This case series documents a greater incidence of degenerative lamellar macular holes subtype in advance stages of age-related macular degeneration.
Key words: age-related macular degeneration, anti–vascular endothelial growth factor, choroidal neovascularization, degenerative lamellar macular hole, epiretinal proliferation, geographic atrophy, lamellar macular holes, macular holes, optical coherence tomography, tractional lamellar macular hole
Abstract
Purpose:
To investigate whether age-related macular degeneration (AMD) has an influence on the prevalence and anatomical characteristics of lamellar macular holes (LMHs).
Methods:
Clinical records and spectral-domain optical coherence tomography images of 756 eyes of 423 consecutive patients diagnosed with AMD were reviewed and analyzed. Spectral-domain optical coherence tomography was used to identify degenerative or tractional LMH subtypes and assess their morphology. The clinical and optical coherence tomography findings of AMD eyes with LMH were compared with those of a control group of eyes with LMH without AMD from a previously published report.
Results:
Lamellar macular holes were identified in 25 eyes of 23 patients (3.3%; 25 of 756). Seventeen of 25 eyes (68%) presented with degenerative LMH and underlying late neovascular AMD. Mean best-corrected visual acuity was worse in eyes with AMD and LMH eyes than in those with AMD and no LMH (20/230 vs. 20/98; P = 0.02). The mean outer diameter was greater in the group with degenerative LMH with concomitant AMD than in the control group of degenerative LMH without AMD (1,323.9 ± 999.1 µm vs. 905.9 ± 356.8 µm, respectively; P = 0.01).
Conclusion:
The incidence of degenerative LMH increased in advanced forms of AMD, whereas the presence of tractional LMH subtype may be unrelated to AMD evolution.
The term lamellar macular hole (LMH) was first introduced by Gass in 1975. Using slit-lamp biomicroscopy, he described a reddish, oval-shaped, macular lesion in a pseudophackic patient with cystoid macular edema and presented histological evidence of foveal tissue loss.1,2 Currently, the evaluation of this clinical entity with optical coherence tomography (OCT) has replaced in vivo histopathology analysis of macular structural changes and evolved into the gold standard for the diagnosis of LMH.3–5 Current anatomical OCT-based features of LMH include an irregular foveal contour and a partial thickness defect of the macula with intact underlying retinal pigment epithelium (RPE) and photoreceptor layers.6–8 With the last generation of OCT imaging, two different subtypes of LMH have been proposed: tractional and degenerative, which may represent unique pathologic entities with different clinical implications.9 Tractional LMH subtype is characterized by the presence of a schitic sharp-edged intraretinal split between the outer plexiform and outer nuclear layers, frequent epiretinal membranes, and intraretinal cystoid spaces. In contrast, degenerative LMH presents with a round-edged intraretinal cavitation, frequent defects in the ellipsoid layer, epiretinal proliferation, and in some cases with a central retinal bump. Although advances in OCT imaging technology have increased our understanding of LMH, the pathophysiology is still not fully understood.3,5,10,11 Many studies have found an increased prevalence of both forms of LMH in older patients, suggesting that age may be a risk factor.9,12–14
Age-related macular degeneration (AMD), the leading cause of irreversible blindness that affects more than eight million individuals in the United States, is an intricate disease with genetic and environmental risk factors.15,16 Several classifications and grading systems for AMD have been proposed.17–19 Ferris et al20 suggested a basic clinical classification 5-point scale including the risk of progression. Age-related macular degeneration begins with the presence of small drusen, which gradually becomes larger and finally coalesce to form confluent drusen, accompanied by retinal hyperpigmentation. At that crucial point, the disease can progress to the advanced stages characterized by choroidal neovascularization and/or geographic atrophy resulting in severe vision loss.21 The advanced form of the disease affects more than 1.75 million individuals.15 Recently, Segal et al22 have focused on LMH associated with end-stage exudative AMD. The mutualistic symbiotic relationship between the components of the photoreceptor, RPE, Bruch membrane, and choriocapillaris complex is altered in AMD.23 Age-related macular degeneration causes apoptosis of RPE cells and loss of the outer retina that could lead to disruption of cell-to-cell adhesions and foveal destabilization, thereby rendering the fovea more susceptible to LMH formation.24
We hypothesize that the structural retinal changes seen in AMD are a predisposition for the development of LMH. Therefore, the purpose of this study is to determine the prevalence and anatomical characteristics of LMH in presence of AMD.
Methods
A retrospective, observational, review of medical records of consecutive patients diagnosed with AMD and seen by a retina specialist (J.-P.H.) at the Stein Eye Institute, University of California Los Angeles from January 1, 2014, to August 31, 2017, was performed. After the approval from the University of California Los Angeles Office of Human Research Protection, cases were identified by performing a search of the medical billing records, using the International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) diagnosis codes 362.5, 362.51, and 362.52 for nonexudative and exudative senile macular degeneration. We included patients with at least one retina office visit and OCT scans confirming the unilateral or bilateral diagnosis of AMD. Exclusion criteria included history of diabetic retinopathy, high myopia, myopic choroidal neovascularization, retinal vein occlusion, uveitis, trauma, and previous pars plana vitrectomy. Demographic and clinical information were reviewed and recorded. The best-corrected visual acuity at the last visit was recorded and reported in Snellen fraction, which was converted into logarithm of the minimal angle of resolution values for statistical analysis.
In all cases, OCT images were obtained with the Spectralis OCT (Heidelberg Engineering GmbH, Heidelberg, Germany) and reviewed with either the Heidelberg Eye Explorer (Version 1.10.0.0) using the HRA/Spectralis Viewing Module (Version 6.8.3.0) or Axis Image Management (Version 11.0.4.1).
Clinical findings and OCT images were reviewed by two independent observers (A.F. and L.Y.), and AMD was graded as, 1) normal aging changes, 2) early AMD, 3) intermediate AMD, and 4) late AMD according to the classification system proposed by Ferris et al.20 Late AMD cases were further categorized into neovascular and/or geographic atrophy subtypes.
Next, the most recent OCT imaging was also used to identify the presence of LMHs. The presence of LMH was defined according to the following OCT findings: presence of irregular foveal contour, separation of the layers of the neurosensory retina, and the absence of full-thickness macular defects.7 Eyes meeting the criteria for LMH were subsequently differentiated into either the degenerative or the tractional subtype using the same criteria as Govetto et al.9 All OCT images were carefully reviewed by two independent observers (A.F. and L.Y.). Using the “caliper” function of the Heidelberg Eye Explorer, we analyzed LMH morphology, including inner diameter, outer diameter, minimum foveal retinal thickness, and perifoveal thickness (Figure 1).
Fig. 1.
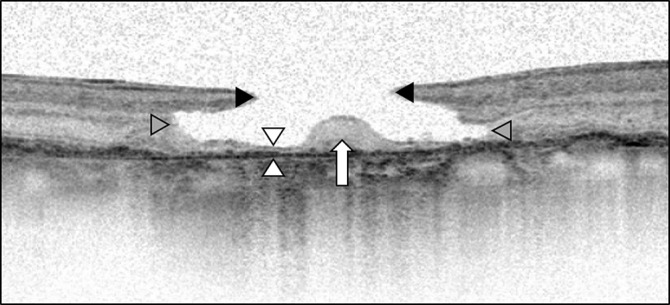
Lamellar macular hole measurements and morphology. The inner diameter (black arrowheads) is the distance between the inner retinal layer at the opening of the hole. The outer diameter (gray arrowheads) is the widest distance of the cavitation. The minimum foveal retinal thickness (white arrowheads) is minimum thickness of the retina at the level of the foveal floor. The foveal bump (white arrow) is the elevation of spared tissue located in the base of the LMH.
The minimum foveal retinal thickness was defined as the minimum thickness of the retina at the level of the foveal floor, typically adjacent to the foveal bump when present. Therefore, the minimum foveal retinal thickness did not always coincide with the exact center of the fovea. The perifoveal thickness was defined as retinal thickness of 750 μm from the center of the macula and was measured both nasally and temporally. As illustrated in Figure 2, we classified ellipsoid zone integrity into three categories: present (no alteration), disrupted (partial disruption) or absent (no ellipsoid zone present) (Figure 2).25 In addition, we also assessed the presence of the foveal bump located at the base of the lamellar macular hole (Figure 1).9
Fig. 2.
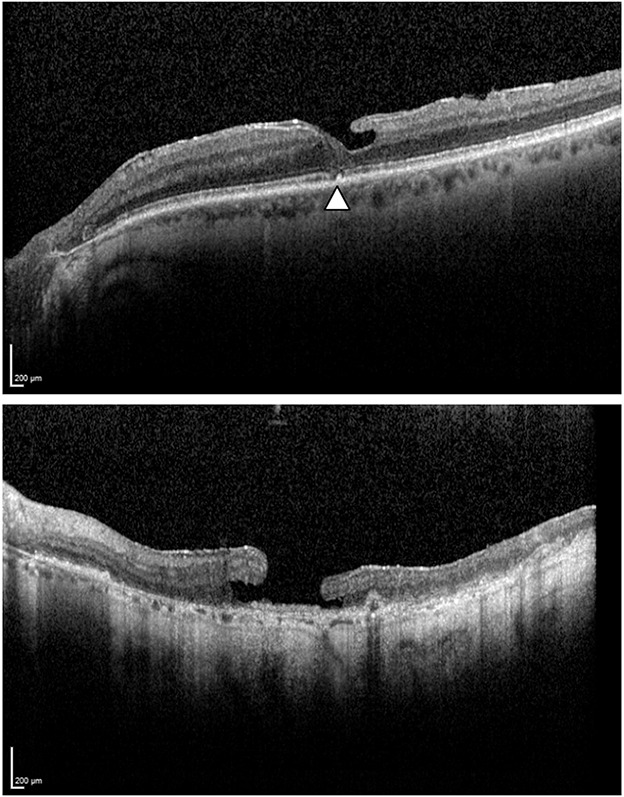
Ellipsoid zone integrity. Top: The arrowhead shows an ellipsoid zone disruption. Bottom: Ellipsoid zone absence with diffuse alteration in the outer retinal layer.
Spectralis OCT scan patterns were used for all measurements. All eyes had at least two images per visit: 20 × 15°, with 19 B-scans spaced 242 µm, and a single high-definition horizontal line at 30°. In addition, 81 of 822 eyes (9.9%) diagnosed with AMD had high-density 15 × 10°, with 97 B-scans spaced at 30 µm. In all cases, the images were adjusted at 1:1 µm. Mean central foveal thickness was obtained using the automated “thickness map” function of the Heidelberg Eye Explorer.
The clinical and OCT findings obtained from eyes with LMH in the presence of AMD from this study population were compared with those of a control group with LMH without AMD published in a previous report.9 Finally, we evaluated the relationship between LMH OCT characteristics and the number of intravitreal injections.
Data collection was performed on Microsoft Excel 2011 (Microsoft Corporation, Redmond, WA). A P value of less than 0.05 was considered statistically significant.
Results
Characteristics of the Study Population
At the end of the review process, 756 eyes from 423 patients diagnosed with AMD were included in the analysis. One hundred sixty-two (38.3%) were male subjects, and 261 (61.7%) were female subjects; the mean age was 81.6 ± 9.62 years. Figure 3 displays the number of eyes classified according to the AMD stage and the number of LMH found in each stage.
Fig. 3.
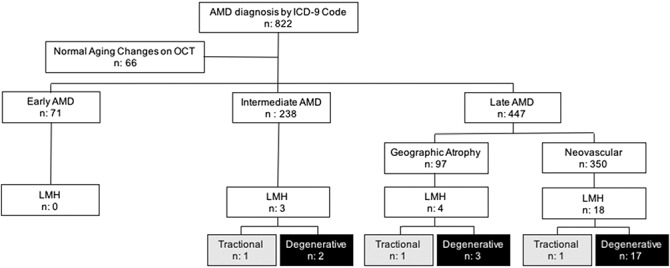
Flow chart of the eyes classified by age-related macular degeneration stage and lamellar macular hole subtype. ICD, International Statistical Classification of Diseases and Related Health Problems; n, number of eyes.
Lamellar Macular Hole Incidence in Eyes With Underlying Age-Related Macular Degeneration
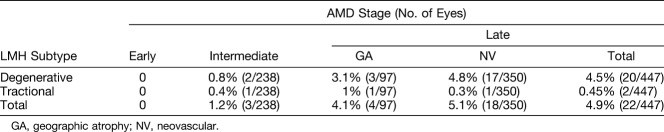
Lamellar macular hole was identified in 25 eyes of 23 patients (25 of 756 eyes; 3.3%) with underlying AMD. The mean age of this subgroup was 83.22 ± 8.86 years; 14 (60.87%) were male subjects and 9 (39.13%) were female subjects. Analysis of the OCT images demonstrated the degenerative LMH subtype in 22 eyes (22 of 25; 88%) and the tractional LMH subtype in 3 eyes (3 of 25; 12%). The incidence of LMH subtypes in each stage of AMD is summarized in Table 1.
Table 1.
Lamellar Macular Hole Subtype Incidence Depending on the Stage of AMD
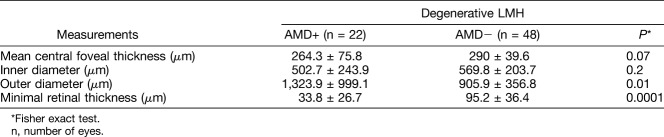
Comparison of Degenerative and Tractional Lamellar Macular Hole Measurements Between Eyes With Age-Related Macular Degeneration and Without Age-Related Macular Degeneration
The differences encountered between degenerative LMH subtype associated with AMD and degenerative LMH subtype from the control group are reported in Table 2. Interestingly, the outer diameter of degenerative LMH subtype in eyes with AMD was significantly larger when compared with that of degenerative LMH subtype from the control group (1,323.91 ± 999.1 µm vs. 905.9 ± 356.8 µm, respectively; P = 0.01). Furthermore, the mean central foveal thickness and minimal retinal thickness were significantly lower in the AMD group (264.3 ± 75.8 µm and 33.8 ± 26.7 µm, respectively) than in the group without AMD (290 ± 39.6 µm and 95.2 ± 36.4 µm, respectively; P = 0.07 and P = 0.0001). In contrast, no statistical difference was found between the eyes with AMD and the control group in the tractional LMH subtype.
Table 2.
Comparison of Degenerative LMH Measurements Between Eyes With Underlying AMD and Eyes Without AMD
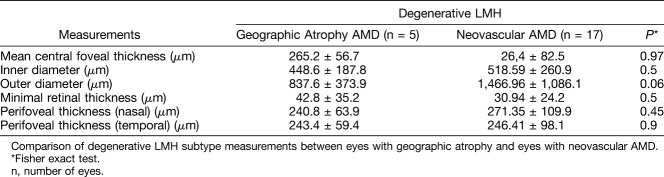
Comparison of Degenerative Lamellar Macular Hole Measurements Between Eyes With Geographic Atrophy and Those With Neovascular Age-Related Macular Degeneration
As shown in Table 3, the outer diameter was greater in eyes with neovascular AMD than in those with geographic atrophy AMD (1,466.96 ± 1,086.1 µm vs. 837.6 ± 373.9 µm), but the difference was not statistically significant (P = 0.06). Eyes with tractional LMH subtype were not analyzed because of the small number of patients.
Table 3.
Late AMD Stage
Correlation Between Measurements of Degenerative Lamellar Macular Holes With Underlying Age-Related Macular Degeneration and the Number of Intravitreal Injections
No statistically significant differences in the mean central foveal thickness, inner diameter, outer diameter, minimal retinal thickness, and perifoveal thickness were encountered. However, there was a trend toward a positive correlation between mean outer diameter and the number of injections (r2 = 0.344; P = 0.19).
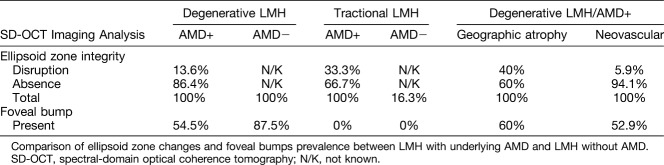
Prevalence of Changes in the Ellipsoid Zone Integrity and the Presence of Foveal Bump
Prevalence of changes in the ellipsoid zone integrity and the presence of foveal bump in eyes with LMH and concomitant AMD and control eyes with LMH without AMD are shown in Table 4.
Table 4.
Spectral-Domain OCT Imaging Analysis
Best-Corrected Visual Acuity
Finally, the mean best-corrected visual acuity was significantly worse in the group of patients with LMH and AMD (25 of 756 eyes) compared with the group of patients with AMD and no LMH (731 of 756) (20/230 vs. 20/98; P = 0.02). Furthermore, the mean best-corrected visual acuity in eyes with degenerative LMH and AMD was also worse than that in eyes with degenerative LMH without AMD (20/235 vs. 20/42; P = 0.0001).
Discussion
Advances in OCT technology allow for a better understanding of pathogenesis, improved monitoring of progression, and assistance in classifying LMHs.3–5,7,10,11 A number of hypotheses have been proposed to explain the formation of LMH, including the union of intraretinal cysts, unroofing of a foveal pseudocyst, aborted formation of a full-thickness macular hole, and centrifugal traction of epiretinal membranes.2–4,26 However, none of these theories seem to unify the spectrum of findings observed within the current classification of LMH. As defined by Govetto et al,9 the presence of epiretinal membrane with signs of traction on the underlying retina may play a pivotal role in the development of tractional LMHs. Conversely, the evolution of degenerative lamellar macular hole may suggest a slow, chronic process causing loss of retinal tissue and the disruption of ellipsoid layer.
The pathophysiology of AMD is also characterized by the progressive dysfunction and changes in the choroid, Bruch membrane, RPE, and outer retina.27–30 Although the exact sites of pathologic and clinical profiles only partially overlap, AMD and LMH may share similarities in disease mechanisms and pathogenic pathways. To our knowledge, there are two studies concerning LMH in eyes with underlying neovascular AMD. Theodossiadis et al31 presented a patient with an epiretinal membrane–associated LMH with coexisting subretinal fluid arising from a choroidal neovascularization in the background of neovascular AMD that was successfully managed with pars plana vitrectomy. Segal et al22 described a consecutive case series of 16 eyes describing the morphologic abnormalities associated with LMH and late AMD as revealed by OCT.
In this study, we hypothesized that AMD could be a predisposition to the development of degenerative LMH. Interestingly, we found that the prevalence of LMH in eyes with underlying AMD is higher than that in eyes without AMD as reported in the literature and also increase with the severity of the AMD. Our data show that 3.3% of eyes had LMH in patients with AMD, whereas published studies reported a prevalence of 0.9% to 2.6% of LMH in the general population.32,33 Furthermore, we found a higher incidence of the degenerative LMH subtype than the tractional LMH subtype and, remarkably, an increase in the incidence of degenerative LMH in the more advanced stages of AMD. Figure 4 illustrates an example of the degenerative LMH subtype with underlying intermediate, geographic atrophy or neovascular AMD. The risk of developing a degenerative LMH seems higher in patients with more advanced forms of AMD. Notably, this trend was not observed in tractional LMH subtype. These findings suggest that the pathophysiologic mechanisms responsible for the development of AMD may be involved in the formation of a degenerative LMH subtype. Many gaps exist in our knowledge of the mechanisms involved in AMD evolution. However, preceding research studies proposed that cell degeneration occurring during AMD may involve toxic products released by photoreceptors,34 RPE and Mṻller cells dysfunction,35 and retinal microglia activation.36 Both in the normal retina and in the brain, quiescent microglia are inconspicuous stellate cells that surround inner retinal blood vessels and function as resident macrophages.37 Activated microglia phagocytose cell debris and destroy normal cells around the primary area of degeneration.36 In advanced form of AMD, the leading edge of apoptosis of retina cells is associated with adjacent retinal degeneration.38 Based on this argument, our study suggests that AMD in advanced stages may present with remodeling of retinal layers that could participate in or lead to the development of degenerative LMH.
Fig. 4.
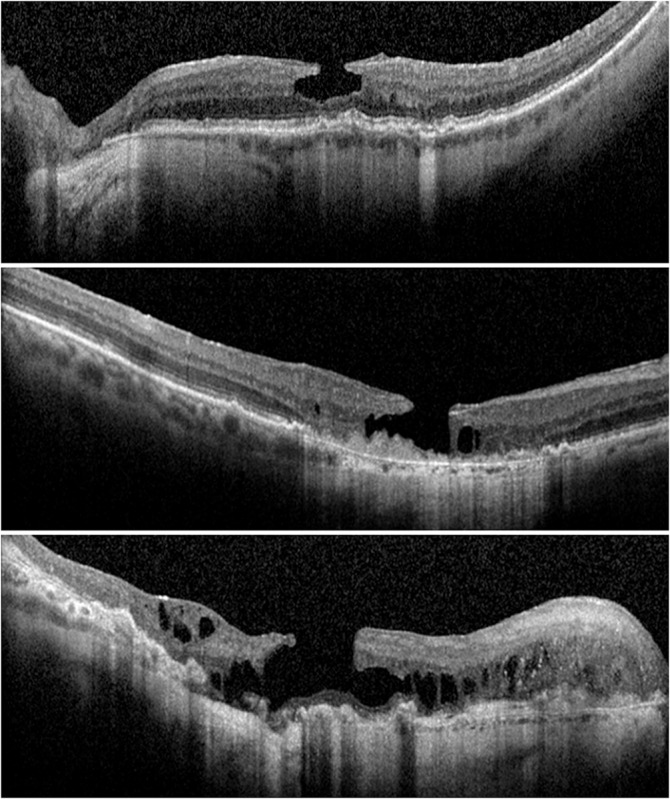
Lamellar macular hole (LMH) and age‐related macular degeneration (AMD) stage. Top: Optical coherence tomographic (OCT) scan of a (instead of AN) LMH with underlying intermediate AMD. Middle: OCT scan of a (instead of AN) LMH with underlying geographic atrophy AMD. Bottom: OCT scan of a (instead of AN) LMH with underlying.
The Beaver Dam Eye Study cohort reported a higher prevalence of LMH in older age groups: 2.1% in 63 to 74 years and 2.6% in 85 years or older.33 This is consistent with our case series in which the mean age of the studied population is 81.6 years. The increased incidence of LMH in older patients with AMD suggests that the impact of aging on retina structures may be a risk factor for the development of degenerative LMH.39,40
In the current study, we assumed that the presence of AMD could contribute to the anatomical features of LMH. Surprisingly, our data showed that the mean outer diameter of the degenerative LMH subtype was significantly larger in patients with underlying AMD compared with the general population. Damage to the retinal architecture induced by AMD formation may be at least partially responsible for the widening of the outer diameter of the LMH. Unlike the degenerative LMH subtype, anatomical features of the tractional LMH subtype do not seem to be largely affected by AMD pathogenesis. This correlation coincides indeed with the theories previously stated about LMH development.4,7,41
In patients with degenerative LMH with underlying late AMD, the mean outer diameter was greater in eyes with underlying neovascular AMD than in eyes with underlying geographic atrophy but not statistically significant. This result may demonstrate a tendency that reinforces the role of the biochemical abnormalities triggered by abnormal angiogenesis.42 Future investigations involving a greater number of eyes are necessary to elucidate the importance of this chronic insult in the development of LMH.
In regard to anti–vascular endothelial growth factor treatment, the number of intravitreal injections seems to have no significant clinical impact on the development and evolution of LMH. No differences were found between the groups divided by the number of injections. However, the division into groups by the number of injections was somewhat arbitrary, and the exact number of injections received by some of our patients was difficult to know because some were not naive when initially evaluated in our center. With the available data, there was a trend toward large mean outer diameter in patients with more injections. Kurihara et al43 revealed an essential role of vascular endothelial growth factor in maintaining choroid vasculature and normal retinal anatomy. These findings suggest that therapeutic approaches to blocking vascular endothelial growth factor signaling in retinal diseases might have unexpected detrimental side effects on the retinochoroidal tissue. Identification of these risks will be important going forward.
It is unknown whether treating LMH with underlying AMD will modify the prognosis of either disease To clarify this issue, long-term clinical studies conducted in individuals diagnosed with AMD are necessary. This population is well defined, and alterations in the disease course can be determined at very early stage because of the increased use of OCT imaging. In addition, studying LMH in the presence of retinal disorders could potentially help to better understand the pathogenesis of the different LMH subtypes.
The strengths of the present study include a randomly selected population-based sample and the availability of medical records and OCT images with a 10-year median length of archive. Previous publications evaluating LMH in eyes with underlying AMD are based on neovascular AMD only; our work includes all stages of the disease.
Weaknesses of this study include its retrospective nature, the limited number of subjects, and the manual method of measuring the LMH features. We based our measurements on horizontal OCT scans; adding oblique and vertical OCT scans would have allowed us to complete a more precise evaluation of LMH diameters. Furthermore, this study was conducted in a tertiary referral center; therefore, some patients had been previously treated and may have a more aggressive form of AMD triggering a selection bias. Moreover, the prevalence of LMH could differ widely from a primary care setting.
In summary, degenerative and tractional LMH subtypes most likely have different pathogenesis. The pathophysiology of degenerative LMH subtype may share common pathways with AMD as supported by the greater incidence of LMH in advance stages of AMD and the greater mean outer diameter in degenerative LMH subtype with underlying AMD.
Footnotes
Supported by an unrestricted institutional grant from the Research to Prevent Blindness (RPB) (J.-P. H.) New York, NY, and by a donation from the Hess Foundation, New York, NY.
J.-P. Hubschman: consultant for Alcon (Fort Worth, TX) and Allergan (Parsippany-Troy Hills, NJ) and receives research support from The Lowy Medical Research Institute (La Jolla, CA). The remaining authors have any conflicting interests to disclose.
The first authors are A. Francone and L. Yun.
References
- 1.Gass JD. Lamellar macular hole: a complication of cystoid macular edema after cataract extraction: a clinicopathologic case report. Trans Am Ophthalmol Soc 1975;73:231–250. [PMC free article] [PubMed] [Google Scholar]
- 2.Gass JD. Lamellar macular hole: a complication of cystoid macular edema after cataract extraction. Arch Ophthalmol 1976;94:793–800. [DOI] [PubMed] [Google Scholar]
- 3.Bottoni F, Deiro AP, Giani A, et al. The natural history of lamellar macular holes: a spectral domain optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol 2013;251:467–475. [DOI] [PubMed] [Google Scholar]
- 4.Haouchine B, Massin P, Tadayoni R, et al. Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am J Ophthalmol 2004;138:732–739. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi H, Kishi S. Tomographic features of a lamellar macular hole formation and a lamellar hole that progressed to a full-thickness macular hole. Am J Ophthalmol 2000;130:677–679. [DOI] [PubMed] [Google Scholar]
- 6.Frangieh GT, Green WR, Engel HM. A histopathologic study of macular cysts and holes. Retina 1981;1:311–336. [PubMed] [Google Scholar]
- 7.Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology 2006;113:388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013;120:2611–2619. [DOI] [PubMed] [Google Scholar]
- 9.Govetto A, Dacquay Y, Farajzadeh M, et al. Lamellar macular hole: two distinct clinical entities? Am J Ophthalmol 2016;164:99–109. [DOI] [PubMed] [Google Scholar]
- 10.Haouchine B, Massin P, Gaudric A. Foveal pseudocyst as the first step in macular hole formation: a prospective study by optical coherence tomography. Ophthalmology 2001;108:15–22. [DOI] [PubMed] [Google Scholar]
- 11.Gaudric A, Haouchine B, Massin P, et al. Macular hole formation: new data provided by optical coherence tomography. Arch Ophthalmol 1999;117:744–751. [DOI] [PubMed] [Google Scholar]
- 12.Compera D, Schumann RG, Cereda MG, et al. Progression of lamellar hole-associated epiretinal proliferation and retinal changes during long-term follow-up. Br J Ophthalmol 2018;102:84–90. [DOI] [PubMed] [Google Scholar]
- 13.Acquistapace A, Cereda MG, Cigada M, et al. Imaging of tangential traction types in lamellar macular holes. Graefes Arch Clin Exp Ophthalmol 2017;255:2331–2336. [DOI] [PubMed] [Google Scholar]
- 14.Ko J, Kim GA, Lee SC, et al. Surgical outcomes of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Acta Ophthalmol (Copenh). 2017;95:e221–e226. [DOI] [PubMed] [Google Scholar]
- 15.Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–572. [DOI] [PubMed] [Google Scholar]
- 16.Sobrin L, Seddon JM. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Prog Retin Eye Res 2014;40:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration: the International ARM Epidemiological Study Group. Surv Ophthalmol 1995;39:367–374. [DOI] [PubMed] [Google Scholar]
- 18.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology 2006;113:260–266. [DOI] [PubMed] [Google Scholar]
- 19.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology 1991;98:1128–1134. [DOI] [PubMed] [Google Scholar]
- 20.Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology 2013;120:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambris JD, Adamis AP. Inflammation and Retinal Disease: Complement Biology and Pathology. Vol 703: New York, NY: Springer; 2010:2. [Google Scholar]
- 22.Segal O, Ferencz JR, Mimouni M, et al. Lamellar macular holes associated with end-stage exudative age-related macular degeneration. Isr Med Assoc J 2015;17:750–754. [PubMed] [Google Scholar]
- 23.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med 2012;33:295–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattacharya S, Chaum E, Johnson DA, Johnson LR. Age-related susceptibility to apoptosis in human retinal pigment epithelial cells is triggered by disruption of p53-Mdm2 association. Invest Ophthalmol Vis Sci 2012;53:8350–8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao LW, Wu Z, Guymer RH, Luu CD. Ellipsoid zone on optical coherence tomography: a review. Clin Exp Ophthalmol 2016;44:422–430. [DOI] [PubMed] [Google Scholar]
- 26.Bottoni F, Carmassi L, Cigada M, et al. Diagnosis of macular pseudoholes and lamellar macular holes: is optical coherence tomography the “gold standard”? Br J Ophthalmol 2008;92:635–639. [DOI] [PubMed] [Google Scholar]
- 27.Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol 1999;117:329–339. [DOI] [PubMed] [Google Scholar]
- 28.McLeod DS, Grebe R, Bhutto I, et al. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci 2009;50:4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogan MJ. Role of the retinal pigment epithelium in macular disease. Trans Am Acad Ophthalmol Otolaryngol 1972;76:64–80. [PubMed] [Google Scholar]
- 30.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol 1976;60:324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theodossiadis P, Charonis A, Panagiotidis D, et al. Vitrectomy for treatment of a lamellar hole in a patient with exudative macular degeneration: the role of vitreous traction elimination. Eur J Ophthalmol 2010;20:1086–1088. [DOI] [PubMed] [Google Scholar]
- 32.Liesenborghs I, Clerck EEBD, Berendschot TTJM, et al. Prevalence of optical coherence tomography detected vitreomacular interface disorders: the Maastricht Study. Acta Ophthalmol 2018;96:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bird AC. Retinal photoreceptor dystrophies LI. Edward Jackson memorial lecture. Am J Ophthalmol 1995;119:543–562. [DOI] [PubMed] [Google Scholar]
- 34.Adler R, Curcio C, Hicks D, et al. Cell death in age-related macular degeneration. Mol Vis 1999;5:31. [PubMed] [Google Scholar]
- 35.Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res 2003;76:463–471. [DOI] [PubMed] [Google Scholar]
- 36.Provis JM, Penfold PL, Edwards AJ, van Driel D. Human retinal microglia: expression of immune markers and relationship to the glia limitans. Glia 1995;14:243–256. [DOI] [PubMed] [Google Scholar]
- 37.Sunness JS. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis 1999;5:25. [PubMed] [Google Scholar]
- 38.Meuer SM, Myers CE, Klein BEK, et al. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: the beaver dam eye study. Ophthalmology 2015;122:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorey CK, Wu G, Ebenstein D, et al. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci 1989;30:1691–1699. [PubMed] [Google Scholar]
- 40.Gao H, Hollyfield JG. Aging of the human retina: differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 1992;33:1–17. [PubMed] [Google Scholar]
- 41.Govetto A, Lalane RA, Sarraf D, et al. Insights into epiretinal membranes: presence of ectopic inner foveal layers and a new optical coherence tomography staging scheme. Am J Ophthalmol 2017;175:99–113. [DOI] [PubMed] [Google Scholar]
- 42.Kent DL. Age-related macular degeneration: beyond anti-angiogenesis. Mol Vis 2014;20:46–55. [PMC free article] [PubMed] [Google Scholar]
- 43.Kurihara T, Westenskow PD, Bravo S, et al. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest 2012;122:4213–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]