To the editor,
The latest version of the criteria for hospital discharge in China and many other countries [1,2] commonly demand (a) normal temperature lasting longer than 3 days, (b) substantially resolved respiratory symptoms, (c) substantially improved acute exudative lesions on chest radiographs and (d) two consecutively negative results of real-time reverse transcription polymerase chain reaction (RT-PCR) tests on respiratory tract samples separated by at least 24 hr. However, negative results of RT-PCR tests in a proportion of patients discharged based on this criteria have, at follow-up examinations, returned positive [[3], [4], [5]], resulting in a raised concern that a proportion of discharged patients may still be virus carriers.
On 11 January 2020, a 66-year-old man with an 8-year history of hypertension was admitted to the Third People's Hospital of Shenzhen, Shenzhen, China, as a probable case of COVID-19 because of his recent 1-week travel history to Wuhan and symptoms of fever and cough. Upon admission, the patient was immediately isolated. Low/high-flow nasal cannula oxygen, antiviral (oseltamivir 75 mg and interferon α1b 30 μg, twice daily), and antibiotic (moxifloxacin 400 mg once daily) therapies were initiated (Fig. S1). He developed shortness of breath and hypoxemia (Table S1), resulting in the use of methylprednisolone and continuous positive airway pressure support.
The diagnosis of COVID-19 was made based on the evidence of chest images and confirmed by the positive result of RT-PCR test (cycle threshold (Ct) 27.6; Ct < 37 represents a positive test) on a throat swab sample on 14 January. Oseltamivir was replaced by ribavirin (500 mg twice daily). His body temperature reduced to ≤37.7°C in the next 5 days with less pulmonary infiltrates apparent on the radiograph (Fig. S2A and S2B) but his respiratory symptoms did not improve. Hypoxaemia reappeared on January 20 (Table S1) and invasive mechanical ventilation was initiated. Superimposed bacterial and fungal infections were observed. Septic shock, consolidation on chest images (Fig. S2C and S2D) and progressive respiratory failure that were refractory to intervention by tracheostomy and therapies of lopinavir, ritonavir and broad-spectrum antibacterial and antifungal drugs developed (Fig. S1). On 30 January, the patient received veno-arterio-venous extracorporeal membrane oxygenation after which his vital signs remained largely stable.
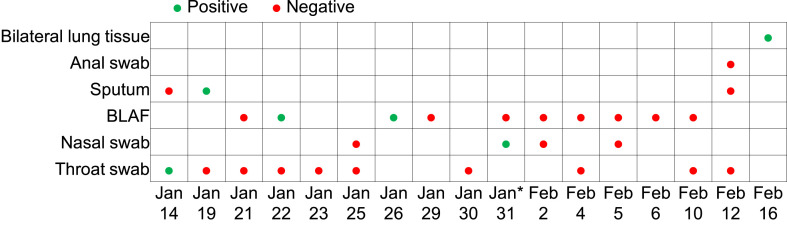
Real-time RT-PCR tests were regularly performed on his multiple clinical samples after the diagnosis (Fig. 1 ). Positive results were found in samples of sputum on January 19 (Ct 26.0), bronchoalveolar lavage fluid on 22 January (Ct 24.2) and 26 January (Ct 20.3), and nasopharyngeal swab on 31 January (Ct 33.5). On 31 January, he received 400 mL of convalescent plasma donated by a recovered COVID-19 patient. Negative RT-PCR test results on various biological samples were detected for the next 12 days.
Fig. 1.
Results of real-time RT-PCR tests for SARS-CoV-2 on multiple clinical samples of the patient. Real-time RT-PCR test was done using the detection kit for 2019 novel coronavirus (2019-nCoV) RNA (DAAN Gene, Guangzhou, China; ce registration no: DE/CA09/0170/D01/IVD/016-03 and CFDA registration no: 20203400063). Cycle threshold (Ct) values of ORF1ab/N on the RT-PCR test were detected, and Ct < 37 represents a positive test (http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html). BALF, bronchoalveolar-lavage fluid; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. ∗400 mL of convalescent plasma was used.
Intrapulmonary bleeding and obstruction, on a ‘white-out’ radiograph of both lungs, were seen on 7 February 2020. The patient suffered from rapidly deteriorating hypoxaemia, complicated with pulmonary hypertension and right ventricular failure, and thus a multidisciplinary team considered organ transplantation. After ethics approval by the admitting hospital and informed consent from the patient's family were obtained, the transplantation was performed on 15–16 February 2020. Unfortunately, the patient died from an uncontrolled loss of blood.
During the transplantation, tissue samples (20 g for each side) were taken from the recipient's bilateral lungs after opening the chest for RT-PCR tests. The results (Fig. 1) were positive with Ct values of 34.2 (the second test 35.3) and 34.3 (the second test 34.9) in the left and right lungs, respectively.
The present study was limited to not having the virus in tissue samples cultured. Even though we tested live lung tissue samples, the detection of part of the viral genome does not necessarily mean that the virus is capable of infection. Data on the contagiousness of these discharged patients retesting as positive are lacking; greater efforts need to be made to solve the existing questions on this population.
Transparency declaration
None.
Funding
There was no funding source for this study.
Ethics committee approval
This study was approved by The First Affiliated Hospital of Guangzhou Medical University review board. As the study was retrospective it was exempt from informed consent.
Author contributions
J. He and X. Xu supervised the overall study and contributed to study concept and design. Y. Zhao, Z. Xia, W. Liang and J. Li collected, analysed and interpreted the data. Y. Zhao and J. Li made the figures. All authors wrote the manuscript and approved its final version.
Acknowledgements
We thank the specialist team in organ transplantation from The First Affiliated Hospital of Guangzhou Medical University. We also thank Sook-San Wong and Mark Zanin (First Affiliated Hospital of Guangzhou Medical University and The University of Hong Kong, China) for their assistance in language improvement and formatting the manuscript.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.05.013.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.National Health Commission & National Administration of Traditional Chinese Medicine Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7) Chin Med J. 2020;E27 http://rs.yiigle.com/yufabiao/1184760.htm In press. Available from: [Google Scholar]
- 2.European Centre for Disease Prevention and Control . 2020. Guidance for discharge and ending isolation in the context of widespread community transmission of COVID-19 – first update.https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-guidance-discharge-and-ending-isolation-first%20update.pdf [Google Scholar]
- 3.Yuan J., Kou S., Liang Y., Zeng J., Pan Y., Liu L. PCR assays turned positive in 25 discharged COVID-19 patients. Clin Infect Dis. 2020:ciaa398. doi: 10.1093/cid/ciaa398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J.-F., Yan K., Ye H.-H., Lin J., Zheng J.-J., Cai T. SARS-CoV-2 turned positive in a discharged patient with COVID-19 arouses concern regarding the present standard for discharge. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.