Abstract
Aims: To understand the different influences of statins on the incidence rate of each stroke subtype in association with low-density lipoprotein (LDL) cholesterol levels, we performed a post hoc analysis on the data from the Japan Statin Treatment Against Recurrent Stroke (J-STARS) study.
Methods: Subjects (n = 1,578) were divided into three groups according to their mean postrandomized LDL cholesterol level (< 100, 100–120, and ≥ 120 mg/dL) until the last observation before the event or the end of follow-up. A Cox proportional hazard model for time to events was used for calculating adjusted hazard ratios, 95% confidence intervals, and the trend tests.
Results: The event rates for atherothrombotic stroke did not decrease in accordance with the postrandomized LDL cholesterol level subgroups of either the control or the pravastatin group (p = 0.15 and 0.33 for the trend, respectively). In the control group, however, no atherothrombotic stroke event was observed in the subgroup of the low postrandomized LDL cholesterol level (less than 100 mg/dL). The event rates for atherothrombotic stroke were lower in the middle postrandomized LDL cholesterol level subgroup (100–120 mg/dL) of the pravastatin group than that of the control group. The event rates for lacunar stroke decreased in the lower postrandomized LDL cholesterol level subgroup of the control group but not of the pravastatin group (p = 0.004 and 0.06 for the trend, respectively).
Conclusions: Statins showed different influences on the risks of atherothromobotic and lacunar stroke according to postrandomized LDL cholesterol levels.
Keywords: Statin, Lacunar stroke, Atherothrombotic stroke, Intracranial hemorrhage, Cholesterol
Introduction
It has been reported that the outcomes of recurrent stroke or cardiovascular disease were reduced with intensive reduction of low-density lipoprotein (LDL) cholesterol by atorvastatin in stroke patients1–3). Stroke is a heterogeneous disease with a range of causes and may present with or without underlying atherosclerotic pathologies. In clinical studies, the composite results with statins could stand on balance on the influences of statins in reducing the risks for each stroke subtype depending on the composite outcomes of stroke or cardiovascular disease based on balances of their risks for stroke subtypes. In clinical settings, treatment would be considered for reducing the risk of the most probable stroke subtypes. In non-cardiogenic ischemic stroke, control of both LDL cholesterol and C-reactive protein has appeared to be effective for preventing recurrent stroke and transient ischemic attack (TIA)4, 5). Previously, we reported that the incidences of stroke or TIA and all vascular events were low in the subgroup of participants with postrandomized LDL cholesterol levels of 80–100 mg/dL adjusted for statin usage6). No desirable postrandomized LDL cholesterol level was detected for the prevention of atherothrombotic stroke and intracranial hemorrhage, although the desirable level was 100–120 mg/dL to prevent lacunar stroke. Although not statistically significant, the incidence of atherothrombotic stroke shifted toward a decrease with statin usage after adjustment for postrandomized LDL cholesterol levels, but the incidences of lacunar stroke and intracranial hemorrhage increased.
Thus, the benefits of statins might be different among at-risk stroke subtypes. We hypothesized that the trends of incidence rates could differ with LDL cholesterol levels among stroke subtypes, separating the pravastatin group and the no statin group. To understand the influence of pravastatin on the incidence rate of each stroke subtype, we conducted a post hoc analysis using J-STARS data.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Details of the rationale, study design, characteristics of the participants, and principal results in J-STARS have been published elsewhere6–8). This study was conducted as a post hoc analysis of J-STARS under the health insurance system of Japan and in accordance with the Declaration of Helsinki and Ethical Guidelines on Clinical Studies of the Ministry of Health, Labour and Welfare of Japan. This study was approved by the Institutional Review Board of each participating center, and written informed consent was obtained from all patients. This trial is registered at ClinicalTrials.gov under number NCT00221104.
Patients
Briefly, patients aged 45–80 years with a history of noncardioembolic ischemic stroke within the preceding 1 month–3 years were enrolled from 123 centers between March 2004 and February 2009. At enrollment, all patients had a total cholesterol level between 180 and 240 mg/dL (4.65 and 6.21 mmol/L) without the use of statins. The exclusion criteria included ischemic stroke of determined rare etiology, ischemic stroke associated with catheterization or surgery, and preferred use of statins for the treatment of comorbid coronary artery disease.
Procedures
The 1,578 patients were randomly allocated to the pravastatin group (10 mg/day) or the control group (793 vs. 785 subjects, respectively). In the randomization process, the prevalence rates of stroke subtype (atherothrombotic stroke), high blood pressure (≥ 150/90 mmHg), and comorbidity of diabetes were dynamically balanced between the treatment groups. The measurements of total cholesterol, LDL cholesterol, triglyceride, and high-density lipoprotein cholesterol levels were performed as previously described7, 8). Treatment compliance was monitored at every clinical visit. The primary endpoint was the onset of stroke or TIA. Secondary endpoints included the onset of each stroke subtype, myocardial infarction, vascular accident, death, or hospitalization.
Statistical Analysis
In accordance with the intention-to-treat (ITT) principle, we defined the analysis set as the ITT population, including all randomized patients. The distribution of the baseline characteristics among the postrandomized LDL cholesterol level groups was compared using analysis of variance (for continuous variables) or χ2 tests (for discrete variables).
In this post hoc analysis, regarding events such as stroke, atherothrombotic stroke, lacunar stroke, and intracranial hemorrhage, we evaluated the associations of three LDL cholesterol levels, the baseline LDL cholesterol level, the postrandomized LDL cholesterol level and the reduced LDL cholesterol level from baseline. The postrandomized LDL cholesterol was defined as the mean value of LDL cholesterol within the patient until the last observation prior to the event or the end of follow-up, excluding the baseline. Subjects were divided into three groups according to both their baseline LDL cholesterol level (< 120, 120–140, and ≥ 140 mg/dL) and postrandomized LDL cholesterol level (< 100, 100–120, and ≥ 120 mg/dL) and divided into tertiles based on their reduced LDL cholesterol level. Patients were excluded from the evaluation of postrandomized LDL cholesterol levels if they experienced an incident of interest before the first follow-up evaluation of LDL cholesterol levels. We used a Cox proportional hazard model with each LDL cholesterol level and selected covariates for time to events to estimate the adjusted hazard ratio (HR), 95% confidence interval (CI), and trend test of the LDL cholesterol levels for the events. Factors with p < 0.20 compared with the distribution of baseline characteristics among the groups of postrandomized LDL cholesterol levels were selected as covariates. In addition, the baseline LDL cholesterol level was added as a covariate to analyze the postrandomized and reduced LDL cholesterol levels. Among the LDL cholesterol level subgroups, the subgroup with the high LDL cholesterol level served as the reference. For the reduced LDL cholesterol level subgroups, the subgroup with the low level served as the reference.
The adjusted HR (95% CI) for the pravastatin group relative to the control group and the interaction test between the randomized group and the LDL cholesterol levels were calculated using a Cox proportional hazard model for time to events after adjusting for the stratification factors at randomization, in particular, stroke subtype (atherothrombotic stroke vs. others), high blood pressure (≥ 150/90 mmHg vs. not), and diabetes mellitus (presence vs. absence). The baseline LDL cholesterol level was also added as a covariate in these analyses of the postrandomized and reduced LDL cholesterol levels. The incidence rates (per 100 person-years) for each event in the pravastatin group and control group were also estimated using the person-years method.
All analyses were conducted using SAS version 9.3 (Cary, NC, USA). The level of significance was set at p < 0.05 (2-tailed).
Data Availability
The data that supported the findings of this study are available from the corresponding author upon reasonable request.
Results
Baseline characteristics are presented in Table 1. Among the postrandomized LDL cholesterol level subgroups, considerable differences were observed across the following characteristics: treatment group, age, gender, hypertension, diabetes mellitus, chronic kidney disease, smoking, and baseline LDL cholesterol (p < 0.20, Table 1).
Table 1. Baseline characteristics with postrandomized LDL cholesterol level for events of stroke.
| Characteristic | Postrandomized LDL cholesterol level |
|||
|---|---|---|---|---|
| < 100 mg/dL n = 408 |
100–120 mg/dL n = 478 |
120 ≤ mg/dL n = 631 |
p value | |
| Group | ||||
| Pravastatin, n (use of statin agents, n) | 338 (331) | 261 (254) | 163 (152) | < 0.001 |
| Control, n (use of statin agents, n) | 70 (10) | 217 (33) | 468 (50) | |
| Age, years | 66.9 ± 8.4 | 66.4 ± 8.4 | 65.7 ± 8.6 | 0.08 |
| Male, n (%) | 295 (72.3) | 337 (70.5) | 413 (65.5) | 0.043 |
| Body mass index, kg/m2 | 23.6 ± 3.0 | 23.9 ± 3.3 | 23.8 ± 2.9 | 0.26 |
| Hypertension, n (%) | 333 (81.6) | 356 (74.5) | 469 (74.3) | 0.013 |
| Diabetes mellitus, n (%) | 109 (26.7) | 124 (25.9) | 116 (18.4) | 0.001 |
| Coronary artery disease, n (%) | 19 (4.7) | 26 (5.5) | 34 (5.4) | 0.84 |
| Chronic kidney disease, n (%) | 113 (27.7) | 109 (22.8) | 145 (23.0) | 0.15 |
| Smoker, n (%) | 232 (56.9) | 267 (55.9) | 318 (50.4) | 0.07 |
| Ischemic stroke subtype | ||||
| Atherothrombotic stroke, n (%) | 100 (24.5) | 115 (24.1) | 163 (25.9) | |
| Lacunar infarction, n (%) | 261 (64.0) | 307 (64.2) | 407 (64.5) | 0.89 |
| Undetermined etiology, n (%) | 47 (11.5) | 56 (11.7) | 61 (9.7) | |
| Use of antiplatelet agents, n (%) | 379 (92.9) | 434 (90.8) | 573 (90.8) | 0.44 |
| LDL cholesterol level at baseline, mg/dL | 115.2 ± 21.1 | 128.1 ± 23.2 | 139.8 ± 22.7 | < 0.001 |
The adjusted HRs for stroke, atherothrombotic stroke, lacunar stroke, and intracranial hemorrhage were not different among the baseline LDL cholesterol level subgroups of either the control or pravastatin groups (data not shown). Furthermore, no significant difference was detected for any events between the treatment groups in any baseline LDL cholesterol level subgroup (data not shown).
In the control group, the adjusted HRs for stroke decreased in the postrandomized LDL cholesterol level subgroup of less than 100 mg/dL compared with those in the subgroup of 120 mg/dL or more after adjustment for the factors, showing a considerable difference among the postrandomized LDL cholesterol level subgroups (p = 0.022 for the trend, Fig. 1B, Supplemental Table 1, 3). However, no significant reduction in the incidence of stroke was detected in the low postrandomized LDL cholesterol level subgroup of the pravastatin group. In the postrandomized LDL cholesterol level subgroup (less than 100 mg/dL), the pravastatin group showed a high adjusted HR of stroke compared with the control group (p = 0.010 for the interaction, Fig. 1C, Supplemental Table 1, 3). No significant trend in the adjusted HR for stroke was observed in accordance with the reduced LDL cholesterol level subgroup of either treatment group (Fig. 2B, Supplemental Table 2, 4).
Fig. 1.
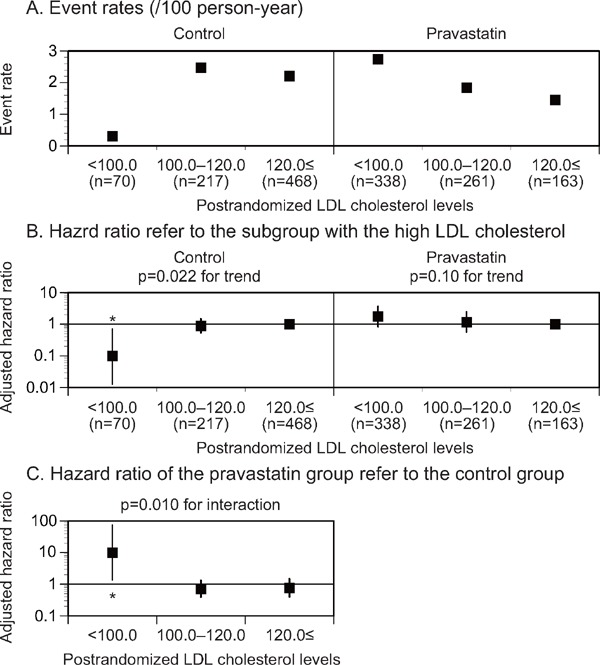
Event rates (A), adjusted HR (B) for stroke stratified by the postrandomized LDL cholesterol levels, and adjusted HR (C) of the pravastatin group in reference to the control group in each postrandomized LDL cholesterol level subgroup
The subgroup with the high postrandomized LDL cholesterol levels was used as a reference (B). LDL, low-density lipoprotein. *p < 0.05.
Supplemental Table 1. Event rates and adjusted hazard ratio stratified by postrandomized LDL cholesterol levels.
| Postrandomized LDL cholesterol | Subjects, n |
Events, n |
Event rate (/ 100 person-yeas) |
Hazard ratio (95% CI) |
P for trend | Hazard ratio (95% CI) |
P for interaction | |
|---|---|---|---|---|---|---|---|---|
| Stroke | ||||||||
| Control | < 100 | 70 | 1 | 0.31 | 0.10 (0.01 to 0.70) | 0.022 | REF | |
| 100.0–120.0 | 217 | 26 | 2.48 | 0.89 (0.53 to 1.49) | REF | |||
| ≥ 120.0 | 468 | 49 | 2.21 | REF | REF | |||
| Pravastatin | < 100 | 338 | 43 | 2.75 | 1.75 (0.83 to 3.69) | 0.10 | 10.04 (1.37 to 73.35) | 0.010 |
| 100.0–120.0 | 261 | 23 | 1.84 | 1.17 (0.56 to 2.45) | 0.72 (0.39 to 1.33) | |||
| ≥ 120.0 | 163 | 11 | 1.45 | REF | 0.77 (0.40 to 1.50) | |||
| Atherothrombotic Stroke | ||||||||
| Control | < 100 | 77 | 0 | 0 | 0.00 | 0.15 | REF | |
| 100.0–120.0 | 219 | 7 | 0.63 | 0.83 (0.32 to 2.18) | REF | |||
| ≥ 120.0 | 467 | 16 | 0.70 | REF | REF | |||
| Pravastatin | < 100 | 337 | 4 | 0.24 | 3.25 (0.26 to 40.26) | 0.33 | - | 0.18 |
| 100.0–120.0 | 266 | 2 | 0.15 | 1.66 (0.14 to 19.78) | 0.15 (0.03 to 0.81) | |||
| ≥ 120.0 | 165 | 1 | 0.13 | REF | 0.21 (0.03 to 1.62) | |||
| Lacunar Stroke | ||||||||
| Control | < 100 | 75 | 0 | 0 | 0.00 | 0.004 | REF | |
| 100.0–120.0 | 220 | 8 | 0.73 | 0.42 (0.18 to 1.00) | REF | |||
| ≥ 120.0 | 467 | 26 | 1.15 | REF | REF | |||
| Pravastatin | < 100 | 342 | 25 | 1.53 | 2.43 (0.84 to 7.03) | 0.06 | - | 0.002 |
| 100.0–120.0 | 259 | 12 | 0.95 | 1.31 (0.45 to 3.82) | 2.16 (0.80 to 5.87) | |||
| ≥ 120.0 | 163 | 5 | 0.64 | REF | 0.66 (0.25 to 1.75) | |||
| Intracranial Hemorrhage | ||||||||
| Control | < 100 | 79 | 1 | 0.27 | 1.03 (0.09 to 11.95) | 0.69 | REF | |
| 100.0–120.0 | 224 | 5 | 0.44 | 2.42 (0.53 to 11.03) | REF | |||
| ≥ 120.0 | 460 | 3 | 0.13 | REF | REF | |||
| Pravastatin | < 100 | 337 | 6 | 0.36 | 2.93 (0.29 to 29.29) | 0.40 | 2.11 (0.24 to 18.71) | 0.84 |
| 100.0–120.0 | 267 | 4 | 0.30 | 2.64 (0.29 to 24.22) | 0.43 (0.10 to 1.77) | |||
| ≥ 120.0 | 165 | 1 | 0.13 | REF | 1.13 (0.11 to 11.23) | |||
| Myocardial Infarction | ||||||||
| Control | < 100 | 80 | 1 | 0.27 | 0.44 (0.04 to 5.42) | 0.29 | REF | |
| 100.0–120.0 | 221 | 0 | 0 | 0.00 | REF | |||
| ≥ 120.0 | 462 | 4 | 0.17 | REF | REF | |||
| Pravastatin | < 100 | 399 | 2 | 0.12 | 1.34 (0.08 to 21.61) | 0.84 | 0.46 (0.04 to 5.22) | 0.81 |
| 100.0–120.0 | 364 | 1 | 0.08 | 0.54 (0.03 to 10.05) | - | |||
| ≥ 120.0 | 166 | 1 | 0.12 | REF | 0.80 (0.09 to 7.26) | |||
| All Vascular Events | ||||||||
| Control | < 100 | 69 | 6 | 1.92 | 0.34 (0.14 to 0.81) | 0.012 | REF | |
| 100.0–120.0 | 203 | 32 | 3.36 | 0.75 (0.48 to 1.18) | REF | |||
| ≥ 120.0 | 475 | 72 | 3.28 | REF | REF | |||
| Pravastatin | < 100 | 335 | 52 | 3.40 | 1.66 (0.97 to 3.17) | 0.12 | 1.84 (0.79 to 4.30) | 0.050 |
| 100.0–120.0 | 258 | 34 | 2.83 | 1.35 (0.72 to 2.51) | 0.83 (0.51 to 1.36) | |||
| ≥ 120.0 | 165 | 15 | 1.97 | REF | 0.66 (0.38 to 1.15) | |||
All vascular events included recurrent stroke, transient ischemic attack, myocardial infarction, and vascular accidents, such as aortic dissection/rupture, pulmonary embolism, cardiac failure, organ/limb infarction, carotid endarterectomy, stenting, extracranial-intracranial bypass, and coronary artery bypass graft/intervention. A Cox proportional hazard model with each LDL cholesterol level and selected covariates for time to events was used to estimate the adjusted hazard ratio, 95% confidence interval (CI), and trend test of the LDL cholesterol levels for the events. The adjusted hazard ratio (95% CI) for the pravastatin group relative to the control group and the interaction test between the randomized group and the LDL cholesterol levels were calculated using a Cox proportional hazard model for time to events after adjusting for the stratification factors at randomization, i.e., stroke subtype (atherothrombotic stroke vs. others), high blood pressure (≥ 150/90 mmHg vs. not), and diabetes mellitus (presence vs. absence).
LDL, low-density lipoprotein; CI, confidence interval; REF, reference.
Supplemental Table 3. Event rates and adjusted hazard ratio stratified by postrandomized LDL cholesterol levels analyzed in per-protocol set.
| Postrandomized LDL cholesterol | Subjects, n |
Events, n |
Event rate (/ 100 person-yeas) |
Hazard ratio (95% CI) |
P for trend | Hazard ratio (95% CI) |
P for interaction | |
|---|---|---|---|---|---|---|---|---|
| Stroke | ||||||||
| Control | < 100 | 60 | 1 | 0.36 | 0.12 (0.02 to 0.95) | 0.08 | REF | |
| 100.0–120.0 | 182 | 19 | 2.18 | 0.99 (0.54 to 1.81) | REF | |||
| ≥ 120.0 | 413 | 32 | 1.63 | REF | REF | |||
| Pravastatin | < 100 | 274 | 31 | 2.25 | 1.29 (0.55 to 3.01) | 0.45 | 6.20 (0.84 to 45.47) | 0.18 |
| 100.0–120.0 | 222 | 18 | 1.61 | 0.97 (0.42 to 2.22) | 0.70 (0.36 to 1.34) | |||
| ≥ 120.0 | 123 | 9 | 1.47 | REF | 0.99 (0.47 to 2.08) | |||
| Atherothrombotic Stroke | ||||||||
| Control | < 100 | 63 | 0 | 0 | 0.00 | 0.15 | REF | |
| 100.0–120.0 | 181 | 5 | 0.55 | 0.73 (0.23 to 2.29) | REF | |||
| ≥ 120.0 | 415 | 11 | 0.54 | REF | REF | |||
| Pravastatin | < 100 | 274 | 2 | 0.14 | 0.99 (0.06 to 106.41) | 0.96 | - | 0.69 |
| 100.0–120.0 | 226 | 2 | 0.17 | 1.38 (0.11 to 16.64) | 0.22 (0.04 to 1.18) | |||
| ≥ 120.0 | 124 | 1 | 0.16 | REF | 0.33 (0.04 to 2.58) | |||
| Lacunar Stroke | ||||||||
| Control | < 100 | 64 | 0 | 0 | 0.00 | 0.05 | REF | |
| 100.0–120.0 | 183 | 6 | 0.66 | 0.61 (0.23 to 1.64) | REF | |||
| ≥ 120.0 | 413 | 17 | 0.85 | REF | REF | |||
| Pravastatin | < 100 | 277 | 18 | 1.26 | 2.00 (0.59 to 6.84) | 0.22 | - | 0.049 |
| 100.0–120.0 | 221 | 11 | 0.97 | 1.35 (0.41 to 4.47) | 1.55 (0.57 to 4.21) | |||
| ≥ 120.0 | 122 | 4 | 0.64 | REF | 0.83 (0.28 to 2.48) | |||
| Intracranial Hemorrhage | ||||||||
| Control | < 100 | 66 | 1 | 0.33 | 0.84 (0.06 to 11.24) | 0.99 | REF | |
| 100.0–120.0 | 186 | 3 | 0.32 | 1.79 (0.28 to 11.55) | REF | |||
| ≥ 120.0 | 409 | 2 | 0.10 | REF | REF | 0.97 | ||
| Pravastatin | < 100 | 275 | 5 | 0.34 | 2.11 (0.19 to 23.48) | 0.38 | 0.98 (0.11 to 8.43) | |
| 100.0–120.0 | 225 | 1 | 0.08 | 0.56 (0.03 to 9.26) | 0.21 (0.02 to 2.14) | |||
| ≥ 120.0 | 124 | 1 | 0.15 | REF | 1.50 (0.13 to 17.00) | |||
A Cox proportional hazard model with each LDL cholesterol level and selected covariates for time to events was used to estimate the adjusted hazard ratio, 95% confidence interval (CI), and trend test of the LDL cholesterol levels for the events. The adjusted hazard ratio (95% CI) for the pravastatin group relative to the control group and the interaction test between the randomized group and the LDL cholesterol levels were calculated using a Cox proportional hazard model for time to events after adjusting for the stratification factors at randomization, i.e., stroke subtype (atherothrombotic stroke vs. others), high blood pressure (. 150/90 mmHg vs. not), and diabetes mellitus (presence vs. absence). Per-protocol set was limited subjects to whom met protocol. Subjects in control group should not take any statin. Subjects in pravastatin group should take pravastatin more than 1/4 of their follow-up duration. LDL, low-density lipoprotein; CI, confidence interval; REF, reference.
Fig. 2.
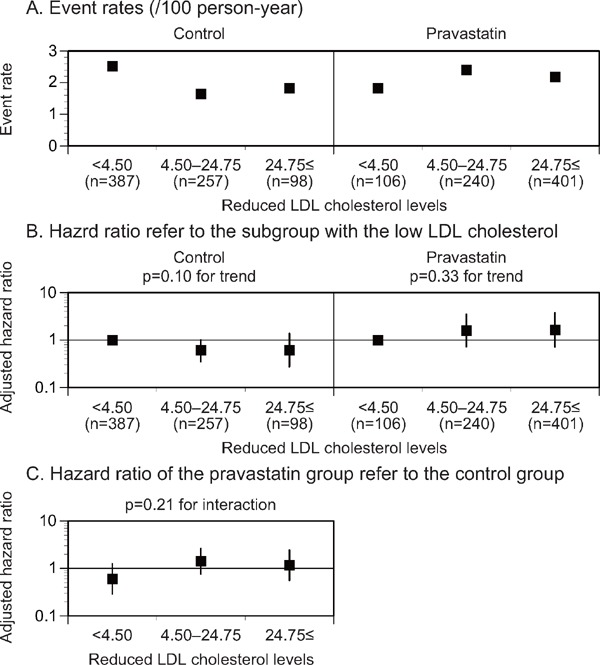
Event rates (A), adjusted HR (B) for stroke stratified by the reduced LDL cholesterol levels, and adjusted HR (C) of the pravastatin group in reference to the control group in each reduced LDL cholesterol level subgroup
The subgroup with the low reduced LDL cholesterol levels was used as a reference (B). LDL, low-density lipoprotein. *p < 0.05.
Supplemental Table 2. Event rates and adjusted hazard ratio stratified by reduced LDL cholesterol levels.
| Reduced LDL cholesterol | Subjects, n |
Events, n |
Event rate (/ 100 person-yeas) |
Hazard ratio (95% CI) |
P for trend | Hazard ratio (95% CI) |
P for interaction | |
|---|---|---|---|---|---|---|---|---|
| Stroke | ||||||||
| Control | < 4.50 | 387 | 46 | 2.52 | REF | 0.10 | REF | |
| 4.50–24.75 | 257 | 20 | 1.65 | 0.61 (0.35 to 1.07) | REF | |||
| ≥ 24.75 | 98 | 9 | 1.82 | 0.61 (0.27 to 1.38) | REF | |||
| Pravastatin | < 4.50 | 106 | 9 | 1.82 | REF | 0.33 | 0.60 (0.29 to 1.26) | 0.21 |
| 4.50–24.75 | 240 | 27 | 2.40 | 1.59 (0.72 to 3.51) | 1.42 (0.76 to 2.63) | |||
| ≥ 24.75 | 401 | 41 | 2.18 | 1.64 (0.71 to 3.78) | 1.17 (0.55 to 2.45) | |||
| Atherothrombotic Stroke | ||||||||
| Control | < 4.63 | 389 | 13 | 0.67 | REF | 0.30 | REF | |
| 4.63–24.88 | 262 | 6 | 0.47 | 0.49 (0.18 to 1.36) | REF | |||
| ≥ 24.88 | 98 | 4 | 0.80 | 0.61 (0.16 to 2.30) | REF | |||
| Pravastatin | < 4.63 | 111 | 1 | 0.18 | REF | 0.69 | 0.32 (0.04 to 2.55) | 0.55 |
| 4.63–24.88 | 239 | 1 | 0.08 | 0.41 (0.02 to 7.04) | 0.15 (0.02 to 1.34) | |||
| ≥ 24.88 | 403 | 5 | 0.25 | 1.08 (0.09 to 12.91) | 0.58 (0.14 to 2.38) | |||
| Lacunar Stroke | ||||||||
| Control | < 4.60 | 388 | 25 | 1.34 | REF | 0.004 | REF | |
| 4.60–25.00 | 263 | 7 | 0.55 | 0.35 (0.14 to 0.86) | REF | |||
| ≥ 25.00 | 97 | 1 | 0.20 | 0.11 (0.01 to 0.90) | REF | |||
| Pravastatin | < 4.60 | 110 | 4 | 0.76 | REF | 0.11 | 0.58 (0.20 to 1.73) | 0.006 |
| 4.60–25.00 | 237 | 13 | 1.13 | 1.99 (0.62 to 6.41) | 2.13 (0.80 to 5.67) | |||
| ≥ 25.00 | 402 | 25 | 1.30 | 2.69 (0.80 to 9.02) | 4.88 (0.65 to 36.58) | |||
| Intracranial Hemorrhage | ||||||||
| Control | < 4.86 | 390 | 6 | 0.31 | REF | 0.80 | REF | |
| 4.86–25.00 | 262 | 1 | 0.08 | 0.34 (0.04 to 3.09) | REF | |||
| ≥ 25.00 | 98 | 2 | 0.40 | 1.98 (0.29 to 13.38) | REF | |||
| Pravastatin | < 4.86 | 111 | 2 | 0.37 | REF | 0.33 | 0.67 (0.13 to 3.38) | 0.90 |
| 4.86–25.00 | 239 | 3 | 0.25 | 1.59 (0.72 to 3.51) | 3.78 (0.35 to 41.19) | |||
| ≥ 25.00 | 404 | 6 | 0.30 | 1.64 (0.71 to 3.78) | 0.91 (0.18 to 4.69) | |||
| Myocardial Infarction | ||||||||
| Control | < 4.86 | 389 | 3 | 0.15 | REF | 0.63 | REF | |
| 4.86–24.88 | 262 | 1 | 0.08 | 0.75 (0.07 to 8.55) | REF | |||
| ≥ 24.88 | 98 | 1 | 0.20 | 2.62 (0.18 to 38.17) | REF | |||
| Pravastatin | < 4.86 | 111 | 0 | 0 | REF | 0.71 | 0.00 | 0.78 |
| 4.86–24.88 | 240 | 2 | 0.17 | - | 2.20 (0.20 to 24.41) | |||
| ≥ 24.88 | 403 | 2 | 0.10 | - | 0.46 (0.04 to 5.09) | |||
| All Vascular Events | ||||||||
| Control | < 4.17 | 388 | 67 | 3.78 | REF | 0.07 | REF | |
| 4.17–24.14 | 254 | 32 | 2.73 | 0.73 (0.46 to 1.14) | REF | |||
| ≥ 24.14 | 92 | 10 | 2.17 | 0.55 (0.26 to 1.15) | REF | |||
| Pravastatin | < 4.17 | 104 | 9 | 1.86 | REF | 0.28 | 0.48 (0.24 to 0.96) | 0.045 |
| 4.17–24.14 | 238 | 37 | 3.40 | 2.06 (0.97 to 4.41) | 1.23 (0.77 to 1.98) | |||
| ≥ 24.14 | 403 | 55 | 2.96 | 1.90 (0.86 to 4.22) | 1.36 (0.69 to 2.68) | |||
All vascular events included recurrent stroke, transient ischemic attack, myocardial infarction, and vascular accidents, such as aortic dissection/rupture, pulmonary embolism, cardiac failure, organ/limb infarction, carotid endarterectomy, stenting, extracranial-intracranial bypass, and coronary artery bypass graft/intervention. A Cox proportional hazard model with each LDL cholesterol level and selected covariates for time to events was used to estimate the adjusted hazard ratio, 95% confidence interval (CI), and trend test of the LDL cholesterol levels for the events. The adjusted hazard ratio (95% CI) for the pravastatin group relative to the control group and the interaction test between the randomized group and the LDL cholesterol levels were calculated using a Cox proportional hazard model for time to events after adjusting for the stratification factors at randomization, i.e., stroke subtype (atherothrombotic stroke vs. others), high blood pressure (. 150/90 mmHg vs. not), and diabetes mellitus (presence vs. absence).
LDL, low-density lipoprotein; CI, confidence interval; REF, reference.
Supplemental Table 4. Event rates and adjusted hazard ratio stratified by reduced LDL cholesterol levels analyzed in per-protocol set.
| Reduced LDL cholesterol | Subjects, n |
Events, n |
Event rate (/ 100 person-yeas) |
Hazard ratio (95% CI) |
P for trend | Hazard ratio (95% CI) |
P for interaction | |
|---|---|---|---|---|---|---|---|---|
| Stroke | ||||||||
| Control | < 4.50 | 353 | 35 | 2.09 | REF | 0.15 | REF | |
| 4.50–24.75 | 227 | 13 | 1.22 | 0.59 (0.30 to 1.17) | REF | |||
| ≥ 24.75 | 64 | 4 | 1.23 | 0.59 (0.19 to 1.82) | REF | |||
| Pravastatin | < 4.50 | 76 | 6 | 1.57 | REF | 0.45 | 0.70 (0.29 to 1.66) | 0.26 |
| 4.50–24.75 | 193 | 22 | 2.29 | 1.71 (0.66 to 4.45) | 1.85 (0.93 to 3.70) | |||
| ≥ 24.75 | 341 | 30 | 1.75 | 1.49 (0.53 to 4.22) | 1.41 (0.50 to 4.02) | |||
| Atherothrombotic Stroke | ||||||||
| Control | < 4.63 | 356 | 9 | 0.51 | REF | 0.84 | REF | |
| 4.63–24.88 | 230 | 5 | 0.45 | 0.78 (0.24 to 2.52) | REF | |||
| ≥ 24.88 | 62 | 2 | 0.63 | 0.97 (0.17 to 5.46) | REF | |||
| Pravastatin | < 4.63 | 80 | 1 | 0.24 | REF | 0.96 | 0.45 (0.06 to 3.55) | 0.89 |
| 4.63–24.88 | 194 | 1 | 0.10 | 0.32 (0.02 to 5.57) | 0.22 (0.03 to 1.85) | |||
| ≥ 24.88 | 341 | 3 | 0.17 | 0.45 (0.06 to 16.41) | 0.36 (0.06 to 2.21) | |||
| Lacunar Stroke | ||||||||
| Control | < 4.60 | 355 | 18 | 1.04 | REF | 0.013 | REF | |
| 4.60–25.00 | 232 | 5 | 0.45 | 0.36 (0.12 to 1.04) | REF | |||
| ≥ 25.00 | 62 | 0 | 0 | 0.00 | REF | |||
| Pravastatin | < 4.60 | 78 | 3 | 0.75 | REF | 0.22 | 0.69 (0.20 to 2.34) | 0.021 |
| 4.60–25.00 | 192 | 10 | 1.01 | 1.76 (0.45 to 6.87) | 2.17 (0.73 to 6.38) | |||
| ≥ 25.00 | 341 | 20 | 1.15 | 2.37 (0.56 to 6.84) | - | |||
| Intracranial Hemorrhage | ||||||||
| Control | < 4.86 | 359 | 5 | 0.28 | REF | 0.93 | REF | |
| 4.86–25.00 | 229 | 0 | 0 | 0.00 | REF | |||
| ≥ 25.00 | 62 | 1 | 0.31 | 2.59 (0.23 to 28.78) | REF | |||
| Pravastatin | < 4.86 | 80 | 1 | 0.24 | REF | 0.85 | 0.79 (0.09 to 6.77) | 0.74 |
| 4.86–25.00 | 193 | 3 | 0.30 | 1.69 (0.14 to 20.03) | - | |||
| ≥ 25.00 | 342 | 3 | 0.17 | 1.50 (0.08 to 28.27) | 0.57 (0.06 to 5.53) | |||
A Cox proportional hazard model with each LDL cholesterol level and selected covariates for time to events was used to estimate the adjusted hazard ratio, 95% confidence interval (CI), and trend test of the LDL cholesterol levels for the events. The adjusted hazard ratio (95% CI) for the pravastatin group relative to the control group and the interaction test between the randomized group and the LDL cholesterol levels were calculated using a Cox proportional hazard model for time to events after adjusting for the stratification factors at randomization, i.e., stroke subtype (atherothrombotic stroke vs. others), high blood pressure (≥ 150/90 mmHg vs. not), and diabetes mellitus (presence vs. absence). Per-protocol set was limited subjects to whom met protocol. Subjects in control group should not take any statin. Subjects in pravastatin group should take pravastatin more than 1/4 of their follow-up duration. LDL, low-density lipoprotein; CI, confidence interval; REF, reference.
Analyses of atherothrombotic stroke, lacunar stroke, and intracerebral hemorrhage were also performed to examine differences in the incidence of stroke subtype influenced by pravastatin treatment. The adjusted HR for atherothrombotic stroke did not decrease in accordance with the postrandomized LDL cholesterol level subgroups of either the control or pravastatin group (p = 0.15 and 0.33 for the trend, Fig. 3B, Supplemental Table 1, 3). In the control group, however, no atherothrombotic stroke event was observed in the subgroup of low postrandomized LDL cholesterol levels (less than 100 mg/dL). The event rates for atherothrombotic stroke were lower in the postrandomized LDL cholesterol level subgroup (100–120 mg/dL) of the pravastatin group than in the same subgroups of the control group (Fig. 3C, Supplemental Table 1, 3). No significant trend in the adjusted HR for atherothrombotic stroke was observed in accordance with the reduced LDL cholesterol level subgroup of either treatment group (Supplemental Table 2, 4).
Fig. 3.
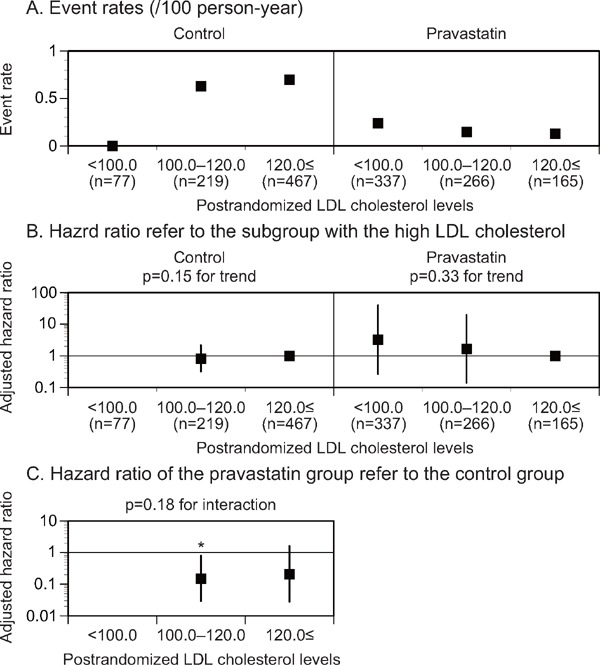
Event rates (A), adjusted HR (B) for atherothrombotic stroke stratified by the postrandomized LDL cholesterol levels, and adjusted HR (C) of the pravastatin group in reference to the control group in each postrandomized LDL cholesterol level subgroup
The subgroup with the high postrandomized LDL cholesterol levels was used as a reference (B). The HR was kept blank when the event rate was 0/100 person-year. LDL, low-density lipoprotein. *p < 0.05.
The event rates for lacunar stroke decreased in the lower postrandomized LDL cholesterol level subgroup of the control group but not in the pravastatin group (p = 0.004 and 0.06 for the trend, respectively, Fig. 4B, Supplemental Table 1, 3). A significant interaction in the adjusted HR for lacunar stroke was observed in accordance with the postrandomized LDL cholesterol level subgroups between the treatment groups (p = 0.002 for the interaction, Fig. 4C, Supplemental Table 1, 3). The event rate of lacunar stroke decreased in accordance with the reduced LDL cholesterol levels in the control group but not in the pravastatin group (p = 0.004 and 0.11 for the trend, respectively, Supplemental Table 2, 4). Moreover, a significant interaction in the adjusted HR for lacunar stroke was observed in accordance with the reduced LDL cholesterol level subgroups between the treatment groups (p = 0.006 for the interaction, Supplemental Table 2, 4). However, no significant trend in the adjusted HR for intracranial hemorrhage was observed in accordance with the postrandomized or reduced LDL cholesterol level subgroups in either treatment group (Supplemental Table 1–4).
Fig. 4.
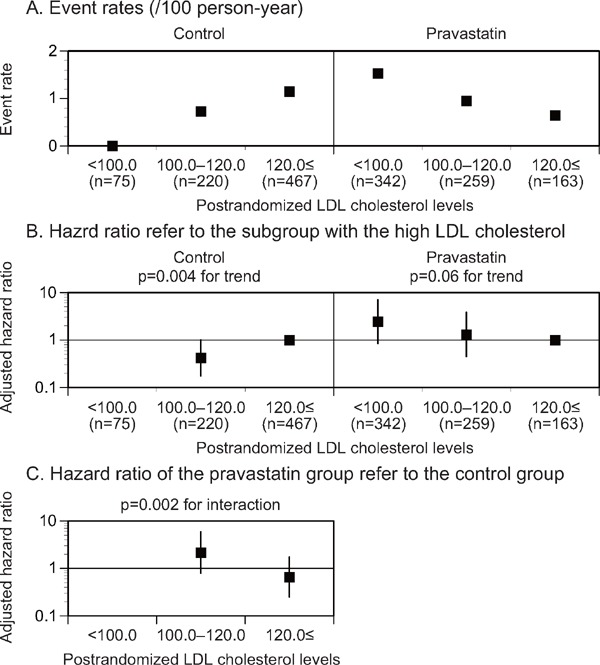
Event rates (A), adjusted HR (B) for lacunar stroke stratified by the postrandomized LDL cholesterol levels, and adjusted HR (C) of the pravastatin group in reference to the control group in each postrandomized LDL cholesterol level subgroup
The subgroup with the high postrandomized LDL cholesterol levels was used as a reference (B). The HR was kept blank when the event rate was 0/100 person-year. LDL, low-density lipoprotein. *p < 0.05.
Discussion
Despite the event rates of major cardiovascular events having been expected to decrease, the event rates of stroke did not decrease in accordance with lower postrandomized LDL cholesterol levels or higher reduced LDL cholesterol levels under pravastatin treatment in our results. Conversely, the event rates of stroke decreased in the low postrandomized LDL cholesterol level (less than 100 mg/dL) in the control group. Stroke is a heterogeneous disease with different etiologies. In our study, stroke events were mostly composite of atherothrombotic stroke, lacunar stroke, and intracranial hemorrhage. Their frequencies and proportions that occurred in the studied cohort may influence the composite results because statin effects may differ among them. In this study, lacunar stroke events consisted of around half of stroke events and atherothrombotic stroke approximately 20%. To understand the influence of statin on composite stroke prevention, we need to consider the influence of statin on each stroke subtypes and their at-risk proportions.
From the Hisayama study, the LDL cholesterol level was associated with the development of atherothrombotic stroke9). We reported that the events of atherothrombotic stroke could be reduced with pravastatin treatment, among the main results of J-STARS8). Pravastatin treatment reduced the progression of carotid intima-media thickness, which could be associated with a reduced risk of atherothrombotic stroke10). In this post hoc analysis of J-STARS, no significant trend was observed for the association of postrandomized LDL cholesterol level with the event of atherothrombotic stroke in the control group. Although no atherothrombotic stroke was observed in the low postrandomized LDL cholesterol level (less than 100 mg/dL) of the control group, the number of patients in this range of postrandomized LDL cholesterol levels in the control group was small. In the middle and high postrandomized LDL cholesterol level subgroups (100–120 mg/dL and 120 mg/dL or more), the pravastatin group showed lower HRs for atherothrombotic stroke than the control group, and the difference was significant in the middle postrandomized LDL cholesterol level subgroup (100–120 mg/dL). From our results, pravastatin treatment showed preventive effect for atherothrombotic stroke independent of postrandomized LDL cholesterol levels. Moreover, non-statin treatment also showed a preventive effect for atherothrombotic stroke in the low postrandomized LDL cholesterol level (less than 100 mg/dL). It could be feasible to control for LDL cholesterol level in the group with less than 100 mg/dL with either statin or non-statin treatment. In addition, even when LDL cholesterol level was maintained between 100 and 120 mg/dL, statins were still beneficial for preventing atherothrombotic stroke.
A post hoc analysis of the Heart Protection Study indicated that the incidence of hemorrhagic stroke shifts toward an increase with simvastatin treatment in patients with a history of cerebrovascular disease11). Furthermore, in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial, the atorvastatin treatment was independently associated with the risk of hemorrhagic stroke after adjusting for covariates, including postrandomization blood pressure12). However, the postrandomization LDL cholesterol level was not independently associated with the risk of hemorrhagic stroke in the atorvastatin group, with a mean postrandomization LDL cholesterol level of 72.9 ± 0.5 mg/dL. In the J-STARS study, a significant increase in the risk of intracranial hemorrhage was not observed in any postrandomized LDL cholesterol level subgroup of either the control or pravastatin group. In addition, no significant differences in the rate of intracranial hemorrhage were observed in any postrandomized LDL cholesterol level subgroup between the pravastatin and control groups. Therefore, we postulate that the risk of intracranial hemorrhage may not increase in individuals with LDL cholesterol levels less than 100 mg/dL who receive pravastatin treatment. We note that a limited number of patients had an outcome of intracranial hemorrhage in the J-STARS study; therefore, conclusive evidence for this hypothesis could not be obtained from our results.
There have been several reports of the association of LDL cholesterol levels with the risk of lacunar stroke. The Hisayama study reported that the incidence rates of both atherothrombotic stroke and lacunar stroke were higher in patients with higher LDL cholesterol levels9). The Atherosclerosis Risk in Communities (ARIC) study reported that the LDL cholesterol level is associated with large lacunar stroke (8 mm or more) but not with small stroke (7 mm or less)13). It has also been reported that elevated LDL cholesterol is more prevalent in nocturnal lacunar strokes, especially when combined with a reduced nocturnal dipping of blood pressure14). However, few studies have evaluated the association of on-treatment LDL cholesterol level or its reduced levels with the risk of lacunar stroke. From our results, the incidence rates of lacunar stroke decreased in association with lower postrandomized and higher reduced LDL cholesterol levels in the control group but not in the pravastatin group. Recently, lacunar stroke has been considered to present an etiology similar to that of intracerebral hemorrhage: so-called cerebral small vessel disease15). In contrast, pericyte degeneration has been reported to cause white-matter dysfunction and to disrupt white-matter microcirculation, triggering losses of myelin, axons, and oligodendrocytes16). Statins induce dose-dependent apoptosis in the pericyte cell line17), and statin-induced apoptosis in pericytes is mediated by cholesterol, caspase-3, and caspase-7. It might be possible that statin treatment diminished the reduction in the risk of lacunar stroke in accordance with lower postrandomized LDL cholesterol levels. However, our post hoc results are inconclusive, and further studies are needed to confirm our results on the influence of statins on the association of LDL cholesterol with the risk of lacunar stroke and to evaluate their pharmacological mechanisms.
The current study has certain limitations. First, this project was a post hoc analysis of a prospective, randomized, open, blinded endpoints (PROBE) design study. Therefore, it is difficult to draw a definitive conclusion. Second, the small study sample size in each subgroup might not provide sufficient statistical power to adequately assess the effects of pravastatin. Third, post hoc analysis may lead to the possibility of type I and II errors.
In conclusion, statins were detected to have different influences on the associations of atherothrombotic stroke and lacunar stroke with LDL cholesterol levels. Although it may still need to be confirmed by other studies, the differences in these associations may influence the effects of statins on reducing stroke recurrence, since at-risk stroke subtypes can differ among patients.
Acknowledgments
The authors thank the patients and their families and appreciate the study participants, physicians, and supporting medical staff and coworkers for their assistance in the preparation and execution of this study.
Conflict of Interest
Dr. Kitagawa reports personal fees from Daiichi Sankyo, during the conduct of the study; personal fees from Bayer Inc., Takeda Pharmaceutical, Nippon Boehringer Ingelheim, Kyowa Hakko Kirin, Sumitomo Dainippon Pharma, Astellas Pharma, and Sanofi, outside the submitted work; and grants from Daiichi Sankyo during the conduct of the study; grants from Bayer Inc., Takeda Pharmaceutical, Nippon Boehringer Ingelheim, Kyowa Hakko Kirin, Sumitomo Dainippon Pharma, Astellas Pharma, and Sanofi, outside the submitted work.
Dr. Maruyama reports grants from Grants-in-Aid for Scientific Research, Eisai, Pfizer, Otsuka Pharmaceutical, Shionogi, Sumitomo Dainippon Pharma, Nihon Medi-Physics, Bayer, MSD, Daiichi Sankyo, Sanofi, and Astellas Pharma, outside the submitted work.
Dr. Minematsu reports personal fees from Bayer Yakuhin, Otsuka Pharmaceutical, Boehringer-Ingelhaeim, AstraZeneca, Pfizer, Mitsubishi Tanabe Pharma Corporation, Japan Stryker, Daiichi Sankyo, Astellas Pharma, Nippon Chemiphar, Fuji Film RI Pharma, CSL Behring, Medicos Hirata, EPS Corporation, HEALIOS K.K., and T-PEC Corporation, outside the submitted work.
Dr. Uchiyama reports personal fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Sanofi, Daiichi Sankyo, Dainippon Sumitomo, Astellas Pharma, AstraZeneca, Sannwa Kagaku, Shionogi, Mitsubishi Tanabe, and Pfizer, outside the submitted work.
Dr. Matsumoto reports personal fees from Kowa Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Sanofi KK, Daiichi Sankyo Co Ltd, Otsuka Pharmaceutical Co Ltd, Astellas Pharma Inc, Astra Zeneca KK, Mochida Pharmaceutical Co Ltd, Sumitomo Dainippon Pharma Co Ltd, Amgen Astellas BioPharma KK, Eisai Co Ltd, and Pfizer Japan Inc, outside the submitted work.
Ms. Nakagawa and Mr. Kagimura are employed at the Translational Research Informatics Center, which contracted with the J-STARS office as a data center and received a data center fee.
The other authors declare that they have no conflicts of interest.
Financial Support
This study was initially supported by a grant from the Ministry of Health, Labour and Welfare of Japan. After the governmental support expired, the study was conducted in collaboration between Hiroshima University Graduate School of Biomedical and Health Sciences and the Foundation for Biomedical Research and Innovation. The latter organization receives unconditional research grants from several pharmaceutical companies, including Daiichi Sankyo Co., Ltd., which markets pravastatin. However, the company was not involved in the design or execution of this study. In addition, the company did not provide pravastatin for this study and did not reviewed the current manuscript.
Author Contributions
MM was the principal investigator. NH, KK, Y Nagai, SA, TN, KM, SU, and MM were responsible for the conception and design of the study. Y Nakagawa, TK, and HO performed the statistical analysis. NH, Y Nagai, TK, and MM interpreted the data. NH, SA, TN, and MM designed the figures. NH, KK, Y Nagai, SA, TN, HM, KM, SU, and MM contributed to drafting the report. All authors participated in the finalization of the report.
References
- 1). Amarenco P, Goldstein LB, Szarek M, Sillesen H, Rudolph AE, Callahan A, 3rd, Hennerici M, Simunovic L, Zivin JA, Welch KM, Investigators S : Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke, 2007; 38: 3198-3204 [DOI] [PubMed] [Google Scholar]
- 2). Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, Messig M, Welch KM, Stroke Prevention by Aggressive Reduction in Cholesterol Levels I : Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke, 2008; 39: 3297-3302 [DOI] [PubMed] [Google Scholar]
- 3). Amarenco P, Benavente O, Goldstein LB, Callahan A, 3rd, Sillesen H, Hennerici MG, Gilbert S, Rudolph AE, Simunovic L, Zivin JA, Welch KM: Results of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial by stroke subtypes. Stroke, 2009; 40: 1405-1409 [DOI] [PubMed] [Google Scholar]
- 4). Kitagawa K, Hosomi N, Nagai Y, Kagimura T, Ohtsuki T, Maruyama H, Origasa H, Minematsu K, Uchiyama S, Nakamura M, Matsumoto M, J-STARS collaborators : Cumulative effects of LDL cholesterol and CRP levels on recurrent stroke and TIA. J Atheroscler Thromb, 2018. in press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Kitagawa K, Hosomi N, Nagai Y, Kagimura T, Ohtsuki T, Origasa H, Minematsu K, Uchiyama S, Nakamura M, Matsumoto M, J-STARS Investigators : Reduction in high-sensitivity C-reactive protein levels in patients with ischemic stroke by statin treatment: Hs-CRP Sub-Study in J-STARS. J Atheroscler Thromb, 2017; 24: 1039-1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Hosomi N, Kitagawa K, Nagai Y, Nakagawa Y, Aoki S, Nezu T, Kagimura T, Maruyama H, Origasa H, Minematsu K, Uchiyama S, Matsumoto M, J-STARS Collaborators : Desirable low-density lipoprotein cholesterol levels for preventing stroke recurrence: a post hoc analysis of the J-STARS Study. Stroke, 2018; 49: 865-871 [DOI] [PubMed] [Google Scholar]
- 7). Nagai Y, Kohriyama T, Origasa H, Minematsu K, Yokota C, Uchiyama S, Ibayashi S, Terayama Y, Takagi M, Kitagawa K, Nomura E, Hosomi N, Ohtsuki T, Yamawaki T, Matsubara Y, Nakamura M, Yamasaki Y, Mori E, Fukushima M, Kobayashi S, Shinohara Y, Yamaguchi T, Matsumoto M, Investigators J-S : Rationale, design, and baseline features of a randomized controlled trial to assess the effects of statin for the secondary prevention of stroke: the Japan Statin Treatment Against Recurrent Stroke (J-STARS). Int J Stroke, 2014; 9: 232-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, Maruyama H, Sunami N, Yokota C, Kitagawa K, Terayama Y, Takagi M, Ibayashi S, Nakamura M, Origasa H, Fukushima M, Mori E, Minematsu K, Uchiyama S, Shinohara Y, Yamaguchi T, Matsumoto M, J-STARS collaborators : The Japan Statin Treatment Against Recurrent Stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine, 2015; 2: 1071-1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Imamura T, Doi Y, Arima H, Yonemoto K, Hata J, Kubo M, Tanizaki Y, Ibayashi S, Iida M, Kiyohara Y: LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: the Hisayama study. Stroke, 2009; 40: 382-388 [DOI] [PubMed] [Google Scholar]
- 10). Koga M, Toyoda K, Minematsu K, Yasaka M, Nagai Y, Aoki S, Nezu T, Hosomi N, Kagimura T, Origasa H, Kamiyama K, Suzuki R, Ohtsuki T, Maruyama H, Kitagawa K, Uchiyama S, Matsumoto M, J-STARS Investigators : Long-term effect of pravastatin on carotid intima-media complex thickness: The J-STARS Echo Study. Stroke, 2018; 49: 107-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Collins R, Armitage J, Parish S, Sleight P, Peto R, Heart Protection Study Collaborative Group : Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet, 2004; 363: 757-767 [DOI] [PubMed] [Google Scholar]
- 12). Goldstein LB, Amarenco P, Szarek M, Callahan A, 3rd, Hennerici M, Sillesen H, Zivin JA, Welch KM, Investigators S : Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology, 2008; 70: 2364-2370 [DOI] [PubMed] [Google Scholar]
- 13). Bezerra DC, Sharrett AR, Matsushita K, Gottesman RF, Shibata D, Mosley TH, Jr., Coresh J, Szklo M, Carvalho MS, Selvin E: Risk factors for lacune subtypes in the Atherosclerosis Risk in Communities (ARIC) Study. Neurology, 2012; 78: 102-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Matz K, Tatschl C, Sebek K, Dachenhausen A, Brainin M: Dyslipidemia, elevated LDL cholesterol and reduced nocturnal blood pressure dipping denote lacunar strokes occurring during nighttime. Eur J Neurol, 2004; 11: 742-748 [DOI] [PubMed] [Google Scholar]
- 15). Moran C, Phan TG, Srikanth VK: Cerebral small vessel disease: a review of clinical, radiological, and histopathological phenotypes. Int J Stroke, 2012; 7: 36-46 [DOI] [PubMed] [Google Scholar]
- 16). Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, Barnes SR, Daianu M, Ramanathan A, Go A, Lawson EJ, Wang Y, Mack WJ, Thompson PM, Schneider JA, Varkey J, Langen R, Mullins E, Jacobs RE, Zlokovic BV: Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med, 2018; 24: 326-337 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17). Boucher K, Siegel CS, Sharma P, Hauschka PV, Solomon KR: HMG-CoA reductase inhibitors induce apoptosis in pericytes. Microvasc Res, 2006; 71: 91-102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supported the findings of this study are available from the corresponding author upon reasonable request.


