Abstract
It has been reported that angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) are the main cell entry proteins for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and play a critical role in causing coronavirus disease 2019 (COVID-19). To investigate the expression level of these SARS-CoV-2 host cell entry genes in the lung airway, we used public gene expression datasets. We have found a differential expression of ACE2 and TMPRSS2 in nasal and bronchial airways relative to age and diseases status. Children were found to have significantly lower expression of COVID-19 receptors in the upper and lower airways (nasal and bronchial). Moreover, the lung airway expression of both ACE2 and TMPRSS2 was found to be significantly upregulated in smokers compared with non-smokers, and in patients with chronic obstructive pulmonary disease (COPD) compared with healthy subjects. No difference was observed in the blood expression levels of ACE2 and TMPRSS2 between children and adults, or in COPD or diabetic patients. However, a significant increase in blood expression levels of these genes was observed in patients with essential hypertension, whereas only ACE2 was upregulated in the blood of asthmatics. These results suggest that the observed difference in COVID-19 severity between children and adults could, in part, be attributed to the difference in ACE2 and TMPRSS2 airways tissue expression levels.
Keywords: ACE2, TMPRSS2, SARS-CoV-2, COVID-19, nasal epithelium, COPD, smoking, hypertension, Children, bronchial
Graphical Abstract
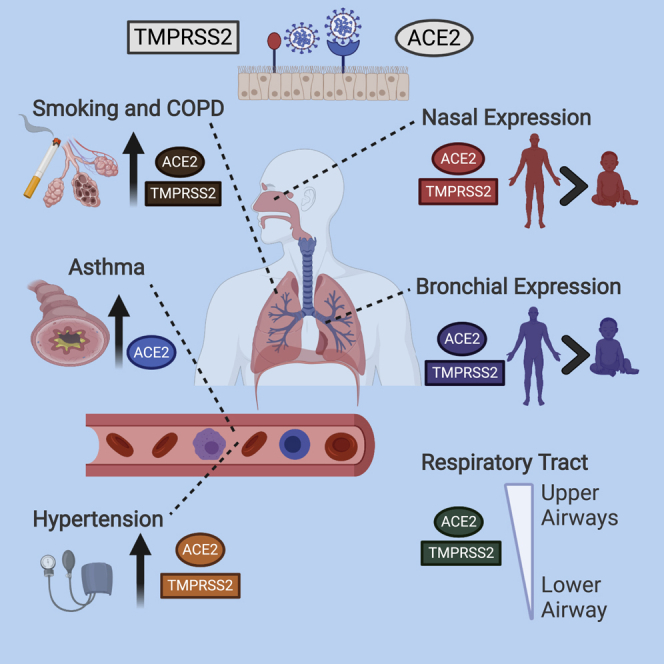
SARS-CoV-2 uses ACE2 and TMPRSS2 proteins to enter human cells. Using gene expression datasets, Halwani and coworkers showed that the airways expression of these receptors is lower in children, but upregulated in smokers and COPD patients. This may contribute to the difference in disease severity observed among these patient categories.
Introduction
Over the last two decades, three waves of coronavirus outbreaks among humans have erupted, severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002,1 Middle East respiratory syndrome (MERS) in 2012,2 and the latest novel SARS-CoV-2 in December 2019. The disease caused by these coronaviruses has pneumonia-like symptoms, such as fever and dry cough, and leads to progressive respiratory failure and even death.3 SARS-CoV1 and MERS-CoV4 caused epidemics in different countries, while the high rate of transmissibility and infectivity of SARS-CoV-2 resulted in a quick escalation of the number of infected cases worldwide, leading to the transformation of this outbreak into a worldwide pandemic (World Health Organization [WHO] situation report 50; https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200310-sitrep-50-covid-19.pdf?sfvrsn=55e904fb_2). As of the preparation of this report, the number of worldwide infections of the virus (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) is estimated to be 3,267,184, while the fatalities reported exceeded 200,000 deaths, according to WHO (WHO situation report 103; https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200502-covid-19-sitrep-103.pdf?sfvrsn=d95e76d8_4).
The new SARS-CoV-2 was found to share 79.6% sequence identity with SARS-CoV.5 Besides, the virus uses the same cell receptor as SARS-CoV, angiotensin-converting enzyme 2 (ACE2), to enter the cell. It was also found to need transmembrane serine protease 2 (TMPRSS2) for priming of the viral spike protein.6 The level of expression of the viral receptor (ACE2) and TMPRSS2, especially in the nasal tissue, may be critical for the ability of the virus to transmit and replicate.7
SARS-CoV-2 is believed to have a much higher rate of transmission compared with SARS-CoV and MERS-CoV.8 The majority of people infected were of older age (approximate age range, 30–79 years) and developed mild symptoms, whereas around 19% developed severe conditions and needed critical care.9 Many of the patients who developed critical conditions had, in fact, chronic diseases such as cardiovascular diseases and diabetes.10 Throughout the three epidemics, low rate of infection was reported in infants and children, with mostly a milder disease profile.11, 12, 13 The reason behind that is still a mystery.
Several theories have been discussed for why children are less susceptible to SARS-CoV-2 infection14,15 and less prone to develop severe conditions following infection with SARS-CoV-2.16 One possibility is their young immune system, which could be more efficient in clearing the infection compared with the adult immune system. Another possibility is the presence of cross immunity due to a previous exposure to a milder widespread form of coronaviruses.11,17 However, one possibility could also be that they express lower levels of the viral receptors that could limit transmission to these young populations and the development of severe conditions. Hence we tested the hypothesis that the expression of viral receptors, ACE2 and TMPRSS2, in children is lower than that in adults. Moreover, because ACE2 is known to be regulated by hypoxia, stress, and several inflammatory mediators,18 we have also tested whether its expression increases because of smoking and chronic inflammatory diseases affecting the lungs, such as asthma, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis (IPF), as well as diabetes and hypertension (HTN).
Results
In this study, we evaluated airway tissue gene expression levels of SARS-CoV-2 receptors, ACE2 and TMPRSS2, using publicly available transcriptomic datasets of nasal and lung airway tissue, as well as blood and saliva. The list of the extracted datasets that passed the quality control (QC) and were included in our analysis is presented in Table S1.
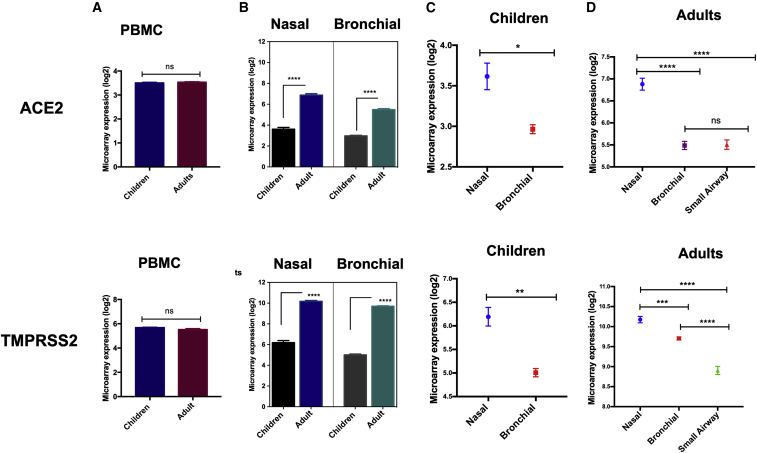
We first evaluated the expression levels of ACE2 and TMPRSS2 in peripheral blood mononuclear cells (PBMCs) of adults versus children and found no significant difference (Figure 1A). However, interestingly, the expression levels of both ACE2 and TMPRSS2 were significantly lower in nasal as well as bronchial epithelial tissue of children compared with those of adults (p < 0.0001; Figure 1B). Notably, these receptors were found to be differentially expressed between upper and lower airways. In children, the expression levels of ACE2 (p = 0.032) and TMPRSS2 (p = 0.002) were both significantly higher in upper respiratory nasal tissue compared with the bronchial epithelial brushing (Figure 1C). Similarly, in adults, we found significantly higher expression of these genes in upper nasal epithelial tissue compared with bronchial and small airway epithelial brushings (p < 0.0001; Figure 1D). Moreover, the expression of these two genes was found to be significantly higher in nasal compared with blood or saliva of adult subjects (p < 0.0001; Figure S2D). This significant differential expression of both ACE2 and TMPRSS2 between children and adults could contribute to the lower infectivity and disease severity observed in younger populations. In addition, the increased expression of these receptors in nasal compared with blood or saliva suggest transmission could be higher through the nasal cavity compared with the oral cavity.
Figure 1.
Microarray Expression Levels of ACE2 and TMPRSS2 in PBMCs, Upper and Lower Respiratory Tract of Children and Adults
(A) There was no difference in expression levels of ACE2 and TMPRSS2 in PBMCs of adults versus children. (B) Nasal and bronchial expression levels of ACE2 and TMPRSS2 were significantly lower in children versus adults. (C) Expression levels of ACE2 and TMPRSS2 were significantly higher in nasal versus bronchial tissue of children. (D) In adults, the expression levels of ACE2 and TMPRSS2 decreased moving from upper to lower airways. The nasal expression of these genes was significantly higher in nasal versus bronchial and in nasal versus small airways. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Results are presented as mean (± SEM) mRNA expression.
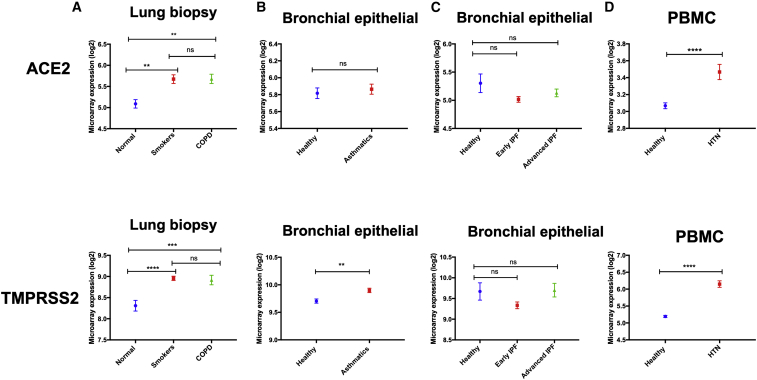
We next determined whether the expression of ACE2 and TMPRSS2 increases in lung tissue in the presence of other comorbidities, especially those associated with a more severe SARS-CoV-2 infection. To do that, we compared the lung tissue expression levels of ACE2 and TMPRSS2 in patients with comorbidities compared with healthy controls. Datasets of blood (PBMCs) were used for patients with HTN and diabetes because no transcriptomic data of lung tissue were available for these groups. Upon analyzing transcriptomic datasets of lung biopsies obtained from COPD adult patients, ACE2 (p = 0.003) and TMPRSS2 (p = 0.0002) were both found to be significantly upregulated (Figure 2A). The expression of these two genes was also significantly increased in the lung tissue of smokers compared with healthy subjects (ACE2, p = 0.002; TMPRSS2, p < 0.0001; Figure 2A). This suggested that the observed increase in the lung tissue of COPD patients could mostly be attributed to the effect of smoking. No difference was observed in the blood expression levels of these genes for COPD compared with healthy controls (Figure S1A).
Figure 2.
ACE2 and TMPRSS2 Are Significantly Higher in Lungs of COPD Patients and PBMCs of Hypertension Patients
(A) Compared with healthy controls, the levels of ACE2 and TMPRSS2 were higher in lungs of COPD patients, as well as in lungs of smokers. (B) No difference was found in expression levels of asthmatics bronchial tissue versus healthy controls. (C) Also, there were no differences in levels of these genes in lung tissue of idiopathic pulmonary fibrosis versus healthy controls. (D) The levels of ACE2 and TMPRSS2 expression were found to be significantly elevated in PBMCs of hypertensive versus healthy controls. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Results are presented as mean (± SEM) mRNA expression.
Although there was no difference in the level of ACE2 expression in asthmatic bronchial epithelial cells compared with healthy controls, the level of TMPRSS2 (p = 0.002) was found to be significantly elevated (Figure 2B). The same pattern was observed upon the assessment of transcriptomic data obtained from the bronchial epithelial cells of asthmatic children. The expression levels of ACE2 were not different compared with healthy controls, but the levels of TMPRSS2 (p = 0.0002) were significantly higher (Figure S2A). This pattern of expression was reversed in the blood of asthmatics, where ACE2 expression was significantly elevated, but no difference in the levels of TMPRSS2 was observed (Figure S1B). In addition, no difference was observed in the expression levels of ACE2 and TMPRSS2, neither in bronchial tissue of patients with IPF (Figure 2C) nor in lung tissue of patients with sarcoidosis (Figure S2B) or scleroderma-associated interstitial lung disease (ILD) (Figure S2C).
Interestingly, the levels of ACE2 and TMPRESS2 were found to be significantly elevated in the blood (PBMCs) of patients with HTN compared with healthy controls (p < 0.0001; Figure 2D). However, no difference in the receptors gene expression was detected in the blood (PBMCs) of patients with diabetes (Figure S1C).
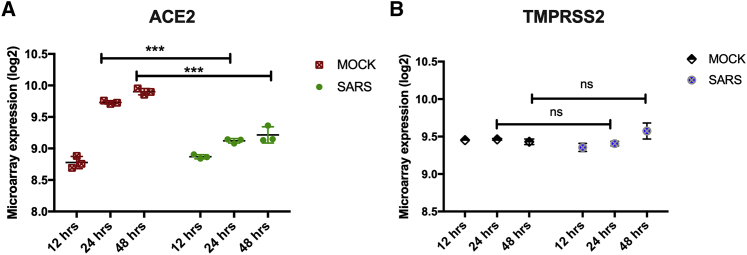
After determining the baseline expression of ACE2 and TMPRSS2 in children, adults, and different COVID-19 high-risk patient groups, we examined how coronavirus infection may affect the expression of ACE2 and TMPRSS2. We hence compared the expressions of these genes in SARS-CoV1-infected lung epithelial cells (Calu-3) compared with Mock-infected Calu-3 controls at different time points. ACE2 expression was significantly decreased at 24- (p < 0.001) and 48-h (p < 0.001) time points following infections. However, no significant change in the expression of TMPRSS2 was observed following infection with SARS-CoV1 (Figure 3).
Figure 3.
Effect of SARS-CoV Infection in Expression Levels of ACE2 and TMPRSS2 in Lung Epithelial Cells (Calu-3)
(A) ACE2 expression was significantly decreased at 24- and 48-h time points following infections. (B) However, no significant change in the expression of TMPRSS2 was observed following infection with SARS-CoV-1. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Results are presented as mean (± SEM) mRNA expression.
Discussion
In this study, using several public gene-expression datasets, we have shown that the SARS-CoV-2 receptor, ACE2, and TMPRSS2 are expressed at significantly higher levels in nasal epithelium compared with blood and saliva, and this expression decreases significantly in lower lung airway tissue. Importantly, the expression of ACE2 and TMPRSS2 in nasal tissue of children is significantly lower than that of adults. Likewise, the bronchial tissue expression of these genes was also lower in children compared with adults. To the best of our knowledge, this is the first study to report lower ACE2 and TMPRSS2 expression in nasal and bronchial epithelial cells of children compared with adults.
The reported rate of SARS-CoV-2 infection and spectrum of symptoms is lower in children than in adults.16 Considering that SARS-CoV-2 cell entry depends on the expression of ACE2 and TMPRSS2 entry genes,6 one can speculate that the transmissibility/clinical manifestations of SARS-CoV-2 could be affected by the levels of ACE2 and TMPRSS2 expression on the cell surface. In fact, by analyzing 7,375 contacts, Zhang et al.15 found that children are less susceptible to SARS-CoV-2 infection than adults. Therefore, the reduced airway tissue expression of ACE2 and TMPRSS2 reported here may contribute to the lower risk for infection14,15 and the reduced disease severity observed in the younger population.
In light of the potentially increased transmissibility of SARS-Cov-2 in smokers and adults with other comorbidities,10,19,20 ACE2 and TMPRSS2 lung airway expression was found to be upregulated upon smoking and in COPD patients, and slightly increased in asthmatics. Of all the chronic lung diseases tested, smokers and COPD had the highest increase in these genes compared with healthy lung tissue. This indicated that smoking could be the reason behind the increased expression observed in COPD, and not the inflammatory mediators. In fact, while preparing this manuscript, Brake et al.21 reported an increase in ACE2 in the lung airways of smokers using immunohistochemistry. Hypoxia is known to regulate ACE2 expression,18,22 which could explain the mechanism by which smoking increases these receptors. The increased expression of ACE2 in smokers may hence justify the observed increase in rate of infection and severity of disease observed in smokers compared with non-smokers.23
No difference in receptors gene expression was observed in lung tissue of patients with IPF, or with sarcoidosis or scleroderma-associated ILD. In addition, no difference in the blood levels of ACE2 and TMPRSS2 expression between children and adults was observed. However, a significant level in blood expression of ACE2 and TMPRSS2 was observed for patients with HTN, while only ACE2 was upregulated in the blood of asthmatics. This may explain the increased risk of these patients for SARS-CoV-2 infection. The absence of difference in blood expression levels of patients with chronic diseases such as diabetes does not exclude the possibility of increased expression of these entry genes in the lung tissue of these patients; however, we had no available data to investigate that. It would hence be interesting to determine the lung expression levels of SARS-CoV-2 receptors in these patients.
The increased expression of ACE2 and TMPRSS2 in the upper respiratory tract compared with blood and lower respiratory tract could contribute to the increased replication of SARS-CoV-2 in this tissue. Recently, Zou et al.7 reported that SARS-CoV-2 viral load is higher in nasal compared with throat swabs. Based on our finding, the reason for that could be the higher expression of ACE2 and TMPRSS2 in nasal compared with throat swaps. In fact, the level of these genes in saliva was significantly lower than that of nasal epithelium (Figure S2D). Interestingly, TMPRSS2 was found to be expressed in nasal and bronchial epithelial cells along with ACE2. SARS-CoV-2 needs this host serine protease for spike protein priming, allowing for viral fusion and cellular entry.5,24 These findings of co-expression of ACE2 and TMPRSS2 on the nasal epithelial cells might provide further insights into the reason for increased infectivity and transmissibility of SARS-CoV-2, and may pave the way for novel therapeutic interventions. It is worth mentioning that serine protease inhibitors such as camostat mesylate, which blocks TMPRSS2 activity, could be used for treatment of SARS-CoV-2-infected patients,6,25 or as a preventive approach, especially for patients identified as high risk based on ACE2 nasal screening.
Of note, infecting human airway epithelial cells with SARS-CoV was found to downregulate ACE2 and TMPRSS2 expression compared with the mock-infected cells. This was in line with a previous similar observation.6,26 The fact that the level of expression of ACE2 is not increased following infection indicates that the baseline level of ACE2 may play a critical role in determining the level of disease severity. Moreover, a dramatic decrease in lung tissue expression of ACE2 was shown to be associated with acute respiratory distress syndrome (ARDS).27 The downregulation of ACE2 expression observed with SARS-CoV infection would hence play a role in the development of ARDS, a common clinical phenotype for patients with COVID-19.
Our results are mainly based on public gene expression datasets. It is warranted that further histological methods and protein validation are performed to confirm our findings. The observed differential level of expression of ACE2 and TMPRSS2 may contribute, at least partially, to our understanding of why elderly adults and patients with other comorbidities are prone to develop a more severe disease compared with children and other younger populations.
Materials and Methods
In this study, bioinformatic analyses were conducted to evaluate the expression levels of ACE2 and TMPRSS2 gene signatures in blood, upper and lower respiratory tract tissue, and saliva for children and adults. The expression was also evaluated in the lung tissue of smokers and patients with chronic conditions, such as COPD, asthma, HTN, diabetes, IPF, sarcoidosis, and scleroderma-associated ILD.23,28
Publicly available gene expression datasets deposited in National Center for Biotechnology Information Gene Expression Omnibus (NCIB GEO; https://www.ncbi.nlm.nih.gov/geo/) and the European Bioinformatics Institute (EMBL-EBI; https://www.ebi.ac.uk) were used. Among the selected studies, all were Affymetrix microarray platforms, and only one study (GEO: GSE118761) was Illumina RNA sequencing (RNA-seq), which was analyzed separately, and it was not used in the microarray comparisons. Before data preprocessing, all data were evaluated for QC, and all poor-quality data were removed.29,30 The details of the final datasets are presented in Table S1, which includes 4 datasets for children groups (healthy and asthmatics), 15 datasets for adults with different comorbidities (asthmatics, COPD, IPF, HTN, diabetes, systemic sclerosis [SSc]-related ILD and sarcoidosis), and 1 dataset to assess the in vitro effect of SARS-CoV infection on lung epithelial cells.
The raw Affymetrix data were normalized and log transformed. Microarray data (CEL files) were pre-processed with Robust Multi-Array Average (RMA) technique using R software.31 The probe set ID with the largest interquartile range (IQR) of expression values was selected to represent the gene. Before including a dataset in the analyses, the expression level of housekeeping genes was used to evaluate whether the batch effect has a significant impact on the results. For the RNA-seq study, the data were pre-processed using the Bioconductor package limma-voom32 and presented as log2 counts per million (log cpm).
Log-transformed normalized intensities were used in the final analyses where all comparisons between two independent groups (children versus adults and healthy versus diseased) were carried out using unpaired Student’s t test.33 Statistical analyses were performed using Prism (v.8; GraphPad Software). For all analyses, p <0.05 was considered significant.
Author Contributions
R.H., Q.H., N.S.S.-A., S.A., and F.S.S.-A. conceived and designed the experiments. N.S.S.-A., F.S.S.-A., and M.A. analyzed the data. All authors contributed to writing and revision of the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This research has been financially supported by Tissue Injury and Repain (TIR) group operational (Grant code: 150317) and by a seed grant (to R.H.), University of Sharjah, UAE, and by Prince Abdullah Ben Khalid Celiac Disease Research Chair, under the Vice Deanship of Research Chairs, King Saud University, Riyadh, Kingdom of Saudi Arabia. The Graphical Abstract was created with biorender.com.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.05.013.
Supplemental Information
References
- 1.Kuiken T., Fouchier R.A.M., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Groot R.J., Baker S.C., Baric R.S., Brown C.S., Drosten C., Enjuanes L., Fouchier R.A.M., Galiano M., Gorbalenya A.E., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J. Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K., Rubin R., Morales-Estrada S., Black S.R., Pacilli M., Illinois COVID-19 Investigation Team First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Bever H.P., Chng S.Y., Goh D.Y. Childhood severe acute respiratory syndrome, coronavirus infections and asthma. Pediatr. Allergy Immunol. 2004;15:206–209. doi: 10.1111/j.1399-3038.2004.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei M., Yuan J., Liu Y., Fu T., Yu X., Zhang Z.-J. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323:1313–1314. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 14.Li W., Zhang B., Lu J., Liu S., Chang Z., Cao P., Liu X., Zhang P., Ling Y., Tao K., Chen J. The characteristics of household transmission of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa450. Published online April 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Litvinova M., Liang Y., Wang Y., Wang W., Zhao S., Wu Q., Merler S., Viboud C., Vespignani A. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020 doi: 10.1126/science.abb8001. Published online April 29, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J., Zhang W., Wang Y., Bao S., Li Y., Chinese Pediatric Novel Coronavirus Study Team SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weng N.P. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke N.E., Belyaev N.D., Lambert D.W., Turner A.J. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin. Sci. (Lond.) 2014;126:507–516. doi: 10.1042/CS20130291. [DOI] [PubMed] [Google Scholar]
- 19.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., Akdis C.A., Gao Y.-D. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. Published online February 29, 2020. [DOI] [PubMed] [Google Scholar]
- 21.Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking Upregulates Angiotensin-Converting Enzyme-2 Receptor: A Potential Adhesion Site for Novel Coronavirus SARS-CoV-2 (Covid-19) J. Clin. Med. 2020;9:841. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi S., Wollenzien H., Leclerc E., Jarajapu Y.P.R. Hypoxic regulation of angiotensin-converting enzyme 2 and Mas receptor in human CD34+ cells. J. Cell. Physiol. 2019;234:20420–20431. doi: 10.1002/jcp.28643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir. Med. 2020;8:e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., Schiergens, T.S., Herrler, G., Wu, N.-H., Nitsche, A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8. [DOI] [PMC free article] [PubMed]
- 25.Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. USA. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy R., Asante I., Liu S., Parikh P., Liebler J., Borok Z., Rodgers K., Baydur A., Louie S.G. Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: A pilot study. PLoS ONE. 2019;14:e0213096. doi: 10.1371/journal.pone.0213096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumor Analysis Best Practices Working Group Expression profiling--best practices for data generation and interpretation in clinical trials. Nat. Rev. Genet. 2004;5:229–237. doi: 10.1038/nrg1297. [DOI] [PubMed] [Google Scholar]
- 30.Galamb O., Sipos F., Spisák S., Galamb B., Krenács T., Valcz G., Tulassay Z., Molnár B. Potential biomarkers of colorectal adenoma-dysplasia-carcinoma progression: mRNA expression profiling and in situ protein detection on TMAs reveal 15 sequentially upregulated and 2 downregulated genes. Cell. Oncol. 2009;31:19–29. doi: 10.3233/CLO-2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughey J.J., Butte A.J. Robust meta-analysis of gene expression using the elastic net. Nucleic Acids Res. 2015;43:e79. doi: 10.1093/nar/gkv229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudoit S., Yang Y.H., Callow M.J., Speed T.P. Statistical methods for identifying differentially expressed genes in replicated cDNA microarray experiments. Stat. Sin. 2002;12:111–139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.