Abstract
Background & Aims
Practices dramatically reduced endoscopy services due to the COVID-19 pandemic. Because practices now are considering reintroduction of elective endoscopy, we conducted a survey of North American practices to identify reactivation barriers and strategies.
Methods
We designed and electronically distributed a web-based survey to North American gastroenterologists consisting of 7 domains: institutional demographics, impact of COVID-19 on endoscopy practice, elective endoscopy resumption plans, anesthesia modifications, personal protective equipment policies, fellowship training, and telemedicine use. Responses were stratified by practice type: ambulatory surgery center (ASC) or hospital-based.
Results
In total, 123 practices (55% ASC-based and 45% hospital-based) responded. At the pandemic’s peak (as reported by the respondents), practices saw a 90% decrease in endoscopy volume, with most centers planning to resume elective endoscopy a median of 55 days after initial restrictions. Declining community prevalence of COVID-19, personal protective equipment availability, and preprocedure severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing availability were ranked as the 3 primary factors influencing reactivation timing. ASC-based practices were more likely to identify preprocedure testing availability as a major factor limiting elective endoscopy resumption (P = .001). Preprocedure SARS-CoV-2 testing was planned by only 49.2% of practices overall; when testing is performed and negative, 52.9% of practices will continue to use N95 masks.
Conclusions
This survey highlights barriers and variable strategies for reactivation of elective endoscopy services after the COVID-19 pandemic. Our results suggest that more widespread access to preprocedure SARS-CoV-2 tests with superior performance characteristics is needed to increase provider and patient comfort in proceeding with elective endoscopy.
Keywords: COVID-19, Endoscopy Operations, Personal Protective Equipment, Safety
Abbreviations used in this paper: ASC, ambulatory surgery center; IQR, interquartile range; PCR, polymerase chain reaction; POC, point-of-care; PPE, personal protective equipment; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
What You Need to Know.
Background
Endoscopy services were reduced markedly because of the COVID-19 pandemic. Although practices now are developing plans to safely reintroduce elective endoscopy, barriers and strategies have not been described.
Findings
Only half of North American practices anticipate preprocedure severe acute respiratory syndrome coronavirus 2 testing; when testing is performed and is negative, approximately 50% of practices will continue to use N95 masks for personal protective equipment.
Implications for patient care
There is significant variability in preprocedure testing and personal protective equipment use in North America, suggesting a need for more widespread availability of tests with superior performance characteristics.
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) throughout North America has massively disrupted the health care system. Because of the high transmissibility and virulence of SARS-CoV-2, hospitals shifted all available resources to preparing for and managing patients with COVID-19. To both protect personnel and preserve the personal protective equipment (PPE) needed to manage the population of patients suffering from COVID-19, most centers stopped performing elective ambulatory procedures during the pandemic.1 Given that a significant volume of endoscopic procedures revolves around disease prevention and screening (and thus are elective), the practice of gastroenterology was impacted dramatically.
In a previous survey, the North American Alliance for the Study of Digestive Manifestations of COVID-19 found that two thirds of practices were performing less than 10% of their usual endoscopic volumes during the pandemic.2 However, endoscopy is essential to the prevention, treatment, and palliation of gastrointestinal illness. This creates a competing need to resume usual clinical operations while ensuring patient and provider safety.
Despite a universal desire to return to usual endoscopic and clinical care, there is a paucity of guidance on how practices should plan to reintroduce elective endoscopy safely. Thus, we conducted a survey of North American gastroenterology groups to assess current and anticipated approaches to recovering from the COVID-19 pandemic.
Methods
Study Design
This was a survey of North American practices conducted between April 24, 2020, and May 8, 2020. At the institutional level this was a cross-sectional analysis, however, given that institutions responded at various points in time during the survey period, this was not a cross-sectional study in aggregate. This study was a web-based survey and was exempt from institutional review board approval. The survey was conducted via the RedCap data capture platform hosted by Washington University School of Medicine (St Louis, MO). The survey was distributed to potential respondents via a web link, without requiring log-in credentials. Survey respondents were asked to confer with institutional or group leaders before filling out the survey. If multiple responses were completed from a single institution, the investigators directly corresponded with respondents to reconcile any inconsistencies.
Survey Development, Validation, and Beta Testing
The initial survey was developed through electronic communication by 4 investigators (V.M.K., Z.L.S., B.J.E., and R.N.K.) and then beta-tested by the remaining authors to establish face and content validity. The survey was modified after this beta testing to improve ease of administration and clarity.
Survey Distribution
Given the rapidly evolving nature of the COVID-19 pandemic, and the need to ensure timely and widespread circulation of the survey, a multifaceted approach to dissemination was used. The survey was distributed via e-mail to gastroenterologists throughout the United States and Canada. The e-mail list was developed by the study team to capture maximum diversity of both geography and practice settings. In addition, all co-investigators directly contacted community and academic colleagues through e-mail, text messaging, and other personal communications to encourage survey participation. The survey also was promoted on social media platforms (Twitter [San Francisco, CA] and physician-only Facebook [Menlo Park, CA] groups). Finally, to ensure a diversity of responses, the investigators worked with a large ambulatory surgery center (ASC) management group (AMSURG, Nashville, TN) to distribute the survey directly to gastroenterology practices within their portfolio.
Survey Items
The survey consisted of 59 questions divided into 7 domains: institutional demographics, impact of COVID-19 on endoscopy practice, logistical plan for resumption of elective endoscopy, impact of COVID-19 on anesthesia services, PPE policies, fellowship training, and use of telemedicine (Appendix 1).
Response Stratification
Responses were stratified according to the primary site of endoscopy practice: hospital-based vs ASC-based. Practices performing 50% or more of their procedures in an ASC were classified as ASC-based, while others were classified as hospital-based.
Statistical Analysis
Individual item survey responses are reported as a proportion of participants completing the question. For example, the number of respondents answering “yes” to a telemedicine question was divided by the total number of responses to the question. Categoric variables were compared using the chi-squared test where appropriate. A 2-sided P value of .05 was required for statistical significance. All analyses were performed using SPSS statistics version 25.0 (IBM Corp, Armonk, NY).
Results
Practice Demographics
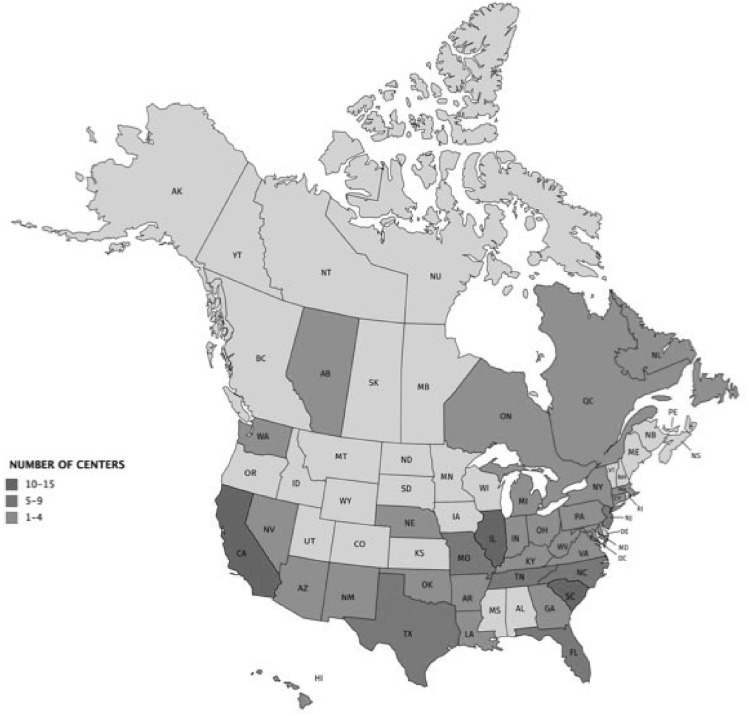
A total of 130 individuals responded to the survey. After reconciling duplicate responses from the same practices, the final sample consisted of 123 unique practices, comprising 1379 gastroenterologists in 32 US states and 4 Canadian provinces (Figure 1 ). Approximately half (50.4%) of the responses were from independent group practices, followed by academic medical center practices (28.5%), nonacademic hospital-employed practices (14.8%), and US Veteran’s Health Administration hospitals (5.7%). The median practice size was 8 physicians (interquartile range [IQR], 5–15). The majority of practices (55%) performed more than 50% of their endoscopic procedures at an ASC. A minority of the respondents (36%) represented practices involved with training gastroenterology fellows. Nearly half (48.8%) of the practice locations were described as urban, followed by suburban (37.7%) and rural (13.8%) practice settings.
Figure 1.
Geographic distribution of participating centers.
Impact of COVID-19 on Endoscopy Practice
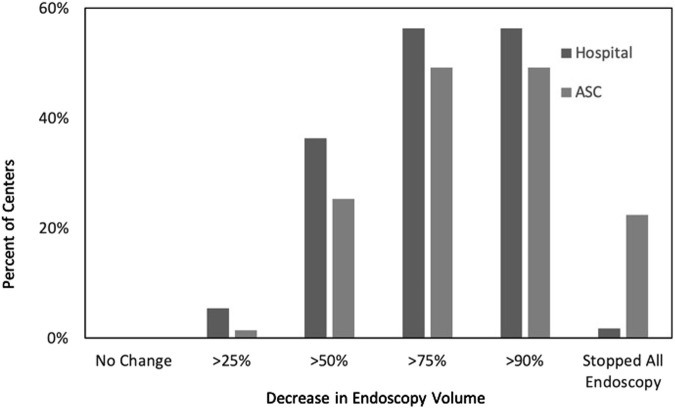
Before the COVID-19 emergency, participating centers performed a median of 150 endoscopic procedures (IQR, 100–250 endoscopic procedures) per week. Complete cessation of elective endoscopy because of the pandemic was undertaken by 98% of hospital-based and 96% of the ASC-based practices. Overall endoscopy volumes decreased markedly because of the pandemic, with practices performing approximately 10% of their usual volume during the peak of the pandemic (median, 15 procedures; IQR, 5–35 procedures). When compared with practices that primarily are hospital-based, practices that work primarily in ASCs experienced a significantly higher decrease in procedure volumes (ASC, 91% decrease; hospital based, 83%; P = .01); furthermore, ASCs were more likely to stop endoscopy completely during the peak of COVID-19 restrictions (Supplementary Figure 1). In the 44 teaching centers, gastroenterology fellows were completely removed from endoscopy in two thirds (66.6%) of programs.
Supplementary Figure 1.
Decreases in endoscopy volume. ASC, ambulatory surgery center.
Planned Resumption of Endoscopy Practice
At the time of survey completion, only 27.6% of practices had resumed elective endoscopy. Practices reported that the median planned (or actual, when elective endoscopy already had resumed) duration of halting nonemergent endoscopy was similar for ASCs (55 d; IQR, 47–62 d) and hospital-based endoscopy units (54 d; IQR, 44–63 d). Respondents were asked to identify the 3 most important factors influencing the decision of when to resume elective endoscopy. The top factors were as follows: decreasing community prevalence of COVID-19 infections (79.5%), increased availability of PPE (74.6%), and availability of preprocedure COVID-19 testing (68.9%) (Table 1 ). ASC-based centers were more likely than hospital-based centers to cite the availability of preprocedure SARS-CoV-2 testing as one of the 3 main factors limiting the resumption of elective endoscopy (56.7% vs 83.6%; P = .001).
Table 1.
Considerations in Resuming Elective Endoscopy
| Total (n = 122) | ASC (n = 67) | Hospital-based (n = 55) | P value | |
|---|---|---|---|---|
| Please select the 3 most important factors that influenced/will influence your decision to resume elective endoscopic procedures? | ||||
| Availability of COVID-19 testing | 84 (68.9%) | 38 (56.7%) | 46 (83.6%) | .001 |
| Community prevalence of COVID-19 | 97 (79.5%) | 53 (79.1%) | 44 (80%) | .9 |
| Patients advocating for resumption of endoscopy | 35 (28.7%) | 21 (31.3%) | 14 (25.5%) | .47 |
| Institutional financial considerations | 29 (23.8%) | 13 (19.4%) | 16 (29.1%) | .21 |
| Physician financial considerations | 17 (13.9%) | 10 (14.9%) | 7 (12.7%) | .7 |
| PPE availability | 91 (74.6%) | 52 (77.6%) | 39 (70.1%) | .39 |
| What do you see as barriers to increasing endoscopic procedure volume once cleared to restart operations by institution/local government? (check all that apply) | ||||
| Inadequate PPE availability | 66 (54%) | 34 (50.7%) | 32 (58.2%) | .4 |
| Limited COVID-19 testing capacity | 85 (69%) | 43 (64.2%) | 42 (76.4%) | .15 |
| Inadequate nursing/support staff | 18 (14.6%) | 5 (7.5%) | 13 (23.6%) | .012 |
| Financial constraints | 12 (9.8%) | 7 (10.4%) | 5 (9.1%) | .8 |
| Patient safety concerns | 80 (65.9%) | 43 (64.2%) | 37 (67.3%) | .72 |
| Staff safety concerns | 45 (36.6%) | 24 (35.8%) | 21 (38.2%) | .79 |
| Limited anesthesia coverage | 13 (10.6%) | 4 (6%) | 9 (16.4%) | .064 |
ASC, ambulatory surgery center; PPE, personal protective equipment.
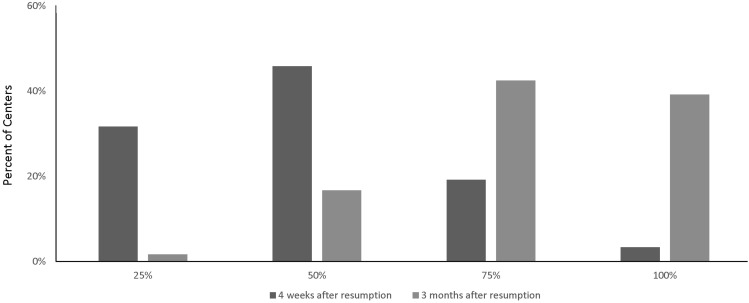
Only 3.3% of centers anticipated returning to 100% of their pre–COVID-19 endoscopy volume within 4 weeks of survey completion; most centers (77.5%) anticipated operating at approximately 25% to 49% of pre–COVID-19 volumes within 4 weeks from the time of survey response. However, 38.2% expected to return to 100% or more of their pre–COVID-19 endoscopy volumes 3 months after survey administration (ie, late July/August) (Figure 2 ). The most frequently cited barriers to increasing procedure volumes after initial resumption of elective endoscopy were limited capacity for COVID-19 testing (69%), patient reluctance to undergo elective endoscopy because of COVID-19–related safety concerns (65.9%), and limited PPE supplies (54%) (Table 1). The projected rebound in endoscopy volumes was similar for hospital-based and ASC-based practices.
Figure 2.
Anticipated recovery of endoscopy volumes after lifting of COVID-19 restrictions.
Rescheduling Procedures Deferred During COVID-19 Restrictions
Most practices anticipated using interventions to catch up on the backlog of elective procedures created by the COVID-19 pandemic. A total of 63.9% of centers planned to extend operating hours on weekdays and 56.6% centers planned to perform outpatient endoscopy on weekends (Table 2 ). ASC-based practices were more likely to offer weekend endoscopy sessions (67.2% vs 43.6%; P = .009); while hospital-based practices were more likely to offer stool-based testing in place of average-risk screening colonoscopy (25.5% vs 4.5%; P = .001) or adjust (lengthen) surveillance colonoscopy intervals based on the 2020 multisociety guidelines (21.8% vs 4.5%; P = .004).
Table 2.
Plans to Reduce Backlog of Elective Procedures After the COVID-19 Pandemic
| Total (n = 122) | ASC (n = 67) | Hospital-based (n = 55) | P value | |
|---|---|---|---|---|
| How do you plan to catch up on procedures postponed because of COVID-19 (check all that apply) | ||||
| Stool-based testing | 17 (13.9%) | 3 (4.5%) | 14 (25.5%) | .001 |
| Adapt colon cancer surveillance intervals to 2020 multisociety guidelines | 15 (12.3%) | 3 (4.5%) | 12 (21.8%) | .004 |
| Extended weekday hours of operations | 78 (63.9%) | 44 (65.7%) | 34 (61.8%) | .66 |
| Weekend endoscopy | 69 (56.6%) | 45 (67.2%) | 24 (43.6%) | .009 |
| Open additional procedure rooms | 28 (23%) | 13 (19.4%) | 15 (27.3%) | .3 |
| Hire additional endoscopy laboratory staff | 4 (3.3%) | 2 (3%) | 2 (3.6%) | .8 |
| Overbook endoscopy time slots | 16 (13.1%) | 8 (11.9%) | 8 (14.5%) | .67 |
| No defined plan | 30 (24.6%) | 16 (23.9%) | 14 (25.5%) | .84 |
ASC, ambulatory surgery center.
COVID-19 Patient and Staff Screening
The vast majority (82.8%) of practices planned to screen all patients for COVID-19 before or on arrival at the endoscopy laboratory. COVID-19 testing by real-time polymerase chain reaction (PCR), point-of-care (POC) testing, and/or serology was planned before all procedures (either on the days preceding or on the day of the procedure) in 49.2% of centers; 45% of responding centers planned to perform real-time PCR testing 2 to 3 days before endoscopy, while 8.1% anticipated offering same-day PCR or POC testing before endoscopy (Table 3 ). Only a minority (10.6%) of centers anticipated serologic testing of staff at the time of their response.
Table 3.
Planned Preprocedure Screening of Patients for COVID-19 Before Endoscopy
| Total (n = 122) | ASC (n = 67) | Hospital-based (n = 55) | P value | |
|---|---|---|---|---|
| Which of the following approaches to screen patients for COVID-19 before endoscopy are being considered? (check all that apply) | ||||
| Symptom screen before arrival for endoscopy | 76 (62.2%) | 52 (77.6%) | 34 (50.7%) | .057 |
| Symptom screen on arrival at endoscopy laboratory | 80 (65.5%) | 50 (74.6%) | 30 (54.5%) | .02 |
| Temperature check on arrival in endoscopy laboratory | 84 (68.8%) | 54 (80.6%) | 30 (54.5%) | .002 |
| COVID-19 real-time PCR testing 2–3 days before procedure | 55 (45.1%) | 25 (37.3%) | 30 (54.5%) | .057 |
| COVID-19 real-time PCR testing on day of procedure | 7 (5.7%) | 3 (4.5%) | 4 (7.3%) | .5 |
| Serologic testing before procedure | 2 (1.6%) | 1 (1.5%) | 1 (1.8%) | .9 |
| Point-of-care testing on day of procedure | 7 (5.7%) | 3 (4.5%) | 4 (7.3%) | .28 |
| Any COVID-19 testing (PCR, serology, and/or point of care) | 60 (49.2%) | 28 (41.8%) | 32 (58.2%) | .07 |
ASC, ambulatory surgery center; PCR, polymerase chain reaction.
Hospital-based practices were more likely to plan COVID-19 testing (real-time PCR, POC testing, and/or serology) before all procedures than ASC-based practices, although this did not reach significance (58.2% vs 41.8%; P = .07). Conversely, more ASC-based practices than hospital-based practices are planning to screen for symptoms of COVID-19 (74.6% vs 54.5%; P = .02) and perform temperature checks (80.6% vs 54.5%; P = .002) on arrival at the endoscopy laboratory. More ASC-based centers plan to routinely screen staff for COVID-19 symptoms (71.6% vs 50.1%; P = .013).
Physical Distancing in Endoscopy Laboratory
Two thirds of centers (67.5%) planned to increase the time allotted for procedures to allow for the anticipated increase in room turnaround time. To allow for physical distancing in the endoscopy unit, the most frequently anticipated changes were as follows: not allowing anyone except for the patient in the endoscopy center (87%), using only every other preprocedure/postprocedure bay (41.8%), and assigning dedicated work stations to each staff member (37.4%) (Table 4 ). There were no significant differences between ASC- and hospital-based centers with regard to anticipated use of physical distancing measures.
Table 4.
Planned Physical Distancing Measures in Endoscopy Laboratory
| Total (n = 122) | ASC (n = 67) | Hospital-based (n = 55) | P value | |
|---|---|---|---|---|
| What social distancing measures will you be implementing/have you implemented in your endoscopy unit? (check all that apply) | ||||
| Allow only patients into the endoscopy center | 107 (87.7%) | 62 (92.5%) | 45 (81.8%) | .073 |
| Use every other preprocedure/postprocedure bay | 51 (41.8%) | 33 (49.3%) | 18 (32.7%) | .66 |
| Assign dedicated nurse to each patient from admission to discharge | 30 (24.9%) | 19 (28.4%) | 11 (20%) | .29 |
| Cohort endoscopy laboratory staff | 43 (35.2%) | 23 (34.3%) | 20 (36.6%) | .82 |
| Limit trainee involvement | 31 (25.4%) | 9 (13.4%) | 22 (40%) | .001 |
| Assign dedicated work stations to each staff member | 46 (37.7%) | 30 (44.8%) | 16 (29.1%) | .075 |
ASC, ambulatory surgery center.
Changes in Airway Management During Endoscopic Procedures
Alterations in airway management (from conventional nasal cannula oxygen delivery) for upper-gastrointestinal endoscopic procedures (esophagogastroduodenoscopy, enteroscopy, endoscopic ultrasound, endoscopic retrograde cholangiopancreatography) were being used/anticipated in 47.2% of centers. In 7 practices (5.8%), general endotracheal anesthesia was being mandated for all upper-endoscopy procedures. Although this rate did not differ between hospital-based vs ASC-based practices, teaching centers were more likely than nonteaching centers to use general endotracheal anesthesia for all upper gastrointestinal endoscopy procedures (13% vs 1.2%; P < .001). In the remaining practices, anesthesia planned to use oxygen delivery masks and/or mechanical barriers to reduce exposure risk via aerosolization. Overall, there was no significant difference in airway management between ASC-based and hospital-based groups.
Personal Protective Equipment
With the resumption of elective endoscopy, universal use of surgical masks in common areas will be required for staff in 94.2% of practices and for patients in 82.6%. For asymptomatic patients who undergo a negative COVID-19 test before the procedure, nearly half (45.5%) of centers are (or anticipate) recommending the use of surgical masks by health care workers during endoscopic procedures; in contrast, 52.9% will continue the use of N95 respirators after negative testing. For those situations where routine COVID-19 preprocedure testing for asymptomatic patients is not being performed, 71.5 % are recommending use of N95 masks, with 20.3% using surgical masks. With regard to eye protection, 97.5% centers required eye protection for all procedures, with full face shields being used in 73.2%. There was no significant difference in planned PPE use between hospital-based and ASC-based centers.
Use of Telemedicine
Before the COVID-19 pandemic, 19% of practices were using telemedicine in some fashion, with hospital-based practices more likely to use telemedicine compared with ASC-based centers (27.2% vs 12.1%; P = .03). This increased to nearly all practices using telemedicine during the pandemic (99.2%). After lifting COVID-19 restrictions, 85.8% of centers anticipate continuing to use telemedicine. Again, hospital-based practices are more likely than ASC-based practices to plan on continued use of telemedicine (92% vs 80%; P = .046) after the pandemic. Most practices (72.3%) were using video calls (vs telephone calls) for the majority of their telemedicine visits.
Discussion
Resumption of elective endoscopy requires a complex, multifaceted approach wherein practices implement systematic SARS-CoV-2 testing, optimize the inventory of PPE, and maintain appropriate physical distancing. Thus, we hypothesized that there would be significant variability in the approach to reactivating elective endoscopy in North America. Indeed, in this survey of 123 North American practices representing 1379 gastroenterologists, we found many barriers to resuming elective endoscopy over the coming months, as well as areas of significant variability in reactivation protocols.
Gastrointestinal endoscopy is a primary modality for the early detection of colorectal, gastroesophageal, and pancreatic disease. Modeling suggests that the prolonged cessation of endoscopic services will result in a significant increase in advanced malignancy3 , 4 and uncontrolled gastrointestinal disease. We found that during the peak of the COVID-19 pandemic, practices were operating at approximately 10% of typical volume. Thus, it is essential for endoscopic practices to increase delivery of endoscopic services while ensuring provider and patient safety. Although most practices expected to restart elective endoscopy after a delay of approximately 8 weeks, only 40% expected re-establishing their usual volumes of endoscopy within the next 3 months. The most common barriers to resuming elective endoscopic services are related to 2 interlocking concerns: the limited capacity for SARS-CoV-2 testing and the parallel concern that patients themselves may be unwilling to return for elective care. In total, this prolonged reduction in elective endoscopy volume will create a backlog of patients, which may negatively impact patient care, unless novel strategies are identified and implemented quickly.
Regarding screening patients before gastrointestinal endoscopy, numerous strategies have been proposed including symptom assessment and/or real-time PCR testing before arrival to the endoscopy center or clinic, or symptom screening and/or testing on arrival.5 We found great variability between endoscopy practices regarding anticipated preprocedure screening protocols. There has been increasing guidance favoring routine screening, generally via real-time PCR, before any endoscopic procedure.6 , 7 The rationale for this preprocedure testing is that any patient who tests positive can have their procedure delayed, reducing exposure to staff and other patients. We found that routine testing by real-time PCR or serology was planned in only 49.2% of practices, with a suggestion of greater use in hospital-based practices. Overall, these data show that there is a need for increased standardization of preprocedure SARS-CoV-2 testing.
There has been extensive international attention regarding optimal PPE strategies for endoscopic procedures and the associated shortage of adequate supplies.8 Thus, despite existing guidelines, we suspected that significant variability in PPE use persists nationally. In fact, we found that practices differed markedly in the type of PPE used for patients who tested negative for SARS-CoV-2. Although recent guidance has suggested that surgical masks can be used in this setting,9 a significant false-negative rate remains for real-time PCR testing and concern for infection between the time of testing and the procedure. This has prompted many practices to advocate for continued N95 use despite a negative test result. We found that 53% of practices were using N95 respirators in patients who tested negative for SARS-CoV-2 whereas 45% of practices recommended use of surgical masks in this setting. We anticipate that development and adoption of POC tests with superior performance characteristics will be needed to change PPE practice patterns substantively. Again, these findings emphasize the need for standardized recommendations and clear guidance on how best to integrate SARS-CoV-2 testing into ambulatory gastroenterology practices.
There are numerous strengths to this survey. We solicited only a single survey response from each practice; thus, our 123 respondents represent the practice patterns of 1379 North American gastroenterologists. Furthermore, we distributed our survey using a variety of modalities (including personal communication) specifically to obtain a diversity of responses from a variety of practice settings; this is highlighted by the fact that the majority of respondents were from independent-group practices, yielding more generalizable findings. However, there are key limitations. The management of COVID-19 is evolving rapidly; thus, select practice patterns may evolve from the time of the survey administration to data analysis. To mitigate this risk, we left our survey open for a short response time (2 weeks). In addition, because this was a survey study, it may be susceptible to recall bias with regard to endoscopy volumes and COVID-19 disease burden. Finally, we were unable to independently assess practice pattern differences of teaching vs nonteaching institutions because these practice types correlated significantly with hospital-based and ASC-based practices, respectively.
In summary, we have shown that there are significant concerns regarding the ability of practices to quickly and safely resume elective procedures and increase endoscopy volume. This is highlighted by variability in how practices have planned for reactivation. Our results suggest that the most pressing need is ready access to preprocedure testing, which can exclude SARS-CoV-2 reliably, so that both providers and patients are comfortable resuming elective endoscopy.
CRediT Authorship Contributions
Vladimir M Kushnir, MD (Conceptualization: Lead; Formal analysis: Lead; Methodology: Equal; Project administration: Lead; Writing – original draft: Lead); Badih Joseph Elmunzer (Conceptualization: Equal; Resources: Equal; Writing – review & editing: Equal); Tyler M Berzin (Conceptualization: Supporting; Investigation: Supporting; Resources: Equal; Validation: Supporting; Writing – review & editing: Supporting); Robin B Mendelsohn (Project administration: Supporting; Writing – review & editing: Supporting); Vaishali Patel (Validation: Supporting; Writing – review & editing: Supporting); Swati Pawa (Validation: Equal; Writing – review & editing: Supporting); Zachary L Smith (Conceptualization: Equal; Resources: Equal; Writing – review & editing: Equal); Rajesh N Keswani (Conceptualization: Equal; Methodology: Lead; Resources: Equal; Writing – original draft: Lead).
Footnotes
Conflicts of interest These authors disclose the following: Vladimir M. Kushnir has consulted for Boston Scientific, and has received research support from Motus GI; B. Joseph Elmunzer has consulted for Takeda Pharmaceuticals; Zachary L. Smith has consulted for US Endoscopy; and Rajesh N. Keswani has consulted for Boston Scientific. The remaining authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.05.030.
Supplementary Data
Supplementary Material
References
- 1.Sethi A., Swaminath A., Latorre M. Donning a new approach to the practice of gastroenterology: perspectives from the COVID-19 pandemic epicenter. Clin Gastroenterol Hepatol. 2020;18:1673–1681. doi: 10.1016/j.cgh.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes N., Smith Z.L., Spitzer R.L. Changes in gastroenterology and endoscopy practices in response to the COVID-19 pandemic: results from a North American Survey. Gastroenterology. 2020 May 4 doi: 10.1053/j.gastro.2020.04.071. S0016-5085(20)30592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones D., Neal R.D., Duffy S.R.G. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21:748–750. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wise J. Covid-19: cancer mortality could rise at least 20% because of pandemic, study finds. BMJ. 2020;369:m1735. doi: 10.1136/bmj.m1735. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S., Shahidi N., Gilroy N. A proposal for the return to routine endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020 Apr 28 doi: 10.1016/j.gie.2020.04.050. S0016-5107(20)34249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-19 - elective surgical procedure guidance. 2020. https://www.dph.illinois.gov/topics-services/diseases-and-conditions/diseases-a-z-list/coronavirus/health-care-providers/elective-procedures-guidance Available from:
- 7.The ASA and APSF Joint Statement on perioperative testing for the COVID-19 virus. 2020. https://www.asahq.org/about-asa/newsroom/news-releases/2020/04/asa-and-apsf-joint-statement-on-perioperative-testing-for-the-covid-19-virus Available from:
- 8.Soetikno R., Teoh A.Y., Kaltenbach T. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020;92:176–183. doi: 10.1016/j.gie.2020.03.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joint AGA/DHPA guidance: recommendations for resumption of elective endoscopy during the COVID-19 pandemic. 2020. https://www.dhpassociation.org/2020/04/27/aga-dhpa-resume-endoscopy-covid19 Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.