Abstract
Aims: Cardiovascular disease (CVD) remains the leading cause of death worldwide despite improvements in the treatment of atherosclerosis, an inflammatory disease and major underlying cause of CVD. Monocytes, an innate immune cell type, are linked to CVD progression; however, given their heterogeneity, the association between distinct monocyte subsets and increased risk of CVD remains unclear. This study investigated the association between peripheral monocyte subpopulation numbers and carotid intima-media thickness (cIMT), a sensitive measure of CVD risk, in a cohort of adults recruited from the general population.
Methods: We used clinical data and peripheral blood mononuclear cell (PBMC) specimens from 67 individuals. cIMT was measured by high-resolution, B-mode, ultrasound images of the right carotid artery. PBMCs were stained with conjugated monoclonal antibodies to define monocyte subpopulations based on CD14 and CD16 co-expressions into classical (CD14++CD16−), intermediate/inflammatory (CD14++CD16+), and non-classical/patrolling (CD14low/+CD16++) monocytes.
Results: We found a higher intermediate monocyte count was significantly correlated with increased right common carotid artery (RCCA) and right carotid bifurcation (RBIF) intima-media thickness (IMT) (p = 0.004 and 0.006, respectively), even after adjusting for CVD-associated clinical data (p = 0.006 and 0.004, respectively).
Conclusion: Our study demonstrated a strong correlation between inflammatory monocyte counts and cIMT. These results suggest that, in the general population, there is a relationship between intermediate monocyte expansion and elevated predictors for CVD risk, and intermediate monocytes may be involved in the development of atherosclerosis and metabolic diseases. Strategies targeting inflammatory monocytes may be needed to slow CVD progression.
Keywords: Intermediate monocyte, cIMT, Atherosclerosis, Inflammation
Introduction
Atherosclerosis is an inflammatory disease characterized by accumulating plaque and associated with cardiovascular disease (CVD) development1). Despite improvements in interventional therapies and treatments for atherosclerosis, CVD remains the leading cause of death in developed countries2). Peripheral monocytes, an innate circulating cell population with inflammatory properties, were suggested to play a key role in atherosclerotic plaque formation1, 3). Based on CD14 and CD16 surface co-expression, monocytes are classified into three different subsets: classical (CD14++CD16−), intermediate or inflammatory (CD14++CD16+), and non-classical or patrolling (CD14low/+CD16++)4). Existing studies have demonstrated monocytes' roles in various disorders, including those that are associated with CVD. Higher levels of classical monocytes have been correlated with insulin resistance, a metabolic disorder closely related to CVD5). In patients with ongoing systemic inflammation, such as HIV infection, an increase in non-classical monocytes was suggested to contribute to CVD progression, even when these individuals were on stable antiretroviral therapy6). Intermediate monocytes were also associated with CVD progression. Increased intermediate monocyte count was independently associated with CVD incidence in non-dialysis, chronic kidney disease (CKD) patients. Non-CKD patients, referred for elective coronary angiography, had higher intermediate monocyte counts as independent predictors of CVD risk7, 8).
However, despite compelling data, another study did not associate monocyte subsets with increased risk of atherosclerosis rupture, rendering their specific role in atherosclerosis and CVD inconclusive9). This contradicting result may be explained by differences in the study population and CVD severity. Our study focused on a relatively healthy population, with no known CKD, and explored the correlation between monocyte subsets, particularly intermediate monocytes, and measurements that predict future CVD risk in healthy individuals. We used carotid intima-media thickness (cIMT), a demonstrated non-invasive method of measuring carotid plaque and predicting future CVD risk10). This study investigated the association between the number of monocyte subpopulations and cIMT indices as a reliable predictive measure of CVD risk among adults recruited within the general population.
Materials and Methods
Study Participants
This is a cross-sectional analysis of data on HIV-negative adult participants who were enrolled as the HIV-uninfected control comparison group in the Hawaii Aging with HIV Cardiovascular (HAHC-CVD) study. The HAHC-CVD cohort was a five-year longitudinal study, which assessed the role of oxidative stress and inflammation in HIV cardiovascular risk. The cohort details have been published previously11). The parent study was approved by the University of Hawaii Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
Clinical Assessment
General medical history, with special emphasis on CVD, was obtained. Clinical parameters, including blood pressure (BP), height, weight, body mass index (BMI), and waist-to-hip ratio, were measured. Blood tests, including fasting for 12 hours, estimated glomerular filtration rate (GFR), high-sensitivity C-reactive protein (hsCRP), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, and glucose, were performed. HIV-seronegative status was confirmed by enzyme-linked immunosorbent assay (ELISA).
Hypertension was defined as systolic BP ≥ 140 mmHg, or diastolic BP ≥ 90 mmHg on entry visit, self-reported history of hypertension, or use of antihypertensive medications. Diabetes mellitus was defined by a fasting blood sugar (FBS) ≥ 126 mg/dL, two-hour oral glucose tolerance test (OGTT) ≥ 200 mg/dL, or self-reported history of diabetes mellitus. Ten-year coronary heart disease (CHD) risk was calculated by Framingham risk score (FRS), using the National Cholesterol Education Program website (http://hp2010.nhlbihin.net/atpiii/calculator.asp)12). Participants with diabetes (as a CVD equivalent) or clinical CVD (history of myocardial infarction, angina, coronary disease-related cardiac surgery, or ischemic stroke) were automatically classified as having ten-year CHD risk by a FRS of 20%. Clinical CVD events were adjudicated by two physicianresearchers (CMS and DCC).
Carotid Artery Intima-Media Thickness
A single, high-resolution, B-mode ultrasound image of the right carotid artery was obtained to measure the cIMT of the far wall of the distal common carotid artery (CCA) and bifurcation (BIF) with automated edge detection. Ultrasound images were acquired at the Queen's Medical Center in Honolulu and analyzed at the University of Southern California Atherosclerosis Research Unit Core Imaging and Reading Center.
Cell Staining and Flow Cytometric Analysis
In brief, cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed and surfacestained with a conjugated antibody panel designed to selectively define and quantify peripheral monocyte subpopulations, as previously described5): V500-conjugated anti-CD3, Qdot605-conjugated anti-CD14, Alexa700-conjugated anti-CD16, PE-Cy7-conjugated anti-CD56, PE-Cy7-conjugated anti-CD19, PE-Cy7-conjugated anti-CD20, and APC-H7-conjugated HLA-DR monoclonal antibodies (mAbs). Monocytes were identified in the HLA-DR positive gate and further into monocyte subpopulations based on CD14 and CD16 expression (Fig. 1). All antibodies were from BD Biosciences, except for Q605-conjugated anti-CD14 and yellow Live/Dead (Life Technologies). Stained PBMCs were acquired by flow cytometry using a 4-laser custom BD-Fortessa instrument (Becton Dickinson) and analyzed with FlowJo software (Treestar Inc Ashland, OR) as previously described5). The total monocyte count was calculated from white blood cell (WBC) count, and percent monocyte values on the complete blood count, conducted as part of entry evaluations on the same blood specimen used for flow cytometry in line with our previous reports5).
Fig. 1.
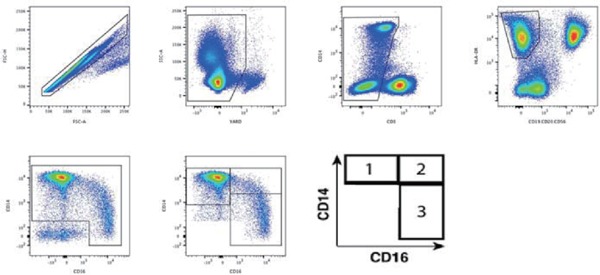
Multiparametric flow cytometry phenotype gating strategy for identification of monocyte subsets
To selectively define and quantify peripheral monocyte subpopulations, a conjugated monoclonal antibody panel was designed to exclude doublets (FSC-H against FSC-A), dead cells (YARD against SSC-A), CD3 positive T-cells, B-cells, and NK-cells (CD19/20 and CD56). Monocytes were identified in the HLA-DR positive gate and further classified into monocyte subpopulations based on CD14 and CD16 expression: (1) classical monocytes (CD14++CD16−), (2) intermediate monocytes (CD14++CD16+), and (3) non-classical monocytes (CD14low/+CD16++)
Statistical Analyses
Demographic and CVD risk characteristics were described using the median, first quartile (Q1), and third quartile (Q3) for continuous variables as well as frequency and percent for categorical variables. Pearson correlation was conducted to correlate demographic and CVD risk characteristics with monocyte subset count. Multivariable linear regression was conducted on each of the intima-media thickness outcomes to assess their association with intermediate monocytes with adjustments for 1) Age, 2) Gender, 3) BMI, 4) LDL cholesterol, 5) HDL cholesterol, 6) Diabetes mellitus, 7) Hypertension, and 8) Current cigarette smoking. Due to the small sample size, manual backward selection was performed on each regression model to determine the most important covariates. A two-sided p-value of less than 0.05 was considered statistically significant. Analyses were conducted using SAS (Version 9.4, 2002–2012 SAS Institute Inc., Cary, NC) and SPSS (IBM, Version 24, Armonk, NY).
Results
There were 67 patients who had available monocyte and cIMT data. The participants' demographic and CVD risk characteristics are presented in Table 1. The participants were primarily male (80%) and Caucasian (65.7%), with a median age of 54 years. The cohort had a median BMI of 26.8 kg/m2, systolic BP of 121 mmHg, diastolic BP of 73 mmHg, and FRS of 4%. The median fasting plasma glucose was 78 mg/dL, GFR was 98.8 mL/min, LDL cholesterol was 115 mg/dL, and HDL cholesterol was 55 mg/dL. Of the 67 participants, 18 (27%) had hypertension, and 4 (6%) were diabetic. Ten patients (15%) were smoking at the time of entry, 39 (58%) had a past history of smoking, and 28 (42%) had never smoked. The median total WBC count was 5230 cells/µL, hsCRP was 1.0 mg/dL, RCCA cIMT was 0.75 mm, and RBIF cIMT was 0.84 mm.
Table 1. Baseline characteristics of study participants.
| N | 67 |
|---|---|
| Age, years [median (Q1, Q3)] | 54.4 (48.0, 59.8) |
| Male gender, n (%) | 54 (80) |
| Ethnicity | |
| Caucasian, n (%) | 44 (65.7) |
| African American, n (%) | 1 (1.5) |
| Native Hawaiian/Pacific Islander, n (%) | 0 (0) |
| Asian, n (%) | 6 (8.9) |
| More than one race, n (%) | 16 (23.8) |
| BMI, kg/m2 [median (Q1, Q3)] | 26.8 (23.5, 29.4) |
| Hypertension, n (%) | 18 (27) |
| Blood pressure | |
| Systolic blood pressure, mmHg [median (Q1, Q3)] | 121 (114, 131) |
| Diastolic blood pressure, mmHg [median (Q1, Q3)] | 73 (68, 79) |
| Fasting plasma glucose, mg/dL [median (Q1, Q3)] | 78 (73, 85) |
| GFR, mL/min [median (Q1, Q3)] | 98.8 (87.5, 121.7) |
| Diabetes mellitus, n (%) | 4 (6) |
| LDL, mg/dL [median (Q1, Q3)] | 115 (94, 139) |
| HDL, mg/dL [median (Q1, Q3)] | 55 (46, 67) |
| Smoking history | |
| Current, n (%) | 10 (14.9) |
| Past, n (%) | 39 (58.2) |
| Never smoked, n (%) | 28 (41.7) |
| 10-year CHD risk estimated by FRS, % [median (Q1, Q3)] | 4 (2, 7) |
| Total WBC count [median (Q1, Q3)] | 5230 (4460, 5950) |
| hsCRP [median (Q1, Q3)] | 1 (1, 2) |
| RCCA cIMT [median (Q1, Q3)] | 0.75 (0.69, 0.84) |
| RBIF cIMT [median (Q1, Q3)] | 0.84 (0.76, 0.93) |
BMI, body mass index; GFR, glomerular filtration rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CHD, coronary heart disease; FRS, Framingham risk score; WBC, white blood cell; hsCRP, high-sensitivity C-reactive protein; RCCA, right common carotid artery; RBIF, right carotid bifurcation; cIMT, carotid intima-media thickness
To selectively define and quantify peripheral monocyte subpopulations, a conjugated monoclonal antibody panel was designed to exclude doublets (FSC-H against FSC-A), dead cells (Yellow Amine Reactive Dye (YARD) against SSC-A), CD3 positive T-cells, B-cells, and NK-cells (CD19/20 and CD56). Monocytes were identified in the HLA-DR positive gate and further classified into monocyte subpopulations based on CD14 and CD16 expression: classical (CD14++CD16−), intermediate (CD14++CD16+), and non-classical (CD14+CD16+) (Fig. 1). The participants' monocyte characteristics are presented in Table 2. The cohort had a median total monocyte count of 450 cells/µL, a classical monocyte count of 295 cells/µL (69%), an intermediate monocyte count of 21 cells/µL (5%), and a non-classical monocyte count of 36 cells/µL (8%).
Table 2. Monocyte characteristics of study participants.
| Total monocyte count [median, (Q1, Q3)] | 450 (325, 544) |
| Classical monocyte count [median, (%), (Q1, Q3)] | 295 (69) (232, 379) |
| Intermediate monocyte count [median, (%), (Q1, Q3)] | 21 (5) (13, 32) |
| Non-classical monocyte count [median, (%), (Q1, Q3)] | 36 (8) (20, 52) |
In unadjusted Pearson correlation, intermediate monocytes were positively correlated with age (r = 0.24, p = 0.05) and FRS (r = 0.37, p = 0.001) and negatively correlated with LDL cholesterol (r = −0.21, p = 0.08) and total cholesterol (r = −0.21, p = 0.08). Similarly, intermediate monocytes were correlated with right common carotid artery intima-media thickness (RCCA; r = 0.34, p = 0.004) and bifurcation intimamedia thickness (RBIF; r = 0.33, p = 0.006). By backward selection, age, BMI, and LDL cholesterol were found to be the most important for both models, as well as HDL cholesterol and diabetes for RCCA and RBIF, respectively. In the adjusted models, shown in Table 3, intermediate monocyte count was significantly associated with both RCCA (standardized β = 0.31, p = 0.006) and RBIF (standardized β = 0.33, p = 0.004).
Table 3. Multivariable linear regression predicting RCCA IMT and RBIF IMT from intermediate monocytes.
| RCCA* |
RBIF* |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Unstandardized Beta | Standardized Beta | p | Parameter | Unstandardized Beta | Standardized Beta | p |
| IM | 0.001 | 0.31 | 0.006 | IM | 0.001 | 0.33 | 0.004 |
| Age | 0.002 | 0.22 | 0.043 | Age | 0.001 | 0.15 | 0.156 |
| BMI | 0.002 | 0.17 | 0.118 | BMI | 0.003 | 0.27 | 0.023 |
| LDL | 0.000 | 0.18 | 0.095 | LDL | 0.000 | 0.14 | 0.191 |
| HDL | −0.001 | −0.24 | 0.033 | Diabetes | 0.05 | 0.16 | 0.170 |
N = 67
RCCA: right common carotid artery; RBIF: right carotid bifurcat ion; Log-10 transformed to correct for normality.
IM, intermediate monocyte; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
No associations were observed with the classical (RCCA p = 0.066, RBIF p = 0.194) or non-classical (RCCA p = 0.182, RBIF p = 0.076) monocyte subsets.
Discussion
In this study, we investigated the association between intermediate monocytes and a marker for atherosclerosis, cIMT, in a group of healthy individuals. As part of the innate immune system, monocytes play a critical role in many inflammatory diseases, represent an important source of cytokines and chemokines, and may be major contributors to CVD1, 13). Monocyte subsets may also be a useful tool to help identify individuals at risk of future CVD3, 7, 8). However, previous data regarding peripheral monocytes' prognostic value remains controversial. Several studies have demonstrated a significant association between monocyte subsets and atherosclerosis plaque severity, vulnerability, and progression of CVD7, 8, 14–17). Additionally, CD14+CD16+ monocytes were associated with restenosis, after percutaneous transluminal angioplasty, in individuals with peripheral artery disease18).
In support of the aforementioned data, our study showed that higher intermediate monocyte subset count with older age was significantly associated with increased cIMT at the CCA region. Similarly, the correlation between intermediate monocyte count and cIMT at the BIF region was also significant. The correlation between intermediate monocytes and cIMT at both the CCA and BIF regions persisted even after adjusting for age, gender, BMI, LDL cholesterol, HDL cholesterol, diabetes mellitus, hypertension, and current cigarette smoking. In summary, this study reported the association between intermediate monocyte numbers with cIMT as predictors of CVD risk in a relatively healthy adult population.
Our study contradicted the results from a previous report that did not associate monocytes and their subsets with the risk of atherosclerosis rupture in patients with severe atherosclerosis9). Additionally, our results contradicted another study, which stated that, unlike non-classical monocytes, there is no significant association between intermediate monocytes and cIMT changes during a two-year period6). These contradicting results may be explained partially by the different study populations. Most of the previous studies involved participants with severe and clinically symptomatic atherosclerosis, whereas our study participants were relatively healthy individuals with subclinical atherosclerosis. Monocyte influx is paramount in the atherogenesis process in mouse studies19). However, monocyte recruitment may be less crucial in more severe and advanced atherosclerotic plaque20). Monocytes' diverse roles in different stages of atherosclerosis development may account for the contradicting results of our study and the study by Meeuwsen et al.9). HIV infection may also contribute to the different results presented by our study and the study by Chow et al.6). Previous reports demonstrated that coronary atherosclerosis was more common in HIV-infected individuals, and that coronary plaque was larger compared to those of HIV-uninfected individuals21). Additionally, immunosuppression and HIV viremia were also associated with more atherogenic lipid profiles in aging individuals with HIV22). Our study focused mainly on exploring monocyte subsets as predictive markers for future atherosclerosis in healthy individuals. Our data showed that intermediate monocytes may be used as potential markers to predict future atherosclerosis establishment in individuals without any current atherosclerotic clinical symptoms.
Monocytes expressing varying degrees of CD16 are important in several diseases, as the subsets' differences in frequency suggest that they may modulate a disease course4, 23). Intermediate monocytes represent only a fraction of the total monocyte population. However, they have been identified as a translational population, sharing phenotypic and functional characteristics as well as expressing surface markers at levels between the classical and non-classical subsets4). Intermediate monocytes also have significant inflammatory properties, increase oxidative stress, and expand in population during chronic inflammation24). A study by Justo-Junior et al. demonstrated that individuals with unstable angina had elevated counts of intermediate monocytes that expressed the highest concentrations of the chemokine receptors CCR2, CCR5, and CX3CR1 as well as the pattern recognition receptors TLR2 and TLR4 compared to the other monocyte subsets25). These receptors are involved in recognizing damage-associated molecular patterns and recruiting immune cells to inflammatory sites; thus, they may contribute to inflammation and the progression of atherosclerosis25). Additionally, this study showed that HSP60, a molecule produced in the presence of oxidized lipids and during hypertension periods, induced the production of IL-12p70 in intermediate monocytes25). The cytokine IL-12p70 promotes Th1 T-lymphocyte differentiation, proinflammatory cytokine release, and macrophage activation, which may thereby increase inflammation and atherosclerotic plaque development26). In individuals with stable atherosclerotic disease, an increased proportion of inflammatory monocytes is associated with elevated levels of lipoprotein a, which is a proatherogenic plasma lipoprotein27). Furthermore, lower HLA-DR expression in intermediate monocytes was associated with metabolic disorders14). These properties, along with their association with increased cIMT in our study, suggested that intermediate monocytes may be involved in the pathogenesis of atherosclerosis.
Heine et al. associated elevated intermediate monocyte count with increased CVD risk in dialysis patients28). Another study, by Rogacev et al, independently associated increased intermediate monocyte count with CVD incidence in non-dialysis patients with CKD7). Both studies could provide a mechanistic model on how CKD, monocyte dysfunction, and atherosclerosis are interrelated. Uremia-related immune dysfunction in dialysis patients leads to increased proinflammatory cytokine production by monocytes28). Additionally, reduced renal cytokine clearance may contribute to elevated cytokine levels in the peripheral blood26). These data put dialysis patients at a higher atherosclerotic risk than the general population. CKD also accelerates atherosclerosis progression and increases CD16-positive monocyte count, compared to healthy controls, which puts them at a higher risk of CVD compared to the general population7). Our study focused on a healthier population, and none of our study participants had CKD based on their estimated GFR. We also did not find any correlation between intermediate monocytes and GFR. Our data suggested that intermediate monocytes may act as independent predictors of future CVD risk.
This study was limited because it was a cross-sectional observation that was restricted by the sample size and demonstrated correlation, not causality. Despite these limitations, our study demonstrated a strong relationship between intermediate monocyte count and cIMT, an atherosclerosis marker, in a well-characterized population of adults with extensive investigation of arterial injury markers. Further investigation involving longitudinal study into the mechanism of monocytes in atherosclerosis development as well as the qualitative cut off and clinical outcomes of cIMT is warranted.
Our study demonstrated a strong correlation between inflammatory monocyte counts and cIMT. These results suggest that the relationship between intermediate monocyte expansion and elevated predictors for CVD risk occurs in the general population, and intermediate monocytes may be involved in the development of atherosclerosis and metabolic diseases.
Acknowledgments
The authors thank the clinical and laboratory staff of the Hawaii Center for AIDS, University of Hawaii, and the many study participants of the HAHC-CVD cohort study who made this study possible. We also thank Dr. Vedbar Khadka for his insight in statistical data analysis. This research was supported by the National Heart, Lung, and Blood Institute under award number R01HL095135 to CMS, 2U54MD007584-04 (RCMI Multidisciplinary and Translational Research Infrastructure eXpansion-II) to CMS, INS, and BS, and the National Institute on Minority Health and Health Disparities under award number U54 MD007601 (Ola HAWAII) to LCN and DCC.
Financial Support
The research reported in this manuscript was supported by the National Heart, Lung, and Blood Institute under award number R01HL095135, RCMI Multidisciplinary and Translational Research Infrastructure eXpansion-II under award number 2U54MD007584-04, and National Institute on Minority Health and Health Disparities U54 MD007601 (Ola HAWAII) of the National Institutes of Health.
Conflict of Interest
None of the authors have any relevant conflicts of interest to disclose.
References
- 1). Ross R: Atherosclerosis--an inflammatory disease. N Engl J Med, 1999; 340: 115-126 [DOI] [PubMed] [Google Scholar]
- 2). Ford ES, Will JC, Mercado CI, Loustalot F: Trends in Predicted Risk for Atherosclerotic Cardiovascular Disease Using the Pooled Cohort Risk Equations Among US Adults From 1999 to 2012. JAMA Intern Med, 2015; 175: 299-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Lassale C, Curtis A, Abete I, van der Schouw YT, Verschuren WMM, Lu Y, Bueno-de-Mesquita HB: Elements of the complete blood count associated with cardiovascular disease incidence: Findings from the EPIC-NL cohort study. Sci Rep, 2018; 8: 3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJM, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobi H, Zembala M, Austyn JM, Lutz MB: Nomenclature of monocytes and dendritic cells in blood. Blood, 2010; 116: e74-80 [DOI] [PubMed] [Google Scholar]
- 5). Shikuma CM, Chow DC, Gangcuangco LMA, Zhang G, Keating SM, Norris PJ, Seto TB, Parikh N, Kallianpur KJ, Nakamoto BK, Nagamine LS, Ndhlovu LC, Barbour JD: Monocytes expand with immune dysregulation and is associated with insulin resistance in older individuals with chronic HIV. PLoS ONE, 2014; 9: e90330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Chow DC, Kagihara JM, Zhang G, Souza SA, Hodis HN, Li Y, Mitchell BI, Nakamoto BK, Kallianpur KJ, Keating SM, Norris PJ, Kohorn LB, Ndhlovu LC, Shikuma CM: Non-classical monocytes predict progression of carotid artery bifurcation intima-media thickness in HIV-infected individuals on stable antiretroviral therapy. HIV Clin Trials, 2016; 17: 114-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH: CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J, 2011; 32: 84-92 [DOI] [PubMed] [Google Scholar]
- 8). Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Große-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Böhm M, Fliser D, Heine GH: CD14++CD16+ Monocytes Independently Predict Cardiovascular Events. J Am Coll Cardiol, 2012; 60: 1512-1520 [DOI] [PubMed] [Google Scholar]
- 9). Meeuwsen JAL, de Vries JJ, van Duijvenvoorde A, van der Velden S, van der Laan SW, van Koeverden ID, van de Weg SM, de Borst GJ, de Winther MPJ, Kuiper J, Pasterkamp G, Hoefer IE, de Jager SCA: Circulating CD14+CD16− classical monocytes do not associate with a vulnerable plaque phenotype, and do not predict secondary events in severe atherosclerotic patients. J Mol Cell Cardiol, 2019; 127: 260-269 [DOI] [PubMed] [Google Scholar]
- 10). de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, Kastelein JJP: Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation, 2004; 109: III33-38 [DOI] [PubMed] [Google Scholar]
- 11). Shikuma CM, Seto T, Liang CY, Bennett K, DeGruttola V, Gerschenson M, Stein JH, Budoff M, Hodis HN, Delaney JAC, Ogata-Arakaki D, Pramyothin P, Chow D: Vitamin D Levels and Markers of Arterial Dysfunction in HIV. AIDS Res Hum Retroviruses, 2011; 28: 793-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) : Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation, 2002; 106: 3143-3421 [PubMed] [Google Scholar]
- 13). Wong KL, Yeap WH, Tai JJY, Ong SM, Dang TM, Wong SC: The three human monocyte subsets: implications for health and disease. Immunol Res, 2012; 53: 41-57 [DOI] [PubMed] [Google Scholar]
- 14). Connaughton EP, Naicker S, Hanley SA, Slevin SM, Eykelenboom JK, Lowndes NF, O'Brien T, Ceredig R, Griffin MD, Dennedy MC: Phenotypic and functional heterogeneity of human intermediate monocytes based on HLA-DR expression. Immunol and Cell Biol, 2018; 96: 742-758 [DOI] [PubMed] [Google Scholar]
- 15). Zhuang J, Han Y, Xu D, Zhu G, Singh S, Chen L, Zhu M, Chen W, Xu Y, Li X: Comparison of circulating dendritic cell and monocyte subsets at different stages of atherosclerosis: insights from optical coherence tomography. BMC Cardiovasc Disor, 2017; 17: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn JF, Veyrie N, Rizkalla S, Fridman WH, Sautes-Fridman C, Clement K, Cremer I: CD14dimCD16+ and CD14+CD16+ Monocytes in Obesity and During Weight Loss: Relationships With Fat Mass and Subclinical Atherosclerosis. Arterioscler Thromb Vasc Biol, 2011; 31: 2322-2330 [DOI] [PubMed] [Google Scholar]
- 17). Yamamoto H, Yoshida N, Shinke T, Otake H, Kuroda M, Sakaguchi K, Hirota Y, Toba T, Takahashi H, Terashita D, Uzu K, Tahara N, Shinkura Y, Kuroda K, Nagasawa Y, Nagano Y, Tsukiyama Y, Yanaka K, Emoto T, Sasaki N, Yamashita T, Ogawa W, Hirata K: Impact of CD14++CD16+ monocytes on coronary plaque vulnerability assessed by optical coherence tomography in coronary artery disease patients. Atherosclerosis, 2018; 269: 245-251 [DOI] [PubMed] [Google Scholar]
- 18). Wildgruber M, Czubba M, Aschenbrenner T, Wendorff H, Hapfelmeier A, Glinzer A, Schiemann M, Zimmermann A, Eckstein HH, Berger H, Wohlgemuth WA, Meier R, Libby P, Zernecke A: Increased intermediate CD14++CD16++ monocyte subset levels associate with restenosis after peripheral percutaneous transluminal angioplasty. Atherosclerosis, 2016; 253: 128-134 [DOI] [PubMed] [Google Scholar]
- 19). Ye D, Zhao Y, Hildebrand RB, Singaraja RR, Hayden MR, Van Berkel TJC, Van Eck M: The Dynamics of Macrophage Infiltration into the Arterial Wall during Atherosclerotic Lesion Development in Low-Density Lipoprotein Receptor Knockout Mice. Am J Pathol, 2011; 178: 413-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M: Monocyte/Macrophage Suppression in CD11b Diphtheria Toxin Receptor Transgenic Mice Differentially Affects Atherogenesis and Established Plaques. Circ Res, 2007; 100: 884-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Vachiat A, McCutcheon K, Tsabedze N, Zachariah D, Manga P: HIV and Ischemic Heart Disease. J Am Coll Cardiol, 2017; 69: 73-82 [DOI] [PubMed] [Google Scholar]
- 22). Levy ME, Greenberg AE, Magnus M, Younes N, Castel A: Immunosuppression and HIV Viremia Associated with More Atherogenic Lipid Profile in Older People with HIV. AIDS Res Hum Retroviruses, 2018; 35: 81-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH: SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood, 2011; 118: e50-61 [DOI] [PubMed] [Google Scholar]
- 24). Stansfield BK, Ingram DA: Clinical significance of monocyte heterogeneity. Clin Transl Med, 2015; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Justo-Junior AS, Villarejos LM, Lima XTV, Nadruz W, Sposito AC, Mamoni RL, Abdalla R, Fernandes JL, Oliveira RTD, Blotta MHSL: Monocytes of patients with unstable angina express high levels of chemokine and pattern-recognition receptors. Cytokine, 2019; 113: 61-67 [DOI] [PubMed] [Google Scholar]
- 26). McLaren JE, Ramji DP: Interferon gamma: A master regulator of atherosclerosis. Cytokine Growth Factor Rev, 2008; 20: 125-135 [DOI] [PubMed] [Google Scholar]
- 27). Krychtiuk KA, Kastl SP, Hofbauer SL, Wonnerth A, Goliasch G, Ozsvar-Kozma M, Katsaros KM, Maurer G, Huber K, Dostal E, Binder CJ, Pfaffenberger S, Oravec S, Wojta J, Speidl WS: Monocyte subset distribution in patients with stable atherosclerosis and elevated levels of lipoprotein(a). J Clin Lipidol, 2015; 9: 533-541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Heine GH, Ortiz A, Massy ZA, Lindholm B, Wiecek A, Martinez-Castelao A, Covic A, Goldsmith D, Süleymanlar G, London GM, Parati G, Sicari R, Zoccali C, Fliser D: Monocyte subpopulations and cardiovascular risk in chronic kidney disease. Nat Rev Nephrol, 2012; 8: 362-369 [DOI] [PubMed] [Google Scholar]


