;35(43):14571–14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill AJ, Mansfield R, Lopez JM, et al. Cellular stress induces a protective sleep-like state in C. elegans. Curr Biol 2014;24(20):2399–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azabou E, Magalhaes E, Braconnier A, et al. Early Standard Electroencephalogram Abnormalities Predict Mortality in Septic Intensive Care Unit Patients. PLoS One 2015;10(10):e0139969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Mizrahi MA, Hartings JA, et al. Continuous Electroencephalography After Moderate to Severe Traumatic Brain Injury. Crit Care Med 2019;47(4):574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourne RS, Minelli C, Mills GH, et al. Clinical review: Sleep measurement in critical care patients: research and clinical implications. Crit Care 2007;11(4):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 11.Schwab KE, Ronish B, Needham DM, et al. Actigraphy to Evaluate Sleep in the Intensive Care Unit. A Systematic Review. Ann Am Thorac Soc 2018;15(9):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trojanowski NF, Raizen DM. Call it Worm Sleep. Trends Neurosci 2016;39(2):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan W, Song Y, Kwak S, et al. Quantitative evaluation of the use of actigraphy for neurological and psychiatric disorders. Behav Neurol 2014;2014:897282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu K, Van Someren EJ, Shea SA, et al. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: Involvement of the circadian pacemaker. Proc Natl Acad Sci U S A 2009;106(8):2490–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu K, Harper DG, Shea SA, et al. Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Sci Rep 2013;3:2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maas MB, Rosenberg NF, Kosteva AR, et al. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology 2013;81(2):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan EP, Abbott SM, Reid KJ, et al. Abnormal environmental light exposure in the intensive care environment. J Crit Care 2017;40:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maas MB, Lizza BD, Abbott SM, et al. Factors Disrupting Melatonin Secretion Rhythms during Critical Illness. Crit Care Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naidech AM, Beaumont JL, Rosenberg NF, et al. Intracerebral hemorrhage and delirium symptoms. Length of stay, function, and quality of life in a 114-patient cohort. Am J Respir Crit Care Med 2013;188(11):1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenthal LJ, Francis BA, Beaumont JL, et al. Agitation, Delirium, and Cognitive Outcomes in Intracerebral Hemorrhage. Psychosomatics 2017;58(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blume C, Santhi N, Schabus M. ‘nparACT’ package for R: A free software tool for the non-parametric analysis of actigraphy data. MethodsX 2016;3:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonçalves BS, Cavalcanti PR, Tavares GR, et al. Nonparametric methods in actigraphy: An update. Sleep Sci 2014;7(3):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruxton GD. Testing for departure from uniformity and estimating mean direction for circular data. Biol Lett 2017;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005;437(7063):1257–1263. [DOI] [PubMed] [Google Scholar]
- 26.Deboer T Sleep homeostasis and the circadian clock: Do the circadian pacemaker and the sleep homeostat influence each other’s functioning? Neurobiol Sleep Circ Rhythms 2018;5:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reasor JD, Poe GR. Learning and memory during sleep and anesthesia. Int Anesthesiol Clin 2008;46(3):105–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schabus M, Wislowska M, Angerer M, et al. Sleep and circadian rhythms in severely brain-injured patients - A comment. Clin Neurophysiol 2018;129(8):1780–1784. [DOI] [PubMed] [Google Scholar]
- 30.Wislowska M, Blume C, Angerer M, et al. Approaches to sleep in severely brain damaged patients - Further comments and replies to Kotchoubey & Pavlov. Clin Neurophysiol 2018;129(12):2680–2681. [DOI] [PubMed] [Google Scholar]
- 31.Watson PL. Measuring sleep in critically ill patients: beware the pitfalls. Crit Care 2007;11(4):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson PL, Pandharipande P, Gehlbach BK, et al. Atypical sleep in ventilated patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med 2013;41(8):1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu-Shore J, Westover MB, Bianchi MT. Power law versus exponential state transition dynamics: application to sleep-wake architecture. PLoS One 2010;5(12):e14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bynum B Phrenitis: what’s in a name? Lancet 2000;356(9245):1936. [DOI] [PubMed] [Google Scholar]
- 35.Maas MB, Naidech AM. Critical Care Neurology Perspective on Delirium. Semin Neurol 2016;36(6):601–606. [DOI] [PubMed] [Google Scholar]
- 36.Doiron KA, Hoffmann TC, Beller EM. Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit. Cochrane Database Syst Rev 2018;3:CD010754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatterjee S, Ma K. Circadian clock regulation of skeletal muscle growth and repair. F1000Res 2016;5:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang R, Lahens NF, Ballance HI, et al. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 2014;111(45):16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354(9188):1435–1439. [DOI] [PubMed] [Google Scholar]
- 40.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471–1472. [DOI] [PubMed] [Google Scholar]
- 41.Tahara Y, Aoyama S, Shibata S. The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci 2017;67(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefta M, Wolff G, Esser KA. Circadian rhythms, the molecular clock, and skeletal muscle. Curr Top Dev Biol 2011;96:231–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyar KA, Hubert MJ, Mir AA, et al. Transcriptional programming of lipid and amino acid metabolism by the skeletal muscle circadian clock. PLoS Biol 2018;16(8):e2005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camera DM. Anabolic Heterogeneity Following Resistance Training: A Role for Circadian Rhythm? Front Physiol 2018;9:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoyama S, Shibata S. The Role of Circadian Rhythms in Muscular and Osseous Physiology and Their Regulation by Nutrition and Exercise. Front Neurosci 2017;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shrestha GS, Suarez JI, Hemphill JC. Precision Medicine in Neurocritical Care. JAMA Neurol 2018;75(12):1463–1464. [DOI] [PubMed] [Google Scholar]
- 47.Kessler R, Knutson KL, Mokhlesi B, et al. Sleep and Activity Patterns in Older Patients Discharged from the Hospital. Sleep 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med 2008;4(1):66–69. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


