Highlights
-
•
Thromboembolic events are frequent in COVID-19 patients
-
•
The patient had no comorbidities and developed a sudden thrombosis of the aorta and consequent occlusion of tibial arteries
-
•
The patient had moderate increase of D-dimer and Fibrinogen values.
Keywords: COVID-19, SARS-CoV-2 infection, Acute limb ischemia, Thrombosis
Abstract
Acute thromboembolic events appear to be frequent in patients with SARS-CoV-2 infection. We report a case of an intubated patient, who developed a threatening lower limb ischemia. Intra-arterial fibrinolysis and intravenous heparin infusion did not lead to complete recanalization of the tibial arteries, which were successfully treated by surgical embolectomy.
Case report
A 58-year-old man was urgently transferred to our COVID-19 intensive care unit from the intensive care unit of a dedicated COVID-19 hospital, due to the sudden onset of ischemia of the right forefoot.
The patient was diagnosed as SARS-CoV-2 infected; contamination occurred two weeks before with progressive worsening of the respiratory function, leading to orotracheal intubation and mechanical ventilation. Only chronic hypertension was found in the clinical history.
At admission in our unit, he showed moderate hypothermia of the right foot with initial skin marbling of the forefoot. Motility and sensibility could not be assessed, due to the deep sedation. The right foot pulses, i.e. dorsalis pedis and posterior tibial, were absent, with all the other easily palpable bilaterally. The ultrasound examination confirmed the thrombotic obstruction of the tibial arteries of the right lower limb.
Table 1 shows blood tests of the patient at admission. The patient had normal white blood cells count, with neutrophilia and lymphocytopenia, normal Procalcitonin and elevated serum Interleukin 6. INR, aPtt and platelets count were normal, with high value of d-dimer and Fibrinogen.
Table 1.
Blood tests at admission.
| Value | UM | Normal Values | |
|---|---|---|---|
| White Blood Cells | 6.07 | 109/L | 3.60–10.50 |
| Neutrophils | > 81.1 | % | 42−77 |
| Lymphocytes | < 16.1 | % | 20−44 |
| Monocytes | 2.6 | % | 2.0 - 9.5 |
| Eosinophils | < 0.0 | % | 0.5 - 5.5 |
| Basophils | 0.2 | % | 0.0 - 1.8 |
| Red Blood Cells | 4.35 | 1012/L | 4.30−5.75 |
| Hematocrit | > 36.5 | % | 39.5 - 50.5 |
| MCV | 84 | fL | 80−99 |
| Platelets | 322 | 109/L | 160−370 |
| INR | 1.17 | < 1.20 | |
| aPtt | < 0.71 | 0.82 - 1.25 | |
| D-Dimer | > 1.19 | mg/L FEU | < 0.55 |
| Fibrinogen | > 524 | mg/dL | 150−400 |
| Procalcitonin | 0.1 | ng/mL | < 0.5 |
| Interleukin 6 | > 1588 | pc/dL | < 5.9 |
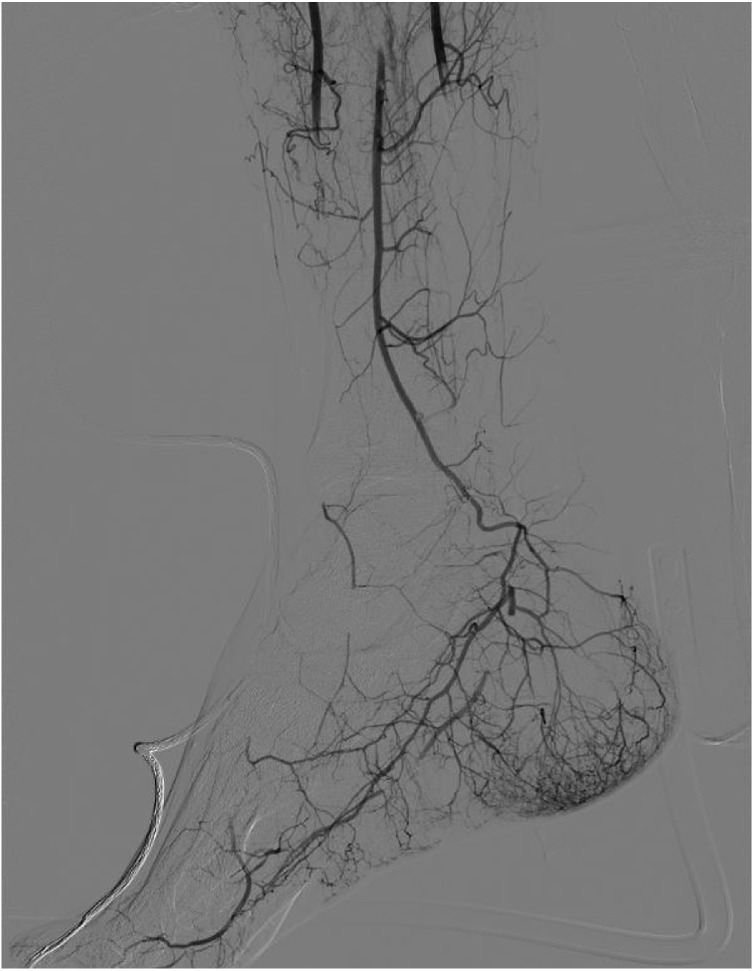
The clinical pattern indicated IIa acute limb ischemia, according with ESVS Guidelines (Björck et al., 2020); therefore, an urgent angiography through percutaneous right common femoral access was performed, in order to place a catheter for intra-arterial thrombolysis. The arteriography showed patent and normal femoro-popliteal axis, with distal occlusion of the anterior and posterior tibial arteries, as well as of the peroneal, which was distally recanalized (Fig. 1 ). A 4 F multi-hole catheter was advanced to the P3 segment of the popliteal artery and the tibio-peroneal trunk, with infusion of a 100.000 UI bolus of urokinase, followed by 50.000 UI per hour. Intravenous sodic Heparin was given at an anticoagulant dose. Serum Fibrinogen, INR, aPtt (target 1.7–2.3), CPK, Mioglobine and Creatinine were strictly monitored (every 6 h), as shown in Table 2 .
Fig. 1.
Lower Limb Arteriography at presentation.
Table 2.
Monitoring of laboratory values during fibrinolysis.
| T0 | T1 (6 h) |
T2 (12 h) | T3(18 h) | T4(24 h) | T5(30 h) | T6(36 h) | T7(42 h) | T8 (48 h) | |
|---|---|---|---|---|---|---|---|---|---|
| Fibrinogen (mg/dL) | 524 | 403 | 320 | 362 | 305 | 284 | 255 | 230 | 201 |
| aPtt | 0.71 | 1.73 | 2.02 | 1.94 | 1.82 | 2.11 | 1.95 | 1.87 | 2.05 |
| INR | 1.17 | 1.21 | 1.27 | 1.19 | 1.11 | 1.35 | 1.21 | 1.08 | 1.12 |
| CPK (U/L) | 3463 | 6478 | 9328 | 7457 | 5321 | 3259 | 2832 | 2038 | 1720 |
| Myoglobin (ng/mL) | 1105 | 2568 | 3347 | 2828 | 1934 | 1078 | 938 | 664 | 550 |
| Creatinine (mg/dL) | 1.09 | 1.17 | 1.18 | 1.11 | 1.08 | 1.02 | 0.99 | 1.05 | 0.69 |
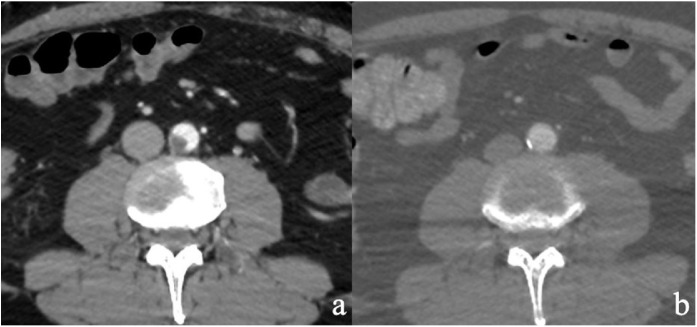
The thoraco-abdominal CT-Scan showed an endoluminal thrombotic apposition (10 × 11 mm) in the distal abdominal aorta (Fig. 2 a).
Fig. 2.
a) CT Scan at presentation; b) Control CT Scan.
After two days of thrombolysis, an arteriographic check was performed, which showed amelioration of the foot vascularization, yet with persisting proximal occlusion of the peroneal artery as well as occlusion of the distal tibial arteries.
Clinically, the right foot was still hypothermic, with evident marbling of the forefoot and toes. The catheter for thrombolysis was therefore removed and the patient was submitted to surgical exposure of the dorsalis pedis and retromalleolar posterior tibial arteries, with Fogarty embolectomy of extended proximally and distally to the pedal arch arteries. Fresh thrombotic material was removed, which was sent for histological assessment. Dorsalis pedis and tibial posterior pulses reappeared, with a triphasic flow pattern of both anterior and posterior tibial arteries at ultrasound evaluation.
Two days after surgery, due to improved respiratory conditions, the patient was extubated, with pedal pulses still present and amelioration of the foot perfusion. At control CT-Scan, aortic thrombus disappeared (Fig. 2b).
Discussion
This report deals with the sudden onset of thrombotic involvement of a healthy aorta of a COVID 19 patient, with subsequent thromboembolic occlusion of the tibial arteries, leading to a limb threatening ischemia. The problem of coagulopathy in COVID-19 is getting increasing interest in the discussion about this pandemic disease. As a matter of fact, the pandemic COVID-19 determined recently a very significant increase of admissions to intensive care unit (ICU) of patients needing ventilation support. Other than an acute respiratory distress syndrome (ARDS), many patients suffered a number of other problems, such as renal failure, cardiac arrhythmia, myocarditis and coagulative disorders (Huang et al., 2020).
Some authors suggested a possible role of disseminated intravascular coagulation; also, elevated d-dimer serum concentration has shown to be an independent risk factors for mortality in different experiences (Tang et al., 2020a, Wu et al., 2020). Although no data are available about the role of a possible hypercoagulable status in severely diseased patients, it is suggested that heparin can play a role in reducing mortality in severe COVID-19 patients. However, the available data on the heparin role concern only patients with ARDS and septic status (Tang et al., 2020b, Thachil, 2020).
The case herein reported is characterized by an anomalous thrombus formation in a healthy abdominal aorta of a 58 year-old COVID-19 patient, which led to peripheral embolization, similarly to the cases reported by Zang et al. (Zhang et al., 2020).
Despite the similarity with those cases, a word of caution should be said before considering the SARS-CoV-2 a highly thrombotic virus. It is true that the thrombus arose suddenly in a healthy aorta in our case, and that anecdotal similar experiences are reported however two conflicting considerations can be made. First of all, the city of Bologna, Italy, where our case occurred, is in the second region for the spread of SARS-CoV-2 infection, with a single vascular surgery unit for all the metropolitan area, accounting for one million citizens. During the first month of COVID-19 pandemic, 2334 patients were found positive for SARS-CoV-2 in the area, and the number of interventions or consultations for acute limb ischemias was similar with that of the same month of 2019, i.e. 2 cases in 2020 vs. 9 cases in 2019. Second important consideration is that both medical and surgical therapies delivered to that COVID-19 patient were successful, with no recurrences as it usually happens in prothrombotic cases.
Antiphospholipid antibodies and alteration in d-dimer concentration have a low-specific value and, even if clearly evident in these patients, their direct cause effect relationship with acute embolism should be demonstrated.
Conflict of interest
The authors have no competing interest to declare.
Ethical approval
This research received the local Ethical Committee approval.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Björck M., Earnshaw J.J., Acosta S., Bastos Gonçalves F., Cochennec F., Debus E.S. European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia. Eur J Vasc Endovasc Surg. 2020;59(2):173–218. doi: 10.1016/j.ejvs.2019.09.006. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 doi: 10.1111/jth.14817. Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14821. Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. Mar 13. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2007575. Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]