The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been spreading throughout the world from its source in Wuhan, China and is responsible for the coronavirus disease 2019 (COVID-19) affecting, in the first-place, the pulmonary system.
Several central nervous system manifestations have been described in patients with COVID-19 and especially those who suffer from a severe illness (Asadi-Pooya and Simani, 2020). Two papers enlightened the presence of abnormal cerebral imaging in COVID-19 patients including one case of acute necrotizing hemorrhagic encephalopathy (Poyiadji et al., 2020) and one case of abnormal findings in the medial temporal lobe suggesting encephalitis (Moriguchi et al., 2020).
However, to the best of our knowledge, no data exist concerning specific investigations with electroencephalography (EEG). We would like to draw attention to unusual and misleading EEG patterns that we have noticed in patients with COVID-19. Thereby, we report here the case of COVID-19 encephalopathy, without abnormalities on MRI but supported by EEG recordings, and will discuss the delicate EEG interpretation we have faced.
An 80-year-old woman was admitted to our hospital for respiratory distress. She had been suffering from dyspnea and fever for two days. Nasal swab was made to test for COVID-19 using the PCR method and was negative. Chest CT showed bilateral bronchial involvement, arguing for a bronchial infection. She was treated by an intravenous antibacterial therapeutic combination of rovamycine, amikacin, and ceftriaxone. After four days with good pulmonary outcome, a restless state with altered consciousness was observed justifying a cerebral non-contrast CT, which was unremarkable. At that time, blood tests showed no issues regarding pCO2, ceftriaxone dosage, or glucose level, neither renal nor liver dysfunction. Amikacin had been discontinued the day before. She then presented with focal motor seizures which were improved by antiepileptic treatment by intravenous sodium valproate (one bolus of 20 mg/kg), lacosamide (100 mg/day for two days intravenously then 200 mg/day orally) and oral clobazam (20 mg/day). But she showed further worsening of her awareness. She did not show at any time signs of visual hallucinations, pyramidal or extrapyramidal features. Analyses of cerebrospinal fluid and brain MRI were unremarkable, but full body CT showed features of COVID-19 lung infection. At that time, the patient was supplied with oxygen 3 L/min. During the whole evolution, she was never artificially ventilated. COVID-19 was confirmed by a second PCR test on nasal swab before her death, on the 25th day after admission.
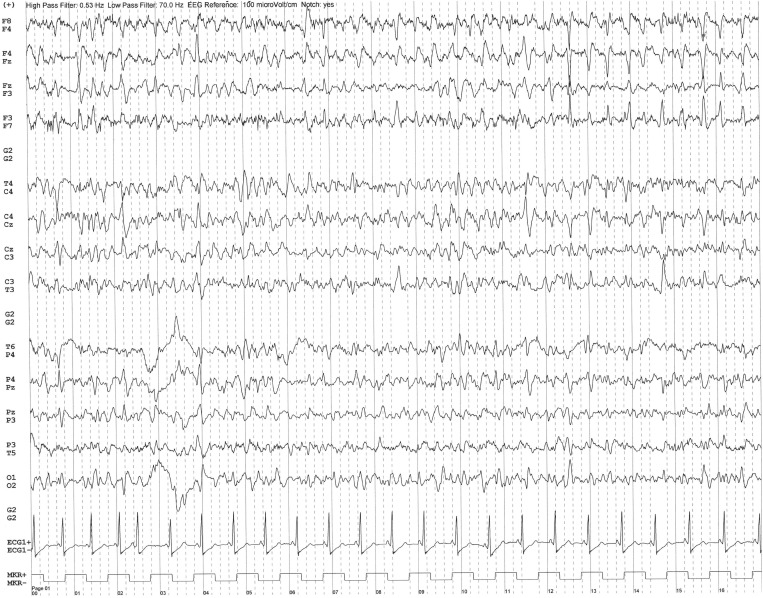
During her stay, several EEGs were performed. The two first EEGs (day 6, day 8) showed background activity slowing and repetitive epileptiform discharges with quasi-rhythmic spatio-temporal evolution mostly in the bilateral frontal areas (Fig. 1a ). Clonazepam 1 mg was administered during recording and led to a clear improvement of EEG. The presence of clonic movements of the left foot, the absence of metabolic disorders or ceftriaxone overdose led us to consider it as a frontal status epilepticus.
Fig. 1a.
EEG recording on day 6 after admission. Transverse bipolar montage. Bandpass: 0.53–70 Hz. 50-Hz notch filter. Sensitivity 10 µV/mm. Note the repetitive epileptiform discharges mostly in the bilateral frontal areas, over a slowing of the background activity.
On the next three EEGs (day 11, day 12, day 13), paroxysms took a more clearly triphasic aspect with sometimes a sharp aspect, suggesting a toxic/metabolic encephalopathy.
On the last two EEGs (day 18, day 21), triphasic activity was clearly periodic with short periods (1–1.5 s) over a worsened background activity (Fig. 1b ). At this moment, osmolarity was 308 mmol/L, glycemia level was 8.9 mmol/L, liver function and ammonia levels were normal, arterial blood gases showed hypoxemia but no hypercapnia, ceftriaxone dosage was in therapeutic range, and there was no hypothermia.
Fig. 1b.
EEG recording on day 21 after admission. Transverse bipolar montage. Bandpass: 0.53–70 Hz. 50-Hz notch filter. Sensitivity 10 µV/mm. Note the periodic triphasic waves with short periods (1–1.5 s) over a worsened background activity.
Triphasic waves have been described in respiratory failure, especially in case of respiratory tract infection (Sutter and Kaplan, 2014). However, oxygen supply was not very high in our patient and could even be stopped after a few days, arterial blood gases did not show hypercapnia while oxygen was no longer dispensed. Besides, neurological worsening took place despite a steady respiratory state, which led us to conclude that the development of the triphasic wave pattern was not caused by respiratory failure.
Moreover, the periodic feature of the triphasic waves in EEG was striking. Even if this peculiar EEG pattern is often associated with Creutzfeldt-Jakob disease, similar patterns have been described in other infections, such as West Nile virus neuro-invasive disease (Parsons et al., 2019) and neurosyphilis (Takagaki et al., 2016). In our case, the medical history, the clinical evolution, the neurological signs, and the absence of metabolic disorders led us to conclude that the evolution of the EEG was indicative of a progressive neurological process, which probably was linked with the SARS-CoV-2 infection.
This case indicates that it is relevant to pay more attention to EEG patterns in patients during the COVID-19 pandemic, especially when initial PCR tests are negative.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413 doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A.M., Grill M.F., Feyissa A.M., Britton J., Hocker S., Crepeau A. EEG in WNV Neuroinvasive Disease. J Clin Neurophysiol. 2019;36:135–140. doi: 10.1097/WNP.0000000000000558. [DOI] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;31 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter R., Kaplan P.W. Uncovering clinical and radiological associations of triphasic waves in acute encephalopathy: a case-control study. Eur J Neurol. 2014;21:660–666. doi: 10.1111/ene.12372. [DOI] [PubMed] [Google Scholar]
- Takagaki K., Morales M.K., Vitantonio D., Berkowitz F., Bell W.L., Kumar P.N., Motamedi G.K. Periodic Lateralized Epileptiform Discharges (PLEDs) in patients with neurosyphilis and HIV infection. Clin EEG Neurosci. 2016;47:247–250. doi: 10.1177/1550059414552704. [DOI] [PubMed] [Google Scholar]