This prospective, three-center, randomized study assessed the efficacy and safety of early vitamin A supplementation in improving the outcomes of retinopathy of prematurity in extremely preterm infants. Two hundred sixty-two extremely preterm infants were randomized to vitamin A and control groups. Vitamin A supplementation reduced its deficiency in these infants.
Key words: early, extremely, infants, preterm, retinopathy of prematurity, supplementation, vitamin A
Abstract
Purpose:
This study assessed the efficacy and safety of early vitamin A (VA) supplementation to improve outcomes of retinopathy of prematurity in extremely preterm infants.
Methods:
A total of 262 eligible extremely preterm infants underwent randomization; of these, 132 were assigned to the VA group and 130 to the control group. The infants were administered a solution of VA (1,500 IU/day), added to their enteral feeds as soon as minimal feeding was introduced and continued for 28 days or until discharge.
Results:
With no adverse effects occurring, serum VA of the VA-supplemented infants on Days 14, 28, and postmenstrual 36 weeks was higher than that of the placebo group (P < 0.001). No signs of VA toxicity or increased intracranial pressure were reported. The VA group had lower unadjusted rates of Type 1 retinopathy of prematurity (1.6 vs. 6.9%, P = 0.030) and bronchopulmonary dysplasia (18.9 vs. 33.8%, P = 0.008) than the control group. Regression analysis revealed an association between serum VA levels and risk of Type 1 retinopathy of prematurity (beta = −2.37).
Conclusion:
Vitamin A supplementation reduced VA deficiency in extremely preterm infants; it was associated with a decreased incidence of Type 1 retinopathy of prematurity and may also have a positive impact on reducing bronchopulmonary dysplasia.
Retinopathy of prematurity (ROP) is a common retinal neovascular disorder and a major cause of visual impairment or blindness in preterm infants, even in the context of current standard care.1 Preventive therapy for ROP is still lacking,2 and visual improvements after treatment are often poor. Retinopathy of prematurity pathogenesis is associated with the regulation of the vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF-1).3 Inhibition of VEGF at the neovascular phase may prevent destructive neovascularization,4 but VEGF as a treatment should be weighted carefully, as VEGF also promotes normal physiological development of blood vessels in many tissues.5–8
Extremely preterm infants are prone to vitamin A (VA) deficiency because of the reduced transplacental transport from their mothers, inadequate intake from enteral feeding for several weeks after birth, and poor gastrointestinal absorption. The inadequate provision and unreliable delivery of parenteral VA may exacerbate the issue of VA deficiency.9 Vitamin A is an essential micronutrient for maintaining a normal visual function.10 Hepatic storage of VA is not so efficient in extremely preterm infants,11–13 and correspondingly, plasma concentrations of retinol are also low.11,12,14 Most of the VA in the body is stored in the liver, but a large amount of VA is also assimilated into the eyes.12,15 Retinaldehyde is an essential constituent of rhodopsin, which is formed by the reversible oxidation of retinol, and is contained within the photoreceptor membrane disks in the outer retina.16 The human retinal rhodopsin content increases several-fold during the third trimester of pregnancy,17 but it is unknown how retinal rhodopsin content increases postnatally in prematurely born infants. The retinal rhodopsin content is dependent on VA sufficiency in the developing rat and is reduced by the early exposure to light.15 Some reports showed that ROP may be prevented by intramuscular supplementation with VA,18 and other showed that in most preterm infants who tolerate feeds, VA deficiency can be corrected safely by supplementing the feeds with 5,000 IU of VA per day.19 However, there have been no previous controlled trials conducted regarding oral VA supplementation in preterm infants in ROP prevention. European guidelines on VA supplementation recommend a daily dose of 1,000 to 3,300 IU/kg body weight in preterm infants.20 This study selected the median dose to assess the efficacy and safety of early VA supplementation for ROP prevention in extremely preterm infants.
Methods
This prospective, three-center, randomized study was performed from August 2015 to December 2017 in neonatal intensive care units in Zhengzhou, Nanjing, and Shangqiu, China. The Life Science Ethics Committee of Zhengzhou University and the local research ethics committees at the participating centers approved the study. Written informed consent was obtained from both parents before participation in the study.
Patient Population
Eligible patients for enrollment included infants admitted to the neonatal intensive care unit at a gestational age of <28 weeks, <96 hours of age. Infants with any of the following were excluded from this study: genetic metabolic diseases; congenital major abnormalities; congenital TORCH infections with overt signs at birth; terminal stage of illness (pH <7.0 or hypoxia with bradycardia >2 hours); or the lack of parental consent. TORCH infections classically comprise toxoplasmosis, syphilis, rubella…21
Following standard procedures, all infants in the study were initially provided with nutrition intravenously (total parenteral nutrition). If the infant was not suffering from any condition that affects the function of the gastrointestinal system, oral feeds were introduced as early as possible through a nasogastric tube using human milk with fortifier or preterm formula. Once the infant was receiving all their nutrition from human milk (“full enteral feeds”), fortifiers (cow's milk–based human milk) were added to each feed. The concentration of VA of the preterm formula milk (Nestle) was 2.2 IU/mL, and for breast milk (3.9 IU/mL) with fortifier (Nestle), it was 5 IU/mL.
Vitamin A Administration
A blocked randomization method stratified by the neonatal intensive care unit size was used to assign infants to either the control or oral VA group. If the parents wished to withdraw consent at any time, all study procedures were ceased, and the infant was fed following current standards of vitamin supplementation as directed by the clinical team.
The infants received the VA (Chinese medicine: H37023082, 1,500 IU/day; Qingdao Double Whale Pharmaceutical, Qingdao, China). The solution was made by the pharmacy and was added to their enteral feeds when minimal feeding started with a very small amount of milk at the beginning and then gradually increasing. The VA was administered at 1,500 IU/day and continued if the infant tolerated the milk, for 28 days or until discharge. An equivalent volume of the placebo solution was provided in the same way in the control group. The placebo solution was prepared with soybean oil by the hospital pharmacist having the same aspect as the VA solution. The medical and nursing teams caring for the infants were thus completely unaware of the content of the solutions, which were only labeled with the study site and infant number. All patients received standard intravenous multivitamin preparation (1 mL/kg/day, containing VA 230 IU/kg/day) within the daily parenteral nutrition. As the amount of milk being fed to the infant increased, the amount of intravenous multivitamin provided was decreased until the infant was receiving all its nutrition from milk 120 mL/kg/day. At this point, the breast milk VA concentration was 2,568 IU/d (VA drop 1,500 IU/d + breast milk VA 468 IU/kg/day + fortifier [Nestle] VA 600 IU/kg/day); the formula milk VA concentration was 1764 IU/d (VA drop 1,500 IU/d + formula VA 264 IU/kg/day).
Retinopathy of Prematurity Screening and Classification
The ROP screening was performed for all extremely preterm infants by qualified ophthalmologists with expertise in ROP according to the Chinese guidelines for examining and treating ROP.22 Retinopathy of prematurity screening frequency was established according to the results of screening, while ceasing ROP screening was conducted according to the vascular development in the retina, or at up to 45 weeks of the postmenstrual age (PMA).22 Retinopathy of prematurity was subdivided into Stages 1 to 5 according to the International Classification of ROP.23
Collection of Serum Samples
Blood samples (500 µL) were obtained from peripheral venous blood on the day before VA supplementation (baseline) and on Days 14, 28, and 36 weeks of the PMA after beginning VA supplementation. Blood samples were wrapped with an aluminum foil to prevent any photodegradation of VA by exposure to light.
Serum samples were obtained from the spontaneous coagulation of blood. Samples were centrifuged at 2,500 rpm at 4°C for 10 minutes to obtain the serum. Hemolyzed samples were excluded. Samples were stored at −80°C until analysis.
Serum VA (retinol) concentrations were detected using the electrode method. Each time samples were tested, an aLK3000V vitamin detector was used to first calibrate standard materials (LANBIAO Tianjin Electronic &Technology Development, Tianjin, China) and to obtain a standard curve. Vitamin A levels of samples were then detected using the LK3000V vitamin detector. The optimal concentration of serum retinol in very low birth weight infants is not known, although it has been suggested that a plasma retinol level >0.70 µmol/L is considered normal, whereas levels of <0.35 µmol/L and 0.35 to 0.7 µmol/L are considered being “deficient” and “low,” respectively.10,24
Data Collection and Study Outcomes
The primary outcome was a composite of mortality or Type 1 ROP, bronchopulmonary dysplasia (BPD), serum VA levels, signs of VA toxicity, vomiting, and increased intracranial pressure. Diagnosis of ROP was conducted by pediatric ophthalmologists. Evaluations of ROP were adjudged as follows: none, immature or mature vascularization; mild ROP; Type 1 ROP (Zone I, any stage ROP with plus disease; Zone I, Stage 3 ROP without plus disease; or Zone II, Stage 2 or 3 ROP with plus disease)25; Type 2 ROP (Zone I, Stage 1 or 2, ROP without plus disease or Zone II, Stage 3 ROP without plus disease)25; treatment-requiring ROP was Type 1 ROP.25 Bronchopulmonary dysplasia was considered to be the need for oxygen therapy at 36 weeks of the PMA.26 The targets of SpO2 saturation parameter were 90% to 95%.27 Sepsis was defined on the basis of a positive blood culture and was treated with antibiotics for ≥5 days.28
Other secondary outcomes were as follows: mortality, ROP, sepsis, necrotizing enterocolitis > Stage 2 according to Bell's criteria29; severe intraventricular hemorrhage ≥Grade 330; and periventricular leukomalacia diagnosed by radiologists using cranial ultrasonography.31
Statistical Analyses
From the sample size of 254 (127 patients per group), we estimated achieving a 80% power to detect at least a 30% relative reduction in the incidence of ROP in the VA group when compared with the control group, a two-sided significance level of 0.05. Data from the study were analyzed on an intention-to-treat basis.
The SPSS software version 19.0 (SPSS, Chicago, IL) was used for statistical analysis and data management. The primary outcomes, secondary outcomes, infant, and maternal characteristics were summarized using descriptive methods and compared using the chi-square test for categorical variables and the t-test for continuous variables. A multivariate logistic regression analysis model was performed to adjust the potential confounding factors for Type 1 ROP. The model was adjusted for gestation, birth weight, duration of intubation, sepsis, BPD, necrotizing enterocolitis, and intraventricular hemorrhage. Based on a multiple logistic regression model, it was adjusted for BPD to determine adjusted odds ratio of the VA group versus control group.
Results
Baseline Characteristics
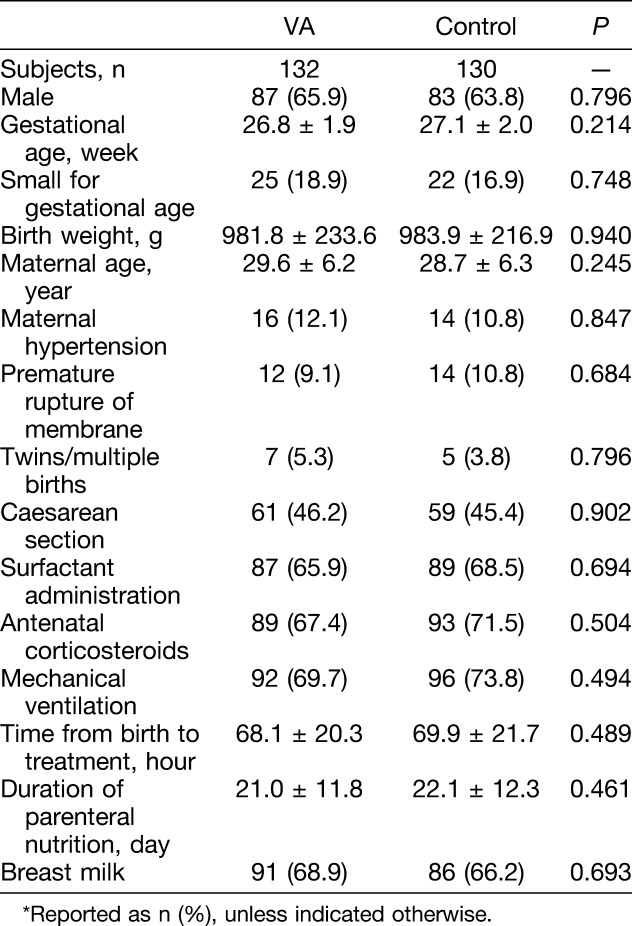
Two hundred sixty-two extremely preterm infants were randomly assigned (Figure 1). Baseline characteristics of the infants in the VA-supplemented and control groups were similar, including birth weight and gestational age (Table 1).
Fig. 1.

Study flow: Schematic flowchart that shows the numbers of infants who were screened for eligibility, randomly assigned to VA or placebo groups and followed up to 45 weeks of the corrected age. Lost to follow-up means that contact with the family was lost during the follow-up period. NEC, necrotizing enterocolitis.
Table 1.
Baseline Characteristics of All Infants Enrolled in the Study*
Safety Analysis
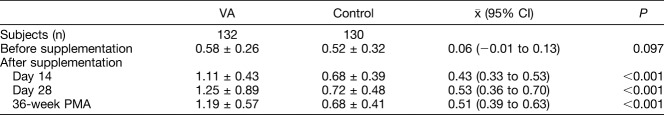
Baseline serum VA (retinol) levels of the VA group and of the control group were similar (Table 2). The serum VA of the VA-supplemented infants on Days 14, 28, and postmenstrual 36 weeks (1.11 ± 0.43, 1.25 ± 0.89, and 1.19 ± 0.57 µmol/L, respectively) was higher than that of the placebo group (0.68 ± 0.39, 0.72 ± 0.48, and 0.68 ± 0.41 µmol/L, all P < 0.001, respectively). No infant in the VA-supplemented group developed hypervitaminosis A (serum VA >2.56 µmol/L). None of the following adverse side effects were observed in either the VA-supplemented group or control group: vomiting, increased intracranial pressure, erythema, liver enlargement, bone local swelling, or bone cortical hyperplasia.
Table 2.
Serum VA Levels of Infants Before and During Supplementation, µmol/L
Study Outcomes
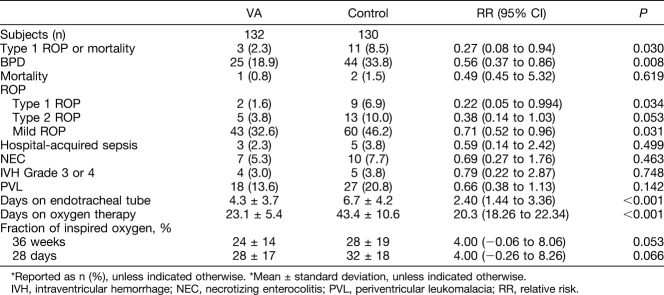
Vitamin A did not impact the mortality rates, which were similar between the VA and control groups (Table 3). The incidence of Type 1 ROP was lower in the VA group. Type 1 ROP occurred in 11 of 262 infants (4.2%), whereas 9 control patients (6.9%) required intervention compared with 2 patients (1.6) from the VA-supplemented group (P = 0.034). A significantly greater percentage of the control group patient required (Type 1) ROP treatment than did the VA-supplemented group, and the same was true for mild ROP. The risk of the composite outcome Type 1 ROP or mortality was significantly lower in the VA group than in the control group. A similar pattern emerged for patients with Type 2 ROP (Table 3). Stages of ROP are shown in Figure 2.
Table 3.
Retinopathy of Prematurity and Other Outcomes of All Study Infants*
Fig. 2.
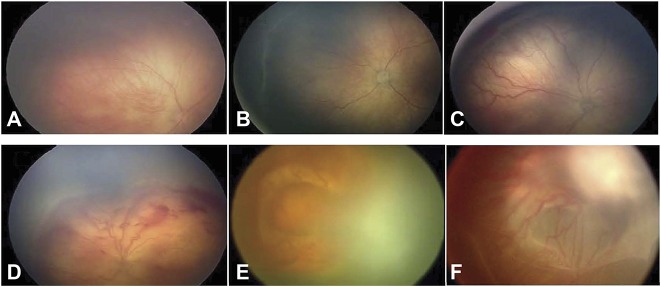
Stages of ROP: (A) Stage 1 of ROP; (B) Stage 2 of ROP; (C) Stage 3 of ROP; (D) Stage 4A of ROP; (E) Stage 4B of ROP; and (F) Stage 5 of ROP.
There were more patients in the control group that developed BPD compared with the VA-supplemented group (VA 18.9 vs. control 33.8%, P = 0.008).
Compared with the control group, the VA-supplemented group required fewer days of intubation and fewer days on oxygen therapy (P < 0.001, respectively). Length of hospital stay was shorter in the VA-supplemented group, 30.1 ± 6.3 days, compared with the control group, 64.2 ± 7.5 days (P < 0.001) (Table 3).
The VA-supplemented and control groups showed no influence in the following outcomes: hospital-acquired sepsis, necrotizing enterocolitis, intraventricular hemorrhage Grade 3 or 4, and periventricular leukomalacia.
Multivariate Analysis for Type 1 Retinopathy of Prematurity Outcome
The multivariate logistic regression analysis conducted to adjust potential confounding factors for Type 1 ROP, revealed that oral VA as compared to control decreased the chance of Type 1 ROP occurrence, with an unadjusted coefficient of −1.58 (odds ratio, 0.21; 95% confidence interval [CI], 0.04–0.98) and an adjusted coefficient of −2.37 (odds ratio, 0.09; 95% CI, 0.01–0.91). Low birth weight was a risk factor for ROP (odds ratio, 0.99; 95% CI, 0.98–1.00) (Table 4).
Table 4.
Logistic Regression Analysis of Risk Factors for Adverse Type 1 ROP Outcomes
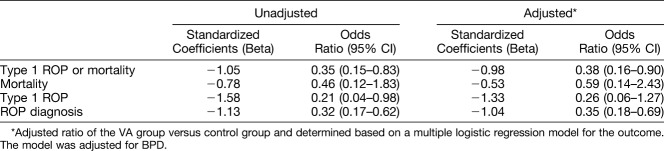
The VA group was compared with the control group and, based on the multiple logistic regression model, was adjusted for BPD. The analysis revealed that VA supplementation decreased the rate of Type 1 ROP or mortality and ROP diagnosis. For Type 1 ROP, the odds ratio was 0.26 (0.06–1.27) after adjusting for BPD (Table 5).
Table 5.
Adjusted Odds Ratios of ROP Outcomes
Discussion
This prospective multicenter study investigated the viability of early VA supplementation for the improvement of ROP outcomes in extremely preterm infants. Infants with a gestational age of less than 28 weeks received a VA supplement or placebo (soybean oil) daily from Day 4 after birth until 28 days of life or discharge. Serum VA levels of the two groups were comparable at baseline, but serum VA of the VA-supplemented group was significantly higher than the control group after in taking the VA supplement.15,32–35 Rates of both mild and severe ROP development were much lower in the VA-supplemented infants compared with the placebo15,33 and more control group patients developed BPD.
Vitamin A is required in the fetal lung for both cellular differentiation and surfactant synthesis.36 In rats, a significant storage of VA in the lungs occurs in the third trimester. These deposits are rapidly depleted during late pregnancy and the early weeks of postnatal life, as the lungs grow and develop.37,38 Vitamin A and steroid hormones have similar effects on prenatal and postnatal lung development, operate through similar cell receptors, and may be interdependent.36 The pathological changes of chronic lung disease are similar to those observed in VA-deficient experimental animals.39 Plasma retinol concentrations were lower9,40 and hepatic deposits fewer41,42 in preterm infants who developed BPD, supporting the hypothesis that VA deficiency contributes to the development of chronic lung disease and/or respiratory tract infections in this type of population.43 Several other reports showed no relationship between VA supplementation in extremely low birth weight infants and the incidence of chronic lung disease.32–34 There is an ongoing trial that aims at evaluating if the early postnatal, additional high-dose enteral VA supplementation for 28 days in extremely low birth weight infants is more efficient in reducing BPD or death than a placebo treatment.44
In this study, supplementation with VA in extremely preterm infants was associated with trends toward a reduced risk of BPD, and decreased the need for oxygen therapy, with similar results reported in other studies.45,46 There are, however, no data on VA supplementation for extremely preterm infants in China. This study showed a lower percentage of infants in the VA-supplemented group requiring oxygen at the PMA of 36 weeks, compared with the control group. In addition, the duration of intubation, oxygen therapy, and length of hospital stay were significantly shorter among the VA-supplemented infants. Supplemental VA improves the respiratory outcome in preterm infants,47 and there is evidence that supplemental VA may also protect against the development of ROP,48 suggesting that higher doses of VA than those associated with an improved respiratory outcome in preterm infants may have a beneficial effect on the incidence of ROP.10
Vascular endothelial growth factor plays an important role in the pathogenesis of ROP,49 and destructive neovascularization in ROP may be prevented by inhibition of VEGF expression in the neovascular phase.4 There is evidence that retinoids exert a highly potent antiangiogenic activity by inhibiting VEGF expression.4 In a Wistar albino rat model, retinoic acid treatment may prevent neovascularization resulting from oxygen-induced retinopathy, by downregulating VEGF expression.4 Thus, retinoic acid treatment may be a safe choice in the clinical practice for ROP prevention.4 Systemic administration of retinoic acid regulates the retinal VEGF expression and supports the retinal vascular development in an oxygen-induced ROP mouse model. It is proposed that systemic administration of retinoic acid to infants with an extremely low birth weight during oxygen therapy may potentially be an effective therapeutic approach for the prevention of ROP.50 The possible mechanism of an improved ROP outcome is conducted by reducing the rate of BPD10 and combined by influencing the VEGF pathway.4
Intramuscular VA supplementation decreases the incidence of BPD in very low birth weight infants48 and may have a beneficial effect on the incidence of ROP. However, the practice of intramuscular VA supplementation is not widely accepted because of the discomfort and risk of trauma associated with repeated intramuscular injections.51 European guidelines on VA supplementation recommend a daily dose of 1,000 to 3,300 IU/kg body weight in preterm infants.20 Therefore, this study selected a median dose to assess the efficacy and safety of oral early VA supplementation for the prevention of ROP in extremely preterm infants. The results of this study support oral VA supplementation with increased serum VA concentrations in this susceptible population. In the control group, 30% of control group infants had a VA concentration of <0.35 µmol/L, considered a VA deficient, compared with 6% in the VA-supplemented group. No major side effects were noted in the VA-supplemented group.
We used the multiple logistic regression analysis for the outcome of ROP to adjust for potential confounding factors. From the model adjusted for gestation, birth weight, duration of intubation, sepsis, BPD, necrotizing enterocolitis, and intraventricular hemorrhage, a low birth weight was associated with ROP and VA supplementation decreased the chance of Type 1 ROP occurrence in extremely preterm infants. From the model adjusted for BPD, VA supplementation was a protecting factor for Type 1 ROP or mortality and ROP diagnosis. For the Type 1 ROP, coefficients decreased after adjusting for BPD.
This study had a few limitations; first, a sex ratio imbalance with more male than female infants, similar to other reported results with Chinese populations, is observed.52 Some reports suggested that male preterm infants have higher morbidity and mortality rates than female preterm infants.53 Second, there may be important characteristics unaccounted for in our model such as clinical changes, shortage of parenteral nutrition, or the nonadministration of intravenous multivitamins, which may result in a significant BPD outcome change. In addition, the level of detail in the data set did not allow for a physiologic definition of BPD,54 which has been shown to reduce variability in reporting this outcome, resulting in a positive effect regarding BPD in the VA group. There were only three hospitals involved in this study. The sample size was not large enough, and bias for the BPD results may exist. So, multicenter, larger-sample studies are needed to further explore the effectiveness of oral VA supplementation.
Conclusions
Vitamin A supplementation increased serum VA concentration in extremely preterm infants, shortened the duration of intubation and oxygen therapy, and reduced the rate of Type 1 ROP.
Acknowledgments
The authors thank the parents for allowing their extremely preterm infants to take part in this study; and the Research Centers of the Children's Hospital affiliated to Zhengzhou University and of Nanjing Medical University, and Shangqiu People's Hospital No 1 for their assistance.
Footnotes
Supported by the Department of Health and Family Planning Commission of Henan Province (201403260).
None of the authors has any conflicting interests to disclose.
H. Sun, R. Cheng, and Z. Wang contributed equally to this work. Concept and study design: H. Sun. Data acquisition and analysis: all authors. Drafting the manuscript and the figures: H. Sun. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Clinical Trial Registration: ClinicalTrials.gov NCT 03154723.
References
- 1.Cavallaro G, Filippi L, Bagnoli P, et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol 2014;92:2–20. [DOI] [PubMed] [Google Scholar]
- 2.VanderVeen DK, Melia M, Yang MB, et al. Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity: a report by the American Academy of Ophthalmology. Ophthalmology 2017;124:619–633. [DOI] [PubMed] [Google Scholar]
- 3.Zhu D, Chen C, Shi W. Variations of vascular endothelial growth factor and pigment epithelial-derived factor are related to retinopathy of prematurity in human babies. West Indian Med J 2015;65:251–255. [DOI] [PubMed] [Google Scholar]
- 4.Ozkan H, Duman N, Kumral A, et al. Inhibition of vascular endothelial growth factor-induced retinal neovascularization by retinoic acid in experimental retinopathy of prematurity. Physiol Res 2006;55:267–275. [DOI] [PubMed] [Google Scholar]
- 5.Good WV. Bevacizumab for retinopathy of prematurity: treatment when pathology is embedded in a normally developing vascular system. Ophthalmology 2016;123:1843–1844. [DOI] [PubMed] [Google Scholar]
- 6.Quinn GE, Darlow BA. Concerns for development after bevacizumab treatment of ROP. Pediatrics 2016;137:e20160057. [DOI] [PubMed] [Google Scholar]
- 7.Wu WC, Lien R, Liao PJ, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol 2015;133:391–397. [DOI] [PubMed] [Google Scholar]
- 8.Morin J, Luu TM, Superstein R, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics 2016;137:e20153218. [DOI] [PubMed] [Google Scholar]
- 9.Kiatchoosakun P, Jirapradittha J, Panthongviriyakul MC, et al. Vitamin A supplementation for prevention of bronchopulmonary dysplasia in very-low-birth-weight premature Thai infants: a randomized trial. J Med Assoc Thai 2014;97:S82–S88. [PubMed] [Google Scholar]
- 10.Mactier H, Weaver LT. Vitamin A and preterm infants: what we know, what we don't know, and what we need to know. Arch Dis Child Fetal Neonatal Ed 2005;90:F103–F108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bental YA, Rotschild A, Cooper PA. Vitamin A supplementation for extremely-low-birth-weight infants. N Engl J Med 1999;341:1697; author reply 8. [DOI] [PubMed] [Google Scholar]
- 12.Woodruff CW, Latham CB, Mactier H, Hewett JE. Vitamin A status of preterm infants: correlation between plasma retinol concentration and retinol dose response. Am J Clin Nutr 1987;46:985–988. [DOI] [PubMed] [Google Scholar]
- 13.Shenai JP, Chytil F, Stahlman MT. Liver vitamin A reserves of very low birth weight neonates. Pediatr Res 1985;19:892–893. [DOI] [PubMed] [Google Scholar]
- 14.Tammela O, Aitola M, Ikonen S. Cord blood concentrations of vitamin A in preterm infants. Early Hum Dev 1999;56:39–47. [DOI] [PubMed] [Google Scholar]
- 15.Mactier H, McCulloch DL, Hamilton R, et al. Vitamin A supplementation improves retinal function in infants at risk of retinopathy of prematurity. J Pediatr 2012;160:954–959.e1. [DOI] [PubMed] [Google Scholar]
- 16.Liu T, Wen R, Lam BL, et al. Depth-resolved rhodopsin molecular contrast imaging for functional assessment of photoreceptors. Sci Rep 2015;5:13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer WJ, Pearring JN, Salinas RY, et al. Progressive rod-cone degeneration (PRCD) protein requires N-terminal S-acylation and rhodopsin binding for photoreceptor outer segment localization and maintaining intracellular stability. Biochemistry 2016;55:5028–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shenai JP, Kennedy KA, Chytil F, Stahlman MT. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J Pediatr 1987;111:269–277. [DOI] [PubMed] [Google Scholar]
- 19.Landman J, Sive A, Heese HD, et al. Comparison of enteral and intramuscular vitamin A supplementation in preterm infants. Early Hum Dev 1992;30:163–170. [DOI] [PubMed] [Google Scholar]
- 20.Agostoni C, Buonocore G, Carnielli VP, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 2010;50:85–91. [DOI] [PubMed] [Google Scholar]
- 21.Neu N, Duchon J, Zachariah P. TORCH infections. Clin Perinatol 2015;42:77–103. [DOI] [PubMed] [Google Scholar]
- 22.Zhu L, Shi WJ, Zhang SL, et al. Evaluation of risk factors for retinopathy of prematurity [in Chinese]. Zhonghua Yi Xue Za Zhi 2011;91:1749–1752. [PubMed] [Google Scholar]
- 23.The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991–999. [DOI] [PubMed] [Google Scholar]
- 24.Shenai JP, Rush MG, Stahlman MT, Chytil F. Plasma retinol-binding protein response to vitamin A administration in infants susceptible to bronchopulmonary dysplasia. J Pediatr 1990;116:607–614. [DOI] [PubMed] [Google Scholar]
- 25.Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121:1684–1694. [DOI] [PubMed] [Google Scholar]
- 26.Stark A, Dammann C, Nielsen HC, Volpe MV. A pathogenic relationship of bronchopulmonary dysplasia and retinopathy of prematurity? A review of angiogenic mediators in both diseases. Front Pediatr 2018;6:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology 2014;105:55–63. [DOI] [PubMed] [Google Scholar]
- 28.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed 2018;103:F182–F189. [DOI] [PubMed] [Google Scholar]
- 30.Calisici E, Eras Z, Oncel MY, et al. Neurodevelopmental outcomes of premature infants with severe intraventricular hemorrhage. J Matern Fetal Neonatal Med 2015;28:2115–2120. [DOI] [PubMed] [Google Scholar]
- 31.Imamura T, Ariga H, Kaneko M, et al. Neurodevelopmental outcomes of children with periventricular leukomalacia. Pediatr Neonatol 2013;54:367–372. [DOI] [PubMed] [Google Scholar]
- 32.Pearson E, Bose C, Snidow T, et al. Trial of vitamin A supplementation in very low birth weight infants at risk for bronchopulmonary dysplasia. J Pediatr 1992;121:420–427. [DOI] [PubMed] [Google Scholar]
- 33.Uberos J, Miras-Baldo M, Jerez-Calero A, Narbona-López E. Effectiveness of vitamin A in the prevention of complications of prematurity. Pediatr Neonatol 2014;55:358–362. [DOI] [PubMed] [Google Scholar]
- 34.Wardle SP, Hughes A, Chen S, Shaw NJ. Randomised controlled trial of oral vitamin A supplementation in preterm infants to prevent chronic lung disease. Arch Dis Child Fetal Neonatal Ed 2001;84:F9–F13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longardt AC, Schmiedchen B, Raila J, et al. Characterization of the vitamin A transport in preterm infants after repeated high-dose vitamin A injections. Eur J Clin Nutr 2014;68:1300–1304. [DOI] [PubMed] [Google Scholar]
- 36.Biesalski HK, Nohr D. Importance of vitamin-A for lung function and development. Mol Aspects Med 2003;24:431–440. [DOI] [PubMed] [Google Scholar]
- 37.Ross AC, Li NQ, Wu L. The components of VARA, a nutrient-metabolite combination of vitamin A and retinoic acid, act efficiently together and separately to increase retinyl esters in the lungs of neonatal rats. J Nutr 2006;136:2803–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shenai JP, Chytil F. Vitamin A storage in lungs during perinatal development in the rat. Biol Neonate 1990;57:126–132. [DOI] [PubMed] [Google Scholar]
- 39.Timoneda J, Rodríguez-Fernández L, Zaragozá R, et al. Vitamin A deficiency and the lung. Nutrients 2018;10:1132–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inder TE, Graham PJ, Winterbourn CC, et al. Plasma vitamin A levels in the very low birthweight infant—relationship to respiratory outcome. Early Hum Dev 1998;52:155–168. [DOI] [PubMed] [Google Scholar]
- 41.Zachman RD, Samuels DP, Brand JM, et al. Use of the intramuscular relative-dose-response test to predict bronchopulmonary dysplasia in premature infants. Am J Clin Nutr 1996;63:123–129. [DOI] [PubMed] [Google Scholar]
- 42.Shenai JP, Rush MG, Parker RA, Chytil F. Sequential evaluation of plasma retinol-binding protein response to vitamin A administration in very-low-birth-weight neonates. Biochem Mol Med 1995;54:67–74. [DOI] [PubMed] [Google Scholar]
- 43.Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 2003;8:63–71. [DOI] [PubMed] [Google Scholar]
- 44.Meyer S, Gortner L. Early postnatal additional high-dose oral vitamin A supplementation versus placebo for 28 days for preventing bronchopulmonary dysplasia or death in extremely low birth weight infants. Neonatology 2014;105:182–188. [DOI] [PubMed] [Google Scholar]
- 45.Londhe VA, Nolen TL, Das A, et al. Vitamin A supplementation in extremely low-birth-weight infants: subgroup analysis in small-for-gestational-age infants. Am J Perinatol 2013;30:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darlow BA, Graham PJ, Rojas-Reyes MX. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst Rev 2016;8:CD000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Araki S, Kato S, Namba F, Ota E. Vitamin A to prevent bronchopulmonary dysplasia in extremely low birth weight infants: a systematic review and meta-analysis. PLoS One 2018;13:e0207730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birthweight infants. Cochrane Database Syst Rev 2011;7:Cd000501. [DOI] [PubMed] [Google Scholar]
- 49.Kwinta P, Bik-Multanowski M, Mitkowska Z, et al. The clinical role of vascular endothelial growth factor (VEGF) system in the pathogenesis of retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2008;246:1467–1475. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Shi P, Xu Z, et al. Up-regulation of VEGF by retinoic acid during hyperoxia prevents retinal neovascularization and retinopathy. Invest Ophthalmol Vis Sci 2014;55:4276–4287. [DOI] [PubMed] [Google Scholar]
- 51.Ambalavanan N, Kennedy K, Tyson J, Carlo WA. Survey of vitamin A supplementation for extremely-low-birth-weight infants: is clinical practice consistent with the evidence? J Pediatr 2004;145:304–307. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Gao X, Liu C, et al. Morbidity and mortality of neonatal respiratory failure in China: surfactant treatment in very immature infants. Pediatrics 2012;129:e731–e740. [DOI] [PubMed] [Google Scholar]
- 53.Binet ME, Bujold E, Lefebvre F, et al. Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol 2012;29:159–166. [DOI] [PubMed] [Google Scholar]
- 54.Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114:1305–1311. [DOI] [PubMed] [Google Scholar]