See Covering the Cover synopsis on page 803.
Coronavirus Disease 2019 (COVID-19) caused 2 million cases and more than 150,000 deaths worldwide as of mid-April 2020.1 Clinical trials are under way to assess the efficacy of a variety of antiviral drugs; however, many of these drugs have toxicities and thus far no drug has been proven to improve outcomes in patients with COVID-19.
Famotidine is a histamine-2 receptor antagonist that suppresses gastric acid production. In vitro, famotidine inhibits human immunodeficiency virus replication.2 Recently, Wu et al.3 used computational methods to predict structures of proteins encoded by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome and identified famotidine as one of the drugs most likely to inhibit the 3-chymotrypsin-like protease (3CLpro), which processes proteins essential for viral replication.4 We hypothesized that famotidine would be associated with improved clinical outcomes among hospitalized patients with COVID-19. To explore this, we performed a retrospective cohort study at a single academic center located at the epicenter of the COVID-19 pandemic in the United States.
Methods
Complete methods are available in the Supplementary Materials. In brief, adults were eligible for the study if they were admitted to our institution from February 25, 2020, to April 13, 2020, and tested positive for SARS-CoV-2 within no more than 72 hours following admission. Patients were excluded if they died or were intubated within 48 hours following hospital admission. The primary exposure was use of famotidine (any dose, form of administration, or duration), classified as present if famotidine was received within 24 hours of hospital admission and otherwise as absent. The primary outcome was a composite of death or endotracheal intubation from hospital day 2 to day 30 (intubation-free survival). This follow-up period avoided immortal time bias because the exposure was classified based on the 24-hour period after hospitalization and the at-risk period began on hospital day 2. Cox proportional hazards modeling was performed on the full cohort, and a matched subset was examined with propensity scoring matching to balance baseline characteristics based on use of famotidine.
Results
Population and Use of Famotidine
A total of 1620 patients met criteria for analysis, including 84 patients (5.1%) who received famotidine within 24 hours of hospital admission. Home use of famotidine was documented on admission medication reconciliation in 15% of those who used famotidine while hospitalized compared with 1% of those who did not (P < .01). Twenty-eight percent of all famotidine doses were intravenous; 47% were 20 mg, 35% were 40 mg, and 17% were 10 mg. Famotidine users received a median 5.8 days of drug for a total median dose of 136 mg (63–233 mg). There were minimal differences comparing patients who used famotidine with those who did not, and balance between the groups was further improved after propensity score matching (Supplementary Table 1).
Death or Intubation
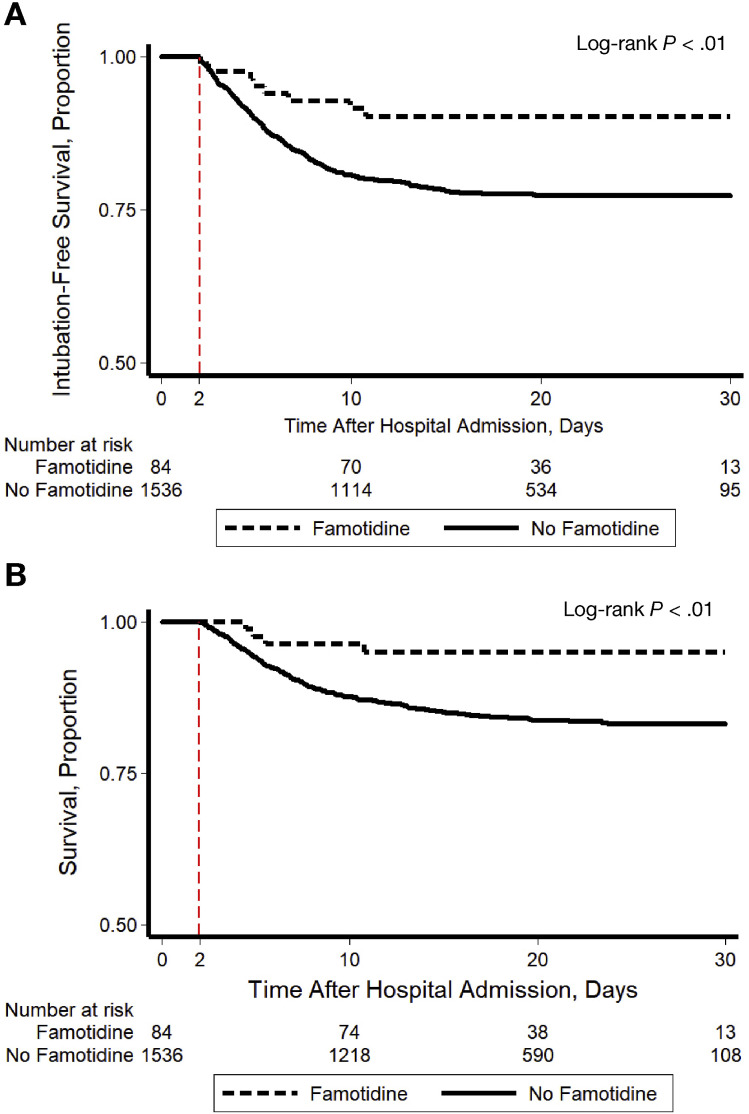
A total of 142 (8.8%) patients were intubated and 238 (15%) died; 340 (21%) patients met the composite study outcome. In crude analysis, use of famotidine was significantly associated with reduced risk for the composite outcome of death or intubation (Figure 1 A, log-rank P < .01). This association was driven primarily by the relationship between famotidine and death (Figure 1 B, log-rank P < .01) and when those who died before intubation were excluded, there was no association between use of famotidine and intubation (log-rank P = .40). After adjusting for baseline patient characteristics, use of famotidine remained independently associated with risk for death or intubation (Supplementary Table 2, adjusted hazard ratio 0.42, 95% confidence interval [CI] 0.21–0.85) and this remained unchanged after propensity score matching to further balance the covariables (hazard ratio 0.43, 95% CI 0.21–0.88).
Figure 1.
Kaplan-Meier plot showing (A) intubation-free survival and (B) survival through a maximum of 30 days after hospital admission, stratified by use of famotidine. Patients were included in the study if they survived without intubation for 2 days following hospital admission. Use of famotidine was classified as present if it was received within the first 24 hours following hospital admission (any dose, form of administration, or duration) and otherwise as absent. The at-risk time began on hospital day 2 (indicated with a dashed red line) and patients were followed until hospital day 30. This study design avoided potential for immortal time bias because the exposure was classified before the start of the at-risk period.
Additional Analyses
Use of proton pump inhibitors (PPIs) was analyzed because PPIs are also gastric acid suppression medications with similar indications as famotidine. There was a no protective effect associated with use of PPIs (adjusted hazard ratio 1.34, 95% CI 1.06–1.69). Next, 784 patients without COVID-19 who were hospitalized during the same study period were analyzed; among these patients, famotidine was not associated with reduced risk for death or intubation (24 deaths or intubations, log-rank P = .70). The maximum plasma ferritin value during the hospitalization was assessed to address the hypothesis that, by blocking viral replication, famotidine reduces cytokine storm during COVID-19. Median ferritin was 708 ng/mL (interquartile range 370–1152) among users of famotidine vs 846 ng/mL (interquartile range 406–1552) among nonusers (rank-sum P = .03).
Conclusions
This retrospective study found that, in patients hospitalized with COVID-19, famotidine use was associated with a reduced risk of clinical deterioration leading to intubation or death. The study was premised on the assumption that use of famotidine represented a continuation of home use, but documentation of why famotidine was given was poor. The results were specific for famotidine (no protective association was seen for PPIs) and also specific for COVID-19 (no protective association in patients without COVID-19). A lower peak ferritin value was observed among users of famotidine, supporting the hypothesis that use of famotidine may decrease cytokine release in the setting of SARS-CoV-2 infection. A randomized controlled trial is currently under way to determine whether famotidine can improve clinical outcomes in hospitalized patients with COVID-19 (NCT04370262).
Famotidine has not previously been studied in patients for antiviral effects, and there are limited relevant prior data. An untargeted computer modeling analysis identified famotidine as one of the highest-ranked matches for drugs predicted to bind 3CLpro,3 a SARS-CoV-2 protease that generates nonstructure proteins critical to viral replication.4 In the 1990s, histamine-2 receptor antagonists including famotidine were shown to inhibit human immunodeficiency virus replication without affecting lymphocyte viability in vitro.2 , 5 , 6
There are limitations to the study. It was observational, and we cannot exclude the possibility of unmeasured confounders or hidden bias that account for the association between famotidine use and improved outcomes. No samples were gathered, and mechanism cannot be directly assessed. Finally, this was a single-center study, which may limit generalizability of the findings.
In sum, in patients hospitalized with COVID-19 and not initially intubated, famotidine use was associated with a 2-fold reduction in clinical deterioration leading to intubation or death. These findings are observational and should not be interpreted to mean that famotidine has a protective effect against COVID-19. Randomized controlled trials are under way.
Acknowledgments
The authors thank Dr Michael Wigler and Dr Richard Axel for useful suggestions.
Appendix
Additional members of the Famotidine Research Group
Magdalena E. Sobieszczyk, MD, MPH (Division of Infectious Diseases, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), David D. Markowitz, MD (Division of Digestive and Liver Diseases, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), Aakriti Gupta, MD, MS (Division of Cardiology, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), Max R. O’Donnell, MD, MPH (Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), Jianhua Li, MD (Department of Medicine, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), David A. Tuveson, MD, PhD (Cancer Center, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York), Zhezhen Jin, PhD (Department of Biostatistics, Columbia University Mailman School of Public Health, New York, New York), William C. Turner, MD (Department of Medicine, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York), and Donald W. Landry, MD, PhD (Department of Medicine, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, New York)
CRediT Authorship Contributions
Daniel E. Freedberg, MD, MS (Conceptualization: Equal; Data curation: Equal; Formal analysis: Equal; Investigation: Equal; Methodology: Equal; Project administration: Equal; Validation: Equal; Writing – original draft: Equal; Writing – review & editing: Equal). Joseph Conigliaro, MD, MPH (Conceptualization: Equal). Timothy C. Wang, MD (Conceptualization: Equal). Kevin J. Tracey, MD (Conceptualization: Equal). Michael V. Callahan, MD (Conceptualization: Equal). Julian A. Abrams, MD, MS (Conceptualization: Equal; Funding acquisition: Equal; Investigation: Equal; Methodology: Equal; Project administration: Equal; Writing – original draft: Equal; Writing – review & editing: Equal).
Footnotes
Conflict of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.05.053.
Contributor Information
Famotidine Research Group:
Magdalena E. Sobieszczyk, David D. Markowitz, Aakriti Gupta, Max R. O’Donnell, Jianhua Li, David A. Tuveson, Zhezhen Jin, William C. Turner, and Donald W. Landry
Supplementary Methods
Population
Adults aged 18 years or older were eligible for the study if they were admitted to Columbia University Irving Medical Center or its affiliate the Allen Pavilion from February 25, 2020, to April 13, 2020, and tested positive for SARS-CoV-2 by nasopharyngeal polymerase chain reaction at presentation or within no more than 72 hours following admission. This 72-hour window was selected because, during the earliest phase of the SARS-CoV-2 pandemic, testing availability was limited and could take up to 72 hours for a result. Patients were excluded if they survived less than 48 hours following hospital admission or if they required urgent or semi-urgent intubation within 48 hours of hospital admission. This study was approved by the institutional review board of the Columbia University Irving Medical Center.
Exposure
The primary exposure was use of famotidine, classified as present if famotidine was received within 24 hours of hospital admission and otherwise classified as absent. Famotidine use was ascertained directly from electronic medical order entry records and could be intravenous or oral, at any dose or duration. Home use of famotidine was examined to understand the reason underlying in-hospital use of famotidine and was classified based on electronic medication reconciliation performed at the time of hospital admission.
Primary Outcome
The primary outcome was a composite of death or endotracheal intubation within 30 days of hospital admission (intubation-free survival). Mortality data were ascertained from the electronic medical record (EMR), which interfaces with the social security death index. Endotracheal intubation was ascertained from EMR documentation of need for mechanical ventilation. The rationale for the combined primary outcome was 2-fold: (1) many patients who deteriorated clinically died without being intubated, often due to transition to palliative care; (2) hospitalization stays for intubated patients with COVID-19 have been very long, and many intubated patients with COVID-19 at the time of the analyses may ultimately not survive.
Covariables
Based on emerging reports of risk factors for COVID-19, the following covariables were selected for inclusion in the analysis: preexisting diabetes, hypertension, coronary artery disease, heart failure, end-stage renal disease or chronic kidney disease, and chronic pulmonary disorders, all classified based on the presence of corresponding International Classification of Diseases, 10th Revision codes at the time of hospital admission; obesity, classified based on body mass index; and age, classified as <50 years, 50 to 65 years, and >65 years. To assess severity of COVID-19, the first recorded form of supplemental oxygen after triage was captured and was classified as room air, nasal cannula oxygen, or non-rebreather/similar. Use of PPIs was classified in the same manner as use of famotidine so that PPIs could be evaluated to test whether any effects of famotidine might be related to acid suppression. The maximum value of plasma ferritin was obtained during the study period for each patient to use as a surrogate for the extent of cytokine storm (normal laboratory range 13.0 to 150.0 ng/mL).
Statistical Approach
Categorical variables were compared across exposure groups using χ2 tests. Full and reduced Cox proportional hazards models were constructed within the complete cohort, with patients followed from the time of hospital admission until the first of the following events: death, intubation, 30 days of follow-up, or the close of the study on April 20, 2020. Because patients were excluded if they died or were intubated before hospital day 2, this effectively meant that patients were followed from day 2 to day 30. This design was selected to avoid immortal time bias (ie, because the exposure was classified based on the 24-hour period after hospitalization and the at-risk period did not begin until hospital day 2). Cox proportional hazards modeling was performed on the full cohort, and a matched subset was examined with propensity scoring matching to balance baseline characteristics based on use of famotidine.
This provided the opportunity for a minimum of 7 days of follow-up time for all patients in the study. The proportional hazards assumption was verified by visual inspection of time-to-event data and by testing for a nonzero slope in the Schoenfeld residuals (11). The full Cox model included all baseline variables. For the reduced model, variables were dropped stepwise unless they had a significant independent relationship with the composite outcome or unless they altered the β-coefficient representing famotidine by at least 10%. Propensity score matching was then performed to balance the baseline characteristics of patients with respect to use of famotidine with a 5:1 nearest-neighbor matching strategy and a caliper of 0.2. The primary analysis was conducted as a time-to-event model within the propensity score–matched cohort, using the same approach. All analyses were performed using STATA statistical software (version 14; StataCorp, College Station, TX) at the α = 0.05 level of significance.
Additional Analyses
Several sensitivity analyses were performed. First, use of PPIs was compared with no PPIs within the complete (unmatched) cohort after excluding those who used famotidine. The purpose of this analysis was to test whether unmeasured patient characteristics related to use of acid suppression rather than famotidine were associated with improved outcomes in COVID-19. Second, an additional study cohort was built including records from patients who tested negative for SARS-CoV-2 during the study period. Within this cohort, use of famotidine was compared with no famotidine to test whether unmeasured patient characteristics related to use of famotidine were associated with improved outcomes regardless of reason for hospitalization (ie, to test whether the observed association with famotidine was specific for patients with COVID-19).
Supplemental Table 1.
Patient Characteristics at the Time of Hospital Admission for COVID-19, Stratified by Use of Famotidine
| Characteristics | Complete cohort |
After propensity score matching |
||||
|---|---|---|---|---|---|---|
| Famotidine (n = 84), n (%) | No famotidine (n = 1536), n (%) | P value | Famotidine (n = 84), n (%) | No famotidine (n = 420), n (%) | P value | |
| Age (y) | .39 | .51 | ||||
| <50 | 13 (15) | 320 (21) | 13 (15) | 57 (14) | ||
| 50–65 | 31 (37) | 483 (31) | 31 (37) | 184 (44) | ||
| >65 | 40 (48) | 733 (48) | 40 (48) | 179 (43) | ||
| Female sex | 39 (46) | 864 (56) | .63 | 39 (46) | 208 (50) | .60 |
| Race/ethnicity | .20 | .90 | ||||
| Hispanic | 25 (30) | 601 (39) | 25 (30) | 127 (30) | ||
| White, non-hispanic | 19 (23) | 336 (22) | 19 (23) | 82 (20) | ||
| Black, non-hispanic | 18 (21) | 322 (21) | 18 (21) | 102 (24) | ||
| Other | 22 (26) | 277 (18) | 22 (26) | 109 (26) | ||
| BMI, kg/m2 | .17 | .97 | ||||
| <25.0 | 15 (18) | 295 (19) | 15 (18) | 66 (16) | ||
| 25.0–29.9 (overweight) | 30 (36) | 388 (25) | 30 (36) | 157 (37) | ||
| ≥30 (obese) | 22 (26) | 434 (28) | 22 (26) | 110 (26) | ||
| Not recorded | 17 (20) | 419 (27) | 17 (20) | 87 (21) | ||
| Comorbidities | ||||||
| Diabetes | 24 (29) | 311 (20) | .07 | 24 (29) | 106 (25) | .52 |
| Hypertension | 29 (35) | 428 (28) | .19 | 29 (35) | 124 (30) | .36 |
| CAD | 9 (11) | 109 (7) | .21 | 9 (11) | 37 (9) | .58 |
| Heart failure | 7 (8) | 85 (6) | .28 | 7 (8) | 26 (6) | .47 |
| ESRD or CKD | 11 (13) | 130 (8) | .14 | 11 (13) | 47 (11) | .62 |
| Chronic pulmonary disorders | 2 (2) | 120 (8) | .07 | 2 (2) | 6 (11) | .52 |
| Initial oxygen requirement | .39 | .85 | ||||
| Room air | 25 (30) | 378 (25) | 25 (30) | 116 (28) | ||
| Nasal canula | 38 (45) | 678 (44) | 38 (45) | 187 (44) | ||
| Non-rebreather or similar | 21 (25) | 480 (31) | 21 (25) | 117 (28) | ||
BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; ESRD, end-stage renal disease.
Supplemental Table 2.
Final Cox Proportional Hazards Model of risk factors for death or Intubation Among Patients With COVID-19
| Characteristics | Death or intubation/n at risk (%) | Hazard ratio (95% CI) |
|
|---|---|---|---|
| Full model | Final model | ||
| Famotidine | |||
| No | 332/1536 (22) | Reference | Reference |
| Yes | 8/84 (10) | 0.43 (0.21–0.86) | 0.42 (0.21–0.85) |
| Age (y) | |||
| <50 | 19/333 (5.7) | Reference | Reference |
| 50–65 | 75/514 (15) | 2.94 (1.77–4.89) | 3.03 (1.83–5.03) |
| >65 | 246/773 (32) | 7.51 (4.66–12.1) | 7.68 (4.79–12.3) |
| Sex | |||
| Male | 197/909 (22) | Reference | — |
| Female | 143/711 (20) | 1.11 (0.89–1.38) | |
| Race/ethnicity | |||
| Hispanic | 129/626 (21) | Reference | — |
| White, non-Hispanic | 84/355 (24) | 0.99 (0.75–1.31) | |
| Black, non-Hispanic | 59/340 (17) | 0.82 (0.60–1.13) | |
| Other | 68/299 (23) | 1.14 (0.85–1.53) | |
| Body mass index, kg/m2 | |||
| <25.0 | 86/310 (28) | Reference | — |
| 25.0–29.9 (overweight) | 92/418 (22) | 0.88 (0.65–1.18) | |
| ≥30 (obese) | 89/456 (20) | 0.97 (0.72–1.31) | |
| Not recorded | 73/436 (17) | 0.67 (0.49–0.92) | |
| Comorbidities | |||
| Diabetes | 72/335 (21) | 1.02 (0.75–1.37) | — |
| Hypertension | 94/457 (21) | 0.72 (0.54–0.97) | 0.74 (0.58–0.94) |
| CAD | 24/118 (20) | 0.77 (0.49–1.21) | — |
| Heart failure | 24/92 (26) | 1.06 (0.67–1.67) | — |
| ESRD or CKD | 33/141 (23) | 1.16 (0.77–1.75) | — |
| Chronic pulmonary disorders | 29/122 (24) | 1.29 (0.87–1.93) | — |
| Initial oxygen requirement | |||
| Room air | 52/403 (13) | Reference | — |
| Nasal canula | 155/716 (22) | 1.60 (1.17–2.19) | 1.63 (1.19–2.24) |
| Non-rebreather | 133/501 (27) | 2.48 (1.79–3.44) | 2.39 (1.73–3.29) |
CAD, coronary artery disease; CKD, chronic kidney disease; ESRD, end-stage renal disease.
References
- 1.Gottschlich M.M., DeLegge M.H., Guenter P. American Society for Parenteral and Enteral Nutrition; Silver Spring, MD: 2007. American Society for Parenteral and Enteral Nutrition. [Google Scholar]
- 2.Bourinbaiar A.S., Fruhstorfer E.C. Life Sci. 1996;59:PL365–370. doi: 10.1016/s0024-3205(96)00553-x. [DOI] [PubMed] [Google Scholar]
- 3.Wu C., Liu Y., Yang Y. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand K., Ziebuhr J., Wadhwani P. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 5.Chen X., Deng H., Churchill M.J. Cell Stem Cell. 2017;21:747–760.e7. doi: 10.1016/j.stem.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Zhang C., Liu L., Gu M. FASEB J. 2020;34:6008–6016. doi: 10.1096/fj.202000502. [DOI] [PMC free article] [PubMed] [Google Scholar]