Abstract
Phosphorus is a common additive used in food processing that is typically consumed in excess of the recommended daily allowance, however our knowledge of its effects on health, in the context of normal renal function, is limited. Unlike phosphorus, calcium intake is generally less than recommended and it has been hypothesized that the calcium to phosphorus ratio may be partly responsible for the proposed negative health consequences. Therefore, this study sought to determine the effects of increased phosphorus additive intake, in the context of high calcium consumption, on endocrine markers of mineral metabolism and cardio-metabolic health. An outpatient feeding study was performed in which healthy adults were fed a run-in control diet for 2 weeks followed by a phosphorus additive enhanced diet with supplemental calcium to an approximate ratio of 1 (experimental diet) for 2 weeks. Blood and urine samples were collected and participants had brachial flow mediated dilatation measured, with analyses comparing follow-up measures to baseline. Two weeks of experimental diet increased serum fibroblast growth factor 23 concentrations but lowered body weight and serum leptin; however, other phosphorus responsive factors such as osteopontin and osteocalcin did not increase. A complementary study in male mice also demonstrated that the regulation of known dietary phosphorus responsive factors was mostly abrogated when dietary calcium was raised in parallel with phosphorus. In conclusion, the study identifies weight, leptin, and insulin as responsive to dietary phosphorus and that certain aspects of the systemic phosphorus response are attenuated by a corresponding high calcium intake.
Keywords: FGF23, Osteopontin, parathyroid hormone, Leptin, insulin, HOMA-IR
1. Introduction
Phosphorus-based additives serve a number of critical functions for food manufacturing, including enhancing taste, appearance, and shelf life of processed foods [1]. Because of this wide diversity of applications, the use of phosphorus additives in the food manufacturing industry is widespread and can substantially augment the total amount of phosphorus in highly processed foods [2–5]. The United States Food and Drug Administration and the European Food Safety Authority have evaluated the use of phosphorus additives and concluded that they are generally safe when consumed in quantities consistent with current industry practice [6, 7]. While the consumption of phosphorus in the American diet is increasing [8] the consumption of calcium remains relatively low. The dietary recommendations for older adults of calcium (1200mg/d) and phosphorus (700mg/d) [9] result in a calcium to phosphorus ratio (Ca:P) of approximately 1.7 and >90% of males and females do not meet these ratios [10]. The health consequences of this, if any, remain largely unknown.
We previously showed that consuming a phosphorus additive enhanced diet that was low in calcium (calcium to phosphorus ratio 0.76) for one week increased fibroblast growth factor 23 (FGF23), osteopontin, and osteocalcin, all factors linked to cardiovascular disease and mineral metabolism [11]. Analogous findings were observed in female mice fed high phosphorus diets for 5 or 15 weeks. These data suggest that phosphorus-based food additives may have deleterious health consequences, at least when consumed in a greater than 1 to 1 ratio with calcium. What is less clear is whether enhancing calcium in the diet may abrogate these effects.
The purpose of this study was to examine the effect of increased phosphorus additive intake on endocrine markers of mineral metabolism and cardio-metabolic health, and whether increased calcium intake modifies the response we previously observed in healthy adults. A mouse model was used to complement and validate the results from the clinical study. Here we used male mice to complement our previous diet study which used female mice [11]. Collectively, the study identified specific phosphorus responses that can be blunted by increasing calcium in humans and mice, such as increased circulating levels of FGF23 and osteopontin (OPN) and others than cannot, such as a decrease in serum insulin. An increase in circulating parathyroid hormone (PTH) in response to a high phosphorus diet was detected only in mice and was not affected by increasing calcium intake. The results provide additional insight into the close relationship of dietary phosphorus and calcium and the potential systemic effects on health and disease.
2. Materials and methods
2.1. Human Studies.
Healthy volunteers 19 to 45 years of age from the Birmingham, AL, USA metropolitan area were recruited to participate in the study. Exclusion criteria included the following: laboratory evidence of kidney disease as indicated by an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 or an abnormal urinalysis; pregnancy or a history of irregular menses for female participants; medical conditions known to impact phosphorus metabolism (such as thyroid disease); current use of medications known to affect phosphorus metabolism (such as phosphorus supplements or high-dose vitamin D compounds); body mass index (BMI) ≥30 kg/m2; or screening laboratory evidence of abnormal serum phosphorus concentrations (serum phosphorus >4.6 or <2.5 mg/dL), abnormal serum calcium concentrations (serum total calcium >10.6 or <8.5 mg/dL), or severe anemia (hemoglobin <8 g/dL for women and <9 g/dL for men). Frequency matching was used to ensure balanced enrollment of men and women, and black and white individuals. The University of Alabama at Birmingham (UAB) Institutional Review Board for Human Use reviewed and approved the study and all participants provided written, informed consent. The study complied with Clinical Trial registration requirements (NCT01394146).
2.2. Study Diets.
Separate menus for a low additive diet (henceforth referred to as the control diet) and an additive enhanced diet (henceforth referred to as the experimental diet) were developed by the Bionutrition Core of the Clinical Research Unit (CRU) in the Center for Clinical and Translational Science at UAB using the Nutrition Data System for Research (NDSR, 2011). Each menu consisted of four separate days of food (breakfast, lunch and dinner). The control menu was designed to provide 15% kilocalories from protein, 55–60% from carbohydrates and 25–30% from fat and 1200–1500 mg of phosphorus per day using minimally-processed foods. The target for phosphorus intake was meant to conform with the average phosphorus intake for US adults according to most recent published estimates [12]. The experimental menu was designed to provide identical energy and nutrient content per day as the low additive menu but substituting highly-processed for minimally-processed foods. The diets were isocaloric with each individual’s estimated caloric needs based upon standard assessments [13]. A copy of sample menus over four days is provided in Supplementary Table 1. In order to confirm that the study diets adequately differed in the main nutrient of interest (phosphorus), the nutrient contents of each menu were measured by sending aliquot samples for each day (half a cup) to Covance Laboratories Inc. (Madison, WI), as detailed previously [4].
2.3. Study Protocol.
After completing a screening visit during which eligibility for study participation was confirmed, study participants entered a two-week run-in period during which two 24-hour urine collections were obtained for measurement of creatinine clearance and urinary excretion of sodium, calcium and phosphorus while consuming ad lib diets (Fig. 1). In addition, a four-day food record was obtained to assess baseline diet intake. At the end of the run-in period, participants were provided study meals prepared by the metabolic kitchen to consume at home over the following four weeks. During the first two weeks, participants consumed the control diet in order to standardize phosphorus intake given wide variability in ad lib phosphorus consumption. Immediately following the last day of the control diet period (considered the baseline visit for all following comparisons), participants switched to consuming the experimental diet for two weeks. The sodium content of the control and experimental diets differed by approximately 65 mmol of sodium a day; therefore, sodium chloride tablets were provided to participants during the control diet period to equalize sodium intake during the two diet intervention periods, so that the two diets only differed in phosphorus additive content. Participants were instructed to take one tablet with each meal. In addition, participants were provided a 600 mg calcium carbonate tablet daily with their dinner to maintain the calcium:phosphorus ratio of each diet ~1.0 (1822 mg/d calcium to 1940 mg/d phosphorus). Participants returned to the CRU every three or four days to pick up packaged meals and to have follow-up weight and blood pressure measurements. In addition, 24-hour urine collections were obtained at the end of each dietary period. No additional foods were allowed during the four-week intervention period. Compliance with interventions was monitored by self-reported intake and measurements of 24-hour urinary phosphorus excretion.
Fig. 1.
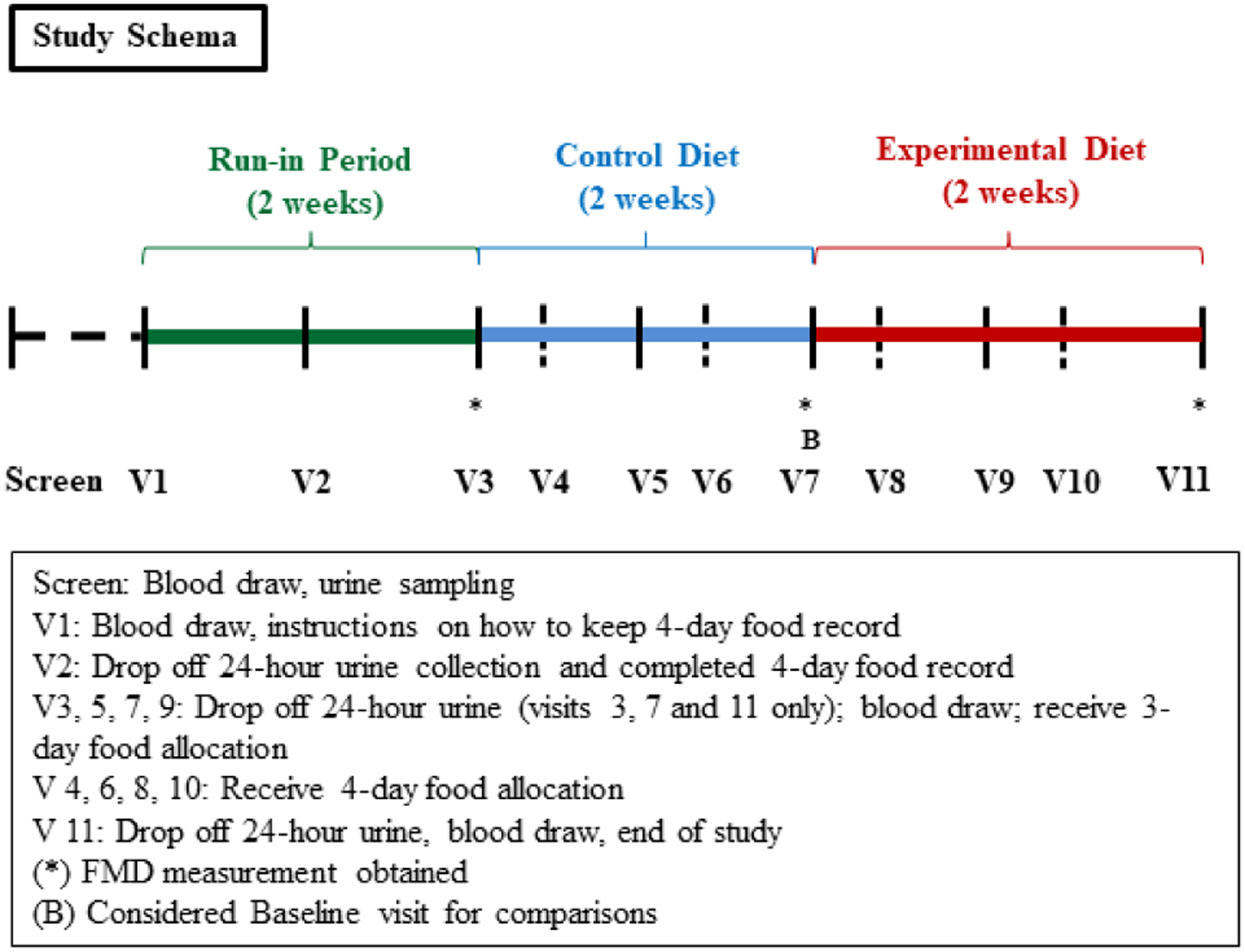
Study design. Time protocol of blood and urine collection as well as food allocation.
2.4. Blood sampling.
Fasting blood and urine specimens were collected at the baseline visit and weekly thereafter. Serum and plasma samples were separated and, along with urine samples, frozen at −80°C for batched analysis. Participants underwent blood sampling after two weeks of consuming the control diet and after two weeks of the experimental diet. Blood concentrations of phosphorus, calcium, insulin, glucose, FGF23 and PTH were measured at baseline and at each follow-up visit. Blood concentrations of P1NP (procollagen type 1 N propeptide), CTX (C-terminal telopeptide of type 1 collagen), OCN (Osteocalcin), OPN, leptin, adiponectin, inflammatory cytokines (interleukin [IL]-1b, IL-6, IL-8, and tumor necrosis factor [TNF] alpha); and urine concentrations of phosphorus, calcium, and creatinine were measured after two weeks of consuming the control diet and after two weeks of consuming the experimental diet. FGF23 was measured using a second-generation c-terminal assay (Immutopics, Santa Clara, CA, CVs < 5%). PTH was measured using an intact PTH assay (ALPCO, Salem, New Hampshire, CVs < 6%). P1NP was measured using the UniQ radioimmunoassay (Orion Diagnostica, Espoo, Finland; CV’s < 10%). CTX was measured using an ELISA (Immunodiagnostic Systems, Fountain Hills, AZ; CV’s < 10%). OPN was measured using quantitative sandwich enzyme immunoassay (R&D Systems, CV’s <6%). OCN was measured using the N-Mid Osteocalcin ELISA (Immunodiagnostic Systems Inc., Gaithersburg, MD, CV’s <4%). The assay detects total osteocalcin (intact and N-Mid fragment). Leptin was measured using the Millipore (Burlington, MA) Human Leptin RIA assay (CV’s < 5%) and adiponectin was measured using the Millipore Adiponectin RIA assay (CV’s < 5%). Inflammatory cytokines were measured using the Meso Scale Discovery Human Proinflammatory Panel II 4-plex Kit (Meso Scale Diagnostics; Rockville, MD), with CV’s <15%. Fasting serum glucose and insulin concentrations were measured using a SIRRUS analyzer (Stanbio Laboratory, Boerne, TX; CV’s < 5%) and the TOSOH AIA-600 II Automated Immunoassay Analyzer (TOSOH Bioscience, South San Francisco CA,; CV’s < 5%), respectively. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated according to the following equation: (fasting insulin concentration [μIU/L])/(fasting glucose concentration [mg/dL]) x 405 [14].
2.5. Flow-mediated dilatation.
All flow-mediated dilatation (FMD) studies were done at the same time of the morning after an overnight fast by the same examiner (AKP). FMD was measured using a high-resolution ultrasound with a 7.5 MHz linear-array probe (Philips HP Agilent Sonos 5500, Andover, MA) after a 30-minute rest in the supine position in a quiet, air-conditioned room according to standard guidelines. FMD was defined as the percentage increase in diameter from baseline to peak dilation after ischemia and reactive hyperemia induced by inflation of a blood pressure cuff 50 mm Hg above the participant’s supine systolic blood pressure for five minutes followed by rapid deflation.
2.6. Animal Studies.
Animal care and experimental procedures were conducted with the approval of the Atlanta Veterans Administration Institutional Animal Care and Use Committee and Emory Institutional Animal Care and Use Committee. Male C57BL/6J mice were obtained at eight weeks of age from The Jackson Laboratories (Bar Harbor, ME) and allowed to acclimate for two weeks. Mice were housed in a facility with controlled conditions (temperature, 21–24°C; humidity, 40–70%; light/dark cycle, 12 h light/12 h dark). At ten weeks of age, mice were randomly assigned (five mice per group) to either a normal phosphorus diet (NPD; 0.6% phosphorus and 0.6% calcium [Cat # TD.110360]), a low phosphorus diet (LPD; 0.2% phosphorus and 0.6% calcium [TD.110360]), a low calcium and phosphorus diet (LCPD; 0.2% phosphorus and 0.2% calcium [TD.140831]) a high phosphorus diet (HPD; 1.8% phosphorus and 0.6% calcium [TD.110362]) or a high phosphorus, high calcium diet (HCPD; 1.8 % phosphorus and 1.8% calcium [Cat # TD.140832]), all manufactured by Envigo (Huntingdon, Cambridgeshire, United Kingdom). Protein, fat, and other mineral elements were matched between the diets providing essentially identical energy and caloric values (3.7–3.9 Kcal/g). Vitamin D was 2.2 IU for each diet. Mice were fed ad libitum. After five weeks on the diets, mice were weighed and serum obtained prior to sacrifice to measure FGF23, PTH, OCN, OPN, leptin, Adiponectin, CTX, P1NP, insulin, and serum chemistries. All groups of mice were sacrificed between 9:00 AM and 12:00 PM (noon) to attempt to limit any differences between the last feed consumption.
2.7. ELISA measurements of murine serum factors.
All assays were performed according to manufacturer’s protocols and quantified by VERSAmax microplate reader (Molecular Devices, San Jose CA). ELISA kits were purchased as follows: FGF23, PTH, and OCN (Quidel (Immutopics) San Diego, CA), OPN, Leptin, and Adiponectin (R and D Systems; Minneapolis, MN), CTX, and P1NP (Immunodiagnostic Systems; Gaithersburg, MD), and Insulin (ALPCO; Salem, NH).
2.8. Statistical Analysis.
Linear mixed-effects models were used to examine changes in study outcome variables over time. In these models, time represented the repeated measures factor and individuals were treated as random effects terms. When we detected a significant effect of time, we localized individually significant changes in post-baseline time points by comparing them with the baseline values. The primary outcome measure was the percent change in FGF23 at the end of the experimental diet as compared to the end of the control diet. FGF23 concentrations were not normally distributed, so log-transformed values were analyzed. Urine analyte excretion was analyzed using values standardized to urinary creatinine excretion to account for incomplete urine collections in study participants. For the animal studies, differences in blood analytes between diet groups were assessed using one-way ANOVA. The data were analyzed using PRISM version 8.1.2 (GraphPad Software, Inc., San Diego, CA). All statistical tests were performed at the 5% significance level.
3. Results
3.1. Clinical study design.
A total of 35 participants were enrolled in the study; one participant was removed due to repeated non-compliance with the study protocol, leaving 34 participants who completed the full four week study protocol. The mean age of study participants was 28.8±8 years, 52% were male, 50% were black, and the average body mass index (BMI) was 25.9 ± 3.5 (Table 1). Diet characteristics of study participants while consuming ad lib diets and measured energy and nutrient contents of the study diets averaged over the four menu days are depicted in Table 2. There were no statistically significant differences in total energy, and sodium was kept constant at ~155 mg/d. As compared to the control diet (low phosphorus additive content, containing 1286 mg/d), the average phosphorus of the experimental diet (enriched with phosphorus additives) was higher by 653 mg/d (1940mg/d) and similar to the total calcium content of 1822 mg/d. After phosphorus intake was standardized on the control diet for two weeks, participants were challenged with the experimental diet for two weeks (Fig. 1). The last day of the control diet was considered the baseline for all following comparisons.
Table 1.
Characteristics of the study sample.
| Variable | Overall (N=34) |
|---|---|
| Age | 28.8 ± 8.0 |
| Male No., (%) | 18 (52) |
| Black No., (%) | 17 (50) |
| Body mass index (kg/m2) | 25.9 ± 3.5 |
| Baseline Blood Values | |
| Phosphorus (mg/dL) | 3.81 ± 0.4 |
| Calcium (mg/dL) | 9.38 ± 0.4 |
| Intact parathyroid hormone (pg/ml) | 40.2 ± 12.9 |
| Fibroblast growth factor 23 (RU/ml) | 70.6 [58.5,85.7] |
| Osteocalcin (ng/ml) | 18.0 ± 8.5 |
| CTX (ng/ml) | 0.56 ± 0.32 |
| P1NP (pg/L) | 64.8 ± 25.0 |
| Osteopontin (ng/ml) | 33.9 ± 12.1 |
| HOMA-IR | 1.87 ± 0.91 |
| Baseline Urine Values | |
| 24-hour urine phosphorus (mg/day) | 766 ± 280 |
| 24-hour urine calcium (mg/day) | 122 ± 67 |
| 24-hour urine sodium (mmol/day) | 139 ± 41 |
| Creatinine clearance (ml/min) | 126 ± 29 |
Values are presented as means ± standard deviation, number (frequency) or median [interquartile range]. CTX, C-terminal telopeptide of type 1 collagen; P1NP, procollagen type 1 N-terminal propeptide; HOMA-IR, homeostatic model assessment-insulin resistance
Table 2.
Diet characteristics of study participants at baseline and measured energy and nutrient contents of control and additive enhanced (experimental) diets.
| Ad Lib | Control diet | Experimental diet | |
|---|---|---|---|
| Estimated* | Measured** | Measured** | |
| Energy (kcal/day) | 2042 ± 457 | 2081 ± 117 | 1951 ± 308 |
| % fat | 34 ± 5 | 28 ± 4 | 24 ± 4 |
| % protein | 15.9 ± 3 | 19 ± 3 | 19 ± 2 |
| % carbohydrates | 48.6 ± 6 | 52 ± 5 | 57 ± 2 |
| Calcium (mg/day) | 845 ± 443 | 1577 ± 319 | 1822 ± 451 |
| Phosphorus (mg/day) | 1234 ± 404 | 1286 ± 223 | 1940 ± 419† |
| Sodium (mmol/day) | 160 ± 50 | 156 ± 28 | 155 ± 27 |
Values are presented as mean ± standard deviation.
Estimated from four-day food record
Based on a 2,000 kcal diet
P<0.05 comparing experimental diet to the control diet measured values.
3.2. Serum and Urine Chemistry.
Twenty-four hour urinary phosphorus excretion standardized to creatinine excretion was significantly higher at the end of the experimental diet as compared to baseline (Fig. 2, Ptime<0.001). Urine calcium and sodium excretion standardized to creatinine did not change from baseline after two weeks of consuming the experimental diet. Changes in blood analyte concentrations are depicted in Table 3. Fasting serum phosphorus concentrations significantly declined from baseline after two weeks of the experimental diet (Ptime=0.002). Serum calcium concentrations significantly increased after one week of the experimental diet but then returned to baseline values after the second week (Ptime=0.02). Geometric mean plasma FGF23 concentrations did not significantly change after two weeks of the experimental diet (Ptime=0.44). When analyzed relative to baseline values, plasma FGF23 concentrations significantly increased by 14% after one week of the experimental diet and remained 11% above baseline after the second week of the experimental diet (P<0.05 for both). There were no other significant changes in other markers of bone or mineral metabolism. Changes in blood and urine markers of mineral metabolism across all time points are depicted in Supplementary Table 2.
Fig. 2.
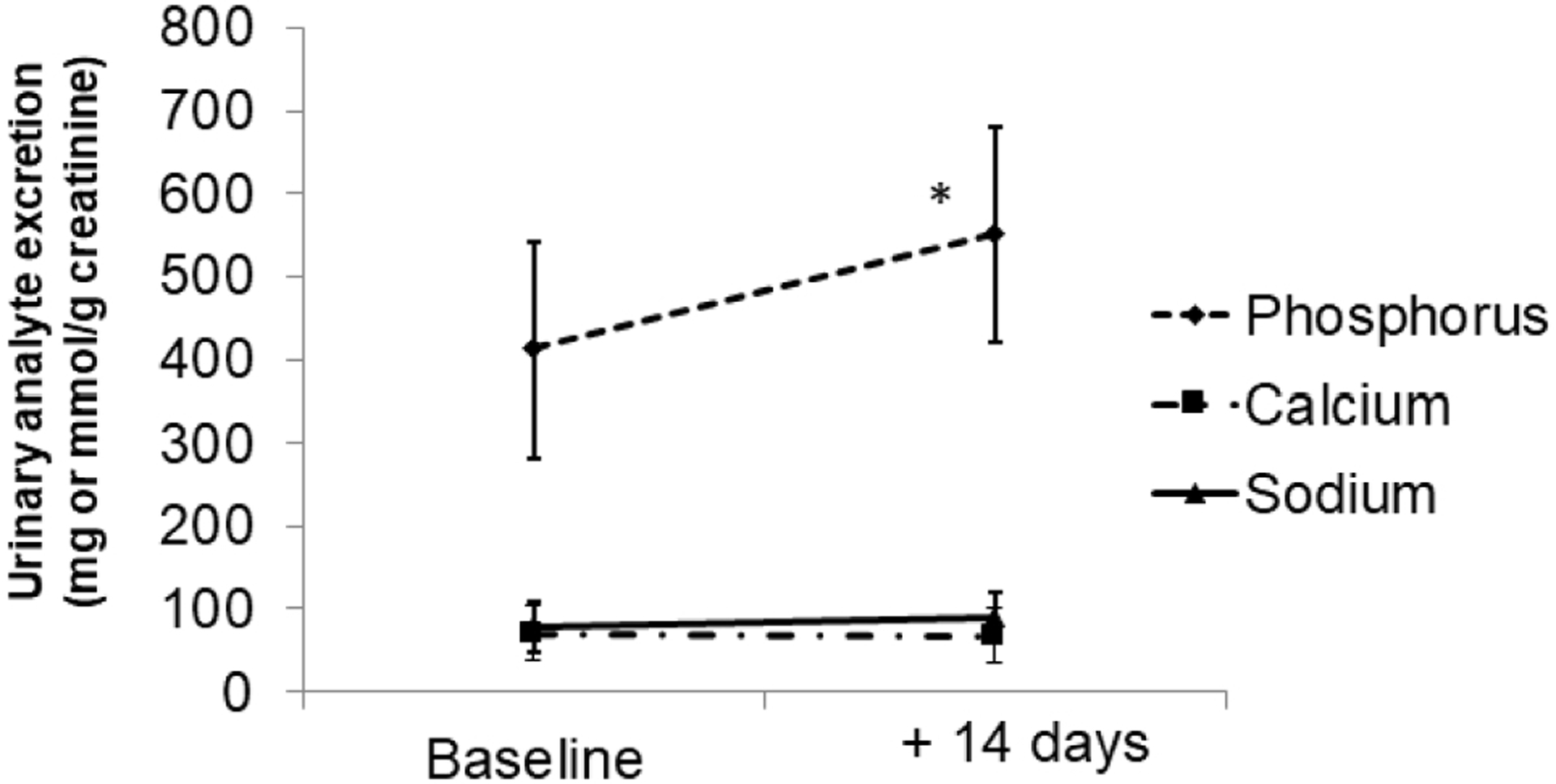
Changes in urinary analytes over time. Values are expressed per gram of creatinine excretion from 24-hour urine collections. Symbols represent mean values and vertical lines represent standard deviation. Asterisk indicates significant (P<0.05) differences from baseline values.
Table 3.
Changes in markers of bone and mineral metabolism after two weeks of consuming the additive enhanced (experimental) diet.
| Baseline | +7 days | +14 days | P value* | |
|---|---|---|---|---|
| Markers of Mineral Metabolism | ||||
| Phosphorus (mg/dL) | 3.95 ± 0.52 | 3.99 ± 0.63 | 3.65 ± 0.46† | 0.002 |
| Calcium (mg/dL) | 9.17 ± 0.31 | 9.31 ± 0.27† | 9.19 ± 0.31 | 0.02 |
| FGF23 (RU/ml) | 74.1 (64.2, 85.5) | 81.6 (70.7, 94.2) | 80.4 (69.7, 92.8) | 0.44 |
| PTH (pg/ml) | 38.4 ± 11.6 | 40.3 ± 13.3 | 39.8 ± 12.0 | 0.62 |
| Markers on Bone Metabolism | ||||
| P1NP (μg/L) | 62.9 ± 25.9 | -- | 62.1 ± 23.9 | 0.70 |
| CTX (ng/ml) | 0.52 ± 0.27 | -- | 0.51 ± 0.28 | 0.35 |
| Osteocalcin (ng/ml) | 18.5 ± 8.8 | -- | 17.5 ± 8.0 | 0.81 |
| Osteopontin (ng/ml) | 33.0 ± 12.0 | -- | 35.4 ± 13.6 | 0.28 |
Values are presented as arithmetic mean ± standard deviation or geometric mean (95 confidence interval). FGF23; fibroblast growth factor 23; PTH, intact parathyroid hormone; CTX, C-terminal telopeptide of type 1 collagen; P1NP, procollagen type 1 N-terminal propeptide
P for time indicating the statistical significance of changes in outcome variables over time
P<0.05 when compared to baseline values
3.3. Cardio-metabolic measurements.
There was a small but statistically significant decline in weight after two weeks of the experimental diet (Table 4). Fasting insulin and HOMA-IR values significantly increased after one week of the experimental diet, but then declined back to values observed at baseline following the second week of the experimental diet (Ptime<0.05 for both). Serum leptin significantly declined from baseline after two weeks of the experimental diet (Ptime=0.01). There were no significant differences in adiponectin or inflammatory cytokine concentrations over time. There was no significant change in percent brachial FMD after two weeks of consuming the experimental diet (11.2±6.3) as compared to baseline values (11.7±5.5) (Ptime=0.69).
Table 4.
Changes in anthropomorphic variables and markers of metabolic health after two weeks of consuming the additive enhanced (experimental) diet.
| Baseline | +7 days | +14 days | P value* | |
|---|---|---|---|---|
| Anthropomorphic Variables | ||||
| Weight (lbs) | 166.1 ± 30.6 | 165.2 ± 30.4 | 164.7 ± 29.9† | 0.002 |
| Systolic blood pressure (mmHg) | 119 ± 13 | 113 ± 22 | 114 ± 12 | 0.10 |
| Diastolic blood pressure (mmHg) | 66 ± 10 | 66 ± 10 | 65 ± 10 | 0.55 |
| Markers of Insulin Resistance and Metabolic Health | ||||
| Fasting glucose (mg/dL) | 79.7 ± 6.2 | 80.5 ± 6.2 | 79.3 ± 6.4 | 0.32 |
| Fasting insulin (μU/L) | 7.6 ± 3.1 | 8.9 ± 4.1† | 6.7 ± 3.2 | 0.002 |
| HOMA-IR | 1.5 ± 0.6 | 1.8 ± 0.9† | 1.3 ± 0.7 | <0.001 |
| Leptin (ng/ml) | 9.7 (6.9, 13.7) | -- | 8.3 (5.8, 11.7)† | 0.01 |
| Adiponectin (μg/ml) | 8.1 ± 3.9 | -- | 7.7 ± 3.6 | 0.11 |
| Inflammatory Cytokines | ||||
| Interleukin 6 (pg/ml) | 0.54 ± 0.23 | -- | 0.61 ± 0.31 | 0.11 |
| Interleukin 8 (pg/ml) | 3.9 ± 1.9 | -- | 4.4 ± 4.4 | 0.54 |
| Tumor necrosis factor a (pg/ml) | 1.7 ± 0.5 | -- | 1.9 ± 0.9 | 0.18 |
HOMA-IR, homeostatic model assessment-insulin resistance. Values are presented as arithmetic mean ± standard deviation or geometric mean (95 confidence interval).
P for time indicating the statistical significance of changes in outcome variables over time
P<0.05 when compared to baseline values
3.4. The dietary phosphorus to calcium ratio alters weight and serum chemistry in male mice.
Mouse models were used to complement the clinical studies because of the ability to perform longer studies and more precisely control the calcium to phosphorus ratio. Male C57BL/6J mice were randomly assigned to low phosphorus (LPD: 0.2% Pi and 0.6% Ca), low phosphorus:low calcium (LCPD: 0.2% Pi and 0.2% Ca), normal phosphorus (NPD: 0.6% Pi and 0.6% Ca), high phosphorus (HPD: 1.8% Pi and 0.6% Ca) or high phosphorus:high calcium (HCPD: 1.8% Pi and 1.8% Ca) diets. The LPD, NPD, and HPD were designed to keep calcium constant at 0.6% and therefore, resulted in calcium to phosphorus intakes of 3:1 (LPD), 1:1 (NPD), and 1:3 (HPD) respectively, while the LCPD and HCPD were designed to achieve calcium to phosphorus intakes of 1:1 (0.2% to 0.2% and 1.8% to 1.8%) (Table 5). The mice were fed the diets ad libitum for 5 weeks. At the completion of the studies the mice were weighed. The mice fed LPD weighed more relative to NPD whereas HPD fed mice weighed less (Fig. 3A). There were no significant differences in body weight when dietary calcium was included in a 1:1 ratio to phosphorus (HCPD vs. NPD vs. LCPD) (Fig. 3A). The results suggested that in male mice the ratio of phosphorus to calcium influences weight which is in contrast to our previous studies in female mice which found no statistical differences in weight after five weeks [11]. Additionally, phosphorus and calcium were measured from serum (non-fasting) collected at the completion of the study. Whereas there was no difference between the NPD and HPD, mice fed the LPD had significantly lower serum phosphorus than mice fed the NPD (Fig. 3B). Interestingly, the HCPD mice also had significantly lower serum phosphorus relative to HPD. No substantial or statistical changes in serum calcium were noted between any of the diets (Fig. 3C).
Table 5:
Diet characteristics for Mouse studies
| Diet | LCPD | LPD | NPD | HPD | HCPD |
|---|---|---|---|---|---|
| % Pi and % Ca | 0.2;0.2 | 0.2;0.6 | 0.6;0.6 | 1.8;0.6 | 1.8;1.8 |
| Ratio | 1:1 | 1:3 | 1:1 | 3:1 | 1:1 |
| Casein (g/Kg) | 210 | 210 | 210 | 210 | 210 |
| L-Cystine | 3 | 3 | 3 | 3 | 3 |
| Corn Starch | 343 | 333 | 325 | 297 | 279 |
| Sucrose | 150 | 150 | 150 | 150 | 150 |
| Maltodextrin | 100 | 100 | 100 | 100 | 100 |
| Cellulose | 47 | 47 | 47 | 47 | 47 |
| Soybean Oil | 50 | 50 | 50 | 50 | 50 |
| Lard | 50 | 50 | 50 | 50 | 50 |
| Vitamin Mix, Teklad (40060) | 10 | 10 | 10 | 10 | 10 |
| TBHQ, antioxidant | .02 | .02 | .02 | .02 | .02 |
| Vitamin D (IU) | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 |
| Mineral Mix, w/o Ca & P (98057) | 13.4 | 13.4 | 13.4 | 13.4 | 13.4 |
| Calcium Carbonate | 5.0 | 14.9 | 14.9 | 14.6 | |
| Sodium Bicarbonate | 9.2 | 9.2 | 3.7 | ||
| Potassium Carbonate | 7.7 | 7.7 | 3.1 | ||
| Potassium Phosphate, monobasic | 1.0 | 1.0 | 10.0 | 16.0 | 16.0 |
| Sodium Phosphate, monobasic, monohydrate | 1.0 | 1.0 | 10.0 | 16.0 | 16.0 |
| Calcium Phosphate, monobasic, monohydrate | 37.5 | ||||
| Calcium Phosphate, dibasic | 41.0 | ||||
| % by weight | |||||
| Protein | 18.6 | 18.6 | 18.6 | 18.6 | 18.6 |
| Carbohydrate | 55.8 | 54.9 | 54.2 | 51.7 | 50.0 |
| Fat | 10.2 | 10.2 | 10.2 | 10.2 | 10.2 |
| Kcal/g | 3.9 | 3.9 | 3.8 | 3.7 | 3.7 |
diet; HPD, high phosphorus diet; HCPD, high phosphorus: high calcium diet; Ca, calcium; P, phosphorus; Kcal, kilocalories
Fig. 3.
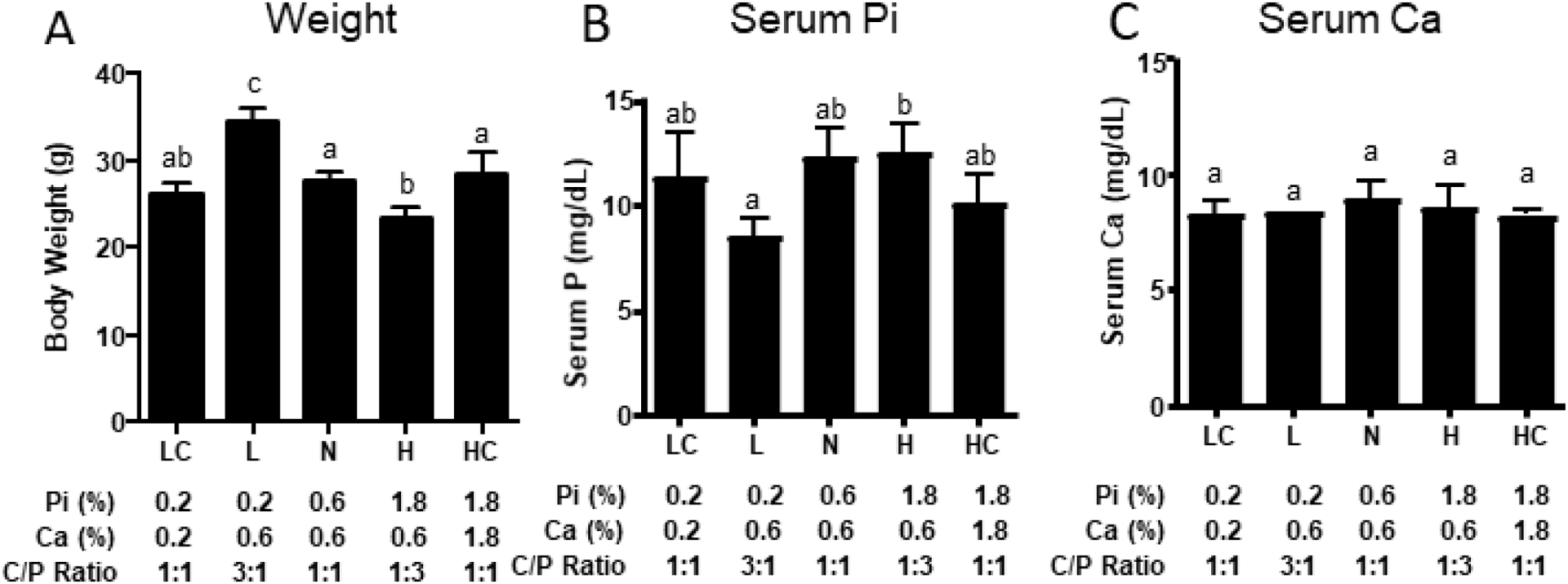
Weight and serum chemistry response of mice to five weeks on the varying phosphorus diets. (A) Weight of 15 wk old male mice fed diets with varying phosphorus and calcium contents for 5 wks. Serum from the same mice were analyzed for (B) phosphorus (Pi) and (C) calcium (Ca). LC (LCPD), L (LPD), N (NPD), H (HPD), HC (HCPD). Different letters denote a significant difference at P < 0.05. N=5.
3.5. The dietary phosphorus to calcium ratio alters phosphorus responsive endocrine factors.
To determine the effects of changing the dietary calcium to phosphorus ratio intakes on phosphorus responsive circulating factors, serum was analyzed by ELISA. As compared to mice fed the NPD, mice fed HPD had significantly higher FGF23 and OPN, whereas mice fed the LPD had slightly lower levels of these analytes, although not statistically significant (Fig. 4). Dietary phosphorus had a significant overall effect on PTH, but multiple comparisons did not reveal any difference between groups that is likely due to large variation and the number of samples (N=5). Addition of calcium to the HPD (HCPD) largely blunted the increase in FGF23 and OPN but interestingly had little effect on the increase in PTH (Fig. 4). Reducing calcium in the LPD diet (LCPD) had little effect on FGF23 or PTH levels but did increase OPN levels above LPD alone. No statistically significant differences between the diets were noted for OCN, CTX, or P1NP although an overall effect of the diets was noted for CTX (Fig. 5). Collectively, the results suggest differences in traditional phosphorus responsive factors, with some responding to the absolute phosphorus intake and others more sensitive to the ratio of calcium to phosphorus intake.
Fig. 4.
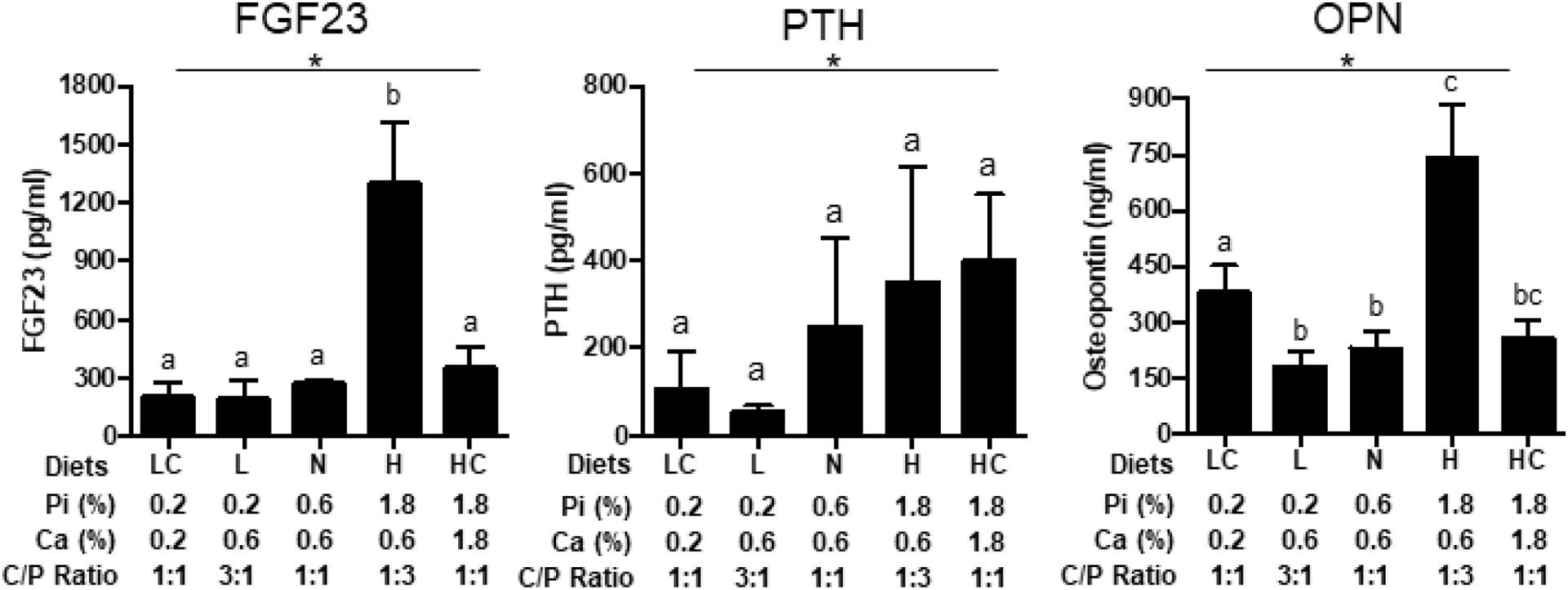
Levels of phosphorus responsive endocrine factors. Serum concentrations of fibroblast growth factor 23 (FGF23), Parathyroid Hormone (PTH) and Osteopontin (OPN) from mice described in Fig.3. LC (LCPD), L (LPD), N (NPD), H (HPD), HC (HCPD). *P≤0.05 for overall effect of diets by ANOVA. Different letters denote a significant difference at P≤0.05 by post-hoc multiple comparisons. N=5.
Fig. 5.
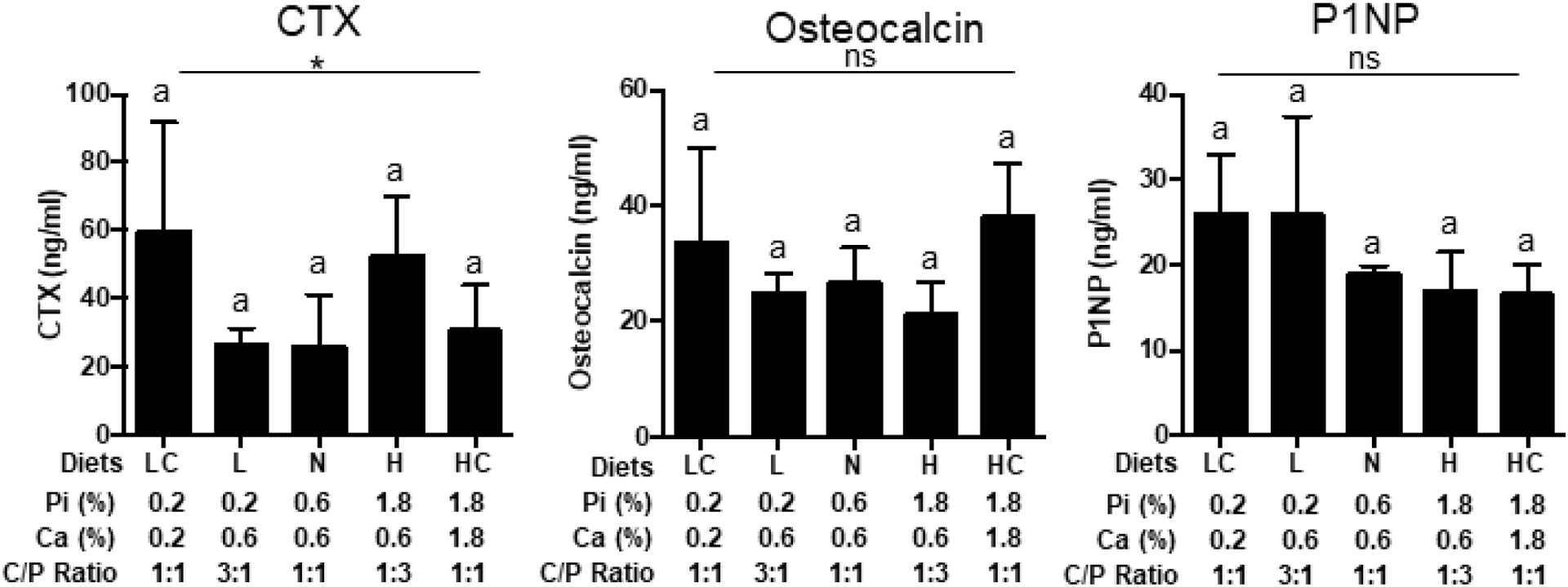
Response of serum mineral metabolism markers factors. Serum concentrations of C-terminal telopeptide of type 1 collagen (CTX), Osteocalcin, and P1NP from mice described in Fig.3. LC (LCPD), L (LPD), N (NPD), H (HPD), HC (HCPD). *P≤0.05 for overall effect of diets by ANOVA. Different letters denote a significant difference at P≤0.05 by post-hoc multiple comparisons. N=5. (ns: not significant).
3.6. The dietary phosphorus to calcium ratio alters circulating markers of metabolic function.
The results from the human study suggested a diet-induced change in both leptin and insulin levels (Table 4). When measured in the serum from the mouse study, leptin levels showed a surprisingly strong induction in response to the LPD and a decrease in response to HPD (Fig. 6). These changes were mostly corrected by balancing phosphorus consumption with the corresponding increase or decrease in calcium consumption suggesting that leptin may be responsive to the ratio of phosphorus to calcium in the diet. Non-fasting insulin was also measured and ELISA results revealed a dose dependent decrease with increasing phosphorus consumption (Fig. 6). Balancing calcium consumption reduced the LPD increase however it did not affect the decrease associated with HPD. Similar to the human study serum adiponectin was not changed (Fig. 6).
Fig. 6.
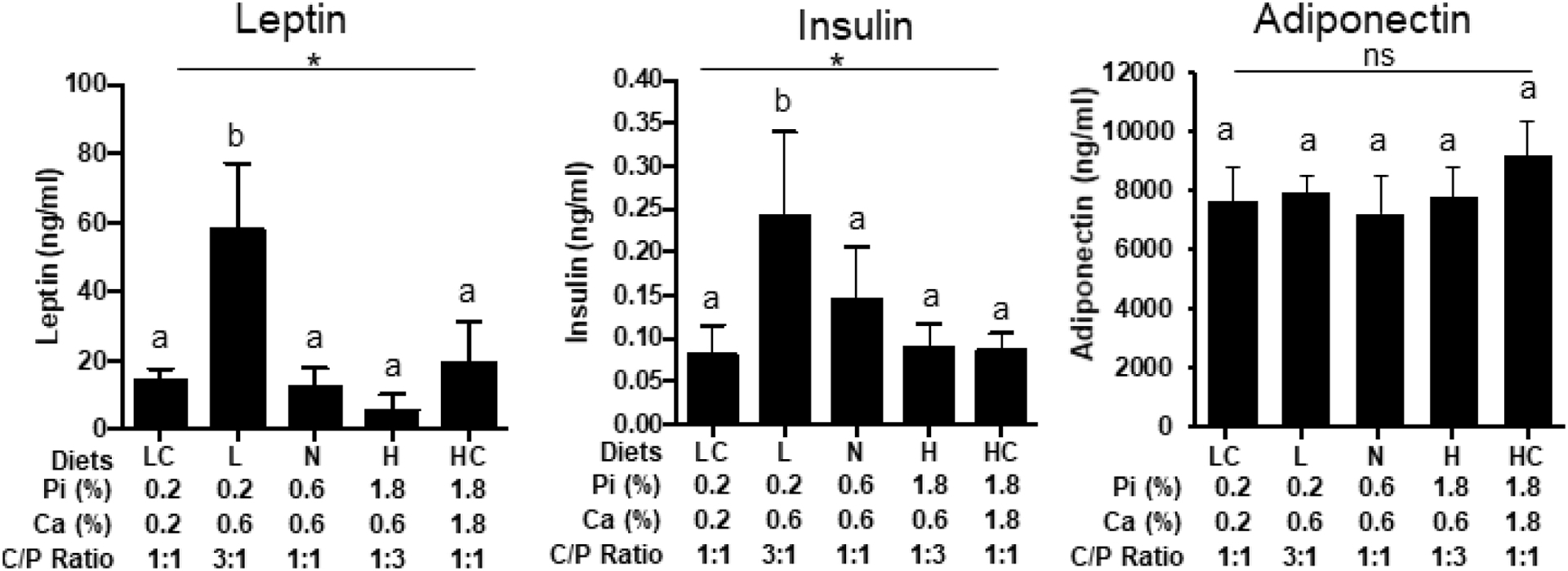
Changes in energy metabolism markers. Serum concentrations of Leptin, Insulin, and Adiponectin from mice described in Fig.3. LC (LCPD), L (LPD), N (NPD), H (HPD), HC (HCPD). *P<0.05 for overall effect of diets by ANOVA. *P≤0.05 for overall effect of diets by ANOVA. Different letters denote a significant difference at P≤0.05 by post-hoc multiple comparisons. N=5. (ns: not significant).
4. Discussion
We recently showed that an additive-enhanced diet containing ~1700 mg of phosphorus and ~700 mg of calcium/day stimulated an increase in circulating FGF23, OPN, and OCN in healthy volunteers and these results were correlated in an associated mouse study [11]. However, a number of studies have suggested that the absolute amount of phosphorus consumed may be less important for influencing health or disease than the ratio of calcium to phosphorus consumption [15–20]. Here we performed a similar study but more carefully controlling for differences in calcium intake between dietary periods. In the current study, the calcium to phosphorus ratios were 0.94:1 in the experimental diet, whereas in our previous study, the difference was greater; 0.76:1 in the experimental diet [11]. The human studies were complemented with studies in mice allowing for an increased number of experimental diets. The effects of the dietary calcium to phosphorus ratio on changes in phosphorus and calcium responsive endocrine and cardiometabolic factors were investigated.
Our clinical study revealed that in healthy volunteers fasting plasma FGF23 concentrations increased relative to baseline values by about 11%, in response to increased phosphorus consumption but had no effect on OPN and OCN. Although the diets were not identical, our previous study in healthy volunteers found that increasing phosphorus consumption while leaving calcium in the normal range significantly increased circulating FGF23, OPN and OCN [11]. In the case of FGF23, circulating levels increased by 23% in the previous study suggesting a blunted response in the current study. In mice, the high phosphorus diet with normal calcium strongly stimulated circulating FGF23 concentrations whereas the effect was mostly blunted in mice fed a diet high in both phosphorus and calcium. Similar results were found with OPN. The reasons for this are unclear, but most likely are related to reduced absorption of phosphorus from dietary sources due to chelation with calcium in the gastrointestinal lumen [21]. This is supported by the decrease in serum phosphorus levels in the mice fed the HCPD relative to HPD. Taken together these findings from humans and mice generally agree and suggest that increasing calcium consumption in parallel with increased phosphorus will attenuate the effect of high diet phosphorus on circulating concentrations of phosphorus responsive factors like FGF23 and OPN.
One difference between the studies was the response of PTH. In the human studies, PTH was not altered by the diets whereas in the animal studies there was an overall effect of diets on PTH which was elevated in both the high phosphorus as well as high phosphorus/calcium. This may be due to the rapid response of PTH to phosphorus consumption reported to occur within 10 hours or less [15, 22–24] or the larger supplements of phosphorus required (1 to 2 g/d) to produce a response [25–27] than were used in the current study (+653 mg/d). It may also be due to the baseline human diets containing higher levels of calcium than the corresponding mouse diets. An interesting finding related to PTH was the failure of the HCPD to reduce serum PTH concentrations to levels comparable to NPD as occurred with FGF23 and OPN. These mice had serum phosphorus and calcium concentrations consistent with control (NPD) and therefore the result suggests the possibility that PTH may be more responsive to phosphorus in the diet and not necessarily changes in serum phosphorus concentrations.
Measurement of circulating bone metabolism markers (P1NP, CTX, and OCN) in human plasma did not identify a significant difference between diets, in contrast to our recent study [11]. Collectively, the results suggest that diet-induced changes in bone metabolism markers are responsive to the calcium to phosphorus ratio and not necessarily the absolute phosphorus content as suggested in prior studies [16, 28–30]. The results of the animal study support this by demonstrating that the adverse effects of high phosphorus consumption on FGF23 and OPN were nearly completely reversed when animals were concurrently fed high calcium. Together, these data suggest that the adverse bone and mineral metabolism effects of phosphorus-based additives may be partly attenuated by consuming higher amounts of calcium in the diet.
We found that HOMA-IR significantly increased after one week of consuming the experimental diet in humans. It is important to note that the carbohydrate, fat, and protein intakes of the control and experimental diets were carefully controlled during the two periods. Since the only major nutrient that changed between the diets was phosphorus, it is possible that higher phosphorus intake resulted in an acute rise in insulin resistance, as has been suggested in other studies [31], though it is possible that other changes in diet intake such as sodium and calcium may have played a role. It remains unclear why HOMA-IR declined back to baseline values after the second week of the experimental diet but could be due to the modest drop in weight observed in study participants at the end of the experimental diet period. This may also explain the drop in leptin concentrations noted at the end of the experimental diet period [32]. The leptin response was more pronounced in the mouse study with the LPD fed mice demonstrating a strong increase in leptin levels relative to NPD which also correlated strongly with weight.
An effect of varying dietary phosphorus on weight was found in the mouse study with the LPD mice weighing more and HPD weighing less than the NPD. These results could be due to changes in feed consumption, activity, or energy metabolism and these possibilities will be addressed in future studies. However, several studies in humans and mice have also described similar effects of phosphorus and calcium to phosphorus ratios on body weight. A previous study in aged female mice (12–24 mo.) examining the effects of increasing the calcium to phosphorus ratio found a diet containing a ratio of 0.6%Ca to 0.3%Pi to result in the greatest weight gain [29]. Our results are also in agreement with a recent randomized controlled study which showed that daily phosphorus supplementation for 12 weeks significantly reduced body weight in healthy adults, perhaps via diminished appetite due to early satiety [33]. Additionally, several studies in male rats have noted a decrease in weight in response to high phosphorus diets and this weight loss was accompanied by reduced food intake [34, 35], reduced visceral adipose tissue accumulation and hepatic lipogenesis and greater insulin sensitivity and increased fat oxidation [36], and enhanced energy expenditure through the utilization of free fatty acids released via lipolysis of white adipose tissue [37, 38] as compared to rats fed low or normal phosphorus diets. Likewise, studies have shown that reduced serum phosphorus levels correlate with increased weight [39, 40]. Interestingly, the changes in weight detected in male mice herein were more dramatic than our previous study in female mice after five weeks on the diets which found a non-statistical difference of 21.3 ± 3.2g [LPD] vs. 19.5 ± 1.3g [HPD][11]. However, unlike the mouse study in which addition of high calcium returned weight to NPD levels, the human arm of this study did find a decrease in weight when both calcium and phosphorus were raised. It is possible that the relatively small decrease in % fat (Table 2) in humans resulted in a decrease in weight which was not related to the Pi:Ca ratio. Taken together, the results suggest that phosphorus consumption may play a role in energy metabolism and points to a potentially sexual dimorphic response to dietary phosphorus which is exaggerated in males.
Our clinical results should be considered in the context of several limitations. First, the length of intervention was only two weeks. Second, although the diets were designed to be isocaloric, participants experienced modest weight loss after two weeks of the experimental diet, which may have impacted the results. The calcium to phosphorus ratio during the control diet was slightly higher than in the experimental diet (1.22 vs. 0.93), which may have impacted the change in some of the outcome measures. Dietary supplements were used to balance nutrient intake and these varied in form, for example sodium in the enhanced diet was in the form of phosphate salts; whereas, sodium chloride was used in the control diet. Likewise, the calcium supplements contained carbonate and these differences in supplements could affect acid-base load [41]. Calcium supplements were taken with dinner to provide an overall daily calcium to phosphorus balance however the breakfast and lunch may have differed in the ratio. The order of the diets was not randomized, which precludes our ability to assess potential effects of the order of diet intake on outcomes. Finally, HOMA-IR is largely a measure of fasting hepatic glucose handling. Future studies using other measures of systemic insulin sensitivity are needed to comprehensively assess the impact of phosphorus additives on glucose handling in humans.
In conclusion, phosphorus-based additives serve a number of critical functions for food manufacturing that make the food supply cheaper and more convenient. However, these beneficial effects may be counter-balanced by persistent stimulation of phosphorus-responsive factors associated with cardiovascular disease, such as FGF23 and OPN. The results of this study broadly show that achieving at least an approximately 1:1 ratio of calcium to phosphorus in the diet will abrogate some of the systemic effects of a dietary phosphorus additive intake typical of a highly processed Western diet including serum phosphorus, circulating FGF23 and OPN, and changes in weight. With the growing appreciation that a diet high in phosphorus may have negative health consequences even in the context of normal kidney function, the results presented herein suggest a relatively simple strategy of increasing calcium consumption to reduce or attenuate some of these health concerns.
Supplementary Material
Acknowledgements:
The authors would like to thank the staff of the Clinical Research Unit in the Center for Clinical and Translational Science at the University of Alabama at Birmingham and all the participants of the study.
Funding support: This study was supported by grants UL1TR00165, P30DK079626, and P30DK056336 from the NIH and by grants from the Biomedical Laboratory Research & Development Service Award Number 1BX001516-05 from the VA Office of Research and Development and an Emory University Research Committee grant (00067461) (GRB). Dr. Gutiérrez was also supported by R03DK095005 and R01NS080850. The content is solely the responsibility of the authors and does not represent the official views of the Department of Veterans Affairs, NIH, or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: NCT01394146
Conflict of Interest Statement: Dr. Gutiérrez reports receiving grant support and consulting fees from Keryx Pharmaceuticals (now Akebia); grant support and consulting fees from Amgen; and grant support from GSK. No other authors declare any competing financial interests.
References
- [1].Gutierrez OM. Sodium- and phosphorus-based food additives: persistent but surmountable hurdles in the management of nutrition in chronic kidney disease. Advances in chronic kidney disease. 2013;20:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Calvo MS, Uribarri J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. The American journal of clinical nutrition. 2013;98:6–15. [DOI] [PubMed] [Google Scholar]
- [3].Calvo MS, Uribarri J. Contributions to total phosphorus intake: all sources considered. Semin Dial. 2013;26:54–61. [DOI] [PubMed] [Google Scholar]
- [4].Carrigan A, Klinger A, Choquette SS, Luzuriaga-McPherson A, Bell EK, Darnell B, et al. Contribution of food additives to sodium and phosphorus content of diets rich in processed foods. J Ren Nutr. 2014;24:13–9, 9e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Leon JB, Sullivan CM, Sehgal AR. The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J Ren Nutr. 2013;23:265–70 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Food and Drug Administration. Evaluation of the health aspects of phosphate as food ingredients. . NTIS PB-262–65. 1975.
- [7].European Food Safety Authority. Assessment of one published review on health risks associated with phosphate additives in food. EFSA Journal. 2013;11:3444. [Google Scholar]
- [8].McClure ST, Chang AR, Selvin E, Rebholz CM, Appel LJ. Dietary Sources of Phosphorus among Adults in the United States: Results from NHANES 2001–2014. Nutrients. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC)1997. [PubMed]
- [10].Adatorwovor R, Roggenkamp K, Anderson JJ. Intakes of Calcium and Phosphorus and Calculated Calcium-to-Phosphorus Ratios of Older Adults: NHANES 2005–2006 Data. Nutrients. 2015;7:9633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gutierrez OM, Luzuriaga-McPherson A, Lin Y, Gilbert LC, Ha SW, Beck GR, Jr. Impact of Phosphorus-Based Food Additives on Bone and Mineral Metabolism. J Clin Endocrinol Metab. 2015;100:4264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].US Department of Agriculture ARS. Nutrient Intakes from Food: Mean Amounts Consumer per Individual, by Race/Ethnicity and Age. What We Eat in America, NHANES 2007–20082010. Available: www.ars.usda.gov/ba/bhnrc/fsrg.
- [13].Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- [14].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- [15].Calvo MS, Kumar R, Heath H, 3rd. Elevated secretion and action of serum parathyroid hormone in young adults consuming high phosphorus, low calcium diets assembled from common foods. The Journal of clinical endocrinology and metabolism. 1988;66:823–9. [DOI] [PubMed] [Google Scholar]
- [16].Clark I Importance of dietary Ca:PO4 ratios on skeletal, Ca, Mg, and PO4 metabolism. The American journal of physiology. 1969;217:865–70. [DOI] [PubMed] [Google Scholar]
- [17].Kemi VE, Karkkainen MU, Rita HJ, Laaksonen MM, Outila TA, Lamberg-Allardt CJ. Low calcium:phosphorus ratio in habitual diets affects serum parathyroid hormone concentration and calcium metabolism in healthy women with adequate calcium intake. The British journal of nutrition. 2010;103:561–8. [DOI] [PubMed] [Google Scholar]
- [18].Brot C, Jorgensen N, Madsen OR, Jensen LB, Sorensen OH. Relationships between bone mineral density, serum vitamin D metabolites and calcium:phosphorus intake in healthy perimenopausal women. Journal of internal medicine. 1999;245:509–16. [DOI] [PubMed] [Google Scholar]
- [19].Teegarden D, Lyle RM, McCabe GP, McCabe LD, Proulx WR, Michon K, et al. Dietary calcium, protein, and phosphorus are related to bone mineral density and content in young women. The American journal of clinical nutrition. 1998;68:749–54. [DOI] [PubMed] [Google Scholar]
- [20].Lee KJ, Kim KS, Kim HN, Seo JA, Song SW. Association between dietary calcium and phosphorus intakes, dietary calcium/phosphorus ratio and bone mass in the Korean population. Nutrition journal. 2014;13:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Heaney RP, Nordin BE. Calcium effects on phosphorus absorption: implications for the prevention and co-therapy of osteoporosis. J Am Coll Nutr. 2002;21:239–44. [DOI] [PubMed] [Google Scholar]
- [22].Karkkainen M, Lamberg-Allardt C. An acute intake of phosphate increases parathyroid hormone secretion and inhibits bone formation in young women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1996;11:1905–12. [DOI] [PubMed] [Google Scholar]
- [23].Reiss E, Canterbury JM, Bercovitz MA, Kaplan EL. The role of phosphate in the secretion of parathyroid hormone in man. The Journal of clinical investigation. 1970;49:2146–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney international. 2006;70:2141–7. [DOI] [PubMed] [Google Scholar]
- [25].Brixen K, Nielsen HK, Charles P, Mosekilde L. Effects of a short course of oral phosphate treatment on serum parathyroid hormone(1–84) and biochemical markers of bone turnover: a dose-response study. Calcified tissue international. 1992;51:276–81. [DOI] [PubMed] [Google Scholar]
- [26].Silverberg SJ, Shane E, Clemens TL, Dempster DW, Segre GV, Lindsay R, et al. The effect of oral phosphate administration on major indices of skeletal metabolism in normal subjects. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1986;1:383–8. [DOI] [PubMed] [Google Scholar]
- [27].Kemi VE, Karkkainen MU, Lamberg-Allardt CJ. High phosphorus intakes acutely and negatively affect Ca and bone metabolism in a dose-dependent manner in healthy young females. The British journal of nutrition. 2006;96:545–52. [PubMed] [Google Scholar]
- [28].Sax L The institute of medicine’s “dietary reference intake” for phosphorus: a critical perspective. J Am Coll Nutr. 2001;20:271–8. [DOI] [PubMed] [Google Scholar]
- [29].Shah BG, Krishnarao GV, Draper HH. The relationship of Ca and P nutrition during adult life and osteoporosis in aged mice. The Journal of nutrition. 1967;92:30–42. [DOI] [PubMed] [Google Scholar]
- [30].Kemi VE, Karkkainen MU, Karp HJ, Laitinen KA, Lamberg-Allardt CJ. Increased calcium intake does not completely counteract the effects of increased phosphorus intake on bone: an acute dose-response study in healthy females. The British journal of nutrition. 2008;99:832–9. [DOI] [PubMed] [Google Scholar]
- [31].Gin H, Combe C, Rigalleau V, Delafaye C, Aparicio M, Aubertin J. Effects of a low-protein, low-phosphorus diet on metabolic insulin clearance in patients with chronic renal failure. Am J Clin Nutr. 1994;59:663–6. [DOI] [PubMed] [Google Scholar]
- [32].Rajaie S, Azadbakht L, Khazaei M, Sherbafchi M, Esmaillzadeh A. Moderate replacement of carbohydrates by dietary fats affects features of metabolic syndrome: a randomized crossover clinical trial. Nutrition. 2014;30:61–8. [DOI] [PubMed] [Google Scholar]
- [33].Ayoub JJ, Samra MJ, Hlais SA, Bassil MS, Obeid OA. Effect of phosphorus supplementation on weight gain and waist circumference of overweight/obese adults: a randomized clinical trial. Nutr Diabetes. 2015;5:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tani Y, Sato T, Yamanaka-Okumura H, Yamamoto H, Arai H, Sawada N, et al. Effects of prolonged high phosphorus diet on phosphorus and calcium balance in rats. Journal of clinical biochemistry and nutrition. 2007;40:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Katsumata S, Matsuzaki H, Uehara M, Suzuki K. Effects of Dietary Calcium Supplementation on Bone Metabolism, Kidney Mineral Concentrations, and Kidney Function in Rats Fed a High-Phosphorus Diet. Journal of nutritional science and vitaminology. 2015;61:195–200. [DOI] [PubMed] [Google Scholar]
- [36].Abuduli M, Ohminami H, Otani T, Kubo H, Ueda H, Kawai Y, et al. Effects of dietary phosphate on glucose and lipid metabolism. American journal of physiology Endocrinology and metabolism. 2016;310:E526–38. [DOI] [PubMed] [Google Scholar]
- [37].Chun S, Bamba T, Suyama T, Ishijima T, Fukusaki E, Abe K, et al. A High Phosphorus Diet Affects Lipid Metabolism in Rat Liver: A DNA Microarray Analysis. PLoS One. 2016;11:e0155386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Imi Y, Yabiki N, Abuduli M, Masuda M, Yamanaka-Okumura H, Taketani Y. High phosphate diet suppresses lipogenesis in white adipose tissue. Journal of clinical biochemistry and nutrition. 2018;63:181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Obeid OA. Low phosphorus status might contribute to the onset of obesity. Obes Rev. 2013;14:659–64. [DOI] [PubMed] [Google Scholar]
- [40].Zhukouskaya VV, Rothenbuhler A, Colao A, Di Somma C, Kamenicky P, Trabado S, et al. Increased prevalence of overweight and obesity in children with X-linked hypophosphatemia. Endocr Connect. 2020;9:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lewis NM, Marcus MS, Behling AR, Greger JL. Calcium supplements and milk: effects on acid-base balance and on retention of calcium, magnesium, and phosphorus. The American journal of clinical nutrition. 1989;49:527–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


