Abstract
Purpose:
The role of vitamin D in bone strength has not been investigated in the African American (AA) population.
Methods:
A 3-year randomized controlled trial designed to examine the effect of vitamin D supplementation on physical performance, bone loss and bone strength in healthy older AA women. 260 postmenopausal AA women, ages ≥60 years were randomized to a vitamin D3 or placebo arm. Vitamin D3 dose was adjusted to maintain serum 25OHD >30 ng/mL. Bone mineral density, femoral axis length and femoral neck (FN) width were measured by dual-energy x-ray absorptiometry. Composite indices of FN strength [compression strength index (CSI), bending strength index (BSI), and impact strength index (ISI)] were computed.
Results:
The mean age of participants was 68.2 ± 4.9 years. Baseline characteristics between groups were similar. The average dose of vitamin D3 was 3490 ± 1465 IU/day in the active group. The mean serum 25OHD was 46.8 ± 1.2 ng/mL versus 20.7 ± 1.1 ng/mL in the active versus placebo group. Serum 25OHD did not correlate with any composite indices. The longitudinal differences observed in FN width, CSI, BSI and ISI in both groups were not statistically significant (all p values >0.05). Further, there was no group × time interaction effect for any of the composite indices (all p values >0.05).
Conclusion:
Maintaining serum 25OHD >30 ng/mL (75 nmol/L) does not affect bone strength in older AA women. There is no evidence to support vitamin D intake greater than the recommended RDA by the Institute of Medicine in this population for bone strength.
Keywords: osteoporosis, fracture prevention, aging, bone strength, clinical trial, vitamin D
Summary
There is controversy over whether African-Americans have higher vitamin D requirements than recommended by the Institute of Medicine. We previously reported that maintaining serum 25(OH)D above 30 ng/mL does not prevent age-related bone loss. Herein, we report that bone strength is also unaffected by maintaining this level in this population.
Introduction
Ethnic differences in the prevalence of osteoporosis and in the accompanying fracture risk are well known. Hip fracture rates among African Americans are lower than rates among Caucasians [1–3]. Vitamin D has an essential role in bone health. Higher serum 25OHD levels are associated with higher bone mineral density (BMD) and vitamin D supplementation reduces rates of bone loss. Contrary to this knowledge, in AAs there is a vitamin D “paradox” [4]. AAs have lower serum 25OHD than Caucasians, yet characteristics which confer advantages for femoral neck bone strength, high bone mineral density and favorable hip geometric structures, have been reported in AA [5–8]. Studies have also reported no reduction in bone loss in postmenopausal AA women with vitamin D3 supplementation compared to placebo [9,10].
In 2011, the Institute of Medicine concluded that serum 25OHD of 20 ng/mL (50 nmol/L) is the level associated with the Recommended Dietary Allowance (RDA) for vitamin D [11]. The IOM report recognized the limited availability of data for subpopulation consideration and concluded that there was no evidence to recommend race- or ethnicity- specific RDA. However, there is controversy over whether AAs should be screened for vitamin D deficiency and have higher vitamin D requirements than recommended by the IOM [12]. It has been suggested that a serum 25OHD of 20 ng/mL (50 nmol/L) may not be high enough to achieve the desired effects of vitamin D in AAs [13,14].
The primary objective of the Physical Performance, Osteoporosis Prevention and Vitamin D in older African Americans (PODA) trial was to assess whether maintaining serum 25OHD above 30 ng/mL (75 nmol/L) prevented bone loss and the decline in physical performance in healthy older AA women [15]. We recently reported that bone loss in this population is unaffected by maintaining serum 25OHD above 30 ng/mL [16]. However, femoral neck (FN) BMD is not the only determinant of fracture risk. FN geometry and body size are important contributors to bone strength [17–19]. Age related decline in BMD is partially compensated by an increase in bone size, indicating that FN geometry contributes to bone strength, independent of the BMD [17,18]. Body size determines the fracture forces in a fall [19,20]. The structural contributions to femoral neck strength can be quantified utilizing a validated concept of composite indices of FN strength integrating FN BMD, FN size and body size [21]. In older women, each SD increment in FN strength indices is associated with 57–66% relative reduction in hip fracture risk over 10 years [21]. However, studies reporting ethnic differences in FN strength did not include older AAs.
We aimed to study the association of serum 25OHD levels and FN strength in older AA women and further hypothesized that vitamin D supplementation to maintain 25OHD concentrations above 30 ng/mL might be associated with higher composite indices of FN strength.
Materials and Methods
The Physical Performance, Osteoporosis Prevention and Vitamin D in older African Americans (PODA) Study is a prospective, randomized, double-blind, placebo controlled, three-year clinical trial of vitamin D3 supplementation in postmenopausal AA women older than 60 years of age. Written informed consent was obtained from each participant. The trial was approved by the Institutional Review Board of Winthrop University Hospital and registered at www.ClinicalTrials.gov as NCT01153568. Detailed study design, study procedures, baseline demographics and laboratory values have been previously reported [16]. The primary objective of the PODA trial was to assess whether maintaining serum 25OHD above 30 ng/mL (75 nmol/L) prevented bone loss and the decline in physical performance in healthy older AA women [15,16,22]. A secondary aim was to determine the effect of high serum 25OHD concentrations on bone strength.
Study Subjects
Study participants were healthy ambulatory volunteers from the Long Island and surrounding communities. Subjects were recruited by direct mail, flyers, and presentations at AA churches and events. African American ancestry of the participants was assessed by self-declaration that both parents and at least 3 of 4 grandparents were African American. The study was conducted in an ambulatory Clinical Research Center of an academic health center. Inclusion criteria included serum 25OHD between 8 ng/mL (20 nmol/L) and 26 ng/mL (65 nmol/L). Exclusion criteria included metabolic bone disease, BMD at total hip below 2.5 standard deviations [using female reference ranges from the dual-energy x-ray absorptiometer (DXA) manufacturer], history of osteoporotic fracture, calcium or parathyroid disorder, previous treatment with bone active agents, medications or chronic illnesses (including neuromuscular disorder) that affect bone metabolism and use of medications known to interfere with vitamin D metabolism.
Study Design
Block randomization of 260 healthy participants to vitamin D3 or placebo group was done through a computer-generated sequence (SAS Proc Plan). Subjects were assigned to one of the two groups: vitamin D3 supplementation (n=130) or placebo (n=130) [15]. Initial vitamin D3 dose was determined by a research pharmacist depending on the baseline serum 25OHD levels. The dose was adjusted further at 3-month intervals to maintain serum 25OHD level between 3069 ng/mL (75–172 nmol/L). The study drug was manufactured by Alcrea Health (Pittsburgh, PA, USA) in two batches. Vitamin D3 was available in doses of 60, 90, and 120mg (2400, 3600, and 4800 IU, respectively). The capsules were analyzed in batches for their actual content at an independent laboratory. The blind was maintained during vitamin D3 titration by adjusting the placebo dose to match the distribution of dose changes in the active group (a double-dummy design). Daily calcium intake was assessed by a food frequency questionnaire (NIH Clinical Center Short Calcium Questionnaire 2002). Calcium supplements, as calcium carbonate were provided as needed in divided doses with meals to both the active and control group to ensure a total calcium intake of at least 1200 mg daily.
Study Procedures
Participants were followed after the baseline visit every 3 months for 36 months. Change in BMD was the primary specified endpoint for this study. BMD was measured at the total hip, femoral neck, non-dominant mid-radius, and anteroposterior lumbar spine with a DXA scanner (model QDR 4500, version 9.80D; Hologic Inc., Waltham, Massachusetts) at baseline and 6-month intervals thereafter. The bone-geometry structural properties were measured by a technologist certified by the International Society for Clinical Densitometry. This included measurement of the 2D projected areal BMD in the FN, the hip axis length (HAL) – the distance on the 2D projected plane along the FN axis from the lateral margin of the base of the greater trochanter to the inner pelvis brim or apex of the femoral head, and the FN width (FNW) – the least thickness of the FN on the 2D projected plane along a line perpendicular to the FN axis.
Composites indices of FN strength:
The compression strength index (CSI), bending strength index (BSI), and impact strength index (ISI) at the FN were computed using algorithms previously described for evaluating the capacity of the FN to endure the load during a fall [21]. CSI reflects the FN ability to withstand an axial compressive load, BSI reflects the FN ability to withstand bending forces, and ISI reflects the FN ability to absorb the energy of impact in a fall from the standing height. The algorithms take into consideration the BMD, body weight, height, FN BMD, FNW and HAL. These algorithms have been validated against 3D methods based on quantitative computed tomography [23]. Equations for the composite indices are as follows:
Laboratory measurements:
Fasting blood samples were collected at baseline and at 3 monthly visits. Serum 25OHD levels were measured using a commercial laboratory (LabCorp, Burlington, NC) for dose adjustment using an immunochemilumetric assay on a DiaSorin Liaison instrument. Samples were analyzed for vitamin D metabolites at baseline and annually by the Department of Laboratory Medicine at the University of Washington (Seattle, WA) using liquid chromatography-tandem mass spectrometry with deuterated internal standards for each analyte. Concentrations of 25OHD3 were standardized to NIST SRM 972a. The % coefficient of variation (CV) of 25OHD3 ranged from 3.54 to 4.41%.
Serum markers of bone turnover and parathyroid hormone (PTH) levels were measured at baseline and at 6-month intervals. Intact PTH was measured by the Immulite 2000 Analyzer assay (Diagnostic Products Corporation, Los Angeles, CA, inter-assay CV: 1.34%). A one-step enzyme-linked immuno-absorbent assay (Micro Vue BAP, Quidel Corp., San Diego, CA and Nordic Bioscience Diagnostics, Herlev, Denmark) was used to measure serum bone turnover markers: bone specific alkaline phosphatase and C-Telopeptide of Type-1 collagen. The intra-assay CV of the bone specific alkaline phosphatase assay is 4–6% and the inter-assay CV is 5–8%. The intra-assay CV of the CrossLap assay is 5.4%, and the inter-assay CV is 6.5%. O-cresolphthalein complex using automated equipment (Dimension-RXL, Dade, DE) was used to measure serum and urinary calcium.
Statistical Analysis
The study was powered for the primary objective: to determine whether maintenance of serum 25(OH)D levels above 75 nmol/L was effective in preventing bone density loss from the total femur compared to placebo in elderly AA women. Specifically, power was determined based on previous studies and a differential bone mineral density rate of change of 0.18% or greater per year along with several other assumptions such as within-patient correlation and variance. It was a priori secondary objective to evaluate the effect of Vitamin D on bone strength.
Any participant that was randomized and received at least one dose of study medication was included in the intention to treat (ITT) population and primary analysis was done according to ITT principle. Descriptive statistics (i.e. mean, median, standard deviation, first quartile and third quartile) were generated and presented as mean (±SD) or median values with interquartile range (IQR) as appropriate for continuous data and as proportion for categorical variables. Normality of distributions of clinical variables and laboratory markers was evaluated using visual observation of histograms and the Kolmogorov-Smirnov test. Differences of each continuous variable between groups were examined using the non-parametric Wilcoxon rank-sum test for non-normally distributed and two independent samples t-test for normally distributed variables. Analyses were performed with and without outliers but output remained similar, so full data were used. Annual percent changes from baseline for all outcomes are presented along with 95% confidence intervals, stratified by treatment group. Correlations between 25OHD and the strength indices were examined via Spearman correlation coefficients and presented with 95% confidence intervals obtained via Fisher’s Z transformation. Fisher’s exact test was used to compare categorical variables between groups.
To test our hypothesis that higher 25OHD concentrations might be associated with higher FNW and composite indices of FN strength (CSI, BSI and ISI), a mixed effects Analysis of Covariance (ANCOVA) model was developed for each endpoint using corresponding baseline values, group, time and the two-way interaction term between group and time. An unstructured correlation structure was used in each model to account for within-patient correlation between the measurements over time. Missing data was assumed to have occurred at random [24,25]. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). A result was considered statistically significant at the p<0.05 level of significance.
Results
Baseline Characteristics
Selected baseline characteristics of the study population are presented in Table 1. The median (IQR) age was 68.2 (65.4–72.5) years and body mass index was 30 (26.5–34.1) kg/m2. Baseline characteristics were similar between groups with most rating their health as good/excellent. The mean dose of vitamin D3 in the active arm was 3490 ± 1465 IU/day. Median daily calcium intake including supplements was 842 (600–1142) mg/d in the vitamin D3 group and 827 (628–1185) mg/d in the placebo group. Overall compliance based on the pill count was 85% for the entire study.
Table 1.
Demographics and Baseline Characteristics
| Active (n=130) | Placebo (n=130) | Overall (n=260) | P value1 | |
|---|---|---|---|---|
| Demographics and behavioral | ||||
| Age (years) † | 67.8 (65.1 – 71.5) | 69.0 (65.4 – 73.4) | 68.2 (65.4 – 72.5) | 0.251 |
| Height (cm) | 162.1 ± 6.3 | 162.6 ± 6.8 | 162.3 ± 6.5 | 0.564 |
| Weight(kg) | 78.2 (68.6 – 90.9) | 80 (68.6 – 90.5) | 79.5 (68.6 – 90.9) | 0.790 |
| BMI (kg/m2) † | 30.2 (26.4 – 34.6) | 30.0 (26.8 – 33.9) | 30.1 (26.6 – 34.1) | 0.867 |
| Calcium intake (mg) † | 842.0 (600 – 1142) | 826.5 (628.0 – 1185) | 828.0 (614.0 – 1164) | 0.857 |
| Smoking History, n (%) | 0.805 | |||
| Present | 7 (5.4) | 5 (3.9) | 12 (4.6) | |
| Past | 28 (21.5) | 31 (23.8) | 59 (22.7) | |
| Never | 95 (73.1) | 94 (72.3) | 189 (72.7) | |
| Alcohol History, n (%) | 0.323 | |||
| Present | 72 (55.4) | 83 (63.9) | 155 (59.6) | |
| Past | 2 (1.5) | 2 (1.5) | 4 (1.5) | |
| Never | 56 (43.1) | 45 (34.6) | 101 (38.9) | |
| Bone Mineral Density | ||||
| Total Hip BMD (g/cm2) | 0.919 ± 0.130 | 0.935 ± 0.134 | 0.927 ± 0.132 | 0.329 |
| Total Hip T-score | −0.185 ± 1.065 | −0.054 ± 1.096 | 0.120 ± 1.080 | 0.329 |
| Femoral neck BMD (g/cm2) † | 0.767 (0.694 – 0.9) | 0.805 (0.718 – 0.9) | 0.785 (0.707 – 0.9) | 0.109 |
| Wrist 1/3 BMD (g/cm2) | 0.689 ± 0.067 | 0.692 ± 0.075 | 0.691 ± 0.071 | 0.684 |
| Spine BMD (g/cm2) | 1.005 ± 0.162 | 1.023 ± 0.171 | 1.014 ± 0.167 | 0.396 |
| Whole body BMD (g/cm2) † | 1.127 (1.076 – 1.2) | 1.156 (1.070 – 1.2) | 1.138 (1.070 – 1.2) | 0.222 |
| Femoral Neck Geometry | ||||
| FNW (cm) | 3.254 ± 0.332 | 3.199 ± 0.323 | 3.226 ± 0.328 | 0.174 |
| Composite Indices of FN Strength | ||||
| CSI (g/kg*m) | 3.233 ±0.747 | 3.239±0.608 | 3.236±0.680 | 0.939 |
| BSI (g/kg*m) | 0.107±0.03 | 0.106±0.025 | 0.107 ±0.027 | 0.797 |
| ISI (g/kg*m) | 1.972±0.481 | 1.959±0.403 | 1.965±0.443 | 0.807 |
| Laboratory (serum) | ||||
| 25OHD3, ng/ml | 21.5 ± 6.5 | 22.2 ± 6.9 | 21.8 ± 6.7 | 0.352 |
| PTH (pg/ml) † | 56.1 (41.0 – 73.6) | 56.4 (39.5 – 73.8) | 56.2 (39.8 – 73.8) | 0.977 |
| Ca (mg/dl) † | 9.5 (9.3 – 9.8) | 9.5 (9.3 – 9.8) | 9.5 (9.3 – 9.8) | 0.943 |
| Cr (mg/dl) † | 0.8 (0.7 – 0.9) | 0.7 (0.6 – 0.9) | 0.8 (0.6 – 0.9) | 0.472 |
| P (mg/dl) † | 3.5 (3.2 – 3.8) | 3.5 (3.2 – 3.8) | 3.5 (3.2 – 3.8) | 0.732 |
| CTX (ng/mL) | 0.52 (0.37–0.67) | 0.50 (0.39–0.73) | 0.51 (0.38–0.69) | 0.289 |
| BSAP (mg/L) | 19.88 (16.7–26.1) | 19.45 (15.4–23.3) | 19.6 (16.2–24.4) | 0.078 |
For continuous data, p-values are from Wilcoxon rank-sum test for non-normally distributed variables and two independent samples t-test for normally distributed variables. For categorical variables, p-values are from Fisher’s exact test.
Not normally distributed; IQR=Inter-quartile range (first quartile – third quartile); SD=Standard Deviation
Normally distributed variables were presented as mean ±SD and not normally distributed variables were presented as median (IQR).
25OHD = 25-hydroxyvitamin D; BMD = bone mineral density; BMI = Body Mass Index; BSAP = bone specific alkaline phosphatase; BSI = bending strength index; CTX = cross-linked C-telopeptide; CSI = compression strength index; FNW = FN width; ISI = impact strength index; PTH = parathyroid hormone
Serum 25OHD concentration was maintained above 30 ng/mL (75 nmol/L) in 90% of the active group. Mean values (mean ± SE) for 12, 24, and 36 months in the active group were 43±9.1, 46±11.0 and 47±11.2 ng/mL, respectively. Corresponding values for the placebo group were 19±8.0, 20±7.9, and 21±10.0 ng/mL. Serum calcium did not change in either group. There was a linear decline in PTH and BSAP in both groups with no interaction effects between study group and time. A marginal association of CTX with time was observed in the active group (p = 0.04), but there was no difference between groups (Table 1).
Associations with serum 25OHD levels
The primary analysis presented was conducted as an intention to treat analysis, thus models included all valid data from all randomized participants whenever possible. On average, the participants who did not complete the study were older than the participants who completed the trial (70.5 vs 68.3 years; p=0.003) and had higher serum PTH (67.96 vs 56.45 mg/dL; p=0.002). No other statistically significant differences were observed between these two groups.
No statistically significant difference was detected at baseline in serum 25OHD concentration, PTH, body mass index, height or calcium intake between assigned groups. Study groups were balanced at baseline with respect to potential confounders (Table 1), thus adjustment for these variables in the model was deemed unnecessary. Serum 25OHD levels showed no correlation with FNW or any of the composite indices of FN strength (CSI, BSI or ISI) in either of the study groups (all p values >0.05), at baseline or at completion of the study (36 months post randomization) [Table 2].
Table 2.
1Correlation between serum 25OHD and the strength indices
| Baseline | Final (36 month) | Δ (Final-Baseline) | |
|---|---|---|---|
| Femoral Neck width (cm) | −0.02(−0.14 0.10) | 0.12(−0.03, 0.26) | 0.004(−0.14, 0.15) |
| Femoral Neck BMD (g/cm2) | 0.02(−0.11, 0.14) | −0.15(−0.29, 0.0004) | 0.10(−0.04, 0.25) |
| Compression Strength Index (g/kg*m) | 0.02(−0.11, 0.14) | 0.02(−0.12, 0.17) | 0.07(−0.08, 0.21) |
| Bending Strength Index (g/kg*m) | 0.03(−0.09, 0.15) | 0.04(−0.11, 0.18) | 0.04(−0.11, 0.19) |
| Impact Strength Index (g/kg*m) | −0.001(−0.12, 0.12) | 0.06(−0.08, 0.21) | 0.06(−0.09, 0.20) |
Spearman’s rho presented with 95% confidence intervals in parentheses. All P values were >0.05
Effects of Vitamin D supplementation on Femoral Neck BMD and Femoral Neck Width
We previously reported that BMD loss was observed in both study groups with the 3-year average decline in total hip BMD of 1.7% in the active group and 2.5% in the placebo group but these differences were not statistically significant [16]. Similar BMD loss at FN was also observed over time in both study groups, 1.28% (95% CI, −2.2 to −0.37) vs 2.01% (95% CI, −2.99 to −1.04) in the active and placebo group respectively (Table 3, Figure 1). FNW increased linearly over time in both groups with the 3-year average increase of 1.12% in the active group and 1.51% in the placebo group. However, these longitudinal changes in FNW were not statistically significant). There was no group x time interaction for FNW suggesting that the longitudinal differences over time between the study groups were non-significant (Table 4).
Table 3.
Average yearly percent change in femoral neck width and the strength indices from baseline (95% CI) by study group arm and year of follow-up
| Active (Vitamin D3) | Placebo | |||||
|---|---|---|---|---|---|---|
| Measure | 1-year | 2-year | 3-year | 1-year | 2-year | 3-year |
| Femoral neck BMD (g/cm2) | 0.77 (− 0.04,1.59) | −0.5 (− 1.3,0.29) | −1.28 (−2.2,− 0.37) | −0.4 (− 1.13,0.32) | −1.27 (−2.1,− 0.44) | −2.01 (−2.99,− 1.04) |
| Femoral Neck width (cm) | 0.66 (− 0.26,1.57) | 1.05 (0.16,1.94) | 1.12 (0.26,1.99) | 1.14 (0.39,1.89) | 1.15 (0.08,2.22) | 1.51 (0.58,2.44) |
| Compression Strength Index, (g/kg*m) | 2.1 (0.59,3.61) | 0.95 (− 0.97,2.88) | 1.46 (− 0.31,3.23) | 1.73 (0.3,3.16) | 1.4 (− 0.35,3.15) | 1.98 (− 0.1,4.06) |
| Bending Strength Index, (g/kg*m) | 2.99 (0.77,5.21) | 2.49 (− 0.13,5.12) | 2.43 (0.08,4.78) | 3.1 (0.97,5.22) | 2.71 (− 0.05,5.47) | 3.6 (0.72,6.48) |
| Impact Strength Index, (g/kg*m) | 2.26 (0.65,3.86) | 0.99 (− 0.96,2.94) | 2.1 (0.27,3.92) | 2.38 (0.89,3.88) | 2.2 (0.37,4.04) | 2.87 (0.67,5.07) |
BMD – bone mineral density; CI – confidence interval
Figure 1.
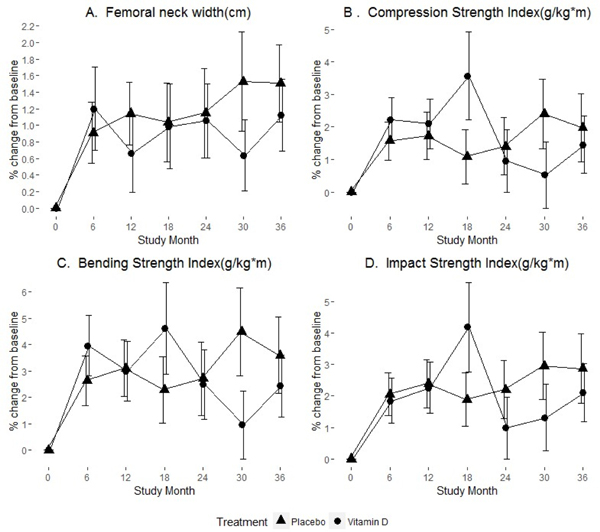
Percent change in femoral neck width and the strength indices over time stratified by treatment group. Group means (circle/triangle) ±1 standard error at each study visit for: (A) Femoral neck width, (B) Compression Strength Index, (C) Bending Strength Index, and (D) Impact Strength Index
Table 4.
Mixed effects ANCOVA models for the outcomes
| Outcomes | Effects | Model Estimate (SE) | p-value |
|---|---|---|---|
| FNW (cm) | Baseline FN width(cm) | 0.8627(0.0208) | <0.0001 |
| Group (Vitamin D vs. Placebo) | 0.0111(0.0183) | 0.545 | |
| Time (month) | 0.0005(0.0005) | 0.326 | |
| time * group (vitamin D) | −0.0004(0.0006) | 0.487 | |
| FN BMD (g/cm2) | Baseline FN BMD | 0.9773(0.0133) | <0.0001 |
| Group (Vitamin D vs. Placebo) | 0.0049(0.0045) | 0.272 | |
| Time (month) | −0.0005(0.0001) | <0.0001 | |
| time * group (vitamin D) | −9.95E-7(0.0001) | 0.995 | |
| CSI (g/kg*m) | Baseline CSI, (g/kg*m) | 0.9054(0.0238) | <0.0001 |
| Group (Vitamin D vs. Placebo) | 0.0292(0.0379) | 0.442 | |
| Time (month) | 0.0006(0.0007) | 0.523 | |
| Time * group (vitamin D) | − 0.0018(0.0010) | 0.079 | |
| BSI (g/kg*m) | Baseline BSI (g/kg*m) | 0.8751(0.0255) | <0.0001 |
| Group (Vitamin D vs. Placebo) | 0.0012(0.0017) | 0.485 | |
| Time (month) | 0.00004(0.00004) | 0.829 | |
| time * group (vitamin D) | −0.0001(0.0001) | 0.096 | |
| ISI (g/kg*m) | Baseline ISI, (g/kg*m) | 0.9089(0.0230) | <0.0001 |
| Group (Vitamin D vs. Placebo) | 0.0073(0.0241) | 0.764 | |
| Time (month) | 0.0006(0.0005) | 0.627 | |
| Time * group (vitamin D) | −0.0008(0.0007) | 0.234 | |
FNW-Femoral Neck Width; FN-Femoral Neck; BMD-Bone Mineral Density; CSI- Compression Strength Index; BSI- Bending Strength Index; Impact Strength Index; ANCOVA-Analysis of Covariance
Effects of Vitamin D supplementation on the Composite Indices of FN strength
Similar to the changes in FNW, longitudinal changes observed in all composite indices (CSI, BSI and ISI) did not meet statistical significance (Table 3, Figure 1). Mixed effects ANCOVA Models on the composite indices of FN strength (CSI, BSI and ISI) revealed that differences over time between the study groups were not statistically significant suggesting no effects of vitamin D3 (see p-values of the interaction terms in the table 4). Outcomes did not change over time (regardless of the study group) either.
However, as expected, the baseline values of FNW and the composite indices were significantly associated with the post baseline measures. Models were adjusted for age to determine the influence of aging on the outcomes. The findings remained unaffected.
Adverse Events
In the per-protocol analysis of participants continuing to take study medication, there were no serious adverse events related to vitamin D. There were 24 incidents of hypercalcemia (>10.5 mg/100 mL), nine in the placebo (9 participants) and 15 in the vitamin D group (11 participants) over 3 years. Hypercalciuria was determined by using 0.222 as the definition of hypercalciuria for fasting urine calcium. Hypercalciuria occurred in 33 subjects: 17 in the vitamin D group vs 16 in the placebo group. There was no statistically significant difference in event rate for either group during the follow up period.
Discussion
Maintaining serum 25OHD levels above 30 ng/mL (75 nmol/L), as recommended by the Endocrine Society guidelines, in healthy older African American women confers no additional benefit in prevention of involutional decline in bone strength. The placebo group had an average serum 25OHD consistent with the RDA associated value 20 ng/mL (50 nmol/L) recommended by the IOM. Femoral neck strength in the active group was not higher than the placebo group. The Dietary Reference Intakes (DRIs) for vitamin D are based solely on skeletal health. We previously reported no prevention of age-related bone loss or falls in healthy older AA women with serum 25OHD level above 30 ng/mL (75 nmol/L) [16,22]. The longitudinal findings of our current study lend further evidence that there is no skeletal health benefit in raising serum 25OHD levels beyond 20 ng/mL (50 nmol/L), the value recommended by the IOM. Therefore, it may be concluded that screening for serum 25OHD levels in healthy older African American women is not required and the recommendations for vitamin D supplementation in this population need not exceed the RDA for vitamin D intake.
The risk of hip fracture is lower in AAs than Caucasians. BMD fails to account for variations in fracture risk among ethnic group [26,27]. The genetic influences on hip shape may also determine fracture risk [28]. AAs have a shorter HAL than Caucasians [29–31]. Longer HAL can lead to increased neck bending and poor adaptation to mechanical loading. This shorter HAL, in addition to high BMD in AAs, is advantageous in fracture risk reduction. A recent study reported other hip structural indices associated with more favorable geometry and greater strength and resistance to fracture in AA and Asian women. Femurs of AA women had a smaller outer diameter and a larger cross-sectional area [30]. This study used the Hip Structural Analysis software to assess these ethnic differences in hip structure and strength. Another study used the same data set and computed composite indices, the methodology we utilized in our study, and reported higher strength indices in AAs concluding that unlike BMD, composite indices provide a more accurate risk stratification, independent of BMD [32]. The study population in these studies were younger women from the Study of Women’s Health Across the Nation who were either premenopausal or early perimenopausal. Our study is the first to report composite strength indices in an older AA population, although the study lacks direct comparison with other ethnic groups. We hypothesized that serum 25OHD concentrations above 30 ng/mL might be associated with higher composite indices of FN strength.
High serum 25OHD levels and vitamin D supplementation may improve bone strength via effects on parameters of hip geometry, such as higher cortical thickness, cross-sectional area, and cross-sectional moment of inertia [33–35]. No intervention studies have explored the relationship of vitamin D supplementation and bone strength in AAs. Although non-significant, in our study there were changes in CSI, BSI and ISI over time in both study groups. This finding must be interpreted with caution. First, in the absence of association of 25OHD with FN BMD or the composite indices of FN strength, it is unlikely that this effect of higher bone strength is mediated by the effect of 25OHD on FN BMD or on hip geometry. Second, it is worth noticing that a parallel increase in FNW was present in both study groups. Increase in FNW is a physiological process observed with aging. Age related decline in BMD is partially compensated by increase in bone size indicating that FN geometry contributes to bone strength, independent of the BMD [20]. Indeed, FNW is one of the variables used in algorithms of all composite indices of FN strength. Therefore, the increase in composite indices is a function of increase in FNW. Third, it is known that hip geometry parameters, specifically HAL and FNW, depend on body height. Thus, body height can indirectly influence bone strength. However, baseline characteristics, including body height and weight were similar in both study arms as a result of randomization. Finally, while the increase in these composite indices is observed in both study groups, there was no group × time interaction effect for any of the composite indices suggesting that the longitudinal differences in CSI, BSI and ISI between groups were not statistically significant. Therefore, this finding is not considered an effect of vitamin D supplementation or high 25OHD concentrations in this healthy older AA population.
Several randomized controlled studies have reported a reduction in fractures with vitamin D and calcium supplementation. In these trials, higher 25OHD levels were associated with improved lower extremity function and physical performance [36]. However, these findings have not been consistent in the literature. Studies have also demonstrated the negative effects of higher doses of vitamin D in older adults [37–39]. Analysis of existing literature has explained the reasons for these discrepant results as the heterogeneity in dosing, the vitamin D assay used and heterogeneity in participant selection [40]. It is noteworthy that studies reporting the benefits of vitamin D often included participants who were vitamin D deficient and it is well established that the benefit of vitamin D supplementation on bone is most pronounced if 25OHD levels are as low as < 12 ng/mL (30 nmol/L). Inclusion of institutionalized participants or participants with multiple medical comorbidities may lead to erroneous conclusions during development of RDAs for the general healthy population. In our study, the placebo group had an average serum 25OHD consistent with the RDA associated value 20 ng/mL (50 nmol/L) recommended by the IOM. Findings of the PODA trial support that the RDA for African American women should be the same as those recommended for the general population.
This is the first study to examine the effect of vitamin D supplementation on hip strength in African Americans while maintaining serum 25OHD levels above 30 ng/mL. We previously reported similar effects on BMD and falls in this population [16,22]. The existing evidence in the literature is supportive of the potential role of vitamin D for skeletal health in those who are deficient in vitamin D. In keeping with this premise, the RDA for older AA women should not be lower than that of the general population. Although an efficient calcium conservation and skeletal resistance to PTH yield distinct advantages in bone mass in AAs [41,42], the skeleton of older AAs is susceptible to the age-associated rise in PTH [43]. Further, in women of all ethnic backgrounds, the decline in estrogen and/or the increase in gonadotropins during menopause results in a reversal of bone remodeling with resorption exceeding formation and resultant decrease in bone mass. There is also a disruption of the bone microarchitecture. Therefore, older AAs may be more vulnerable to vitamin D deficiency and the subsequent changes in bone and mineral metabolism. If a lower RDA is considered for this population, the median serum 25OHD would shift the distribution curve increasing the AA population at risk for vitamin D deficiency and consequent rickets and osteomalacia.
Our study has its strengths and limitations. Findings from the PODA trial should not be extrapolated to men or women of other ethnic groups. Our study population was relatively healthy and highly functioning. These findings should not be generalized to institutionalized or sick individuals or those with vitamin D deficiency. This analysis of secondary outcomes did not capture microscopic features of bone: mineralization and micro-architecture and focused on the composite indices of hip strength which are based on the macroscopic measurements from DXA. This study was not designed to investigate other beneficial effects of vitamin D beyond the reported musculoskeletal outcomes. It is possible that the optimal range of serum 25OHD levels with respect to extraskeletal health benefits may be quite different among various disorders. However, recently reported large RCTs found no evidence that vitamin D is protective against cardiovascular disease or cancer [44,45]. Strengths of the study include its randomized control double blind design, 3-year duration and maintenance of serum 25OHD above 30 ng/mL (75 nmol/L). We also ensured adequate daily calcium intake. Although this study was not powered to detect statistically significant differences in adverse effects, the relatively high dosage of vitamin D did not appear to be associated with frequent adverse events.
In conclusion, this study demonstrated that, independent of BMD, maintaining serum 25OHD above 30 ng/mL (75 nmol/L) does not affect bone strength in older African American women. Accumulating findings of no effect of higher serum 25OHD levels on bone strength, involutional bone loss, physical performance and falls in vitamin D-sufficient older African Americans have important clinical implications. There is no evidence to support vitamin D intake greater than the recommended RDA by the Institute of Medicine in this population for bone health. Information from larger clinical trials exploring extraskeletal outcomes has also yielded no benefit thus far. Whether these extraskeletal outcomes should influence vitamin D recommendations is yet to be elucidated.
Acknowledgements
This research was supported by grants from the National Institute of Health (NIH) and Office of Dietary Supplements (ODS); R01- AG032440-05. We thank the study participants and staff at the Bone Mineral Research Center for their contribution, and the Data Safety and Monitoring Board Committee Members: Munro Peacock, MD, DSc; Bess Dawson-Hughes, MD; Lynette Smith, MS; Judy Hannah, PhD; Rebecca Costello, PhD; Christopher Sempos, PhD; Andy Hoofnagle MD, PhD, Director, Nutrition and Obesity Research Center, University of Washington.
Abbreviations:
- 25OHD
25-hydroxy vitamin D
- BMD
bone mineral density
- BSI
bending strength index
- CI
confidence interval
- CSI
compression strength index
- DXA
Dual-energy X-ray Absorptiometry
- FN
femoral neck
- FNW
FN width
- HAL
hip axis length
- HSA
hip structure analysis
- ISI
impact strength index
- IU
international units
- PTH
parathyroid hormone
Footnotes
Disclosures:
The authors, Ruban Dhaliwal, Shahidul Islam, Mageda Mikhail, Louis Ragolia and John Aloia declare that they have no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Fang J, Freeman R, Jeganathan R, Alderman MH. Variations in hip fracture hospitalization rates among different race/ethnicity groups in New York City. Ethn Dis 2004;14(2):280–284. [PubMed] [Google Scholar]
- 2.Griffin MR, Ray WA, Fought RL, Melton LJ 3rd. Black-white differences in fracture rates. Am J Epidemiol. 1992;136(11):1378–1385. [DOI] [PubMed] [Google Scholar]
- 3.Karagas MR, Lu-Yao GL, Barrett JA, Beach ML, Baron JA. Heterogeneity of hip fracture: age, race, sex, and geographic patterns of femoral neck and trochanteric fractures among the US elderly. Am J Epidemiol 1996;143(7):677–682. [DOI] [PubMed] [Google Scholar]
- 4.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr 2008;88(2):545S–550S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 2008;88(6):1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson DA, Barondess DA, Hendrix SL, Beck TJ. Cross-sectional geometry, bone strength, and bone mass in the proximal femur in black and white postmenopausal women. J Bone Miner Res 2000;15(10):1992–1997. [DOI] [PubMed] [Google Scholar]
- 7.Nelson DA, Beck TJ, Wu G, et al. Ethnic differences in femur geometry in the women’s health initiative observational study. Osteoporos Int 2011;22(5):1377–1388. [DOI] [PubMed] [Google Scholar]
- 8.Peacock M, Buckwalter KA, Persohn S, Hangartner TN, Econs MJ, Hui S. Race and sex differences in bone mineral density and geometry at the femur. Bone 2009;45(2):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med 2005;165(14):1618–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieves JW, Cosman F, Grubert E, Ambrose B, Ralston SH, Lindsay R. Skeletal effects of vitamin D supplementation in postmenopausal black women. Calcif Tissue Int 2012;91(5):316–324. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross ACTC, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US) 2011. [PubMed] [Google Scholar]
- 12.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96(7):1911–1930. [DOI] [PubMed] [Google Scholar]
- 13.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res 2011;26(3):455–457. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. The D-batable Institute of Medicine report: a D-lightful perspective. Endocr Pract 2011;17(1):143–149. [DOI] [PubMed] [Google Scholar]
- 15.Dhaliwal R, Mikhail M, Usera G, et al. The relationship of Physical performance and Osteoporosis prevention with vitamin D in older African Americans (PODA). Contemp Clin Trials 2018;65:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aloia J, Fazzari M, Islam S, et al. Vitamin D Supplementation in Elderly Black Women Does Not Prevent Bone Loss: A Randomized Controlled Trial. J Bone Miner Res 2018;33(11):1916–1922. [DOI] [PubMed] [Google Scholar]
- 17.Allolio B. Risk factors for hip fracture not related to bone mass and their therapeutic implications. Osteoporos Int 1999;9 Suppl 2:S9–S16. [DOI] [PubMed] [Google Scholar]
- 18.Alonso CG, Curiel MD, Carranza FH, Cano RP, Perez AD. Femoral bone mineral density, neck-shaft angle and mean femoral neck width as predictors of hip fracture in men and women. Multicenter Project for Research in Osteoporosis. Osteoporos Int 2000;11(8):714–720. [PubMed] [Google Scholar]
- 19.Heaney RP, Barger-Lux MJ, Davies KM, Ryan RA, Johnson ML, Gong G. Bone dimensional change with age: interactions of genetic, hormonal, and body size variables. Osteoporos Int 1997;7(5):426–431. [DOI] [PubMed] [Google Scholar]
- 20.Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women’s health initiative-observational study. J Bone Miner Res 2009;24(8):1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlamangla AS, Barrett-Connor E, Young J, Greendale GA. Hip fracture risk assessment using composite indices of femoral neck strength: the Rancho Bernardo study. Osteoporos Int 2004;15(1):62–70. [DOI] [PubMed] [Google Scholar]
- 22.Aloia JF, Rubinova R, Fazzari M, Islam S, Mikhail M, Ragolia L. Vitamin D and Falls in Older African American Women: The PODA Randomized Clinical Trial. J Am Geriatr Soc 2019;67(5):1043–1049. [DOI] [PubMed] [Google Scholar]
- 23.Danielson ME, Beck TJ, Karlamangla AS, et al. A comparison of DXA and CT based methods for estimating the strength of the femoral neck in post-menopausal women. Osteoporos Int 2013;24(4):1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin DB. Inference and missing data. Biometrika 1976;63(3):581–592. [Google Scholar]
- 25.Ibrahim JG, Molenberghs G. Missing data methods in longitudinal studies: a review. Test (Madr) 2009;18(1):1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res 2005;20(2):185–194. [DOI] [PubMed] [Google Scholar]
- 27.Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 2001;286(22):2815–2822. [DOI] [PubMed] [Google Scholar]
- 28.Kaptoge S, Beck TJ, Reeve J, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res 2008;23(12):1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings SR, Cauley JA, Palermo L, et al. Racial differences in hip axis lengths might explain racial differences in rates of hip fracture. Study of Osteoporotic Fractures Research Group. Osteoporos Int 1994;4(4):226–229. [DOI] [PubMed] [Google Scholar]
- 30.Danielson ME, Beck TJ, Lian Y, et al. Ethnic variability in bone geometry as assessed by hip structure analysis: findings from the hip strength across the menopausal transition study. J Bone Miner Res 2013;28(4):771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikhail MB, Vaswani AN, Aloia JF. Racial differences in femoral dimensions and their relation to hip fracture. Osteoporos Int 1996;6(1):22–24. [DOI] [PubMed] [Google Scholar]
- 32.Ishii S, Cauley JA, Greendale GA, Danielson ME, Safaei Nili N, Karlamangla A. Ethnic differences in composite indices of femoral neck strength. Osteoporos Int 2012;23(4):1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang S, Choi HS, Kim KM, Rhee Y, Lim SK. Associations between serum 25-hydroxyvitamin D and bone mineral density and proximal femur geometry in Koreans: the Korean National Health and Nutrition Examination Survey (KNHANES) 2008–2009. Osteoporos Int 2015;26(1):163–171. [DOI] [PubMed] [Google Scholar]
- 34.Jackson RD, Wright NC, Beck TJ, et al. Calcium plus vitamin D supplementation has limited effects on femoral geometric strength in older postmenopausal women: the Women’s Health Initiative. Calcif Tissue Int 2011;88(3):198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin EN, Haney EM, Shannon J, et al. Femoral volumetric bone density, geometry, and strength in relation to 25-hydroxy vitamin D in older men. J Bone Miner Res 2015;30(3):562569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhaliwal R, Aloia JF. Effect of Vitamin D on Falls and Physical Performance. Endocrinol Metab Clin North Am 2017;46(4):919–933. [DOI] [PubMed] [Google Scholar]
- 37.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly High-Dose Vitamin D Treatment for the Prevention of Functional Decline: A Randomized Clinical Trial. JAMA Intern Med 2016;176(2):175–183. [DOI] [PubMed] [Google Scholar]
- 38.Cauley JA, Danielson ME, Boudreau R, et al. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women’s Health Initiative (WHI). J Bone Miner Res 2011;26(10):2378–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faulkner KA, Cauley JA, Zmuda JM, et al. Higher 1,25-dihydroxyvitamin D3 concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int 2006;17(9):1318–1328. [DOI] [PubMed] [Google Scholar]
- 40.Beaudart C, Buckinx F, Rabenda V, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2014;99(11):4336–4345. [DOI] [PubMed] [Google Scholar]
- 41.Aloia JF, Vaswani A, Ma R, Flaster E. Body composition in normal black women: the four-compartment model. J Clin Endocrinol Metab 1996;81(6):2363–2369. [DOI] [PubMed] [Google Scholar]
- 42.Kritchevsky SB, Tooze JA, Neiberg RH, et al. 25-Hydroxyvitamin D, parathyroid hormone, and mortality in black and white older adults: the health ABC study. J Clin Endocrinol Metab 2012;97(11):4156–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cauley JA, Lui LY, Stone KL, et al. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American women. J Am Geriatr Soc 2005;53(2):183–189. [DOI] [PubMed] [Google Scholar]
- 44.Manson JE, Cook NR, Lee IM, et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med 2019;380(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urashima M, Ohdaira H, Akutsu T, et al. Effect of Vitamin D Supplementation on Relapse-Free Survival Among Patients With Digestive Tract Cancers: The AMATERASU Randomized Clinical Trial. JAMA 2019;321(14):1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]


