Abstract
Low birth weight (LBW) infants have higher risk of developing insulin resistance and its comorbidities later in life. The concept of “developmental origins of health and disease” suggests that intrauterine and postnatal environments have an important role in increasing these risks. The risk of such adult-onset diseases in LBW infants might be associated with adipose tissue maldevelopment including altered body composition and increased amount of visceral fat, which is the same mechanism as that in children and adults with metabolic syndrome. However, LBW infants often have different characteristics: they are not always overweight or obese over their life course. The inconsistency might be associated with the thrifty phenotype, which is produced in response to impaired growth potential and decreased lean body mass. LBW infants tend to be obese within the limits of impaired growth potential. Through our previous investigations evaluating longitudinal changes in adiponectin levels at an early stage of life, we speculated that probably, the intrauterine life of term infants or the period up to term-equivalent age in preterm infants might be the key age for the development of adipose tissues including fat cells. Because of that, we hypothesized that the smaller number of adipocytes in LBW infants might be associated with overloading of single adipocytes and impaired adipose tissue expandability. The possible mechanisms are discussed from the perspective of adipose tissue maldevelopment in LBW infants.
Keywords: Adipose tissue maldevelopment, Low birth weight infant, Developmental origins of health and disease, Thrifty phenotype, Overloaded adipocyte hypothesis
Introduction
Low birth weight (LBW) is defined as a birth weight of less than 2,500 g, regardless of gestational age. LBW is considered an important indicator of neonatal and infantile morbidity and mortality1, 2). In addition, an accumulating evidence showed that LBW infants have higher risk of developing insulin resistance and its comorbidities such as diabetes mellitus, hypertension, and cardiovascular disease later in life3). Therefore, several international organizations, including the World Health Organization and United Nations International Children's Emergency Fund, have adopted strategies to reduce the incidence of LBW. A recent systematic review showed that the estimated worldwide LBW prevalence decreased from 17.5% to 14.6% between 2000 and 2015 4). On the other hands, in Japan, birth weight had fallen rapidly during the period from 1980s to 2000s, which had been associated with a reduction in family size, increased maternal smoking, decreased maternal body mass index before pregnancy resulting from dieting, and aggressive management of weight gain in pregnancy5). Japan's obsession with slender women is still an important problem for society and the media are paying more attention to the problem of LBW6).
LBW is mainly induced by intrauterine growth restriction and preterm birth. Preterm birth is defined as the birth of a baby at fewer than 37 weeks' gestational age, and it is the most common cause of LBW in both developed and developing countries. In the United States, the rates of preterm births had increased from 10% to 12.5% in the past 25 years7). Advances in perinatal care have resulted in higher survival rates in premature babies. This tendency is particularly evident in very premature babies such as extremely low birth weight (ELBW) infants whose birth weight is less than 1,000 g8–10). In Japan, the mortality rates of ELBW infants admitted to the neonatal intensive care unit (NICU) according to their birth weights demonstrated definite improvement compared to before8), and more than 50% of the infants who were born with a birth weight of less than 500 g survived until discharge from the NICU9). With this background, it is important to improve the long-term prognosis of these infants including the neurodevelopmental outcomes and risks of adult-onset diseases later in life11). The concept of “developmental origins of health and disease (DOHaD)” suggests that intrauterine and postnatal environments have an important role in increasing the risks of adult-onset disease. This article provides an overview of DOHaD concept, focusing on the maldevelopment of fat tissues and insulin resistance in LBW infants later in life.
Developmental Origins of Health and Disease
In the late 1980s to early 1990s, Barker et al. reported that LBW infants have higher risks of diabetes mellitus and cardiovascular disease later in life12–18). Barker provided a personal account of such findings from epidemiological observations, which led to the fetal origin or fetal programming hypothesis (often called “Barker's hypothesis”)19). According to the hypothesis, fetuses who experience malnutrition during intrauterine life would often present intrauterine growth restriction and simultaneously acquire the thrifty phenotype with good energy efficiency through altered mechanisms in endocrinology and metabolism. They tend to be obese due to comparatively excessive nutrition after birth and are at higher risk for glucose intolerance and cardiovascular diseases20). Barker's hypothesis stimulated a great deal of worldwide interest and activity in the area of developmental plasticity.
After that, the hypothesis had been supported by many animal studies21) and epidemiological surveys such as that of Dutch famine22–27), suggesting that LBW was linked with an increased risk of not only lifestyle-related diseases but also other chronic diseases including microalbuminuria, obstructive airway disease, increased stress responsiveness, some types of cancer, and mental and neurological disorders24–27). Moreover, some previous studies showed that rapid weight gain during infancy was also associated with increased risk of subsequent obesity28) and visceral fat accumulation in adulthood29). With this background, the concept of DOHaD evolved to resolve some of the limitations of Barker's hypothesis30, 31). One of the most important types of fetal responses to various environments is “predictive adaptive responses.” The responses are not simply the effects of constraint in utero, but rather mechanisms by which the fetus uses an early environmental cue to predict its future. What is essential in the concept is a mismatch between prenatal and postnatal environments. It is advantageous if the predicted future and actual future environments match. On the contrary, it is disadvantageous if the predicted future and actual future environments do not match. Hence, the greater the mismatch, the greater the risk. The mechanisms underlying the predictive adaptive responses are likely to involve epigenetic changes such as DNA methylation32). Some previous studies showed that altered DNA methylation links intrauterine events with the onset of diseases later in life33). However, the concept of predictive adaptive response can explain only one component of the risks of chronic disease later in life in LBW infants. For example, it cannot explain the risk of metabolic disorder in individuals born with higher birth weight, although the relation of the prevalence of diabetes with birth weight is U shaped34). In addition, a previous systematic review showed that high, but not LBW is associated with increased risk of obesity later in life. No previous study has reported a linear inverse relation between birth weight and obesity risk later in life35). These facts imply that a higher risk of metabolic disorders in LBW infants might be induced by different mechanisms, although obesity usually has the most important role in the development of insulin resistance in adults with metabolic syndrome.
The LBW phenotype is produced in response to the adaptive changes occurring in utero such as “trade-off,” which is another type of developmental response to environmental influences. The advantage of an immediate alteration in developmental pattern ensures short-term survival in utero. It must be traded off against potential disadvantages in a later environment30, 31). For example, a general response of the fetus to maternal nutritional deprivation is to reduce feral growth, and an impaired growth trajectory may become irreversible if fetal undernutrition is prolonged. This change is clearly of benefit the fetus, helping it optimize the use of limited nutrients, but it may have postnatal costs30). Premature delivery following maternal infection is one of the typical examples of “trade-off,” in which the benefit is leaving the infected intrauterine environment early, while the cost is permanent stunting with maldevelopment of organs. For example, both prematurity and LBW are the most consistent clinical surrogates for a low nephron number, which is associated with an increased risk of hypertension later in life36). A previous study suggested that the kidney continued to form postnatally in preterm neonates, but glomerulogenesis ceased after 40 days37). Decreased nephron number and subsequent risk of hypertension and chronic kidney disease are induced by “trade-off,” but not the “predictive adaptive response.” Similarly, it is highly likely that an increased risk of insulin resistance and its comorbidities in LBW infants might be induced by many factors associated with the maldevelopment of organs.
Clinical Feature of Thrifty Phenotype
LBW infants develop postnatal growth problems. First, persistent short stature is one of the most frequent complications of being small for gestational age (SGA)38). SGA and being moderately preterm are associated with short stature during the first 5 years of life39). SGA children are at high risk of developing permanent short stature, and 10% continue to fall below the 3rd percentile of height until adulthood40). Some previous studies showed that the final height of very premature babies is shorter even if they reach adulthood41, 42). Morisaki et al. reported that adult height in Japan has started to decline for those born after 1980, a trend that may be attributed to an increase in the prevalence of LBW births over time43). Although the mechanism underlying the short stature in SGA infants remains controversial, it is probably a result of the abnormalities in the growth hormone-insulin-like growth factor (IGF) axis44). The cord blood and placenta of SGA infants have lower concentrations of IGF-1, and the IGF-1 levels remain low in SGA children with short stature45). Second, LBW infants have altered body composition. For example, postnatal catch-up growth in growth-restricted infants resulted from head growth and adipose tissue accumulation46). Rapid weight gain during infancy in SGA children seemed to be associated with increased fat mass rather than lean body mass41). According to a recent meta-analysis, the free fat mass of preterm LBW infants decreased by 460 g and their body fat percentage increased by 3% at term- equivalent age47). In other words, preterm LBW infants have relatively large amount of fat tissue at term-equivalent age compared with term infants at birth. LBW is associated with lower lean mass even in adults48). Some previous studies showed that nutrition intake in premature infants during their NICU stay has an impact on body composition in infants49), children50), and adults51). Sufficient protein and calorie intake during the first several weeks of life in premature infants might be important for optimistic body composition throughout their life49, 51). In addition, chronic cortisol exposures alter body composition through lean tissue catabolism and fat deposition52). Cortisol levels are higher in preterm infants than in term infants; therefore, they have an impact on their body composition. As a matter of fact, stress attenuation by massage therapy for male preterm infants during their NICU stay improved growth quality by decreasing body fat deposition53). Third, LBW infants tend to have higher accumulation of visceral adipose tissue. Uthaya et al.54) reported that preterm infants had a highly significant decrease in subcutaneous adipose tissue and significantly increased intra-abdominal adipose tissue. Some previous studies showed that LBW was associated with greater visceral adiposity in adolescents55) and adults56, 57). Moreover, ELBW survivors in early adulthood have higher proportion of liver and pancreatic fat than individuals born with normal birth weight58).
It remains controversial why LBW infants have higher risk of insulin resistance development later in life, but some of the abovementioned characteristics can be associated with such risk in LBW infants as shown in Fig. 1. First, the development of insulin resistance is mostly associated with the accumulation of excessive fat in the body, especially in the perivisceral area of the abdomen in children and adolescents59). Therefore, the accumulation of visceral adipose tissue is probably associated with insulin resistance development in LBW infants. Second, both short stature and altered body composition such as decreased lean body mass are beneficial for the effective utilization of energy, because resting energy expenditure, which usually accounts for more than 60% of the total energy, is directly related to the amount of lean body mass and is more active metabolically than fat mass60). Lower lean body mass was associated with lower resting energy expenditure in young adults born with very low birth weight (VLBW)52, 61).
Fig. 1.
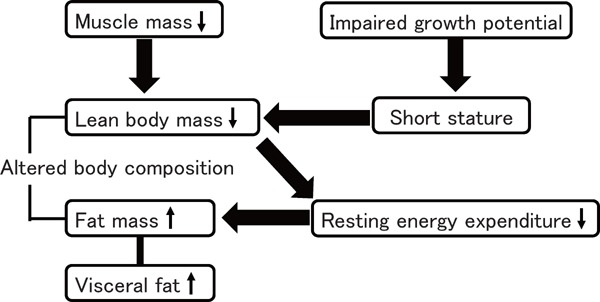
Clinical feature of a thrifty phenotype
Both impaired growth potential and decreased lean body mass may be essential factor that triggers the production of a thrifty phenotype. Thrifty phenotype is beneficial to acquiring adipose tissue by lowering the resting energy expenditure, but it may occur within the limits of impaired growth potential.
Additionally, there is a measurable association between insulin sensitivity and preterm birth in infancy and early childhood; however, in late childhood and adulthood, the strength of this association reduces and current body composition becomes the variable most strongly associated with insulin sensitivity62). One possible explanation why LBW infants with relatively lower body weight and body mass index have higher risk of insulin resistance is that both impaired growth potential and decreased lean body mass may be essential in the production of thrifty phenotype, and LBW infants tend to be obese within the limits of impaired growth potential, because the good energy efficiency has an important role in acquisition of fat mass by lowering the resting energy expenditure. This finding is consistent with those of previous studies, showing that shorter final height was associated with less favorable metabolic profiles in young adults with VLBW42) and that adults who were small at birth and thin during infancy had a higher risk of coronary event63, 64), implying that impaired growth potential might be one of the clinical surrogate markers of thrifty phenotype.
Adipose Tissue Development at the Early Stage of Life
The fat mass can expand by increasing the average fat cell volume and/or the number of adipocytes. Not only fat cell size but also adipocyte number are important for adipose tissue accumulation and insulin resistance development65). For example, individuals with a very small number of adipocytes might become lean adults with an increased risk for glucose intolerance similar to those with lipoatrophic diabetes mellitus66). On the contrary, individuals with a morbidly large number of adipocytes have higher risk of obesity later in life with comparatively low risk of glucose intolerance due to the increased amount of adipose tissue; this finding is similar to that of a previous study using a mouse model lacking leptin while overexpressing adiponectin67). The numbers of fat cells remained constant in adulthood in lean and obese individuals, even after a marked weight loss. The number of adipocytes increases during childhood and adolescence and remains constant during adulthood in both lean and obese individuals. Major weight loss by bariatric surgery results in a significant decrease in cell volume; however, it fails to reduce the adipocyte cell number68). These results indicate that the number of adipocytes is set at least until childhood and adolescence. By contrast, a fetus usually has smaller adipocytes. In term infants, the adipose tissue rapidly expands mainly due to the increased number of small adipocytes during the second half of fetal life. Fat cells enlarge during the first 12 months of life after birth, while the number of fat cells remains unchanged69). Probably, intrauterine life in term infants, or the period up to term-equivalent age in preterm infants, might be the key age when adipose tissues develop and the number of fat cells increases.
Adiponectin consists of a carboxyl-terminal globular domain and an amino-terminal collagenous domain, and it circulates in the serum to form several different molecular weight species including low-molecular-weight adiponectin (LMW-Ad), middle-molecular-weight adiponectin (MMW-Ad), and high-molecular-weight adiponectin (HMW-Ad)70). Adiponectin is secreted exclusively by adipocytes and has a beneficial role in insulin sensitivity. HMW-Ad is considered the active form of this protein71), because the HMW-Ad level is a better indicator of glucose intolerance than total adiponectin (T-Ad) levels72). Paradoxically decreased levels of adiponectin in individuals with obesity are considered to be a result of adipocyte hypertrophy73). On the contrary, the accumulation of fat tissue induced by an increase in the number of smaller adipocytes is associated with increased production of adiponectin67). With this background, we previously investigated the longitudinal changes in the serum levels of adiponectin and its multimers during infancy in term and preterm infants with LBW74–79). Combined results obtained in these studies are shown in Table 1. As a result, HMW- Ad is the main form of this protein found in both term and preterm infants with LBW at birth74–76). The serum levels of T-Ad and HMW-Ad are much higher than the levels in children and adults as shown in some previous investigations. Comparing between term and preterm infants with LBW, the serum levels of T-Ad and HMW-Ad in preterm infants with LBW were significantly lower than the levels in term infants at birth. In addition, birth weight and gestational age were positively associated with both T-Ad and HMW-Ad levels in term and preterm infants with LBW74, 76). An increase in the number of small adipocytes during the second half of fetal life may contribute to the increased levels of adiponectin; therefore, this is probably associated with the drastically increased adiponectin levels seen during this period. Furthermore, we evaluated the adipose tissue accumulation and distribution by performing a computed tomography scan at the levels of umbilicus; results showed that the accumulation of subcutaneous fat, but not visceral fat, was a predictor of adiponectin levels in preterm infants with LBW at term-equivalent age79). These results suggest that there might be a room for an increase in fat cell number at least up to term- equivalent age in preterm infants with LBW. In addition, the serum levels of T-Ad and HMW-Ad at 6 month-equivalent age were almost the same as those in term-equivalent age. However, the T-Ad and HMW-Ad levels decreased until the 12 month-equivalent age in both term and preterm infants75, 78). HMW-Ad levels at term-equivalent age were only a significant determinant of the changes in HMW-Ad between the term- and 12 month-equivalent ages in term and preterm infants with LBW, suggesting that the changes in HMW-Ad levels during infancy might be determined at least to a certain degree up to term-equivalent age in term and preterm infants with LBW75, 78). Interestingly, T-Ad levels were significantly higher in preterm infants with LBW at term- equivalent age than the levels observed in term infants at birth, probably because they have larger amounts of fat mass if they did not experience extrauterine growth restriction at term-equivalent age76). In addition, the serum levels of T-Ad and HMW-Ad had not only a positive association with the subcutaneous fat tissue in preterm infants with LBW at term-equivalent age but also a negative association with gestational age in the multiple regression analysis79). Taken together, very preterm infants may have higher amounts of fat mass and comparatively low levels of T-Ad and HMW-Ad at term-equivalent age. As mentioned, in term infants, the adipose tissue rapidly expands mainly because of the increased number of small adipocytes during the second half of fetal life, and this is probably associated with the drastically increased adiponectin levels observed during this period. The fat cells enlarge during the first 12 months of life after birth, while the number of fat cells remains unchanged69). If we apply this to preterm infants, fat cell hypertrophy might have occurred to a certain degree, although the fat cell numbers increase between birth and term-equivalent age in preterm infants with LBW. Adipocyte hypertrophy may induce comparatively low T-Ad and HMWAd levels in very preterm infants at term-equivalent age. The current hypothesis, however, is not supported by sufficient data. Hence, further studies are warranted to clarify the period of fat tissue development including the increase in the number and size of adipocyte.
Table 1. Adiponectin levels and its correlation with adipose tissue accumulation during infancy.
| At birth | At term* | At 6 months* | At 12 months* | ||
|---|---|---|---|---|---|
| Term infants | Serum levels | High | High | Middle | |
| Correlation | Yes (but not strong) | No | No | ||
| Preterm infants | Serum levels | Low | High | High | Middle |
| Correlation | Yes | Yes | No | No | |
Presented as corrected age in preterm infants
Overloaded Adipocyte Hypothesis
The “adipose tissue expandability” hypothesis, proposed by Virtue and Vidal-Puig, explains the development of insulin resistance in obesity and the apparent paradox that insulin resistance might occur when there is a deficit of adipose tissue80). This hypothesis proposes that adipose tissue has a limited maximal capacity to increase in mass, which is determined on an individual basis by environmental and genetic factors81). In the hypothesis, insulin sensitivity remains high as long as adipose tissue can expand. Once the point of maximal adipose tissue expansion is reached, metabolic complications ensue rapidly80). This is caused by the overfilling of subcutaneous adipocytes, resulting in lipid deposition in non-subcutaneous adipose tissue and in non- adipose organs such as the liver, muscle, or pancreas82). If this hypothesis applies to LBW infants, they will possibly develop impaired adipose tissue expandability, because they tend to be relatively lean and have higher risk of insulin resistance later in life.
Here, we propose one hypothesis as a possible explanation for impaired adipose tissue expandability in LBW infants, called the “overloaded adipocyte hypothesis” (Fig. 2). As previously stated, the number of adipocytes throughout their lives is probably set during the early stage of life68), especially during the second half of fetal life in term infants69) and up to term-equivalent age in preterm infants. LBW infants, whether term infants or preterm infants, are often exposed to malnutrition during these periods.
Fig. 2.
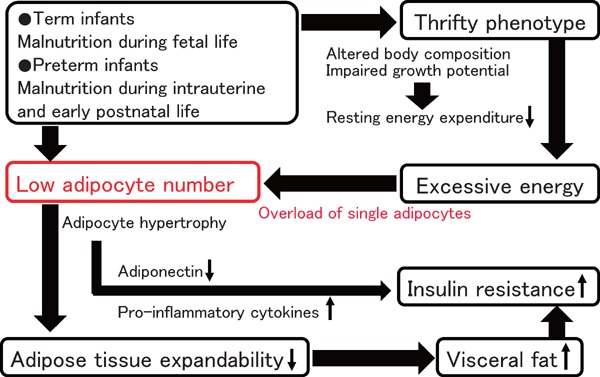
Overloaded adipocyte hypothesis
The number of adipocytes throughout their life is probably set during the early stage of life. Smaller number of adipocytes may be associated with an overload of single adipocytes and impaired adipose tissue expandability. Once the point of maximal adipose tissue expansion is reached, metabolic complications easily occur.
Therefore, LBW infants may have smaller number of adipocytes throughout their life, which is influenced by malnutrition during these periods. The number of adipocytes is likely a strong determinant of adipose tissue expandability, because not only fat cell size but also fat cell number is important for adipose tissue accumulation65). Furthermore, LBW infants with thrifty phenotype easily gain excessive energy. However, smaller number of adipocytes in LBW infants would induce an overload of energy in a single adipocyte. There is a limit to maximal hypertrophy in a single adipocyte; therefore, it would be associated with impaired adipose tissue expandability in total. As a matter of fact, our pilot study aimed to test the hypothesis showed that the adipocyte size of LBW male infants during the infantile period is larger than that of term appropriate-for- gestational-age (AGA) infants; nevertheless, LBW male infants were rather smaller and lighter than term AGA infants (unpublished data). Other than that, adipose tissue fibrosis is possibly associated with impaired adipose tissue expandability in LBW infants, because adipose tissue fibrosis limited adipocyte hypertrophy83, 84). Further studies are required to test the “overloaded adipocyte hypothesis.”
Summary
The mechanisms underlying the higher risk of insulin resistance in LBW infants remain controversial. Adipose tissue maldevelopment such as altered body composition, increased amount of visceral fat, and altered number and/or size of adipocyte is one of the possible mechanisms of insulin resistance in LBW infants. Impaired growth potential and decreased lean body mass may explain at least a part of thrifty phenotype in LBW infants. Through our previous investigations, we speculated that intrauterine life in term infants, or the period up to term-equivalent age in preterm infants, might be the key age for fat tissue development including the increase in the number of fat cells throughout their life. The Smaller number of adipocytes in LBW infants might be associated with overloading of single adipocytes and impaired adipose tissue expandability, which can explain the coincidence of being comparatively lean and the higher risk of insulin resistance development in LBW infants.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP16K19695.
Conflict of Interest
I declare that no financial support or relationship will pose a conflict of interest, except for a grant from JSPS KAKENHI Grant Number JP16K19695.
References
- 1). McCormick MC: The contribution of low birth weight to infant mortality and childhood mortality. N Eng J Med, 1985; 312: 82-90 [DOI] [PubMed] [Google Scholar]
- 2). Mclntire DD, Bloom SL, Casey BM, Leveno KJ: Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med, 2999; 340: 1234-1238 [DOI] [PubMed] [Google Scholar]
- 3). Gluckmann PD, Hanson MA, Cooper C, Thomburg KL: Effects of in utero and early life conditions on adult health and disease. N Eng J Med, 2008; 359: 61-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Blencowe H, Krasevec J, de Onis M, Blavk RE, An X, Stevens GA, Borghi E, Hayashi C, Estevez D, Cegolon L, Shiekh S, Ponce Hardy V, Lawn JE, Cousens S: National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health, 2019: 7: e849-e860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Gluckman PD, Seng CY, Fukuoka H, Beedle AS, Hanson MA: Low birthweight and subsequent obesity in Japan. Lancet, 2007; 369: 1081-1082 [DOI] [PubMed] [Google Scholar]
- 6). Normile D: Staying slim during pregnancy carries a price. Science, 2018; 361: 440. [DOI] [PubMed] [Google Scholar]
- 7). Goldenberg RL, Culhane JF: Low birth weight in the United States. Am J Clin Nutr, 2007; 85: 584S-590S [DOI] [PubMed] [Google Scholar]
- 8). Itabashi K, Horiuchi T, Kusuda S, Kabe K, Itani Y, Nakamura T, Fujimura M, Matsuo M: Mortality rates for extremely low birth weight infants born in Japan in 2005. Pediatrics, 2009; 123: 445-450 [DOI] [PubMed] [Google Scholar]
- 9). Inoue H, Ochiai M, Yasuoka K, Tanaka K, Kurata H, Fujiyoshi J, Matsushita Y, Suga S, Nonaka K, Taguchi T, Kato K, Ohga S, Neonatal Research Network of Japan (NRNJ) : Early mortality and morbidity in infants with birth weight of 500 grams or less in Japan. J Pediatr, 2017; 190: 112-117 [DOI] [PubMed] [Google Scholar]
- 10). Iglesias-Leboreiro J, Bernardez-Zapata I, Ramírez-Haua J, González-Morán R, Rendón-Macías ME: Mortality in extremely low-birth-weight neonates in México city (1985-2009). Int J Pediatr, 2010; 2010: 265146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Roberts G, Cheong JL: Long-term growth and general health for the tiniest or most immature infants. Semin Fetal Neonatal Med, 2014; 19: 118-124 [DOI] [PubMed] [Google Scholar]
- 12). Barker DJ, Osmond C: Infant mortality, childhood nutrition, and ischaemic heart disease in Enfland and Wales. Lancet, 1986; 1: 1077-1081 [DOI] [PubMed] [Google Scholar]
- 13). Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ: Weight in infancy and death from ischaemic heart disease. Lancet, 1989; 2: 577-580 [DOI] [PubMed] [Google Scholar]
- 14). Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS: Fetal nutrition and cardiovascular disease in adult life. Lancet, 1989; 341: 938-941 [DOI] [PubMed] [Google Scholar]
- 15). Barker DJ, Hale CN, Fall CH, Osmond C, Phipps K, Clark PM: Type 2 (non-insulin- dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia, 1993; 36: 62-67 [DOI] [PubMed] [Google Scholar]
- 16). Phipps K, Barker GJ, Hales CN, Fall CH, Osmond C, Clark PM: Fetal growth and impaired glucose tolerance in men and women. Diabetologia, 1993; 36: 225-228 [DOI] [PubMed] [Google Scholar]
- 17). Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD: Fetal and infant growth and impaired glucose tolerance at age 64. BMJ, 1991; 303: 1019-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Fall CH, Osmond C, Barker DJ, Clark PM, Hales CN, Stirling Y, Meade TW: Feral and infant growth and cardiovascular risk factors in women. BMJ, 1995; 310: 428-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Barler DJ: The origins of the developmental origins theory. J Intern Med, 2007; 261: 412-417 [DOI] [PubMed] [Google Scholar]
- 20). Ozanne SE, Hales CN: The long-term consequences of intra-uterine protein malnutrition for glucose metabolism. Proc Nutr Soc, 1999; 58: 615-619 [DOI] [PubMed] [Google Scholar]
- 21). MuMullen S, Mostyn A: Animal models for the study of the developmental origins of health and disease. Proc Nutr Soc, 2009; 68: 306-320 [DOI] [PubMed] [Google Scholar]
- 22). Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP: Glucose tolerance in adults after prenatal exposure to famine. Lancet, 1998; 351: 173-177 [DOI] [PubMed] [Google Scholar]
- 23). de Rooij SR, Painter RC, Phillips DI, Osmond C, Michels RP, Godsland IF, Bossuyt PM, Bleker OP, Roseboom TJ: Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care, 200; 29: 1897-1901 [DOI] [PubMed] [Google Scholar]
- 24). Roseboom T, de Rooij S, Painter R: The Dutch famine and its long-term consequences for adult health. Early Hum Dev, 2006; 82: 485-491 [DOI] [PubMed] [Google Scholar]
- 25). Painter RC, Roseboom TJ, Belker OP: Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol, 2005; 20: 345-352 [DOI] [PubMed] [Google Scholar]
- 26). Kyle UG, Pichard C: The Dutch famine of 1944-1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care, 2006; 9: 388-394 [DOI] [PubMed] [Google Scholar]
- 27). de Rooij SR, Painter RC, Phillips DI, Osmond C, Michels RP, Bossuyt PM, Bleker OP, Roseboom TJ: Hypothalamic-pituitary-adrenal axis activity in adults who were prenatally exposed to the Dutch famine. Eur J Endoclinol, 2006; 155: 153-160 [DOI] [PubMed] [Google Scholar]
- 28). Ong KK, Loos RJ: Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr, 2006; 95: 904-908 [DOI] [PubMed] [Google Scholar]
- 29). Demerath EW, Reed D, Choh AC, Scloway L, Lee M, Czerwinski SA, Chumlea WC, Siervogel RM, Towne B: Rapid postnatal weight gain and visceral adiposity in adulthood: the Fels Longitudinal Study. Obesity (Silver Spring), 2009; 17: 2060-2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Gluckman PD, Hanson MA, Spencer HG, Bateson P: Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc Biol Sci, 2005; 272: 671-677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Gluckman PD, Hanson MA, Beedle AS: Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol, 2007; 19: 1-19 [DOI] [PubMed] [Google Scholar]
- 32). Qiu J: Epigenetics: unfinished symphony. Nature, 2006; 441: 143-145 [DOI] [PubMed] [Google Scholar]
- 33). Reynolds RM, Jacobsen GH, Drake AJ: What is the evidence in humans that DNA methylation changes link events in utero and later life disease? Clin Endocrinol (Oxf), 2013; 78: 814-822 [DOI] [PubMed] [Google Scholar]
- 34). McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH: Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ, 1994; 306: 942-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Harder T, Schellong K, Stupin J, Dudenhausen JW, Plagemann A: Where is the evidence that low birthweight leads to obesity? Lancet, 2007; 369: 1859. [DOI] [PubMed] [Google Scholar]
- 36). Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, Vikse BE: Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet, 2013; 382: 273-283 [DOI] [PubMed] [Google Scholar]
- 37). Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE: Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol, 2004; 7: 17-25 [DOI] [PubMed] [Google Scholar]
- 38). Labarta JI, Ruiz JA, Molina I, De Arriba A, Mayayo E, Longás AF: Growth and growth hormone treatment in short stature children born small for gestational age. Pediatr Endocrinol Rev, 2009; 6: 350-357 [PubMed] [Google Scholar]
- 39). Lindström L, Ahlsson F, Lundgren M, Bergman E, Lampa E, Wikström AK: Growth patterns during early childhood in children born small for gestational age and moderate preterm. Sci Rep, 2019; 9: 11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Cho WK, Suh BK: Catch-up growth and catch-up fat in children born small for gestational age. Korean J Pediatr, 2016; 59: 1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Roberts G, Cheong JL: Long-term growth and general health for the tiniest or most immature infants. Semin Fetal Neonatal Med, 2014; 19: 118-124 [DOI] [PubMed] [Google Scholar]
- 42). Sato R, Maekawa M, Genma R, Shirai K, Ohki S, Morita H, Suda T, Watanabe H: Final height and cardiometabolic outcomes in young adults with very low birth weight (< 1,500 g). PLoS One, 2014; 9: e112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Morisaki N, Urayama KY, Yoshii K, Subramanian SV, Yokoya S: Ecological analysis of secular trends in low birth weight births and adult height in Japan. J Epidemiol Community Health, 2017; 71: 1014-1018 [DOI] [PubMed] [Google Scholar]
- 44). Cianfarai S, Ladaki C, Geremia C: Hormonal regulation of postnatal growth born small for gestational age. Horm Res, 2006; 65: 70-74 [DOI] [PubMed] [Google Scholar]
- 45). Renes JS, van Doorn J, Hokken-Koelega ACS: Current insights into the role of the growth hormone-insulin-like growth factor system in short children born small for gestational age. Horm Res Paediatr, 2019; 11: 1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Modi N, Thomas EL, Harrington TA, Uthaya S, Doré CJ, Bell JD: Determinants of adiposity during preweaning postnatal growth in appropriately grown and growth-restricted term infants. Pediatr Res, 2006; 60: 345-348 [DOI] [PubMed] [Google Scholar]
- 47). Johnson MJ, Wootton SA, Leaf AA, Jackson AA: Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics, 2012; 130: e640-e649 [DOI] [PubMed] [Google Scholar]
- 48). Ylihärsilä H, Kajantie E, Osmond C, Forsén T, Barker DJ, Eriksson JG: Birth size, adult body composition and muscle strength in later life. Int J Obes (Lond), 2007; 31: 1392-1399 [DOI] [PubMed] [Google Scholar]
- 49). Ramsel SE, Gray HL, Christiansen E, Boys C, Georgieff MK, Demerath EW: Greater early gains in fat free mass, but not fat mass, are associated with improved neurodevelopment at 1 year corrected age for prematurity in very low birth weight preterm infants. J Pediatr, 2016; 173: 108-115 [DOI] [PubMed] [Google Scholar]
- 50). Stutte S, Gohlke B, Peiler A, Schreoner F, Born M, Bartmann P, Woelfle J: Impact of early nutrition on body composition in children aged 9.5 years born with extremely low birth weight. Nutrients, 2017; 9: E124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Matinolli HM, Hovi P, Männistö S, Sipola-Leppänen M, Eriksson JG, Mäkitie O, Järvenpää AL, Andersson S, Kajantie E: Early protein intake is associated with body composition and resting energy expenditure in young adults born with very low birth weight. J Nutr, 2015; 145: 2084-2091 [DOI] [PubMed] [Google Scholar]
- 52). Savage MO, Scommegna S, Carroll PV, Ho JT, Monson JP, Besser GM, Grossman AB: Growth in disorders of adrenal hyperfunction. Horm Res, 2002; 58: 39-43 [DOI] [PubMed] [Google Scholar]
- 53). Moyer-Mileur LJ, Haley S, Slater H, Beachy J, Smith SL: Massage improves growth quality by decreasing body fat deposition in male preterm infants. J Pediatr, 2013; 162: 490-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Uthaya S, Thomas EL, Hamilton S, Doré CJ, Bell J, Modi N: Altered adiposity after extremely preterm birth. Pediatr Res, 2005; 57: 211-215 [DOI] [PubMed] [Google Scholar]
- 55). Stanfield BK, Fain MB, Bhatia J, Gutin B, Nguyen JT, Pollock NK: Nonlinear relationship between birth weight and visceral fat in adolescents. J Pediatr, 2016; 174: 185-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Rolfe Ede L, Loos RJ, Druet C, Stolk RP, Ekelund U, Griffin SJ, Forouhi NG, Wareham NJ, Ong KK: Association between birth weight and visceral fat in adults. Am J Clin Nutr, 2010; 92: 347-352 [DOI] [PubMed] [Google Scholar]
- 57). Ronn RF, Smith LS, Andersen GS, Carstensen B, Bjerregaard P, Jorgensen ME: Birth weight and risk of adiposity among adult Inuit in Greenland. PLoS One, 2014; 9: e115976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Crane JD, Yellin SA, Ong FJ, Singh NP, Konyer N, Noseworthy MD, Schmidt LA, Saigal S, Morrison KM: ELBW survivors in early adulthood have higher hepatic, pancreatic and subcutaneous fat. Sci Rep, 2016; 6: 31560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59). Maffeis C, Morandi A: Body composition and insulin resistance in children. Eur J Clin Nutr, 2018; 72: 1239-1245 [DOI] [PubMed] [Google Scholar]
- 60). Tur JA, Bibiloni MDM: Anthropometry, body composition and resting energy expenditure in human. Nutrients, 2019; 11: E1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Sipola-Leppänen M, Hovi P, Andersson S, Wehkalampi K, Vääräsmälo M, Strang-Karlsson S, Jävenpää AL, Mäkitie O, Eriksson JG, Kajantie E: Resting energy expenditure in young adults born preterm — the Helsinki study of very low birth weight adults. PLoS One, 2011; 6: e17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62). Tinnion R, Gillone J, Cheetham T, Embleton N: Preterm birth and subsequent insulin sensitivity: a systematic review. Arch Dis Child, 2014; 99: 362-368 [DOI] [PubMed] [Google Scholar]
- 63). Barker DJ, Osmond C, Forsén TJ, Kajantie E, Erikson JG: Trajectories of growth among children who have coronary events as adults. N Engl J Med, 2005; 353: 1802-1809 [DOI] [PubMed] [Google Scholar]
- 64). Eriksson JG: Epidemiology, genes and the environment: lessons learned from the Helsinki Birth Cohort Study. J Intern Med, 2007; 261: 418-425 [DOI] [PubMed] [Google Scholar]
- 65). Vishvanath L, Gupta RK: Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest, 2019; 129: 4022-4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Cortés VA, Fernández-Galilea M: Lipodystrophies: adipose tissue disorders with severe metabolic implications. J Phjysiol Biochem, 2015; 71: 471-478 [DOI] [PubMed] [Google Scholar]
- 67). Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE: Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest, 2007; 117: 2621-2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68). Spalding KL, Arner E, Westermark PO, Bernard S, Bernard S, Buchholz BA, Bergmann O, Mlomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P: Dynamics of fat cell turnover in humans. Nature, 2008; 453: 783-778 [DOI] [PubMed] [Google Scholar]
- 69). Kiess W, Petzold S, Töpfer M, Garten A, Blűher S, Kapellen T, Körner A, Kratzsch J: Adipocytes and adipose tissue. Best Pract Res Clin Endocrinol Metab, 2008; 22: 135-153 [DOI] [PubMed] [Google Scholar]
- 70). Heiker JT, Kosel D, Beck-Sickinger AG: Molecular mechanisms of signal transduction via adiponectin and adiponectin receptors. Biol Chem, 2010; 391: 1005-1018 [DOI] [PubMed] [Google Scholar]
- 71). Hada Y, Yamauchi T, Waki H, Tsuchida A, Hara K, Yago H, Miyazaki O, Ebinuma H, Kadowaki T: Selective purification and characterization of adiponectin multimer species from human plasma. Biochem Biophys Res Commun, 2007; 356: 487-493 [DOI] [PubMed] [Google Scholar]
- 72). Araki S, Dobashi K, Kubo K, Asayama K, Shirahara A: High molecular weight, rather than total, adiponectin levels better reflect metabolic abnormalities associated with childhood obesity. J Clin Endocrinol Metab, 2006; 91: 5113-5116 [DOI] [PubMed] [Google Scholar]
- 73). Ito A, Suganami T, Miyamoto Y, Yoshimasa Y, Takeya M, Kamei Y, Ogawa Y: Role of MAPK phosphatase-1 in the induction of monocyte chemoattractant protein-1 during the course of adipocyte hypertrophy. J Biol Chem, 2007; 282: 25445-25452 [DOI] [PubMed] [Google Scholar]
- 74). Inoue M, Itabashi K, Nakano Y, Nakano Y, Tobe T: High-molecular-weight adiponectin and leptin levels in cord blood are associated with anthropometric measurements at birth. Horm Res, 2008; 70: 268-272 [DOI] [PubMed] [Google Scholar]
- 75). Hibino S, Itabashi K, Nakano Y, Inoue M, Tanaka D, Maruyama T: Longitudinal changes in high molecular weight serum adiponectin levels in healthy infants. Pediatr Res, 2009; 65: 363-366 [DOI] [PubMed] [Google Scholar]
- 76). Nakano Y, Itabashi K, Sakurai M, Aizawa M, Dobashi K, Mizuno K: Preterm infants have altered adiponectin levels at term-equivalent age even if they do not present with extrauterine growth restriction. Horm Res Paediatr, 2013; 80: 147-153 [DOI] [PubMed] [Google Scholar]
- 77). Nakano Y, Itabashi K, Nagahara K, Sakurai M, Aizawa M, Dobashi K, Muzuno K, Tanaka D: Cord serum adiponectin is positively related to postnatal body mass index gain. Pediatr Int, 2012; 54: 76-80 [DOI] [PubMed] [Google Scholar]
- 78). Nakano Y, Itabashi K, Dobashi K, Mizuno K: Longitudinal changes in adiponectin multimer levels in preterm infants. Early Hum Dev, 2016; 95: 29-33 [DOI] [PubMed] [Google Scholar]
- 79). Nakano Y, Itabashi K, Sakurai M, Aizawa M, Dobashi K, Mizuno K: Accumulation of subcutaneous fat, but not visceral fat, is a predictor of adiponectin levels in preterm infants at term-equivalent age. Early Hum Dev, 2014; 90: 213-217 [DOI] [PubMed] [Google Scholar]
- 80). de Zegher F, Lopez-Bermejo A, Ibánez L: Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab, 2009; 20: 418-423 [DOI] [PubMed] [Google Scholar]
- 81). Virtue S, Vidal-Puig A: It's not how fat you are, it's what you do with it that counts. PLoS Biol, 2008; 6: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82). Garg A: Adipose tissue dysfunction in obesity and lipodystrophy. Clin Cornerstone, 2006; 8: S7-S13 [DOI] [PubMed] [Google Scholar]
- 83). Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clément K: Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes, 2010; 59: 2817-2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84). Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, WAshabaugh AR, Varban OA, Finks JF, Zamarron BF, Flesher CG, Chang JS, DelProposto JB, Geletka L, Martinez-Santibanez G, Kaciroti N, Lumeng CN, O'Rourke RW: Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring), 2016; 24: 597-605 [DOI] [PMC free article] [PubMed] [Google Scholar]


