Highlights
-
•
In this commentary, we discuss some controversial issues about the performance-enhancing role of the lactate-consuming bacteria Veillonella.
-
•
The relative abundance of Veillonella in human stool samples was not associated with training status or endurance performance.
-
•
In studying the effect on performance of Veillonella supplementation in mice, we found that Lactobacillus bulgaricus creates a biased control due to its interference in lactate metabolism and its influence on endurance performance.
-
•
In the absence of a vehicle-treated mouse group and ignoring the response to exercise of the native microbiota, the role of Veillonella atypica administration in maximal endurance performance in nontrained mice cannot be elucidated.
1. Introduction
Regular exercise induces changes in the overall diversity and in the relative abundance of certain gut microbiota phyla and families in humans1,2 and in animal models.3,4 This relationship has attracted a great deal of interest because it is one of the mechanisms involved in the health benefits of regular exercise3 that could also influence performance.5 A recent publication by Scheiman et al.6 provides a multidimensional approach to the effects of acute exercise on gut microbiota composition in humans as well as on the influence of bacterial supplementation on performance in mice. Scheiman et al.6 conclude that Veillonella atypica (V. atypica) is a performance-enhancing microbe that functions via the utilization of lactate and the production of propionate.
The aim of this commentary is to discuss some of the controversial issues raised in the study by Scheiman et al.6 and to provide original data to support alternative explanations.
2. Main concerns
2.1. The relative abundance of Veillonella in human stool samples was not associated with training status or endurance performance
It is remarkable that Scheiman et al.6 found no significant differences in the relative abundance of Veillonella between runners and sedentary individuals. Also, the correlation between marathon performance and Veillonella abundance was not explored by Scheiman et al.,6 and the study did not investigate the effect of Veillonella administration on physical performance. Therefore, in light of these results, the Veillonella genus appears not to be a good biomarker of training status, and its influence on performance remains unexplored.
2.2. The native gut microbiota of gavaged mice and its response to exercise was not analyzed
Despite the fact that the results from the human study by Scheiman et al.6 did not support the idea of a performance-enhancing effect of Veillonella, the authors decided to gavage nontrained mice with the species V. atypica (isolated from runners’ samples) in order to analyze its potential effect on acute endurance performance. This species was selected for its high prevalence of enzymes involved in lactate metabolism. A control group was administered Lactobacillus bulgaricus (L. bulgaricus) due to its supposed incapacity to impact lactate metabolism.6
The authors administered these bacteria over the animals’ resident gut microbiota, whose composition was not analyzed. The native gut microbiota of mice shows a dynamic response to training,5 although the effect of acute exercise has not been explored. Furthermore, the relationship between performance improvements in response to training and gut microbiota composition are not associated with a single microbial population but with a set of changes in the microbiome.7,8
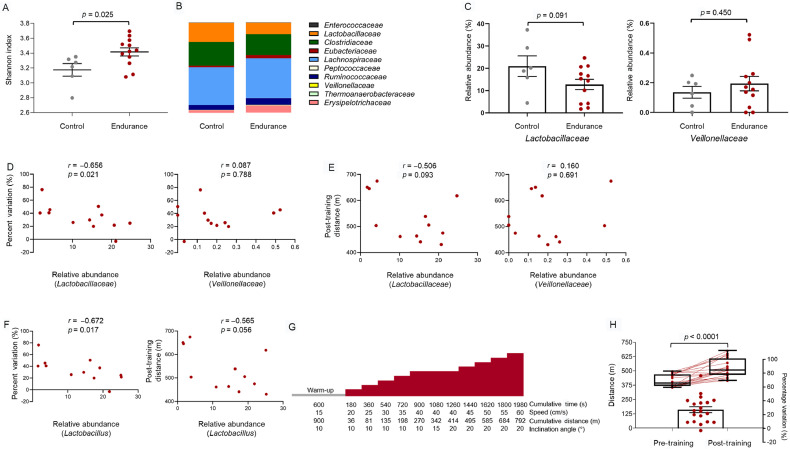
Thus, we have explored the effect on the gut microbiota composition of progressive treadmill endurance training for 5 days/week for 4 weeks in a group of 8-week-old C57BL/6N male mice. After 16S ribosomal RNA sequencing, α-diversity analysis of cecal samples showed a higher gut microbiota diversity in endurance-trained mice (n = 12) vs. sedentary control mice (n = 6) (Fig. 1A and Supplementary Table 1). However, considering the relative abundance at the family level in the phylum Firmicutes (Fig. 1B), where V. atypica and L. bulgaricus are included, we observed that the relative abundance of the Lactobacillaceae and Veillonellaceae families (Fig. 1C and Supplementary Table 2) were not significantly different between endurance-trained mice and nontrained control mice (p = 0.091 for Lactobacillaceae; p = 0.450 for Veillonellaceae).
Fig. 1.
Gut Lactobacillus relative abundance is negatively correlated with endurance performance in trained mice. (A) α-diversity analysis of bacterial communities in cecal content of control mice (sedentary; n = 6) and endurance trained mice (n = 12). Shannon index (richness and evenness) shows differences in diversity between control and endurance groups (p = 0.025) as determined by unpaired t test. Each mouse is represented by an independent point in the scatter dot plot. A bar represents the mean, and error is represented as S.E.M. (B) Average relative abundance of Firmicutes phylum at the family level in control (n = 6) mice and endurance mice (n = 12). (C) Relative abundance of Lactobacillaceae and Veillonellaceae families for control mice and endurance mice is shown (mean ± S.E.M.), and differences between groups were determined by unpaired t test. (D) Pearson correlation analysis indicates a negative correlation between Lactobacillaceae family abundance and percent variation for total distance in endurance-trained mice (n = 12). A nonsignificant positive correlation was found between Veillonellaceae family abundance and percent of variation in the same group of mice. (E) When post-training distance was considered, the Pearson correlation showed a nonsignificant negative association with Lactobacillaceae family relative abundance in endurance-trained mice (n = 12). In the case of Veillonellaceae family relative abundance, no correlation was found in the same group of mice. (F) At the genus level, Pearson correlation analysis showed a significant association between the genus Lactobacillus relative abundance and the percent variation for total distance in endurance-trained mice (n = 12). This association was not seen when the post-training total distance was analyzed for the same genus and the same group of mice. (G) For the assessment of endurance capacity, mice performed a 10-min warm-up at 15 cm/s and 10° slope. Then, at the same conditions established for the warm-up, speed was increased 5 cm/s every 3 min until reaching 40 cm/s. At this speed, the slope was increased from 10° to 15° and from 15° to 20° in 2 consecutive 3-min stages. After that, speed was increased again 5 cm/s every 3 min at 20° slope till exhaustion. Maximum speed (cm/s) and total time (s) were recorded, and total distance (m) was calculated as measurements of endurance capacity. (H) Total distance covered in pretraining and post-training tests. Eight-week-old male mice (n = 20) performed a pretraining maximal test before training intervention. After a progressive endurance training intervention of 5 days/week for 4 weeks, the same protocol was applied for post-training performance assessment. The before-after graph for distance (left Y-axis) shows each individual as an independent point. A box and whiskers (maximum to minimum) plot is also shown, where a bar represents the median; p < 0.0001 was obtained by Wilcoxon matched-pairs signed rank test. Percentage variation for total distance (calculated as: (post-training − pre-training) × 100/pre-training) is shown in the right Y-axis. The scatter box plot shows the mean and S.E.M. Each point represents 1 mouse.
2.3. The effect on performance of the supplementation with V. atypica is confounded by the use of L. bulgaricus as a control
As mentioned above, Scheiman et al.6 selected V. atypica from the human study based on its capacity for lactate catabolism which, in turn, produces propionate. The authors observed an association between propionate administration and improvements in run-to-exhaustion time in mice. On the contrary, the Lactabacillaceae family is characterized by high rates of lactate production.9 Specifically, L. bulgaricus, which was used as a control, is a homofermentative bacterium, generating 1 mol of lactate for each mol of glucose.9 This is particularly important considering the high carbohydrate content of standard rodent chow (usually more than 40% by weight, excluding fiber). Furthermore, Scheiman et al.6 did not explore the mutual metabolic interactions between the supplemented bacteria and the native microbiota.
However, we have observed a significant negative correlation between the relative abundance of the Lactobacillaceae family or Lactobacillus genus in trained animals and endurance capacity improvements in response to training (Fig. 1D and 1F, respectively, and Supplementary Table 3). A clear trend toward a significant negative correlation (r = –0.565, p = 0.056) was also observed between the relative abundance of Lactobacillus genus and a post-training run-to-exhaustion test (Fig. 1F). These data indicate that the relative abundance of Lactobacillus in the native gut microbiota of trained mice is related to a lower endurance performance. On the other hand, no differences in the relative abundance of the Veillonellaceae family were observed between trained and untrained mice (Fig. 1C), and no relationship with physical performance was found (Fig. 1D and 1E). Therefore, it is possible that what Scheiman et al.6 actually observed was, in fact, a decrease in performance induced by L. bulgaricus administration.
Thus, the choice of L. bulgaricus-gavaged mice as a control against which comparisons were made may be a confounding factor due to the influence of L. bulgaricus on intensive lactate metabolism and its negative relationship with endurance performance.
2.4. The absence of a vehicle-treated group does not allow elucidation of the role of V. atypica administration on maximal endurance performance in nontrained mice when L. bulgaricus is used as control
To determine whether what Scheiman et al.6 observed was an increase in endurance performance due to V. atypica administration or a decrease associated with L. bulgaricus, it would have been necessary to have a new experimental group of mice to which only vehicle was administered, without bacteria supplementation. However, this group was not included in the study by Scheiman et al.6
The need for this additional group is also evident considering that V. atypica-treated animals did not perform better in a run-to-exhaustion treadmill test than has been previously described in the literature. In fact, both V. atypica- and L. bulgaricus-treated animals showed considerably lower performance than nontrained mice of the same strain and age under similar exercise protocols (Fig. 1G), as observed by our research group (Pretraining, and Supplementary Table 4 and Fig. 1H) and others.10, 11, 12 These differences are even greater when compared to trained animals (Post-training) (Fig. 1H). A lower performance in the run-to-exhaustion tests would have been expected if Scheiman et al.6 had pretreated their animals with antibiotics, as previously described by Hsu et al.5 Therefore, these discrepancies with other studies, which could be explained by the differences in the run-to-exhaustion tests used, highlight the fact that the improvements in performance reported by Scheiman et al.6 may not be attributable to V. atypica but, instead, may have been determined by their experiment’s design.
In summary, it is not clear whether what Scheiman et al.6 report is an improvement in performance attributable to V. atypica administration or a decrease associated with L. bulgaricus. In the absence of a vehicle-treated group, both explanations are possible. Considering the wide pharmacological and dietary use of probiotics, particularly those containing members of the Lactabacillaceae family, additional research is needed to confirm the relationship between microorganism administration, gut microbiota composition, and performance.
3. Data availability
Sequence raw data have been uploaded to NCBI and SRA as Bioproject PRJNA558220. Other raw data are available as a Supplementary Tables file. Additional data are available on request from the authors. Methods are available as Supplementary Data.
Acknowledgments
Acknowledgments
This work was supported by Ministerio de Economía y Competitividad under Grant DEP2015-69980-P to BFG, and by Programa de Ayudas a Grupos de Investigación del Principado de Asturias to FLB (FC-GRUPIN-IDI/2018/000120). The authors acknowledge the technical support provided by Servicios Científico-Técnicos de la Universidad de Oviedo.
Authors’ contributions
MFS and JF performed the experiments, analyzed the data, and wrote the manuscript; CTZ analyzed the data, prepared the figures, and wrote the manuscript; BFG designed and supervised the study; CJV analyzed the data; FL and EIG designed and supervised the study and wrote the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of the presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2020.02.005.
Appendix. Supplementary materials
References
- 1.Barton W., Penney N.C., Cronin O., Garcia-Perez I., Molloy M.G., Holmes E. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67:625–633. doi: 10.1136/gutjnl-2016-313627. [DOI] [PubMed] [Google Scholar]
- 2.Petersen L.M., Bautista E.J., Nguyen H., Hanson B.M., Chen L., Lek S.H. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome. 2017;5:98. doi: 10.1186/s40168-017-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mailing L.J., Allen J.M., Buford T.W., Fields C.J., Woods J.A. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev. 2019;47:75–85. doi: 10.1249/JES.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 4.Allen J.M., Berg Miller M.E., Pence B.D., Whitlock K., Nehra V., Gaskins H.R. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol (1985) 2015;118:1059–1066. doi: 10.1152/japplphysiol.01077.2014. [DOI] [PubMed] [Google Scholar]
- 5.Hsu Y.J., Chiu C.C., Li Y.P., Huang W.C., Huang Y.T., Huang C.C. Effect of intestinal microbiota on exercise performance in mice. J Strength Cond Res. 2015;29:552–558. doi: 10.1519/JSC.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 6.Scheiman J., Luber J.M., Chavkin T.A., MacDonald T., Tung A., Pham L.D. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25:1104–1109. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu Y.J., Huang W.C., Lin J.S., Chen Y.M., Ho S.T., Huang C.C. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients. 2018;10 doi: 10.3390/nu10070862. pii: E862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai Z.L., Tseng C.H., Ho H.J., Cheung C.K.Y., Lin J.Y., Chen Y.J. Fecal microbiota transplantation confers beneficial metabolic effects of diet and exercise on diet-induced obese mice. Sci Rep. 2018;8:15625. doi: 10.1038/s41598-018-33893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garvie E.I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980;44:106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcaletti S., Thomas C., Feige J.N. Exercise performance tests in mice. Curr Protoc Mouse Biol. 2011;1:141–154. doi: 10.1002/9780470942390.mo100160. [DOI] [PubMed] [Google Scholar]
- 11.Conner J.D., Wolden-Hanson T., Quinn L.S. Assessment of murine exercise endurance without the use of a shock grid: an alternative to forced exercise. J Vis Exp. 2014:e51846. doi: 10.3791/51846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougherty J.P., Springer D.A., Gershengorn M.C. The treadmill fatigue test: a simple, high-throughput assay of fatigue-like behavior for the mouse. J Vis Exp. 2016:e54052. doi: 10.3791/54052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence raw data have been uploaded to NCBI and SRA as Bioproject PRJNA558220. Other raw data are available as a Supplementary Tables file. Additional data are available on request from the authors. Methods are available as Supplementary Data.