Abstract
Background
Low cardiorespiratory fitness is an independent predictor of all-cause and cardiovascular mortality, and interventions that increase fitness reduce risk. Water-walking decreases musculoskeletal impact and risk of falls in older individuals, but it is unclear whether water-walking improves aerobic fitness in the same way as weight-dependent land-walking. This randomized controlled trial involved 3 intervention groups—a no-exercise control group (CG), a land-walking (LW) group, and a water-walking (WW) group—to investigate the comparative impacts of LW and WW to CG on fitness.
Methods
Both exercise groups attended individually tailored, center-based, intensity-matched 3 × weekly sessions for 24 weeks, which progressed to 150 min of exercise per week. This was followed by a 24-week no-intervention period. Maximal graded exercise tests were performed on a treadmill at Weeks 0, 24, and 48.
Results
Maximal oxygen uptake increased from Week 0 to Week 24 in both exercise groups (0.57 ± 0.62 mL/kg/min, 0.03 ± 0.04 L/min for LW; 0.93 ± 0.75 mL/kg/min, 0.06 ± 0.06 L/min for WW, mean ± SE) compared to the CG (–1.75 ± 0.78 mL/kg/min, –0.16 ± 0.05 L/min) (group × time, p < 0.05). Time to exhaustion increased significantly following LW only (123.4 ± 25.5 s), which was significantly greater (p = 0.001) than the CG (24.3 ± 18.5 s). By Week 48, the training-induced adaptations in the exercise groups returned to near baseline levels.
Conclusion
Our study supports current physical-activity recommendations that 150 min/week of moderate-intensity exercise produces improvements in fitness in previously sedentary older individuals. Also, LW and WW elicit similar improvements in fitness if conducted at the same relative intensities. Exercise-naïve older individuals can benefit from the lower impact forces and decreased risk of falls associated with WW without compromising improvements in cardiorespiratory fitness.
Keywords: Cardiorespiratory fitness, Graded exercise test, Physical activity, Water exercise
Graphical abstract
1. Introduction
Low cardiorespiratory fitness is a potent independent predictor of all-cause and cardiovascular mortality in apparently healthy individuals and those with established cardiovascular disease.1,2 The optimal measure of cardiorespiratory fitness is maximal oxygen uptake (VO2max), the integrative capacity to transport and utilize O2 for the provision of cellular energy, usually determined in response to a graded exercise test (GXT). An increase in VO2max of 3.5 mL/kg/min (1 metabolic equivalent) translates to a 13% decrease in all-cause mortality and a 15% reduction in mortality due to cardiovascular disease.3
Historically, exercise science research has focused on the impact of interventions involving bouts of large-muscle-group dynamic exercise such as walking, running, and cycling,4,5 the outcomes of which have driven health guideline development such as the recommendation that individuals accrue 150 min of moderate-to-vigorous-intensity physical activity (MVPA) per week.6 Such studies have also fostered the notion that a dose-response relationship exists between the intensity (or volume) of exercise undertaken and the consequent fitness gain.7 At the same time, an appropriate clinical exercise prescription recognizes individual differences in the capability to undertake exercise, with improved adherence and adaptation in older individuals resulting from exercise that is progressively titrated to avoid musculoskeletal injury and cardiovascular risk.8 In essence, an optimal exercise prescription in older individuals maximizes the beneficial adaptation whilst minimizing potential risk.
Water-based exercise performed in the upright posture is often used for older populations9 and those at elevated injury risk,10 recognizing the decreased gravitational forces and musculoskeletal impact on bone and soft tissue structures.11 To date, the majority of water-based exercise training interventions have focused on water-aerobics, which involve a range of distinct movement patterns, sometimes with the inclusion of some resistance exercises, and usually performed whilst stationary.9 In contrast, walking in water (WW) is associated with drag force and resistance and induces distinct gait patterns comparable to land walking (LW) (i.e., shorter stride length, slower walking speed, and smaller range of motion in the knees), particularly in older individuals.12 We recently reported changes in body composition favoring increased lower limb lean body mass as a consequence of WW compared to LW, suggesting that the greater resistance during WW could be responsible for muscle mass adaptations that are at least as beneficial as LW, if not more so.13 This is of particular importance for older adults vulnerable to age-related muscle wasting;14 and WW also provides some benefit in terms of reduced skeletal loading and joint stress,15 in addition to reducing potential injury as a result of falls. However, to our knowledge, a direct comparison of the impact of LW vs. WW conducted at the same relative intensity (% heart rate reserve (HRR)) over a 6-month intervention period concerning cardiorespiratory fitness has not previously been undertaken in older adults. We, therefore, designed a randomized controlled trial comparing the impact of a 24-week supervised, monitored, and center-based LW or WW intervention in previously sedentary older participants, followed by an additional 24-week no-intervention period for all groups. Maximal cardiorespiratory fitness was measured at Weeks 0, 24, and 48. We hypothesized that both forms of exercise would enhance aerobic capacity relative to a nonintervention control group (Week 0 vs. Week 24) and that the adaptation would be reversed following cessation of exercise (Week 24 vs. Week 48).
2. Materials and methods
This study conformed to the Declaration of Helsinki and was granted approval by The University of Western Australia Human Research Ethics Committee. All participants provided written informed consent prior to participation. The study was prospectively registered, and a detailed methodologic paper that includes a consort flow diagram has been published.16 The study was registered as a clinical trial (ACTRN12614000017628).
A variety of recruitment strategies, including flyers and advertisements in local newspapers and on local radio broadcasts, was utilized to encourage community-dwelling individuals aged 50 years and over (females must have been post-menopausal) to contact the research team for study details. An initial phone screening session by questionnaire was conducted to ascertain whether potential participants met the predetermined inclusion criteria.16 These inclusion criteria required individuals to be relatively physically inactive (less than 60 min/week of purposeful physical activity); nonsmokers (> 12 months); alcohol intake lower than 280 g/week (and/or drinking < 40 g ethanol in 1 session); no injuries or illnesses that would prohibit participation in exercise; no current or previous disease, such as cardiovascular disease, inclusive of diabetes, heart attack, or stroke; and no associated medical procedures such as stenting. The use of medications such as beta-blockers, blood pressure drugs, or lipid-lowering drugs were permitted, provided that they had been taken regularly for at least 6 months prior to entering the study. Individuals meeting these criteria attended the university laboratory to undergo a physical examination to further determine suitability for inclusion in the study. This included height and body mass (body mass index < 40 kg/m2), resting blood pressure (systolic blood press < 160 mmHg, diastolic blood press < 100 mmHg), fasting blood tests (total cholesterol < 7.0 mmol/L), and urea and creatinine to rule out the presence of abnormal kidney function (<11 mmol/L in men and <9 mmol/L in women). Individuals satisfying all of these criteria were entered into the study and attended a session that familiarized them with the fitness-testing equipment and treadmill walking.
As part of the study, all participants took part in a maximal exercise test on a treadmill at 3 time points: Week 0 (baseline), after Week 24 (immediately post-intervention), and after Week 48 (24 weeks post-intervention). Following the baseline test at Week 0, participants were randomized to one of 3 groups for interventions that were 24 weeks in duration: LW, WW, or control group (CG). Participants in the CG were asked to maintain their usual level of physical activity and daily living habits across the 24 weeks. Following the initial 24-week intervention period and repeated GXT, participants in all groups were thanked for their involvement and had no contact with the university or researchers for an additional 24 weeks (Weeks 24–48). Participants were provided with no deliberate instruction to either continue or desist from exercise.
2.1. Exercise training interventions
Participants randomized to the 2 exercise groups (LW and WW) attended the university 3 times/week for 24 consecutive weeks to take part in either LW or WW, as per group randomization.16 All sessions were supervised by an experienced exercise physiologist. The LW group took part in outdoor walking in and around the university grounds and nearby river foreshore, which consisted of a combination of paved and short grass terrain made up primarily of flat surfaces (i.e., no/minimal grade). WW was conducted in a heated (28°C–30°C) swimming pool (20 m width × 30 m length) at the university. Participants walked at a depth approximately reaching the xiphoid process, which results in 30% weight bearing compared to that on land.17 All sessions commenced with a brief warm-up, including light aerobic activity and dynamic and static stretches, and concluded with a cool-down. The exercise intensity was based on individual HRR values (%),16 with resting heart rate (HR) derived from a 20-min period of supine rest and maximum HR (HRmax) derived from the initial GXT. Studies of the acute effects of WW have demonstrated that, at matched HRs, there is a similar oxygen cost relative to LW,18,19 suggesting that the use of HR as a tool for exercise prescription is valid and feasible. HRR takes into account differences in resting HR among participants and has been recommended in preference to percentage of HRmax by the American College of Sports Medicine (ACSM).6 WW and LW groups performed 1 interval and 2 continuous exercise sessions per week at the same HR intensity. HR was measured continuously during each session using a Polar RS300X HR monitor (Polar Electro Oy, Helsinki, Finland) and was documented every 5 min by the supervising exercise physiologist. Walking pace was dictated by the exercise physiologist to ensure that the target HR was reached and maintained.
The exercise sessions initially comprised 15 min of exercise at an HRR of 40%–45%, building to 50 min at 55%–65% HRR over the course of the study. Mean HR over the exercise session was used to determine the intensity (%HRR) achieved for each individual at every session. Participants were given the opportunity to make up any missed sessions in order to maximize adherence by attending extra sessions in the following 2 weeks of exercise. Apart from the addition of the exercise sessions as part of the intervention, the LW and WW groups were asked to maintain their usual lifestyle behaviors throughout the study. After the 24-week exercise intervention and subsequent assessments, the study continued for an additional 24-week period. During this phase, the LW and WW groups were free to exercise or not, of their own volition.
2.2. Control group
Participants randomized to the CG were advised not to change their prestudy physical activity routine throughout the intervention period. Once every 6 weeks, participants in the CG attended the university to participate in seminars that were unrelated to physical activity or health promotion. The purpose of these seminars was to avoid a possible Hawthorne effect biasing the interpretation of the study results.16
2.3. GXT and oxygen consumption assessment
A familiarization session exposing participants to the equipment, the treadmill walking procedures, and the GXT protocol was undertaken before the first formal oxygen-uptake assessment. On a separate day, aerobic fitness was assessed using a GXT on a treadmill, in line with ACSM recommendations at the time of the study's commencement.20 The protocol comprised continuous, incremental exercise, with 3-min stages that continued until volitional exhaustion.16 Respiration was measured continuously throughout the test by using indirect calorimetry (applied electrochemistry oxygen analyzer S-3A and carbon dioxide analyzer CD-3A; AEI Technologies Inc., Pittsburgh, PA, USA), and these data were averaged every 15 s. HR was recorded using a 12-lead electrocardiogram (Mortara Instrument X-Scribe, Milwaukee, WI, USA) in the last 30 s of each stage and at the end of exercise if the test was completed midstage. VO2max was determined as the maximum oxygen consumption measured for 1 entire minute during the test.
2.4. Physical activity assessment
Daily physical activity was objectively assessed every 15 s for 8 consecutive days using an ActiGraph accelerometer (ActiGraph GT1M, Pensacola, FL, USA), worn on the right hip and attached to an elasticized strap. This measurement was included to determine whether our results were caused by the intervention or whether changes in daily physical activity may have been a contributing factor. Participants were instructed to wear the device during waking hours, but the devices were removed during participation in the exercise sessions that were part of the intervention. Monitors were worn at Weeks 0, 24, and 48 to determine whether any changes in lifestyle physical activity occurred around the prescribed center-based activities. Participants were also asked to complete a continuous 8-day diary at each time point to record hours of wear. Data were downloaded using ActiLife software (Version 6.13.1; ActiGraph, Pensacola, FL, USA), and a member of the research team, who was blinded to group allocation, processed the data using a customized Excel macro (Version 2013; Microsoft Excel, Redmond, WA, USA). Nonwear time was defined as 60 min of consecutive zeros. Freedson adult cut-points were used to quantify time spent in light-intensity physical activity (LPA), moderate-intensity physical activity (MPA), and vigorous-intensity physical activity (VPA) per day. MPA and VPA were summed to obtain daily MVPA.21 Data were included if the ActiGraph was worn for ≥ 10 h/day on at least 4 days.22
2.5. Statistics
Analyses were conducted using SPSS Version 23.0 (IBM Corp., Armonk, NY, USA) and STATA Version 15.0 (STATA Corp., College Station, TX, USA). Data were analyzed on an intention-to-treat basis. Unadjusted means, SDs, and confidence intervals were calculated for the fitness outcome variables at Weeks 0, 24, and 48. Due to the correlated and repeated-measure nature of the data, separate linear mixed models were used to investigate the relationship between the fitness variables (VO2 data at max, treadmill time to exhaustion, and peak HR), groups (CG, LW, and WW), and time (baseline and Week 24) using 2-tailed tests. These analyses accounted for time invariant covariates, including age and sex, and an interaction between group and time. A random intercept was included in each model to account for the repeated nature of the data. These analyses were repeated to test the maintenance of the intervention (Week 24 to Week 48). Analyses were also conducted with daily physical activity data by Actigraph (LPA, MPA, MVPA, and VPA) because these outcomes would examine whether any changes in fitness might be attributable to changes in habitual and incidental physical activity. The statistical approach described above for fitness variables was also implemented for Actigraph outcomes, with all models additionally adjusted for wear time. Statistical significance was set at p < 0.05.
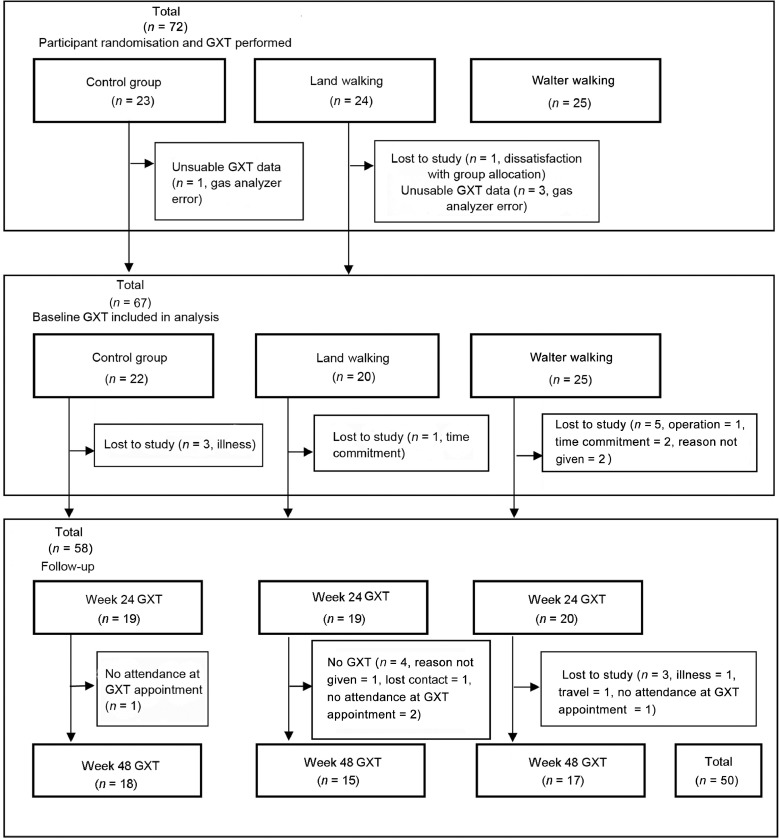
A sample size power calculation was conducted using G*Power Software Version 3.1.9.4,23 which indicated that with an α of 0.05, and assuming no change in VO2max in the CG and a difference of 1 mL/kg/min in the 2 exercise groups (LW and WW) with an SD of 0.8 mL/kg, 11 participants per group would be required to test our hypotheses. A full consort diagram, including the recruitment and screening process, can be found in our associated methodology paper.16 A consort diagram that relates specifically to the GXT outcomes following randomization can be found in Fig. 1.
Fig. 1.
Consort diagram showing participants randomized to control group, land-walking group, or water-walking group, and completion of a graded exercise test (GXT) included in statistical analysis for Weeks 0, 24, and 48.
3. Results
A total of 72 participants were randomized into the study and completed baseline measures, although 1 participant withdrew for personal reasons and requested that data be deleted. Baseline characteristics of the participants, including age and gender, are described in Table 1. Although all 71 participants completed a baseline GXT, data for 4 participants were not included in the analysis (Table 2) due to technical issues with the gas analyzer (n = 3), and 1 participant did not approach maximum exertion. Overall, 12 participants withdrew at various stages of the study (Fig. 1). A total of 58 participants completed a postintervention GXT (CG, n = 19; LW, n = 19; WW, n = 20), and 50 participants attended testing at Week 48 (CG, n = 18; LW, n = 15; WW, n = 17). Noncompletion of the GXT at either Week 24 or Week 48 was due to reasons that included illness, lack of interest in remaining in the study, and overseas travel. In accordance with current statistical practice related to the use of intention-to-treat analysis, the data in the figures are based on the mixed-model analysis of the 71 participants who attended the baseline assessment. Table 2 summarizes the data for all participants who completed GXT at each time-point.
Table 1.
Baseline characteristics of participants (mean ± SD).
| Control | Land walking | Water walking | |
|---|---|---|---|
| (n = 23:6M, 17F) | (n = 23:6M, 17F) | (n = 25:7M, 18F) | |
| Age (year) | 62.1 ± 7.0 | 62.7 ± 7.0 | 62.6 ± 6.7 |
| Height (cm) | 167.8 ± 9.6 | 165.1 ± 8.0 | 166.9 ± 7.2 |
| Body mass (kg) | 73.8 ± 13.6 | 74.4 ± 11.1 | 76.8 ± 19.8 |
| BMI (kg/m2) | 26.2 ± 4.1 | 27.3 ± 3.4 | 27.3 ± 5.6 |
Note: No significant differences were found between groups; n = 71 overall.
Abbreviations: BMI = body mass index; F = female; M = male.
Table 2.
Peak exercise performance before and after a 24-week intervention consisting of either no-intervention control, land walking, or water walking (mean ± SD).
| Control |
Land walking |
Water walking |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 24 | Week 48 | Baseline | Week 24 | Week 48 | Baseline | Week 24 | Week 48 | |
| (n = 22) | (n = 19) | (n = 18) | (n = 20) | (n = 19) | (n = 15) | (n = 25) | (n = 20) | (n = 17) | |
| VO2max (mL/kg/min)*† | 29.7 ± 4.41 | 28.0 ± 3.02 | 27.7 ± 2.99 | 28.4 ± 7.85 | 29.5 ± 6.71 | 26.4 ± 5.55 | 28.8 ± 5.05 | 30.1 ± 5.30 | 27.2 ± 3.16 |
| VO2 (L/min)*† | 2.19 ± 0.50 | 1.99 ± 0.42 | 2.02 ± 0.42 | 2.12 ± 0.73 | 2.19 ± 0.68 | 1.97 ± 0.58 | 2.19 ± 0.63 | 2.27 ± 0.76 | 1.97 ± 0.54 |
| Exercise duration (s)*† | 1046 ± 89 | 1071 ± 111 | 1095 ± 88 | 986 ± 236 | 1149 ± 203 | 1119 ± 210 | 992 ± 179 | 1074 ± 178 | 1059 ± 171 |
| HRmax (beats/min) | 166.0 ± 17.0 | 162.0 ± 13.4 | 169.0 ± 13.7 | 164.0 ± 21.0 | 167.0 ± 20.5 | 167.0 ± 20.1 | 166.0 ± 12.7 | 165.0 ± 14.4 | 165.0 ± 13.1 |
Notes: VO2 in relative (mL/kg) and absolute (L/min) values, exercise duration, and HRmax at baseline (Week 0), following the intervention (Week 24) and after a further 24-week no-intervention period (Week 48).
Mixed-models analysis revealed significant group × time interactions for these variables as discussed in the text (*intervention phase, 0–24 weeks; †follow-up phase, 24–48 weeks). Abbreviations: HRmax = maximum heart rate; VO2 = volume of oxygen; VO2max = maximal oxygen uptake.
Adherence to the exercise program was similar across the LW and WW groups, with mean attendance rates of 83.2% ± 4.7% (mean ± SD) for the LW group and 79.2% ± 4.8% for the WW group. Training intensity did not differ between groups over the course of the 24 weeks and was in accordance with the HR intensities dictated by the protocol.
3.1. Impact of the intervention period (Week 0 to Week 24)
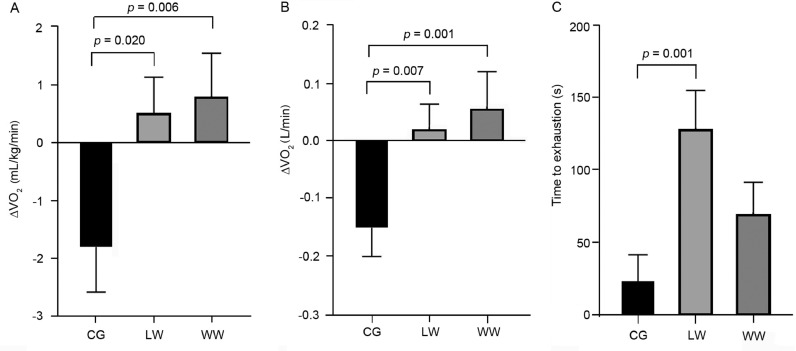
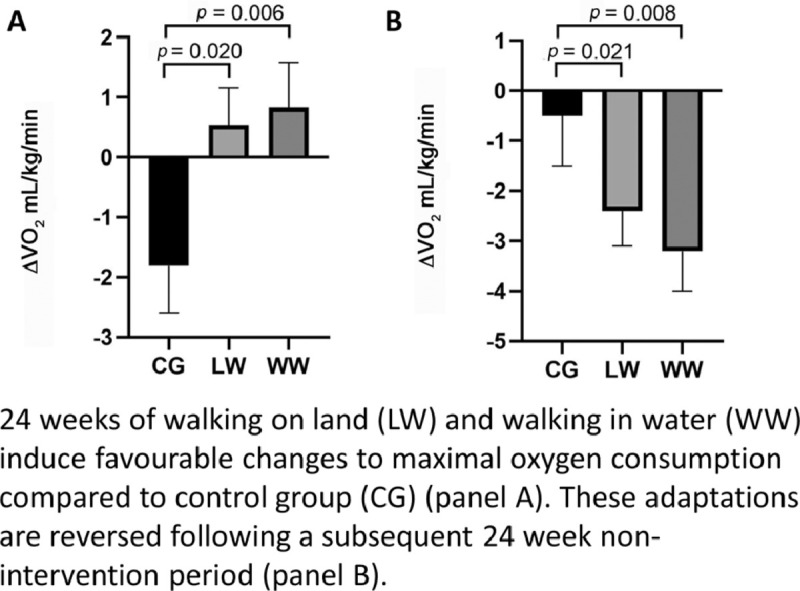
There was a significant main effect for time from Week 0 to Week 24 (p = 0.012). Given that VO2max (mL/kg/min) increased from Week 0 to Week 24 in both exercise groups but decreased in the CG, there was also a significant interaction effect between group and time for both exercise groups vs. the CG (LW vs. CG, p = 0.020; WW vs. CG, p = 0.006) (Fig. 2A). The responses and statistical results from Week 0 to Week 24 time points for relative VO2max (mL/kg/min) were consistent when VO2 was expressed in absolute terms (L/min) (Table 2) (Fig. 2B).
Fig. 2.
Changes in maximal oxygen consumption (VO2max) in mL/kg/min (A), L/min (B), and treadmill time to exhaustion (C), from maximal treadmill tests conducted in male and female participants as a result of 24 weeks of either no exercise (CG), land walking (LW), or water walking (WW). Delta were calculated as Week 24 minus Week 0. All data are presented as mean ± SE. A linear mixed model analysis was conducted.
An interaction effect was also observed for time to exhaustion during the GXT because post hoc analysis indicated significant differences between LW and CG (p = 0.001) but not between WW and CG (p = 0.103) or between LW and WW (p = 0.079) (Fig. 2C). There were no significant between-group differences in HRmax from Week 0 to Week 24—LW and CG (p = 0.505), WW and CG (p = 0.875), or LW and WW (p = 0.595) (Table 2)—nor were there any significant interaction effects for HRmax (all p > 0.05). However, there was a significant time effect (p = 0.040) because HRmax decreased slightly overall from Week 0 (166 ± 16 beats/min) to Week 24 (164 ± 17 beats/min).
No significant main effects for group or time were observed for daily MVPA between baseline and Week 24 (p > 0.05) (Table 3), nor were there any significant interactions for any of the physical activity intensities (p > 0.05).
Table 3.
Physical activity domains before and after a 24-week walking intervention consisting of either no-intervention control, land walking, or water walking (mean ± SD).
| Control |
Land walking |
Water walking |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 24 | Week 48 | Baseline | Week 24 | Week 48 | Baseline | Week 24 | Week 48 | |
| (n = 22) | (n = 17) | (n = 19) | (n = 19) | (n = 19) | (n = 17) | (n = 22) | (n = 20) | (n = 19) | |
| LPA (min/day)a | 194.0 ± 54.6 | 191.9 ± 57.1 | 202.7 ± 54.7 | 214.2 ± 41.3 | 198.8 ± 46.0 | 215.4 ± 44.8 | 200.2 ± 49.8 | 210.9 ± 54.4 | 207.7 ± 44.3 |
| MPA (min/day) | 31.6 ± 15.5 | 32.6 ± 16.2 | 32.3 ± 18.0 | 34.3 ± 16.9 | 38.0 ± 15.3 | 45.8 ± 18.2 | 27.8 ± 14.5 | 28.5 ± 14.1 | 30.9 ± 16.5 |
| MVPA (min/day) | 31.8 ± 15.7 | 33.2 ± 16.8 | 32.9 ± 18.8 | 34.7 ± 17.2 | 38.7 ± 16.2 | 46.5 ± 18.8 | 29.2 ± 14.6 | 31.1 ± 17.5 | 33.2 ± 16.5 |
| VPA (min/day) | 0.28 ± 0.41 | 0.61 ± 1.46 | 0.60 ± 1.64 | 0.52 ± 0.55 | 0.75 ± 1.36 | 0.71 ± 0.98 | 1.30 ± 3.80 | 2.58 ± 7.65 | 2.21 ± 6.30 |
Note: Physical activity was measured at baseline (Week 0), following the intervention (Week 24), and after a further 24-week no-intervention period (Week 48).
Mixed models analysis revealed a significant interaction effect for this variable from the end of the intervention to the end of the follow-up period, as discussed in the text. Abbreviations: LPA = light physical activity; MPA = moderate physical activity; MVPA = moderate-to-vigorous physical activity; VPA = vigorous physical activity.
3.2. Impact of the post-intervention period (Week 24 to Week 48)
After completion of the 24-week (exercise/control group) interventions, a final follow-up was conducted after an additional 24 weeks of no intervention, such that participants were reassessed at 48 weeks. This enabled assessment of the maintenance or reversal of any fitness changes as a result of the initial 24-week intervention.
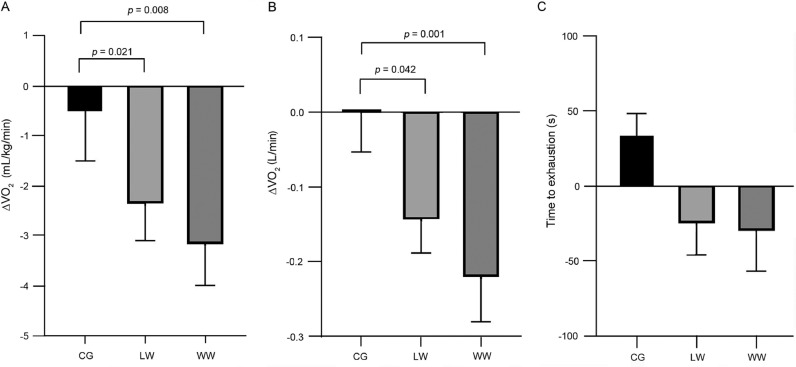
There were no significant between-group effects for VO2max in mL/kg/min (CG vs. LW, p = 0.382; CG vs. WW, p = 0.140; LW vs. WW, p = 0.558) or time effects (p = 0.533) between Week 24 and Week 48 (Table 2). These results were consistent when VO2 was presented in absolute terms (L/min, all p > 0.05). However, due to the reduction in VO2max from Week 24 to Week 48 in the 2 exercise groups (LW and WW), there were significant interaction effects between both exercise groups and the CG for VO2max in relative (mL/kg/min) terms (CG vs. WW, p = 0.008; CG vs. LW, p = 0.021) (Fig. 3A) and VO2max in absolute (L/min) terms (LW vs. CG, p = 0.042; WW vs. CG, p = 0.001) (Fig. 3B).
Fig. 3.
Changes in maximal oxygen consumption (VO2max) in mL/kg/min (A), L/min (B), and treadmill time to exhaustion (s) (C), from maximal treadmill tests conducted in male and female participants in a no-exercise control group (CG), land walking (LW) group, or water walking (WW) group. Delta were calculated as Week 48 minus Week 24. All data are presented as mean ± SE. A linear mixed model analysis was conducted.
No significant group effects for time to exhaustion were evident between Week 24 and Week 48, and no main time effects were found (p > 0.05, Fig. 3C). There was a significant interaction effect between the WW group and the CG (p = 0.031) but not between the CG and the LW group (p = 0.088) or between the LW group and the WW group (p = 0.233) from Week 24 to Week 48 (Table 2). From Week 24 to Week 48, no significant between-group differences were observed in HRmax: LW and CG (p = 0.683), WW and CG (p = 0.757), or between LW and WW (p = 0.924), but there was a significant (p = 0.012) overall time effect from Week 24 (164 ± 17 beats/min) to Week 48 (167 ± 15 beats/min).
No significant group or time effects for LPA, MPA, MVPA, or VPA were observed between Week 24 and Week 48 (all p > 0.05) (Table 3). A significant interaction effect was found for LPA between the LW group and the WW group (p = 0.031). The remaining interaction effects were not significant (p > 0.05).
4. Discussion
Cardiorespiratory fitness is a strong independent predictor of all-cause and cardiovascular disease-related mortality, and increases in fitness are associated with reduced cardiovascular risk.2 The aim of the current study was to investigate the impacts of walking exercise, conducted either in water (WW) or on land (LW) at identical relative HR-based intensities, on aerobic fitness. We found that VO2max improved following both of the 24-week, supervised, and center-based walking interventions, compared to a no-exercise CG. The volume of exercise undertaken was intentionally prescribed to meet current exercise guidelines.6,24 Our investigation supports these recommendations because improvements in fitness were observed in our previously sedentary cohort. Our study also indicates that walking on land or in water elicits similar improvements in aerobic fitness if conducted at the same relative intensities. This may be particularly relevant for individuals who are older and/or new to exercise, given that these participants may benefit from the lower impact forces associated with WW. Our findings also suggest that these modalities could potentially be used independently, or interchangeably, to induce similar cardiovascular adaptations.
Recent reviews indicate that the majority of previous water-based exercise studies are of relatively short duration (e.g., 8 weeks) and have included a variety of exercises performed in water as opposed to WW, which is directly comparable to LW.9,25,26 Some studies involving water-based exercise did not assess changes in cardiorespiratory fitness27,28 or did not include a comparison to a land-based exercise group.29,30 In 1 study, 8 weeks of interval running in water whilst wearing a buoyancy vest increased maximal exercise performance measured on a bicycle ergometer in older women, but land-based exercise was not included in the study.29 Treadmill walking in water and on land for 12 weeks improved aerobic fitness similarly in middle-aged obese adults,31 and we confirm these findings in our longer study in an older cohort. It is pertinent that stationary WW (i.e., treadmill in water or aqua aerobics) may have distinct effects compared to walking forward against water resistance, although some effort has been made to address this issue through the use of resistance jets.31 A study involving older women found that 12 weeks of LW for 60 min, 5 times/week increased VO2max, but 60 min of water-based exercise, including aerobic and resistance-based movements 3 times/week, induced additional improvements in fitness.26 The apparent superior results from water-based exercise in the latter study26 may be due to differences in the frequency and/or modality of exercise between the land and water groups, as opposed to the influence of the exercise being performed in water or on land. We have previously shown that, at matched HRs, there is a similar oxygen cost during WW relative to LW,19 and this supports our approach in the present study to using HR to prescribe and match exercise intensity. Our study is novel and translatable to the general public, in that we included only WW, as opposed to treadmill WW, which has been previously utilized in other studies reported in the scientific literature.31 Our results suggest that, although there are distinctions between LW and WW, aerobic fitness improvements can occur in inactive older adults with both forms of training, and both forms preserve functional capacity compared to doing no level of exercise training.
An interesting aspect of the fitness adaptation to LW and WW in the present study relates to the changes observed in the LW group vs. the WW group in time to exhaustion and VO2max. The LW group increased exercise duration by an average of 50 s more than the WW group, whereas the larger improvement in VO2max was evident in the WW group. This apparent discrepancy may be due to the specificity of training on land. Walking efficiency may have improved in the LW group, reflected by the enhanced time to exhaustion, whereas the VO2max gains were not different and, indeed, tended to be larger in the WW group.11,12 The trend for enhanced VO2max in WW compared to LW may, in turn, reflect the larger impact of the WW modality on lower limb lean body mass (i.e., skeletal muscle mass). In fact, in our recent companion paper pertaining to the body composition impacts of this study, we observed greater increases in lean mass of the lower limbs following WW compared to both LW and CG.13 Indeed, WW may represent a form of combined aerobic and resistance exercise. In this regard, the benefits of WW in older exercise-naive individuals may exceed those associated with land-based training, notwithstanding the apparent weight support of exercising in the water.
It may be expected that the improvements in aerobic fitness in response to engagement in 3 bouts of 50 min of walking per week (150 min/week) would be associated with more substantive fitness gain than we observed. The intensity of exercise conducted by participants was approximately 55%–65% HRR and is, therefore, considered to be of moderate intensity.6 Moderate-intensity exercise is associated with better compliance compared to vigorous exercise,32 and our findings indicate that moderate-intensity exercise is capable of having some positive impact on fitness. A larger benefit in this older cohort might have been observed if the training had continued in the longer term. There is a well-established dose-response curve relating exercise volume to health and fitness benefits,7 and a volume of exercise that exceeded the minimum recommendation for exercise would, therefore, likely incur additional benefit.6 We chose our exercise interventions carefully to represent those recommended in current guidelines, and our aim was to compare the impacts of distinct modalities (WW vs. LW) when both were matched. Although it may appear that the benefits in the 2 exercise groups were relatively modest in absolute terms, it is important to emphasize that, on average, the net difference between the changes we observed in the 2 intervention groups (LW and WW) and the CG was ∼2.5–3 mL/kg/min, which translates to an approximately 13% reduction in cardiovascular mortality.3
Our study design allows some inference to be drawn regarding the maintenance of exercise-training effects. Across the 24- to 48-week time period, participants were no longer in contact with the study staff and no longer attended the supervised exercise sessions. No specific direction was given to the participants in relation to this follow-up stage; they were free to exercise or not. It was of interest to see whether these formerly inactive participants maintained the change in activity levels associated with the supervised training interventions, particularly as we observed excellent adherence during the interventional phase. However, the fitness adaptations measured at Week 24 were completely reversed following the subsequent 24-week no-intervention period (Week 48). This reversal strongly supports the causal link between our exercise interventions and the fitness changes we observed between Week 0 and Week 24. It also confirms that exercise must be continued to maintain fitness levels and, by extension, health benefits. Interestingly, the objective physical activity data we collected suggested that there were no systematic changes in free-living physical activity over the intervention and postintervention phases of the study. Hence, the decreases in fitness we observed between Week 24 and Week 48 were likely due to cessation of the exercise interventions per se, with no evidence for systematic alteration or carry-over of volitional exercise. These data suggest that the maintenance of benefits associated with fitness gain requires ongoing exposure to the stimulus of exercise.
A strength of this study was that it included supervised, center-based exercise training tailored for each individual based on HRR, which ensures strict adherence to the intervention and documentation of nonadherence. A potential limitation of this study is that we focused only on the impact of exercise and did not assess other factors such as diet that may have changed during the intervention. This limitation is mitigated, to some degree, by the fact that participants were randomized to 2 exercise interventions and CG, which assumes some similarity in the nonexercise behaviors. Our study supports existing data showing that adherence to exercise is greater when conducted in a group setting and progresses gradually to support exercise self-efficacy.33 Future research might incorporate psychological methods for promoting behavior change and exercise adherence. Our study included the minimum dose of exercise recommended to induce some health benefit,6 but a greater volume of exercise, achieved by a higher frequency, intensity or duration, may have resulted in more pronounced adaptations in the exercise groups, and this may be the subject of future investigations. Due to the nature of the exercise interventions, it was not possible for the exercise physiologists to be blinded to group allocation during the time of the intervention, and this may also be a limitation of the study. However, each participant was provided with a de-identifying code, so that the person subsequently collating, processing, and analyzing the data output from the GXT indirect calorimetry output file was blinded to group allocation, eliminating bias in the analysis. We arranged for the CG participants to attend seminars once every 6 weeks (a strength of the study), but this did not equal the number of exercise sessions undertaken by the 2 exercise groups, so this discrepancy in contact hours may represent a limitation in our methodology.
5. Conclusion
In summary, we found that 24 weeks of walking increases aerobic capacity, a strong predictor of overall health, compared to remaining inactive. The adaptations were similar regardless of whether walking was conducted in water or on land. Because fitness returned to baseline levels following a subsequent 6-month nonintervention period, it is important that exercise adherence be maintained if the positive adaptations derived from exercise are to be preserved. This study has particular implications for the elderly and for those who may benefit from low-impact musculoskeletal exercise. Older individuals who are not regular exercisers can benefit from the lower impact forces and decreased risk from falls associated with WW, without compromising improvements in cardiorespiratory fitness.
Acknowledgments
Acknowledgments
The authors thank our research participants, exercise supervision staff, and the research staff who contributed to the study. This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (1045204). The NHMRC did not have any involvement in the study design, data collection, or analysis or in the results or writing of the report. DJG is supported by an NHMRC Principal Research Fellowship (APP1080914). NR is supported by a National Heart Foundation of Australia Future Leader Fellowship (ID 101895).
Authors' contributions
DJG, NTL, and KLC conceived the study and obtained grant funding; ER, ALS, HHC, BAM, and LHN performed the exercise training, administered the outcome measure tests, and collected the data; AH analyzed the data, interpreted the results, and wrote the first draft of the manuscript; NDR analyzed and interpreted the Actigraph physical activity data. All authors reviewed and contributed to the final manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Blair S.N., Kampert J.B., Kohl H.W., 3rd, Barlow C.E., Macera C.A., Paffenbarger R.S., Jr Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 2.Ross R., Blair S.N., Arena R., Church T.S., Després J.P., Franklin B.A. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 3.Kodama S., Saito K., Tanaka S., Maki M., Yachi Y., Asumi M. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 4.Herrod P.J.J., Doleman B., Blackwell J.E.M., O'Boyle F., Williams J.P., Lund J.N. Exercise and other nonpharmacological strategies to reduce blood pressure in older adults: a systematic review and meta-analysis. J Am Soc Hypertens. 2018;12:248–267. doi: 10.1016/j.jash.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher G.F., Balady G., Blair S.N., Blumenthal J., Caspersen C., Chaitman B. Statement on exercise: benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1996;94:857–862. doi: 10.1161/01.cir.94.4.857. [DOI] [PubMed] [Google Scholar]
- 6.Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.M. American College of Sport Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 7.Sisson S.B., Katzmarzyk P.T., Earnest C.P., Bouchard C., Blair S.N., Church T.S. Volume of exercise and fitness nonresponse in sedentary, postmenopausal women. Med Sci Sports Exerc. 2009;41:539–545. doi: 10.1249/MSS.0b013e3181896c4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price K.J., Gordon B.A., Bird S.R., Benson A.C. A review of guidelines for cardiac rehabilitation exercise programmes: is there an international consensus? Eur J Prev Cardiol. 2016;23:1715–1733. doi: 10.1177/2047487316657669. [DOI] [PubMed] [Google Scholar]
- 9.Bergamin M., Zanuso S., Alvar B.A., Ermolao A., Zaccaria M. Is water-based exercise training sufficient to improve physical fitness in the elderly. Eur Rev Aging Phys Act. 2012;9:129–141. [Google Scholar]
- 10.Verhagen A.P., Cardoso J.R., Bierma-Zeinstra S.M. Aquatic exercise & balneotherapy in musculoskeletal conditions. Best Pract Res Clin Rheumatol. 2012;26:335–343. doi: 10.1016/j.berh.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Barela A.M., Stolf S.F., Duarte M. Biomechanical characteristics of adults walking in shallow water and on land. J Electromyogr Kinesiol. 2006;16:250–256. doi: 10.1016/j.jelekin.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Barela A.M., Duarte M. Biomechanical characteristics of elderly individuals walking on land and in water. J Electromyogr Kinesiol. 2008;18:446–454. doi: 10.1016/j.jelekin.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Naylor L.H., Maslen B.A., Cox K.L., Spence A.L., Robey E., Haynes A. Land-versus water-walking interventions in older adults: effects on body composition. J Sci Med Sport. 2020;23:164–170. doi: 10.1016/j.jsams.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Breen L., Phillips S.M. Skeletal muscle protein metabolism in the elderly: interventions to counteract the “anabolic resistance” of ageing. Nutr Metab. 2011;8:68. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruoti R.G., Troup J.T., Berger R.A. The effects of nonswimming water exercises on older adults. J Orthop Sports Phys Ther. 1994;19:140–145. doi: 10.2519/jospt.1994.19.3.140. [DOI] [PubMed] [Google Scholar]
- 16.Green D.J., Cox K.L., Badcock J.C., Ainslie P.N., Pestell C., Maslen B.A. Does manipulation of arterial shear stress enhance cerebrovascular function and cognition in the aging brain? Design, rationale and recruitment for the Preventia randomised clinical trial. Ment Health Phys Act. 2018;15:153–163. [Google Scholar]
- 17.Harrison R., Bulstrode S. Percentage weight-bearing during partial immersion in the hydrotherapy pool. Physiother Theory Pract. 1987;3:60–63. [Google Scholar]
- 18.Evans B.W., Cureton K.J., Purvis J.W. Metabolic and circulatory responses to walking and jogging in water. Res Q. 1978;49:442–449. [PubMed] [Google Scholar]
- 19.Pugh C.J., Sprung V.S., Ono K., Spence A.L., Thijssen D.H., Carter H.H. The effect of water immersion during exercise on cerebral blood flow. Med Sci Sports Exerc. 2015;47:299–306. doi: 10.1249/MSS.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 20.Gordon N.F., Pescatello L.S. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. ACSM's guidelines for exercise testing and prescription. [Google Scholar]
- 21.Freedson P.S., Melanson E., Sirard J. Calibration of the Computer Science and Applications, Inc., accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Troiano R.P., Berrigan D., Dodd K.W., Masse L.C., Tilert T., McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 23.Faul F., Erdfelder E., Lang A.G., Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 24.Department of Health, London, UK . The Department of Health; London: 2011. Start Active, Stay Active: a report on physical activity for health from the four home countries’ Chief Medical Officers. [Google Scholar]
- 25.Igarashi Y., Nogami Y. The effect of regular aquatic exercise on blood pressure: a meta-analysis of randomized controlled trials. Eur J Prev Cardiol. 2018;25:190–199. doi: 10.1177/2047487317731164. [DOI] [PubMed] [Google Scholar]
- 26.Bocalini D.S., Serra A.J., Murad N., Levy R.F. Water-versus land-based exercise effects on physical fitness in older women. Geriatr Gerontol Int. 2008;8:265–271. doi: 10.1111/j.1447-0594.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- 27.Shibata Y., Goto Y., Ojima T., Hayasaka S. Effects of water exercise on physiological and psychological health in the Japanese: Kawane Spa Study. Int SportMed J. 2012;13:190–202. [Google Scholar]
- 28.Arca E.A., Martinelli B., Martin L.C., Waisberg C.B., Franco R.J. Aquatic exercise is as effective as dry land training to blood pressure reduction in postmenopausal hypertensive women. Physiother Res Int. 2014;19:93–98. doi: 10.1002/pri.1565. [DOI] [PubMed] [Google Scholar]
- 29.Broman G., Quintana M., Lindberg T., Jansson E., Kaijser L. High intensity deep water training can improve aerobic power in elderly women. Eur J Appl Physiol. 2006;98:117–123. doi: 10.1007/s00421-006-0237-2. [DOI] [PubMed] [Google Scholar]
- 30.Tsourlou T., Benik A., Dipla K., Zafeiridis A., Kellis S. The effects of a twenty-four-week aquatic training program on muscular strength performance in healthy elderly women. J Strength Cond Res. 2006;20:811–818. doi: 10.1519/R-18455.1. [DOI] [PubMed] [Google Scholar]
- 31.Greene N.P., Lambert B.S., Greene E.S., Carbuhn A.F., Green J.S., Crouse S.F. Comparative efficacy of water and land treadmill training for overweight or obese adults. Med Sci Sports Exerc. 2009;41:1808–1815. doi: 10.1249/MSS.0b013e3181a23f7f. [DOI] [PubMed] [Google Scholar]
- 32.Perri M.G., Anton S.D., Durning P.E., Ketterson T.U., Sydeman S.J., Berlant N.E. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. 2002;21:452–458. [PubMed] [Google Scholar]
- 33.Schutzer K.A., Graves B.S. Barriers and motivations to exercise in older adults. Prev Med. 2004;39:1056–1061. doi: 10.1016/j.ypmed.2004.04.003. [DOI] [PubMed] [Google Scholar]