Abstract
Objective:
The circadian system modulates many important physiologic processes, synchronizing tissue-specific functions throughout the body. We sought to characterize acute alterations of circadian rhythms in critically ill patients and to evaluate associations between brain dysfunction, systemic multiorgan dysfunction, environmental stimuli that entrain the circadian rhythm (zeitgebers), rest-activity rhythms, and the central circadian rhythm-controlled melatonin secretion profile.
Design:
Prospective study observing a cohort for 24–48 hours beginning within the first day of intensive care unit admission.
Setting:
Multiple specialized intensive care units within an academic medical center.
Patients:
Patients presenting from the community with acute onset of either intracerebral hemorrhage (ICH) as a representative neurologic critical illness or sepsis as a representative systemic critical illness. Healthy control patients were studied in using modified constant routine in a clinical research unit.
Interventions:
None.
Measurements and Main Results:
Light, feeding, activity, medications and other treatment exposures were evaluated along with validated measures of encephalopathy (Glasgow Coma Scale), multiorgan system function (Sequential Organ Failure Assessment Score), and circadian rhythms (profiles of serum melatonin and its urinary metabolite aMT6s). We studied 112 critically ill patients, including 53 with sepsis and 59 with ICH. Environmental exposures were abnormal, including light (dim), nutritional intake (reduced or absent and mistimed) and arousal stimuli (increased and mistimed). Melatonin amplitude and acrophase timing were generally preserved in awake patients but dampened and delayed with increasing encephalopathy severity. Melatonin hypersecretion was observed in patients exposed to catecholamine vasopressor infusions, but unaffected by sedatives. Change in vasopressor exposure was the only factor associated with changes in melatonin rhythms between days 1 and 2.
Conclusions:
Encephalopathy severity and adrenergic agonist medication exposure were the primary factors contributing to abnormal melatonin rhythms. Improvements in encephalopathy and medical stabilization did not rapidly normalize rhythms. Urinary aMT6s is not a reliable measure of the central circadian rhythm in critically ill patients.
Keywords: circadian, melatonin, aMT6s, critical illness, intensive care, encephalopathy
Introduction
The circadian system modulates many important physiologic functions throughout the body, including brain arousal, sympathetic tone, cardiovascular function, coagulation, immune system activity, glycemic control and metabolism.(1) The ability to synchronize cellular functions with sleep, activity, feeding and the environment is an important adaptive mechanism for health preservation, whereas dyssynchronization creates acute and cumulative deleterious effects on health.(2) The suprachiasmatic nucleus (SCN) functions as a central pacemaker for the circadian rhythm, relaying through the subparaventricular zone of the hypothalamus to other brain targets to organize sleep, locomotor activity, temperature, and autonomic tone, and projecting to the pineal gland to regulate melatonin secretion.(3) Approximately 43% of mammalian protein-encoding genes demonstrate circadian transcription rhythms, mostly in an organ-specific pattern.(4) Those peripheral tissue rhythms are synchronized with the central clock by melatonin and autonomic signaling, and melatonin is the most commonly used and robust marker of circadian phase in humans.(5)
Given the important homeostatic role of the circadian system, circadian rhythm disruption may exacerbate multiorgan dysfunction and disrupt recovery in acute illness. Prior studies in critically ill patients have mostly measured urinary metabolite 6-sulphatoxymelatonin (aMT6s) as a surrogate for serum melatonin and have reported inconsistent and conflicting findings such that the factors associated with circadian disruption are not clearly established. The objective of this study was to systematically characterize patterns and risk factors for circadian rhythm disturbance during critical illness.
Methods
Study Patients
Patients admitted to intensive care units (ICUs) at Northwestern Memorial Hospital between April 2014 and December 2018 were prospectively enrolled in an observational cohort study. The study was approved by the Institutional Review Board (IRB). Written informed consent was obtained from the patient or their legally authorized representative. Given role of the brain in efferent expression of the central circadian rhythm, we had prespecified separate consideration for critical illness from brain disease and multisystem critical illness. We enrolled patients ≥18 years old with either (1) spontaneous intracerebral hemorrhage (ICH) as a representative neurologic critical illness, or (2) acute sepsis as a representative systemic critical illness. ICH and sepsis were chosen because of their high morbidity and well-established literature identifying the factors with major influence on outcomes, including validated prognostic scores.(6, 7) We restricted inclusion to patients presenting emergently from the community with demonstrably acute onset of symptoms to reduce confounding by pre-hospitalization factors, and excluded patients unlikely to survive for at least 24 hours, unlikely to require at least 48 hours of ICU care, those with baseline need for renal replacement therapy, and with hemoglobin concentrations less than 7 g/dL. Intracerebral hemorrhage was diagnosed by a board certified neurologist, excluding hemorrhages attributed to trauma, hemorrhagic conversion of ischemic stroke, structural lesions, or vascular malformations, as we have described elsewhere in detail.(8) Sepsis was diagnosed by a board certified intensivist as organ dysfunction caused by an acute infection evidenced by an increase in the Sequential Organ Failure Assessment (SOFA) score of 2 points or more, consistent with the current consensus definition.(9) Sample size calculations estimated that enrollment of 32 subjects would be needed to observe an association between melatonin rhythm suppression and encephalopathy severity, although enrollment was designed to be greater to address analyses of separate aims.
Exposures and Outcomes
We characterized the severity of the primary exposure (critical illness) with the Sequential Organ Failure Assessment (SOFA) score for all patients, calculated using the worst values for each parameter in the 24-hour period as previously described and validated, along with the ICH Score as a global measure of severity for patients with ICH as we have previously described.(8–10) Given the role of the brain in efferent expression of the central circadian rhythm and the predominant prognostic importance of encephalopathy severity in patients with sepsis and ICH, measuring the severity of encephalopathy and altered arousal was an exposure of interest.(6, 11) Other covariates of interest included sedative medications that may alter brain arousal rhythms, antihypertensive (e.g. beta blockers) and vasopressor medications (e.g. norepinephrine) that may alter β-adrenergic receptor mediated melatonin release from the pineal gland, altered kidney function which may affect clearance of melatonin from blood and the reliability of urinary aMT6s as a surrogate measurement for melatonin, and external stimuli with clearly established effects on melatonin rhythms (“zeitgebers”) including retinal light exposure, nutritional intake, activity and physical stimulation/arousals. The outcome of interest, melatonin rhythms, were characterized by profiling serum melatonin and urinary aMT6s to characterize strength and timing. We characterized the baseline characteristics of patients at the time of initial assessment in the emergency department, then measured exposures and melatonin rhythm characterization over a minimum of 24 hours and up to 48 hours immediately following initial stabilization. The organization of the study measurements are detailed in Online Supplemental Table 1.
Measurement Methods
Study measurements were initiated within 24 hours of emergency department presentation and within two hours of enrollment and continued until death, ICU discharge or for 48 hours, whichever occurred first. Vital signs and Glasgow Coma Scale were recorded hourly and Richmond Agitation Sedation Scale every four hours, with sedatives held if applicable and safe per the treating team, performed by trained critical care nurses.(8) An activity-light monitor (Actiwatch-L; Philips/Respironics, Bend, OR, USA) was affixed to the hospital bed at the patients’ eye level to record illuminescence and another to the patients’ dominant wrist (or non-dominant wrist in the context of dominant extremity hemiparesis) to record physical activity in 30 second intervals, as we have described in greater detail elsewhere.(12, 13) Blood was sampled every two hours for serum melatonin and urine every four hours for urinary aMT6s, both measured by radioimmunoassay (IBL International GmbH, Hamburg, Germany, and Stockgrand Ltd, Guildford, Surrey, UK, respectively). Kidney function and impairment was graded using the KDIGO criteria.(14) Baseline glomerular filtration rate (GFR) was estimated using the CKD-EPI Creatinine formula.(15) When patients were grouped according to encephalopathy severity, we followed the conventional interpretation of the Glasgow Coma Scale which identifies scores from 3 to 8 as coma, and for which the maximum score of 15 is applied to normal patients, using the patients’ median measurement. Catecholamine vasopressor exposure was ordinalized as none, low dose, or high dose according to its use in calculating the SOFA score, using the worst value for this parameter in the 24-hour period. Control melatonin data were obtained from healthy, similar age community dwelling volunteers who underwent melatonin sampling in a clinical research unit under modified constant routine that included an adaptation night followed by a cay of physical inactivity in a dim light environment with regularly spaced isocaloric snacks, as we have previously described elsewhere.(16) Constant routine protocols reduce the “masking”, or confounding, of circadian rhythms by exogenous components like light, activity, nutritional intake and sleep.(17) Wrist actigraphy recording was also performed in a group of pharmacologically paralyzed patients to evaluate the frequency and intensity of physical stimuli related to patient care.
Analyses
We used data from the first 24 hours of circadian characterization measurements, which were complete in all patients included in this study, for the main analyses. Secondary analyses that compared serum melatonin amplitude and area under the curve between the first and second 24 hour intervals were also performed in the subset of patients with 48 hours of complete data. We defined dim light melatonin onset as a >2 standard deviation rise above baseline.(5) Given that many patients’ melatonin trends were irregular, when selecting the melatonin acrophase for timing analyses, we chose the highest value which occurred within the greatest mean consecutive sequence of three samples in order to exclude isolated outlier values. Melatonin acrophase calculated by this method was identical to the absolute maximum in 137 cases and different in 13. We used nonparametric univariate tests for continuous or ordinal variables. We selected variables for inclusion into multivariable models by a priori selection based on published associations, and assessed inclusion of other covariates based on univariate associations and covariate removal for parsimony based on model fit using Akaike Information Criteria. Melatonin amplitude and urinary aMT6s amplitude were log-transformed for use as dependent variables in linear regression. Melatonin acrophase timing was assessed using Rayleigh z and Rao spacing tests to evaluate for unimodal versus uniform distribution and was compared to the healthy patients using Watson’s U2 and Rao tests, consistent with recommendations for complementary tests for circular data analyses.(18) These circular statistical tests evaluate the hypothesis that the distribution of melatonin acrophase timing is distributed randomly and uniformly throughout the 24 hour day, or when comparing two groups, that the distribution of the acrophase timing over the day is the same in both groups with respect to mean time and the dispersion of individual acrophase times around that mean. Statistical analyses were performed in R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
We studied 112 patients, including 53 with sepsis and 59 with ICH. The mean age was 63.7 (standard deviation ±16.7), 65 (58.6%) were male, and 73 (65.2%) were white. Premorbid dementia was uncommon (6.3%) and most patients had no baseline functional impairment (baseline mRS 0 in 65.1%). Median GCS at the time of initial presentation was 13 [interquartile range 10, 15]. The median ICH Score was 2 [1, 3] for patients with ICH and median SOFA was 7 [3, 11] for patients with sepsis, of whom 88.5% had shock requiring vasopressor infusion at the time of initial presentation. By the time the circadian rhythm characterization began, after initial stabilization but within the first 24 hours of hospitalization, 31.3% of patients remained vasopressor dependent. The most common sources of sepsis were lung (34.6%), gastrointestinal tract (32.7%) and urinary tract (26.9%). Patient demographic and clinical characteristics are shown in the Table with further details provided in the Online Supplemental Table 2. Melatonin measurements were also obtained in 43 healthy control participants.
Table:
Patient Characteristics
| Baseline Characteristics on Admission | N, Mean or Median |
|---|---|
| Number of critically ill patients | 112 |
| Sepsis (%) | 53 (47.3) |
| Intracerebral hemorrhage (%) | 59 (52.7) |
| Age (mean (SD)) | 63.74 (16.66) |
| Sex = male (%) | 65 (58.6) |
| Race (%) | |
| Asian | 4 (3.6) |
| Black | 35 (31.2) |
| White | 73 (65.2) |
| Ethnicity (%) | |
| Hispanic or Latino | 13 (11.7) |
| Not Hispanic or Latino | 97 (87.4) |
| Unknown or Not Reported | 1 (0.9) |
| Pre-existing dementia | 7 (6.3) |
| Baseline disability by modified Ranking Scale (median [IQR]) | 0 [0, 2] |
| Glasgow Coma Scale at initial presentation (median [IQR]) | 13.00 [10.00, 15.00] |
| ICH Score (median [IQR], only reported for patients with ICH) | 1.00 [1.00, 2.00] |
| Sepsis patients in shock at initial presentation (%) | 46 (88.5) |
| Exposures During Circadian Characterization Interval | |
| Median Glasgow Coma Scale score over 24 hours (median [IQR]) | 14.00 [10.00, 15.00] |
| Glasgow Coma Scale score nadir (median [IQR]) | 14.00 [8.00, 15.00] |
| Median Richmond Agitation Sedation Scale score over 24 hours (median [IQR]) | 0.00 [−2.00, 0.00] |
| Richmond Agitation Sedation Scale score nadir (median [IQR]) | −1.00 [−3.25, 0.00] |
| Delirium by CAM-ICU (%) | 10 (8.9) |
| Sequential Organ Failure Assessment score (SOFA; median [IQR]) | 5 [2.75, 9] |
| SOFA for sepsis patients only | 7 [3, 11] |
| Mechanical ventilation (%) | 43 (38.4) |
| Intravenous sedatives (%) | 46 (41.1) |
| SOFA blood pressure categories (%) | |
| 0: No hypotension | 57 (50.9) |
| 1: MAP <70 mmHg | 20 (17.9) |
| 3: Dopamine >5, epinephrine ≤0.1, or norepinephrine ≤0.1 mcg/kg/min | 12 (10.7) |
| 4: Dopamine >15, epinephrine >0.1, or norepinephrine >0.1 mcg/kg/min | 23 (20.5) |
| Acute kidney injury by KDIGO category (%) | |
| No AKI | 79 (71.2) |
| Stage 1 | 15 (13.5) |
| Stage 2 | 11 (9.9) |
| Stage 3 | 6 (5.4) |
| Renal replacement therapy (%) | 2 (1.8) |
Exposures
Over the initial 24 hours of sample collection, 43 (38.4%) patients received invasive mechanical ventilation. Acute kidney injury occurred in 33 (28.8%) and two individuals required emergent renal replacement therapy. No patient with ICH developed sepsis during the time of ICU data collection, and no patient with sepsis developed a primary neurologic condition such as stroke or seizures. Regarding pharmacologic exposures, 46 (41.1%) patients received intravenous sedatives, 35 (31.3%) received catecholamine vasopressors and 16 (14.3%) received intravenous vasopressin concurrently with a catecholamine infusion. All patients who received catecholamine vasopressors received norepinephrine, in some case with concurrent phenylephrine or dopamine. Nicardipine infusions were used exclusively for blood pressure control in patients with intracerebral hemorrhage; no beta-adrenergic antagonist exposure occurred.
Hourly average light intensity is shown in Figure 1 for the critically ill study patients with reference comparison to the age-matched control participants’ light exposure in their home environments during usual life. The median light exposure experienced by critically ill patients showed a diurnal pattern (p<0.001), but median daytime light intensity did not surpass 100 lux in any hour. Light exposure did not differ significantly between individuals (p=0.9) or according to illness severity (p=0.37 for light amplitude vs ICH Score in ICH subjects, p=0.95 for light amplitude vs SOFA in sepsis subjects). Tactile stimuli and arousals, estimated using the actigraphy records of the pharmacologically paralyzed control patients, showed that care-related stimuli were spread uniformly through day and night. Nutritional intake was reduced and irregular. Nutritional records showed that 43 (38.4%) patients received no nutrition, another 40 (35.7%) received less than 50% of their total daily caloric needs, with the remaining 29 (25.9%) receiving 50–100% of their caloric needs. Among the 69 patients who received some nutritional intake, 42 (61%) were on NPO status for part of the day or received nutrition by continuous tube feeding, whereas the remaining 27 (39%) received daytime nutrition in bolus form, either orally or by tube.
Figure 1.
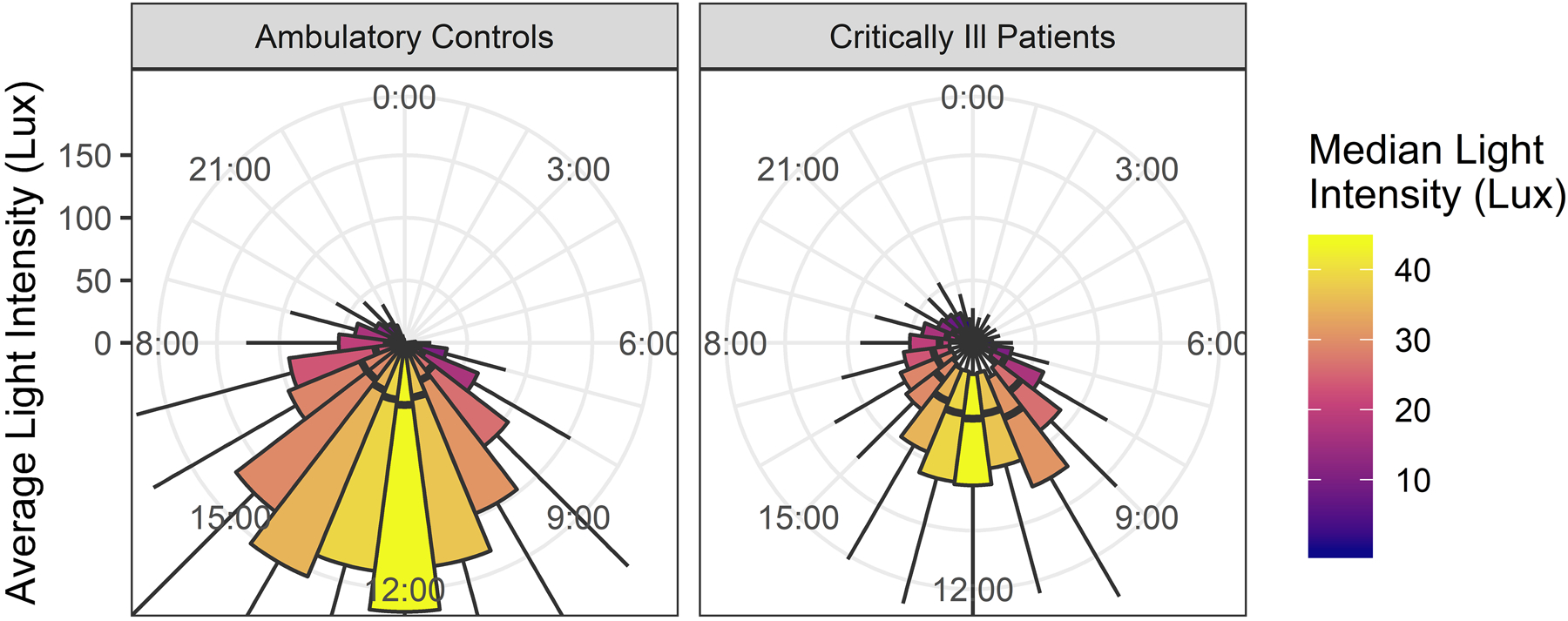
shows light exposure levels in healthy ambulatory control participants (left panel) and critically ill patients (right panel) across the day in hourly boxplots (thick line: median, box: interquartile range). Illuminescence was measured at eye level in critically ill patients and from the wrist in ambulatory patients.
Melatonin Rhythm Amplitude
Melatonin rhythms of the critically ill patients are compared to healthy control participants in Figure 2. The two primary effects observed were a dampening of melatonin amplitudes with increasing neurologic dysfunction (measured by GCS; Spearman’s rank correlation rho 0.30, p=0.0015), and a marked amplification of melatonin secretion in patients exposed to catecholamine vasopressors (rho 0.29, p=0.043). Significant univariate differences in melatonin amplitude were also found by sex and sedative exposure, but not for age or other SOFA score elements. All critically ill patient groups, as divided by GCS score category, showed evidence of temporal fluctuation in melatonin secretion over the day, both with and without adjustment for vasopressor exposure (all p<0.002). A parsimonious multivariable model confirmed that GCS and vasopressor exposure were independently associated with melatonin amplitude (p=0.0067 and p<0.0001, respectively), but not sedative exposure (p=0.066), age (p=0.11) or sex (p=0.10). No independent effect was seen for vasopressin, and no patients received angiotensin II. Melatonin trough levels were increased by vasopressor exposure (p<0.0001) as well as acute kidney injury (p=0.0036), and diminished by sedative exposure (p=0.044) and male sex (p=0.044). Type of critical illness (ICH versus sepsis) was not an independent predictor of melatonin amplitude, and there was no correlation between melatonin amplitude and any measure of light exposure.
Figure 2.
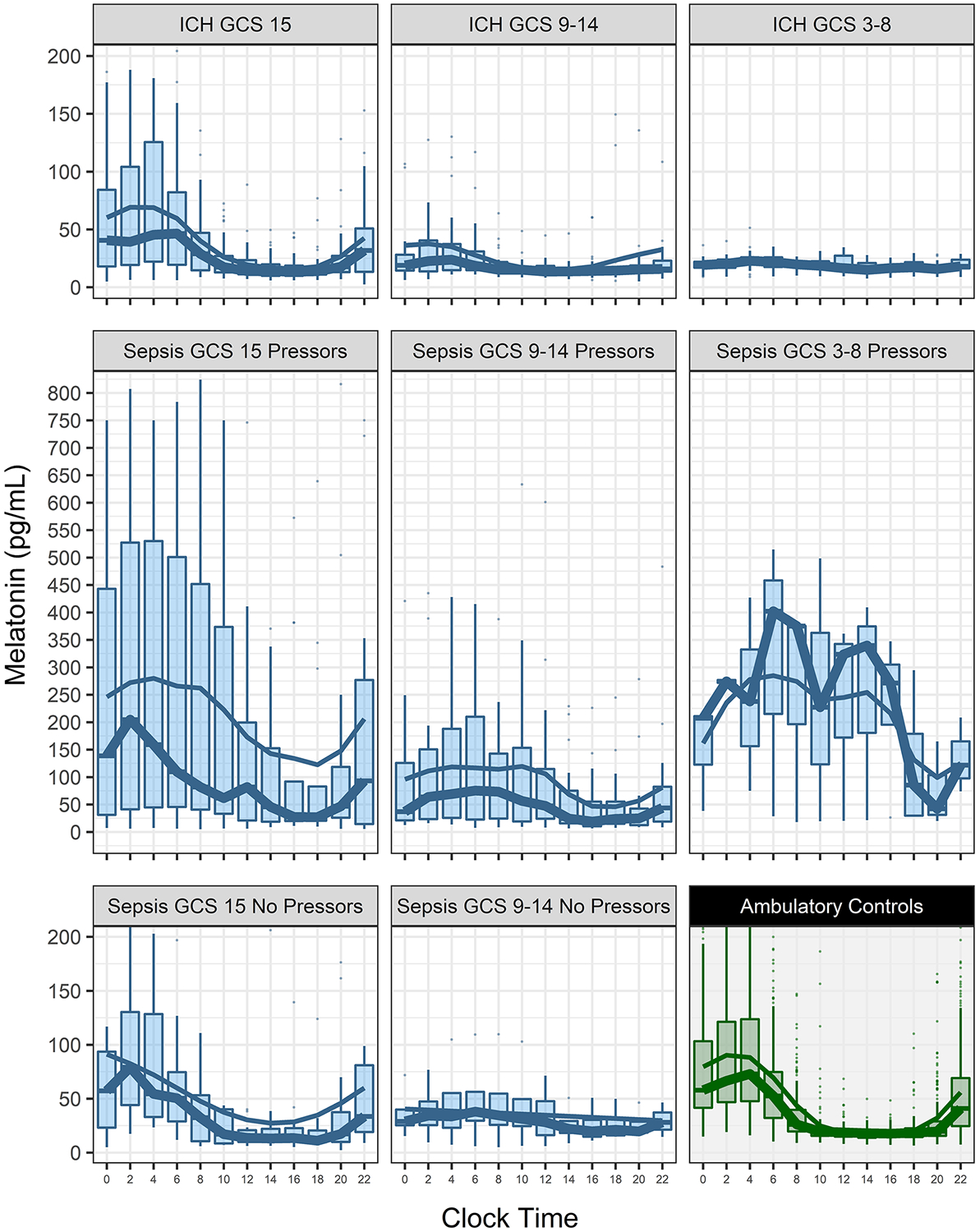
shows melatonin levels in critically ill patients (blue) and healthy control patients (green) across the day. Critically ill patients are separated into groups by Glasgow Coma Scale scores (GCS) and vasopressor exposure. There were no patients with sepsis and coma (GCS ≤8) who did not receive vasopressors. The thick trendline is the hourly median values and the thin trendline a moving average. Note that the melatonin plot scale is reduced 50% in the middle row panels.
Serum melatonin samples were obtained through 48 hours in 61 patients. There was no significant change in melatonin amplitude or total daily melatonin secretion between day 1 and 2. There was no association between a change in any of the clinical variables measured and the change in melatonin amplitude from day 1 to 2, although the change in total daily melatonin secretion was associated with changes in pressor dosing (β 547, 95% CI [100,995], p=0.018) after adjusting for age, change in GCS and kidney function. Specifically, improvement in encephalopathy severity as measured by change in median GCS between day 1 and 2 did not correlate with change in melatonin amplitude.
Melatonin Rhythm Timing
Dim light melatonin onset time could not be determined for many critically ill patients due to large magnitude, irregular fluctuations in melatonin concentration. Melatonin rhythm timing based on melatonin acrophase is shown in Figure 3A. All critically ill groups showed evidence of a groupwise circadian pattern of melatonin timing (all p<0.01 vs uniform time distribution), although there was a difference in acrophase timing (healthy group mean time 02:57, critically ill mean time 04:20, p=0.039) and dispersion (p<0.001) compared to healthy patients. Compared to healthy participants, patients with less severe neurologic dysfunction (GCS 9–15) differed only by dispersion of individual timing but not by average timing as a group, whereas comatose patients’ (GCS 3–8) melatonin timing was more dispersed (p=0.028) and delayed (06:51; p=0.0044).
Figure 3.
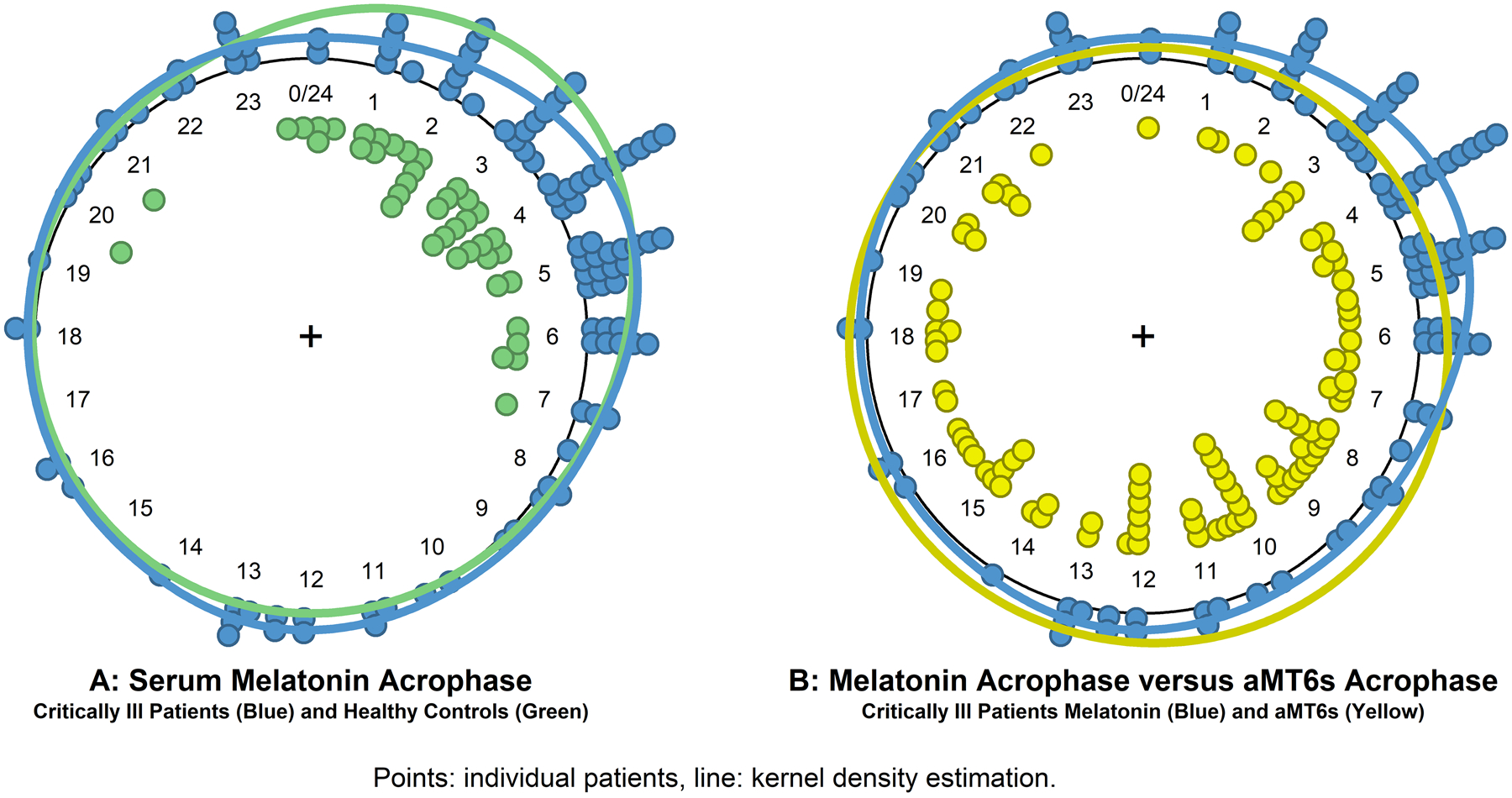
shows the timing of serum melatonin acrophase (panel A) for critically ill patients (blue) and healthy control participants (green), and a comparison of the timing of serum melatonin acrophase and urinary aMT6s acrophase (panel B) for critically ill patients. Points indicate individual measurements and the solid line is the circular kernel density estimation plot.
Urinary aMT6s and Serum Melatonin
The correlation between serum melatonin amplitude and urinary aMT6s amplitude was significant but weak (rho 0.36, p=0.00026). Multivariable models found that urinary aMT6s amplitudes varied significantly by age (p=0.037) and GFR (p=0.00010) in addition to serum melatonin amplitude. The correlation between serum melatonin amplitude and urinary aMT6s amplitude was moderate in patients with no acute kidney injury (rho 0.48, p<0.0001), but no correlation was seen in patients with acute kidney injury (rho 0.33, p=0.091). The temporal distribution of serum melatonin and urinary aMT6s is shown in Figure 3B. Urinary aMT6s acrophase showed greater temporal dispersion and later timing than serum melatonin acrophase (09:47 versus 04:20, p=0.02).
Discussion
This detailed characterization of central circadian rhythms in a large group of critically ill patients found that serum melatonin secretion patterns become rapidly disrupted within the first day of hospital care in high severity neurologic and systemic illnesses. Melatonin rhythms were generally preserved in awake patients but increasingly delayed and dampened with greater encephalopathy severity. Superimposed supraphysiologic melatonin secretion was observed in patients exposed to catecholamine vasopressor infusions. External stimuli were markedly abnormal, including light exposure (dim), nutritional intake patterns (reduced or absent and mistimed) and arousal stimuli (increased and mistimed to normal activity rhythms). Excessive nighttime light was rare and light exposure was not a factor in melatonin amplitude suppression. Urinary aMT6s was an unreliable surrogate for serum melatonin for measurement of circadian strength and timing, especially in patient with acute kidney injury.
Consistent zeitgebers- external, environmental stimuli that entrain and reinforce the central circadian rhythm- are crucial for circadian stability. Light has long been recognized as a potent zeitgeber and melatonin secretion inhibitor, although feeding and other behavioral rhythms are also well established as entraining stimuli. The abnormal zeitgeber exposures we found in these critically ill patients are consistent with findings we and others have reported previously with respect to abnormally dim lighting, frequent nursing care arousal stimuli, nighttime noise pollution and negative caloric balances, yet as to date there is limited evidence for therapeutically modifying these factors during acute illness aside from limited but promising data recommending cyclical instead of continuous enteral nutrition.(12, 19–21)
The central circadian clock is generally stable in an individual at a consistent time interval (phase angle) from usual environmental cues like light/darkness. Two types of stimuli are known to disrupt circadian rhythms. First, exposure to strong zeitgebers out of phase can destabilize the rhythm, either shifting it or dampening it. Second, some “critical stimuli” have a suppressive effect, and when applied with sufficient intensity, the resulting perturbation will cause a complete loss of rhythm expression. Circadian singularity, a state with no circadian phase (timing) or amplitude (signal strength) can be induced in humans from bright, mistimed light exposure alone.(22, 23) Other critical stimuli like abrupt temperature shifts, molecular toxins, ionizing radiation, hypertonicity and tissue damage have only been systematically studied in simple animal models (e.g. Drosophila, C. elegans) and cell cultures due to feasibility and ethical considerations. In this study, we leveraged the onset of critical illness to explore the circadian effects of intense physiologic stressors in humans, and found evidence that increasingly intense brain injury or multisystem derangements due to infection may represent critical stimuli to the melatonin rhythm, which at high severity were associated with singularity in melatonin rhythms.
A recent review of studies reporting melatonin or aMT6s patterns in critical illness characterized the literature as heterogeneous and contradictory.(24) Our findings provide clarifying insights. Although most studies reported abnormal rhythms in critically ill patients, numerous studies found intact hormonal rhythms. Although the patients in studies reporting intact rhythms were in intensive care units, they were generally non-septic, received no adrenergic agonists or corticosteroids, and had a comparatively low burden of encephalopathy and multiorgan failure.(25–27) By enrolling a wide spectrum of severity in neurologic and systemic critical illness, we were able to show that melatonin dysregulation is not ubiquitous during critical illness, but rather a feature of severe brain dysfunction or severe multiorgan dysfunction. While most studies of critically ill patients have reported suppressed aMT6s or melatonin amplitudes, several studies have conversely reported increased amplitudes, particularly among patients with shock. One study that observed both elevation and suppression patterns in patients who developed shock at different timepoints attributed the finding to differences in how pro-inflammatory cytokines may block pineal endocrine production of melatonin.(28) A more likely explanation for the divergent shock and non-shock critical illness melatonin patterns is the role of adrenergic stimulation by catecholamine pressors that we observed. Melatonin is released by β-adrenergic stimulation on pineal cell membranes.(29) Nearly 80% of published studies used aMT6s as a surrogate for serum melatonin, however, prior research has shown irregularities in patients with end-stage kidney disease suggesting aMT6s may not be reliable in the context of abnormal clearance, a finding we confirmed in this study.(24, 30) Given that acute kidney injury occurs in approximately 57% of critically ill patients, circadian profiling directly with serum melatonin is methodologically superior.(31)
These data suggest that normalization of circadian rhythms is a therapeutic opportunity, but the appropriateness of interventions must be carefully considered. Administration of melatonin could normalize timing and reinforce alignment between the central and peripheral rhythms, but doses used for many studies cause supraphysiologic serum melatonin concentrations, and our data show that many patients on vasopressors already have iatrogenically elevated melatonin levels.(32) A recent systematic review of studies using melatonin to improve sleep in the intensive care unit found no evidence for efficacy, perhaps because nocturnal melatonin deficiency was not present in many of the subjects treated or multiple other impediments to sleep hygiene and sleep generation persisted.(33) Chronotherapies may be more effective if they are targeted according to risk: environmental and sleep hygiene interventions for patients with intact mental status and a low burden of multiorgan failure, cyclical feeding and nighttime melatonin supplementation in select cases with severe illnesses, and interventions to reduce excessive daytime melatonin for patients with catecholamine pressor exposure or acute kidney injury. Bright light strongly inhibits pineal secretion of melatonin, as does clonidine, an alpha2 adrenoceptor agonist, a relevant consideration given the increasing utilization of dexmedetomidine.(34)
There are important limitations to these data. Melatonin is one of several important central circadian rhythm signals. Cortisol, core body temperature and autonomic activity are other measurable outputs of the central circadian rhythm, but intense physiologic stimuli overwhelm and obscure circadian contributions to those systems during critical illness. Sleep, another important circadian-entrained process, is of high interest because sleep deprivation interacts with circadian misalignment to destabilize health. Measuring sleep as a circadian phenomenon during critical illness, however, is challenging because it is also influenced by separate homeostatic mechanisms and disrupted by many other stimuli.(35) Moreover, sleep becomes indeterminable by electroencephalography when encephalopathic patterns predominate, as in the case with severe neurologic or systemic illness.(36–38) We sought to reduce confounding in this study by initiating study measurements within the first day of hospitalization to avoid cumulative effects over time, studying a relatively uniform physical environment, studying diseases treated using uniform care protocols, and by studying only patients with acute onset of illness who presented from the community to increase the likelihood of a stable premorbid circadian rhythm. While the strict design enabled us to better analyze the effects of important clinical and treatment factors, it is uncertain how well findings in patients with ICH and sepsis generalize to other critical illness and the extent to which other important variables were unmeasured. Understanding the relationship between central and peripheral tissue clocks during critical illness and the dynamics of circadian rhythm normalization is of particular interest moving forward.
Conclusions
Encephalopathy severity and adrenergic agonist medication exposure were the primary factors contributing to abnormal melatonin rhythms. Although melatonin rhythms became rapidly aberrant, improvements in encephalopathy and medical stabilization did not result in similarly rapid rhythm normalization. After accounting for other clinical characteristics, no differences were identified in between patients with neurologic and systemic critical illness. Finally, urinary aMT6s is not a reliable measure of the central circadian rhythm in critically ill patients.
Supplementary Material
Funding
Dr. Maas received support from National Institutes of Health grants K23NS092975 and L30NS080176, and a Dixon Translational Research Grant from the Northwestern Memorial Foundation. Dr. Liotta received support from the National Institutes of Health National Center for Advancing Translational Sciences grant KL2TR001424. Dr. Naidech received support from Agency for Healthcare Research and Quality grant K18HS023437. Drs. Abbott and Reid received support through the Northwestern Center for Circadian and Sleep Medicine. Drs. Reid and Zee receive support from National Institutes of Health grants UM1HL112856, R01HL140580 and P01AG011412. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences grant UL1TR000150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
Copyright form disclosure: Drs. Maas, Reid, and Zee’s institution received funding from National Institutes of Health (NIH). Dr. Maas’ institution received funding from Northwestern Memorial Foundation, Agency for Healthcare Research and Quality (AHRQ), and Northwestern Center for Circadian and Sleep Medicine, and he received support for article research from the AHRQ. Drs. Maas, Liotta, Naidech, Reid, and Zee received support for article research from the NIH. Dr. Abbott’s institution received funding from American Sleep Medicine Foundation and she received funding from American Board of Internal Medicine (Sleep Medicine Exam Writing Committee Member) and UptoDate. Dr. Liotta’s institution received funding from NIH National Center for Advancing Translational Sciences grant KL2TR001424; he received funding from NIH medical school loan repayment grant L30 NS098427; and he received support from NIH grants K23 NS092975. Dr. Naidech’s institution received funding from NIH R01 NS110779, and he received funding from the Society of Critical Care Medicine (speaking fees). Dr. Reid received funding from Sleep Research Foundation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Disclosures
All authors declare they have no conflicts of interest.
References
- 1.Chan MC, Spieth PM, Quinn K, et al. Circadian rhythms: from basic mechanisms to the intensive care unit. Crit Care Med 2012;40(1):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005;437(7063):1257–1263. [DOI] [PubMed] [Google Scholar]
- 3.Vujovic N, Gooley JJ, Jhou TC, et al. Projections from the subparaventricular zone define four channels of output from the circadian timing system. J Comp Neurol 2015;523(18):2714–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang R, Lahens NF, Ballance HI, et al. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 2014;111(45):16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med 2008;4(1):66–69. [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt FA, Liotta EM, Prabhakaran S, et al. Assessment and comparison of the max-ICH score and ICH score by external validation. Neurology 2018;91(10):e939–e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 8.Maas MB, Rosenberg NF, Kosteva AR, et al. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology 2013;81(2):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286(14):1754–1758. [DOI] [PubMed] [Google Scholar]
- 11.Knox DB, Lanspa MJ, Pratt CM, et al. Glasgow Coma Scale score dominates the association between admission Sequential Organ Failure Assessment score and 30-day mortality in a mixed intensive care unit population. J Crit Care 2014;29(5):780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan EP, Abbott SM, Reid KJ, et al. Abnormal environmental light exposure in the intensive care environment. J Crit Care 2017;40:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maas MB, Lizza BD, Kim M, et al. Stress-Induced Behavioral Quiescence and Rest-Activity Dysrhythmia during Critical Illness. Crit Care Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun R, Kath WL, Iwanaszko M, et al. Universal method for robust detection of circadian state from gene expression. Proc Natl Acad Sci U S A 2018;115(39):E9247–E9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms 2002;17(1):4–13. [DOI] [PubMed] [Google Scholar]
- 18.Ruxton GD. Testing for departure from uniformity and estimating mean direction for circular data. Biol Lett 2017;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bion V, Lowe AS, Puthucheary Z, et al. Reducing sound and light exposure to improve sleep on the adult intensive care unit: An inclusive narrative review. J Intensive Care Soc 2018;19(2):138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyko Y, Jennum P, Nikolic M, et al. Sleep in intensive care unit: The role of environment. J Crit Care 2017;37:99–105. [DOI] [PubMed] [Google Scholar]
- 21.Saito M, Nishimura K, Kato H. Modifications of circadian cortisol rhythm by cyclic and continuous total enteral nutrition. J Nutr Sci Vitaminol (Tokyo) 1989;35(6):639–647. [DOI] [PubMed] [Google Scholar]
- 22.Czeisler CA, Kronauer RE, Allan JS, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science 1989;244(4910):1328–1333. [DOI] [PubMed] [Google Scholar]
- 23.Jewett ME, Kronauer RE, Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature 1991;350(6313):59–62. [DOI] [PubMed] [Google Scholar]
- 24.Oldham MA, Lee HB, Desan PH. Circadian Rhythm Disruption in the Critically Ill: An Opportunity for Improving Outcomes. Crit Care Med 2016;44(1):207–217. [DOI] [PubMed] [Google Scholar]
- 25.Riutta A, Ylitalo P, Kaukinen S. Diurnal variation of melatonin and cortisol is maintained in non-septic intensive care patients. Intensive Care Med 2009;35(10):1720–1727. [DOI] [PubMed] [Google Scholar]
- 26.Boyko Y, Holst R, Jennum P, et al. Melatonin Secretion Pattern in Critically Ill Patients: A Pilot Descriptive Study. Crit Care Res Pract 2017;2017:7010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mundigler G, Delle-Karth G, Koreny M, et al. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med 2002;30(3):536–540. [DOI] [PubMed] [Google Scholar]
- 28.Sertaridou EN, Chouvarda IG, Arvanitidis KI, et al. Melatonin and cortisol exhibit different circadian rhythm profiles during septic shock depending on timing of onset: a prospective observational study. Ann Intensive Care 2018;8(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore RY. Neural control of the pineal gland. Behav Brain Res 1996;73(1–2):125–130. [DOI] [PubMed] [Google Scholar]
- 30.Lüdemann P, Zwernemann S, Lerchl A. Clearance of melatonin and 6-sulfatoxymelatonin by hemodialysis in patients with end-stage renal disease. J Pineal Res 2001;31(3):222–227. [DOI] [PubMed] [Google Scholar]
- 31.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41(8):1411–1423. [DOI] [PubMed] [Google Scholar]
- 32.Galley HF, Lowes DA, Allen L, et al. Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res 2014;56(4):427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis SR, Pritchard MW, Schofield-Robinson OJ, et al. Melatonin for the promotion of sleep in adults in the intensive care unit. Cochrane Database Syst Rev 2018;5:CD012455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muñóz-Hoyos A, Fernández-García JM, Molina-Carballo A, et al. Effect of clonidine on plasma ACTH, cortisol and melatonin in children. J Pineal Res 2000;29(1):48–53. [DOI] [PubMed] [Google Scholar]
- 35.Deboer T. Sleep homeostasis and the circadian clock: Do the circadian pacemaker and the sleep homeostat influence each other’s functioning? Neurobiol Sleep Circ Rhythms 2018;5:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azabou E, Magalhaes E, Braconnier A, et al. Early Standard Electroencephalogram Abnormalities Predict Mortality in Septic Intensive Care Unit Patients. PLoS One 2015;10(10):e0139969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H, Mizrahi MA, Hartings JA, et al. Continuous Electroencephalography After Moderate to Severe Traumatic Brain Injury. Crit Care Med 2019;47(4):574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourne RS, Minelli C, Mills GH, et al. Clinical review: Sleep measurement in critical care patients: research and clinical implications. Crit Care 2007;11(4):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


