Abstract
Transition-metal-catalyzed tandem Heck/carbonylation reaction has emerged as a powerful tool for the synthesis of structurally diverse carbonyl molecules, as well as natural products and pharmaceuticals. However, the asymmetric version was rarely reported, and remains a challenging topic. Herein, we describe a palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes. Alcohols, phenols and amines are employed as versatile coupling reagents for the construction of multifunctional chiral bicyclo[3.2.1]octanes with one all-carbon quaternary and two tertiary carbon stereogenic centers in high diastereo- and enantioselectivities. This study represents an important progress in both the asymmetric tandem Heck/carbonylation reactions and enantioselective difunctionalization of internal alkenes.
Subject terms: Asymmetric catalysis, Asymmetric synthesis, Synthetic chemistry methodology
Tandem Heck/carbonylation reaction gives access to ubiquitous carbonyl molecules, however the asymmetric version is rarely studied. Here, the authors synthesize chiral bicyclo[3.2.1]octanes with a palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes with alcohols, phenols and amines.
Introduction
Transition-metal (TM)-catalyzed carbonylation reaction1–16, especially palladium-catalyzed tandem Heck/carbonylation reaction, presents an efficient method to construct a variety of synthetically versatile carbonyl compounds from readily accessible organic halides and alkenes17–21. Moreover, these methods have been applied as key steps in the total synthesis of natural products and bioactive molecules22–28. Very recently, Reisman and co-workers realized the total synthesis of (+)-Perseanol employing palladium-catalyzed tandem Heck/carbonylation to assemble the vital tetracyclic core (Fig. 1a)29. However, the asymmetric version of tandem Heck/carbonylation reactions was rare, and remains a challenging topic. Some inherent issues, such as the strong π-acidity and coordination ability of CO, would hamper the oxidative addition of organohalides towards low-valent metal species30. In addition, the harsh reaction conditions (high temperature and high CO pressure), the incidental racemization31, the β-hydrogen elimination of alkylpalladium intermediates, the direct carbonylation of organohalides, and other competitive side-reactions make the asymmetric progress more difficult and complicated. Recently, three representative works on palladium-catalyzed asymmetric tandem Heck/carbonylation reaction of 1,1-disubstitueted alkenes to synthesize dihydrobenzofurans, oxindole derivatives, and 3,4-dihydroisoquinolines have been realized by Correia’s group32, Zhu and Luo’s group33, and Zhang’s group34, respectively. In contrast to the success of 1,1-disubstituted alkenes (the alkylpalladium intermediates lack eliminable β-hydrogen), the TM-catalyzed asymmetric tandem Heck/carbonylation reaction of unactivated internal alkenes has not been developed until now.
Fig. 1. Palladium-catalyzed tandem Heck/carbonylation reactions of alkenes.
a Pd-catalyzed tandem Heck/carbonylation as key step to the total synthesis of (+)-Perseanol. b Palladium-catalyzed asymmetric tandem Heck/carbonylation of internal alkenes towards bicyclo[3.2.1]octanes (this work). c Hypothesis of mechanism for the tandem Heck/carbonylation reaction and foreseeable side reactions.
On the other hand, bicyclo[3.2.1]octanes are found in several natural products with antibacterial, antioxidant, antithrombosis, and antitumor activities35,36. However, constructing such intricate polycyclic bridge ring compounds with multiple chiral centers simultaneous implementation remains a challenging project37–46. Based on our research interest in Heck reactions47–50, herein, we describe a palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes to construct multifunctional chiral bicyclo[3.2.1]octanes bearing one all-carbon quaternary and two tertiary carbon stereogenic centers in excellent diastereoselectivities and enantioselectivities (Fig. 1b). Suppressing the foreseeable side reactions, such as β-hydrogen elimination of alkylpalladium intermediates, and the direct CO insertion or nucleophiles insertion reaction (Fig. 1c), is the key to the success of this reaction.
Results
Reaction optimization
After systematic evaluation of the reaction conditions, the desired chiral bicyclo[3.2.1]octane product 3aa was achieved in 81% yield and 96% ee employing Pd2dba3·CHCl3 (5 mol%) as the catalyst, (S)-Difluorphos L1 (20 mol%) as the ligand, K2CO3 (2 equiv) as the base, and mixed 1,2-dichloroethane (DCE)/dichloromethane (DCM) (10/1) as the solvent at 100 °C (Table 1, entry 1). Other catalysts, such as Pd(OAc)2 and Pd2(dba)3 were less effective (entries 2 and 3). BINAP L2, SEGPHOS L3, DM-SEGPHOS L4 offered 3aa in 52–80% ee (entries 4–6), while BOX-type ligand L5, PHOX-type ligand L6, bis(phosphine-amide) ligand L7 and phosphoramidite ligand L8 caused the reaction inactivation (entries 7–10). Decreasing the amount of ligand resulted 3aa in diminished yield, diastereo- and enantioselectivity (entry 11). Screening the additives revealed that K2CO3 was optimal, and AgOAc delivered racemic 3a′ in 83% yield, which was formed via β-hydrogen elimination (entries 1 and 12–14). The choice of solvent, also the ratio of the mixed solvent, was crucial to the reaction efficiency (entries 15–20). Adjusting the reaction temperature was inconducive to improve the outcome of the reaction (entries 21–22). The structure and absolute configuration of 3aa were confirmed by single-crystal X-ray diffraction analysis (see the Supplementary Note 3 for details).
Table 1.
Optimization of reaction conditionsa.
 | |||||
|---|---|---|---|---|---|
| Entry | Deviation of standard conditions | Yield of 3aa (%)b | Dr of 3aac | Ee of 3aa (%)d | Yield of 3a′ (%)b |
| 1 | None | 81 | >20:1 | 96 | <2 |
| 2 | Pd(OAc)2 instead of Pd2dba3·CHCl3 | 71 | >20:1 | 66 | 6 |
| 3 | Pd2dba3 instead of Pd2dba3·CHCl3 | 31 | >20:1 | 85 | 7 |
| 4 | L2 instead of L1 | 82 | >20:1 | 52 | <2 |
| 5 | L3 instead of L1 | 75 | 15:1 | 79 | 9 |
| 6 | L4 instead of L1 | 60 | >20:1 | 80 | <2 |
| 7 | L5 instead of L1 | 59 | >20:1 | −5 | <2 |
| 8 | L6 instead of L1 | 21 | >20:1 | 5 | <2 |
| 9 | L7 instead of L1 | <2 | - | - | 13 |
| 10 | L8 instead of L1 | <2 | - | - | 21 |
| 11 | 10 mol% of L1 instead of 20 mol% of L1 | 63 | 11:1 | 36 | 24 |
| 12 | KHCO3 instead of K2CO3 | 57 | 4:1 | 80 | <2 |
| 13 | Na2CO3 instead of K2CO3 | 32 | 7:1 | 53 | <2 |
| 14 | AgOAc instead of K2CO3 | <2 | – | – | 83 |
| 15 | DCE instead of DCE/DCM | 78 | 4:1 | 80 | <2 |
| 16 | DCM instead of DCE/DCM | 75 | >20:1 | 88 | <2 |
| 17 | Toluene instead of DCE/DCM | 69 | 5:1 | 53 | <2 |
| 18 | CH3CN instead of DCE/DCM | 90 | 8:1 | 77 | <2 |
| 19 | DCE/DCM = 1/1 instead of 10/1 | 71 | 10:1 | 96 | 8 |
| 20 | DCE/DCM = 1/10 instead of 10/1 | 38 | 12:1 | 93 | 38 |
| 21 | 80 °C instead of 100 °C | 71 | >20:1 | 77 | <2 |
| 22 | 120 °C instead of 100 °C | 38 | 13:1 | 94 | <2 |
aReaction conditions: 1a (0.1 mmol), 2a (1 mmol), [Pd] (10 mol%), ligand (20 mol%), base (0.2 mmol) in 1 mL solvent, 100 °C, 36 h, under CO (1 atm).
bIsolated yield.
cDetermined by 1H NMR analysis.
dDetermined by HPLC analysis on a chiral stationary phase.
Substrate scope
With the optimized reaction conditions in hand, we then tested the substrate scope of alcohols in this asymmetric Heck/carbonylation reaction, and the results were summarized in Fig. 2. Simple primary alcohols, such as ethanol, n-propanol and benzyl alcohol afforded the products 3aa–ad in moderate to good yields with high enantioselectivities. It is noted that aryl bromine derivative was also a good candidate, delivering 3ab in 50% yield with 94% ee after prolonging the time to 48 h. Besides, other primary alcohols with various functional groups, such as alkenyl, trifluoromethyl, halogen, trimethylsilyl, even highly sterically hindered adamantly group, all performed well, offering 3ae–ai in 88–96% ee. Cyclic and acyclic secondary alcohols delivered the corresponding products 3aj–ao in good efficiency. Products 3ba–da with different substituents on the benzene ring were produced in good yields with high enantioselectivities. Product 3eb with two ester groups was achieved in 68% yield with 95% ee.
Fig. 2. Substrate scope.
a Substrate scope of primary alcohols. b Substrate scope of secondary alcohols. c Substrate scope of the benzoylcyclopentenes. Reaction conditions: X = I, 1 (0.1 mmol), 2 (1 mmol), Pd2dba3•CHCl3 (5 mol%), L1 (20 mol%), K2CO3 (0.2 mmol) in 1 mL solvent, 100 °C, 36 h, under CO (1 atm). Yields of isolated products are given. The dr values were determined by 1H NMR analysis. The ee values were determined by HPLC analysis on a chiral stationary phase. aX = Br. b48 h.
Phenol esters are important skeletons in pharmaceuticals and bioactive compounds. Although phenols as nucleophilic reagents have been employed in some carbonylation reactions51,52, they have not met with the success in asymmetric tandem Heck/carbonylation reactions, because the two potential nucleophilic sites at O and C of phenols would increase the complexity of the reaction. Herein, phenols as versatile components were performed in our asymmetric Heck/carbonylation reactions with KHCO3 as the base and toluene as the solvent (Fig. 3). Electron donating groups (−Me, −tBu, and −OMe), a halogen group (−Cl), an electron withdrawing group (−COMe), as well as a phenyl group at the para-position of phenols offered the corresponding products 5aa–ag in 91–95% ee. meta-Chlorine substituted phenol 4h and 3,5-dimethylphenol 4i could also fulfill the reaction well, and no significant steric hindrance effect was observed. 1-Naphthol delivered 5aj in 95% ee. Moreover, monobenzone, as a potent skin lightener drug, could give the adduct 5ak in 91% ee.
Fig. 3. Substrate scope of phenols.
Reaction conditions: 1a (0.1 mmol), 4 (0.25 mmol), Pd2dba3•CHCl3 (5 mol%), L1 (20 mol%), KHCO3 (0.2 mmol) in toluene (1 mL), 100 °C, 36 h, under CO (1 atm). Yields of isolated products are given. The dr values were determined by 1H NMR analysis. The ee values were determined by HPLC analysis on a chiral stationary phase.
To further exhibit the robustness and generality of this reaction, scope of nitrogen nucleophiles was investigated with Pddba2 (10 mol%) as the catalyst, L1 (20 mol%) as the ligand, K2HPO4 (0.2 mmol) as the base in CH3CN (1 mL) at 100 °C (Fig. 4). Acyclic secondary alkylamines, such as diethylamine and dibenzylamine delivered products 7aa (see the Supplementary Note 4 for details on the X-ray crystal structure) and 7ab in 93 and 94% ee. Cyclic secondary alkylamines furnished products 7ac–ag in 92–94% ee. Alkylarylamines, such as N-methylaniline and indoline, provided 7ah and 7ai in 92 and 91% ee. Primary alkylamines, such as n-propylamine, benzylamines, and thiophenylmethanamine offered products 7aj–am in 92–94% ee. Primary arylamines were also qualified to work in this reaction, delivering products 7an–ap in good enantioselectivities with KHCO3 as the base after prolonging the reaction time to 48 h. 5-OMe-substituted cyclopentene 1f performed smoothly to give 7fb in 97% ee. Finally, pharmaceuticals including Vortioxetine, Trimetazidine and Riluzole were all well late-stage functionalized with bicyclo[3.2.1]octanes to offer 7aq–as in 88–95% ee.
Fig. 4. Substrate scope.
a Substrate scope of amines. b Application to asymmetric late-stage functionalization of pharmaceuticals. Reaction conditions: 1a (0.1 mmol), 6 (0.2 mmol), Pddba2 (10 mol%), L1 (20 mol%), K2HPO4 (0.2 mmol) in CH3CN (1 mL), 100 °C, 36 h, under CO (1 atm). Yields of isolated products are given. The dr values were determined by 1H NMR analysis. The ee values were determined by HPLC analysis on a chiral stationary phase. aK2HPO4 was replaced by KHCO3, 48 h. b48 h.
Further study
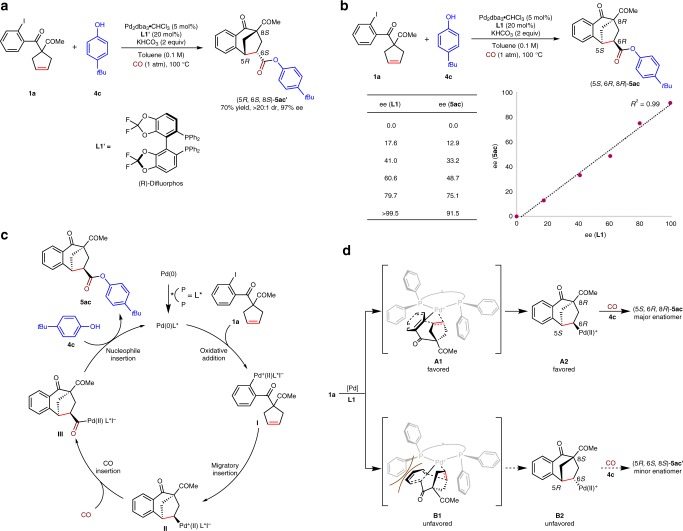
The enantiodivergent synthesis of (5R, 6S, 8S)-5ac′ was also realized in 70% yield and 97% ee employing (R)-Difluorphos as the ligand (Fig. 5a). To demonstrate the mechanism of this reaction, study on nonlinear effect of the enantioselectivity of 5ac was carried out (Fig. 5b). The linear correlation (R2 = 0.99) between the enantioselectivities of the product 5ac and the enantiopurities of the ligand L1 revealed the involvement of one active catalyst species in the stereo-determining transition states of the migratory insertion process. On the basis of the above-mentioned results and previous literatures31,32,47, a proposed mechanism of this reaction is figured in Fig. 5c. Firstly, oxidative addition of the active palladium catalyst with 1a delivers the cationic Pd(II) intermediate I. Intramolecular syn-migratory insertion of I results in the alkylpalladium intermediate II, which followed by the insertion of CO delivers the intermediate III. Finally, the nucleophile insertion of the phenol 4c to the intermediate III produces the product 5ac. It is noted that the high diastereoselectivity was arisen from the stereospecific syn-migratory insertion step, which has been confirmed in our previous work by the deuterium-labeling experiments47. The observed stereochemical outcome of the reaction with the C2-symmetric, (S)-configured ligand L1 can be rationalized based on the two diastereomeric intermediates A1 and B1 (Fig. 5d). The transition state B1 is notable for the severe steric repulsion between the benzoyl moiety of the cyclopentene 1a and the benzene ring of the ligand L1, a factor which is not present in the transition state A1; this may account for the predominance of the (5S, 6R, 8R) enantiomer of 5ac in the product.
Fig. 5. Further study on the reaction.
a Enantiodivergent synthesis of (5R, 6S, 8S)-5ac′. b Linear correlation between the ee values of 5ac and L1. c Proposed mechanism. d Model for enantioselectivity.
Discussion
In summary, we have developed a Pd-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes. Various bicyclo[3.2.1]octanes bearing one chiral all-carbon quaternary and two tertiary carbon stereogenic centers were obtained in moderate to good yields with excellent diastereoselectivities and enantiomeric excess. This method provided a general and practical route for the enantioselective difunctionalization of unactivated internal alkenes and chiral bicyclo[3.2.1]octanes.
Methods
General procedure for the catalytic reactions with alchohols
A sealed tube was charged with the cyclopentenes 1 (0.1 mmol, 1 equiv), Pd2dba3•CHCl3 (5 mol%), L1 (20 mol%), and K2CO3 (0.2 mmol, 2 equiv). The vial was thoroughly flushed with CO, and alchohols 2 (1 mmol, 10 equiv), as well as DCE/DCM (10/1, 1 mL) were added under CO atmosphere. The reaction mixture was stirred at 100 °C for 36 h. After the reaction vessel was cooled to room temperature, the solution was concentrated in vacuo and purified by careful chromatography on silica gel (200–300 mesh) (PE/EA = 4/1) to afford the desired products 3.
General procedure for the catalytic reactions with phenols
A sealed tube was charged with the cyclopentenes 1 (0.1 mmol, 1 equiv), phenols 4 (0.25 mmol, 2.5 equiv), Pd2dba3•CHCl3 (5 mol%), L1 (20 mol%), and KHCO3 (0.2 mmol, 2 equiv). The vial was thoroughly flushed with CO, and toluene (1 mL) was added under CO atmosphere. The reaction mixture was stirred at 100 °C for 36 h. After the reaction vessel was cooled to room temperature, the solution was concentrated in vacuo and purified by careful chromatography on silica gel (200–300 mesh) (PE/EA = 4/1) to afford the desired products 5.
General procedure for the catalytic reactions with amines
A sealed tube was charged with the cyclopentenes 1 (0.1 mmol, 1 equiv), Pddba2 (10 mol%), L1 (20 mol%), and K2HPO4 (0.2 mmol, 2 equiv). The vial was thoroughly flushed with CO, and amines 6 (0.2 mmol, 2 equiv), as well as CH3CN (1 mL) were added under CO atmosphere. The reaction mixture was stirred at 100 °C for 36 h. After the reaction vessel was cooled to room temperature, the solution was concentrated in vacuo and purified by careful chromatography on silica gel (200–300 mesh) (PE/EA = 2/1) to afford the desired products 7.
Supplementary information
Acknowledgements
The authors thank the National Natural Science Foundation of China (NSFC21572272), the Innovation Team of “the Double-First Class” Disciplines (CPU2018GY04 and CPU2018GY35), and the Foundation of the Open Project of State Key Laboratory of Natural Medicines (SKLNMZZCX201818) for the financial support.
Author contributions
Z.Y. planned and conducted most of the experiments; Z.Y., Y.Z., Z.F., and Z. G. prepared substrates for the reaction scope evaluation; A.L. and H.Y. directed the projects and cowrote the manuscript. All authors contributed to the discussion.
Data availability
The authors declare that all the data supporting the findings of this study are available within the article and Supplementary Information files, and are also available from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Center (CCDC) under deposition numbers 1936197 (3aa) and 1936198 (7aa). These data could be obtained free of charge from The Cambridge Crystallographic Data Center via http://www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Zhuangzhi Shi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aijun Lin, Email: ajlin@cpu.edu.cn.
Hequan Yao, Email: hyao@cpu.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-16221-9.
References
- 1.Brennfuehrer A, Neumann H, Beller M. Palladium-catalyzed carbonylation reactions of aryl halides and related compounds. Angew. Chem. Int. Ed. 2009;48:4114–4133. doi: 10.1002/anie.200900013. [DOI] [PubMed] [Google Scholar]
- 2.Liu Q, Zhang H, Lei A. Oxidative carbonylation reactions: organometallic compounds (R-M) or hydrocarbons (R-H) as nucleophiles. Angew. Chem. Int. Ed. 2011;50:10788–10799. doi: 10.1002/anie.201100763. [DOI] [PubMed] [Google Scholar]
- 3.Sumino S, Fusano A, Fukuyama T, Ryu I. Carbonylation reactions of alkyl iodides through the interplay of carbon radicals and Pd catalysts. Acc. Chem. Res. 2014;47:1563–1574. doi: 10.1021/ar500035q. [DOI] [PubMed] [Google Scholar]
- 4.Wu X-F, et al. Transition-metal-catalyzed carbonylation reactions of Olefins and alkynes: a personal account. Acc. Chem. Res. 2014;47:1041–1053. doi: 10.1021/ar400222k. [DOI] [PubMed] [Google Scholar]
- 5.Wu L, et al. Palladium-catalyzed carbonylative transformation of C(sp3)−X bonds. ACS Catal. 2014;4:2977–2989. [Google Scholar]
- 6.Chen J, et al. Base-controlled selectivity in the synthesis of linear and angular fused quinazolinones by a palladium-catalyzed carbonylation/nucleophilic aromatic substitution sequence. Angew. Chem. Int. Ed. 2014;53:7579–7583. doi: 10.1002/anie.201402779. [DOI] [PubMed] [Google Scholar]
- 7.Lin M, Li F, Jiao L, Yu Z-X. Rh(I)-catalyzed formal [5+1]/[2+2+1] cycloaddition of 1-Yne-vinylcyclopropanes and two CO units: one-step construction of multifunctional angular tricyclic 5/5/6 compounds. J. Am. Chem. Soc. 2011;133:1690–1693. doi: 10.1021/ja110039h. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Song W, Tang W. Rhodium-catalyzed tandem annulation and (5+1) cycloaddition: 3-hydroxy-1,4-enyne as the 5-carbon component. J. Am. Chem. Soc. 2013;135:16797–16800. doi: 10.1021/ja408829y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J, Qi X, Li M, Chen P, Liu G. Palladium-catalyzed intermolecular aminocarbonylation of alkenes: efficient access of β-amino acid derivatives. J. Am. Chem. Soc. 2015;137:2480–2483. doi: 10.1021/jacs.5b00719. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Yu F, Qi X, Chen P, Liu G. Cooperative strategy for the highly selective intermolecular oxycarbonylation reaction of alkenes using a palladium catalyst. Angew. Chem. Int. Ed. 2016;55:13843–13848. doi: 10.1002/anie.201607248. [DOI] [PubMed] [Google Scholar]
- 11.Qi X, Yu F, Chen P, Liu G. Palladium-catalyzed intermolecular oxidative fluorocarbonylation of unactivated alkenes: efficient access of β-fluorocarboxylic esters. Angew. Chem. Int. Ed. 2017;56:12692–12696. doi: 10.1002/anie.201706401. [DOI] [PubMed] [Google Scholar]
- 12.Ding D, Zhu G-H, Jiang X-F. Ligand controlled Pd(II)-catalyzed regiodivergent carbonylation of alkynes for syntheses of indolo[3,2-c]coumarins and benzofuro[3,2-c]quinolinones. Angew. Chem. Int. Ed. 2018;57:9028–9032. doi: 10.1002/anie.201804788. [DOI] [PubMed] [Google Scholar]
- 13.Wu L-J, Song R-J, Luo S, Li J-H. Palladium-catalyzed reductive [5+1] cycloaddition of 3-acetoxy-1,4-enynes with CO: access to phenols enabled by hydrosilanes. Angew. Chem. Int. Ed. 2018;57:13308–13312. doi: 10.1002/anie.201808388. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Chen P, Liu G. Pd-catalyzed intermolecular arylcarbonylation of unactivated alkenes: incorporation of bulky aryls at room temperature. Angew. Chem. Int. Ed. 2018;57:15871–15876. doi: 10.1002/anie.201810405. [DOI] [PubMed] [Google Scholar]
- 15.Zheng W-F, et al. Tetrasubstituted allenes via the palladium-catalysed kinetic resolution of propargylic alcohols using a supporting ligand. Nat. Catal. 2019;2:997–1005. [Google Scholar]
- 16.Yang J, et al. Direct synthesis of adipic acid esters via palladium-catalyzed carbonylation of 1,3-dienes. Science. 2019;366:1514–1517. doi: 10.1126/science.aaz1293. [DOI] [PubMed] [Google Scholar]
- 17.Negishi E-i, et al. Palladium-catalyzed carbonylative cyclization of 1-iodo-2-alkenylbenzenes. J. Am. Chem. Soc. 1996;118:5904–5918. [Google Scholar]
- 18.Aggarwal, V. K., Davies, P. W. & Moss, W. O. A palladium catalysed cyclisation-carbonylation of bromodienes: control in carbonylation over facile β-hydride elimination. Chem. Commun.9, 972–973 (2002). [DOI] [PubMed]
- 19.Seashore-Ludlow B, Somfai P. Domino carbopalladation-carbonylation: generating quaternary stereocenters while controlling β-hydride elimination. Org. Lett. 2010;12:3732–3735. doi: 10.1021/ol1009703. [DOI] [PubMed] [Google Scholar]
- 20.Seashore‐Ludlow B, Danielsson J, Somfai P. Domino carbopalladation‐carbonylation: investigation of substrate scope. Adv. Synth. Catal. 2012;354:205–216. [Google Scholar]
- 21.Liu X, Gu Z. Pd-catalyzed heck cyclization and in situ hydrocarboxylation or hydromethenylation via a hydrogen borrowing strategy. Org. Chem. Front. 2015;2:778–782. [Google Scholar]
- 22.Gehrtz PH, Hirschbeck V, Ciszek B, Fleischer I. Carbonylations of alkenes in the total synthesis of natural compounds. Synthesis. 2016;48:1573–1596. [Google Scholar]
- 23.Bai Y, Davis DC, Dai M. Natural product synthesis via palladium-catalyzed carbonylation. J. Org. Chem. 2017;82:2319–2328. doi: 10.1021/acs.joc.7b00009. [DOI] [PubMed] [Google Scholar]
- 24.Ma K, Martin BS, Yin X, Dai M. Natural product syntheses via carbonylative cyclizations. Nat. Prod. Rep. 2019;36:174–219. doi: 10.1039/c8np00033f. [DOI] [PubMed] [Google Scholar]
- 25.Artman GD, Weinreb SM. An approach to the total synthesis of the marine ascidian metabolite perophoramidine via a halogen-selective tandem Heck/carbonylation strategy. Org. Lett. 2003;5:1523–1526. doi: 10.1021/ol034314d. [DOI] [PubMed] [Google Scholar]
- 26.Bai Y, Shen X, Li Y, Dai M. Total synthesis of (-)-spinosyn A via carbonylative macrolactonization. J. Am. Chem. Soc. 2016;138:10838–10841. doi: 10.1021/jacs.6b07585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu. L, Wang C, Gao Z, Zhao Y-M. Total Synthesis of (±)-cephanolides B and C via a palladium-catalyzed cascade cyclization and late-stage sp3 C–H bond oxidation. J. Am. Chem. Soc. 2018;140:5653–5658. doi: 10.1021/jacs.8b03015. [DOI] [PubMed] [Google Scholar]
- 28.Ma K, Yin X, Dai M. Total syntheses of bisdehydroneostemoninine and bisdehydrostemoninine by catalytic carbonylative spirolactonization. Angew. Chem. Int. Ed. 2018;57:15209–15212. doi: 10.1002/anie.201809114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han A, Tao Y, Reisman SE. A 16-step synthesis of the isoryanodane diterpene (+)-perseanol. Nature. 2019;573:563–567. doi: 10.1038/s41586-019-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanti, G. & Peeters, D. DFT study of small palladium clusters Pdn and their interaction with a CO ligand (n = 1–9). Eur. J. Inorg. Chem.2009, 3904–3911 (2009).
- 31.Noonan GM, et al. Regioselective and enantioselective hydroformylation of dialkylacrylamides. Adv. Synth. Catal. 2010;352:1047–1054. [Google Scholar]
- 32.Carmona RC, Koster OD, Duarte Correia CR. Chiral N,N ligands enabling palladium-catalyzed enantioselective intramolecular Heck-Matsuda carbonylation reactions by sequential migratory and CO insertions. Angew. Chem. Int. Ed. 2018;57:12067–12070. doi: 10.1002/anie.201805831. [DOI] [PubMed] [Google Scholar]
- 33.Hu H, et al. Enantioselective synthesis of 2-oxindole spiro-fused lactones and lactams by Heck/carbonylative cyclization: method development and applications. Angew. Chem. Int. Ed. 2019;58:9225–9229. doi: 10.1002/anie.201904838. [DOI] [PubMed] [Google Scholar]
- 34.Cheng C, Wan B, Zhou B, Gu Y, Zhang Y. Enantioselective synthesis of quaternary 3,4-dihydroisoquinolinones via Heck carbonylation reactions: development and application to the synthesis of Minalrestat analogues. Chem. Sci. 2019;10:9853–9858. doi: 10.1039/c9sc03406d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filippini MH, Rodriguez J. Synthesis of functionalized bicyclo[3.2.1]octanes and their multiple uses in organic chemistry. Chem. Rev. 1999;99:27–76. doi: 10.1021/cr970029u. [DOI] [PubMed] [Google Scholar]
- 36.Presset M, Coquerel Y, Rodriguez J. Syntheses and applications of functionalized bicyclo[3.2.1]octanes: thirteen years of progress. Chem. Rev. 2013;113:525–595. doi: 10.1021/cr200364p. [DOI] [PubMed] [Google Scholar]
- 37.Bashore CG, et al. Enantioselective synthesis of nicotinic receptor probe 7,8-difluoro-1,2,3,4,5,6-hexahydro-1,5-methano-3-benzazocine. Org. Lett. 2006;8:5947–5950. doi: 10.1021/ol0623062. [DOI] [PubMed] [Google Scholar]
- 38.Grachan ML, Tudge MT, Jacobsen EN. Enantioselective catalytic carbonyl-ene cyclization reactions. Angew. Chem. Int. Ed. 2008;47:1469–1472. doi: 10.1002/anie.200704439. [DOI] [PubMed] [Google Scholar]
- 39.Rueping M, Kuenkel A, Tato F, Bats JW. Asymmetric organocatalytic domino Michael/Aldol reactions: enantioselective synthesis of chiral cycloheptanones, tetrahydrochromenones, and polyfunctionalized bicyclo-[3.2.1] octanes. Angew. Chem. Int. Ed. 2009;48:3699–3702. doi: 10.1002/anie.200900754. [DOI] [PubMed] [Google Scholar]
- 40.Huwyler N, Carreira EM. Total synthesis and stereochemical revision of the chlorinated sesquiterpene (±)-gomerone C. Angew. Chem. Int. Ed. 2012;51:13066–13069. doi: 10.1002/anie.201207203. [DOI] [PubMed] [Google Scholar]
- 41.Zhu S, Zhang Q, Chen K, Jiang H. Synergistic catalysis: metal/proton-catalyzed cyclization of alkynones toward bicyclo[3.n.1]alkanones. Angew. Chem. Int. Ed. 2015;54:9414–9418. doi: 10.1002/anie.201504964. [DOI] [PubMed] [Google Scholar]
- 42.Ando Y, Hori S, Fukazawa T, Ohmori K, Suzuki K. Toward naphthocyclinones: doubly connected octaketide dimers with a bicyclo[3.2.1]octadienone core by thiolate-mediated cyclization. Angew. Chem. Int. Ed. 2015;54:9650–9653. doi: 10.1002/anie.201503442. [DOI] [PubMed] [Google Scholar]
- 43.Burns AR, Madec AGE, Low DW, Roy ID, Lam HW. Enantioselective synthesis of bicyclo[3.n.1]alkanes by chiral phosphoric acid-catalyzed desymmetrizing Michael cyclizations. Chem. Sci. 2015;6:3550–3555. doi: 10.1039/c5sc00753d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu C, Wang D, Zhao Y, Sun W-Y, Shi Z. Enantioselective palladium-catalyzed intramolecular α-arylative desymmetrization of 1,3-diketones. J. Am. Chem. Soc. 2017;139:16486–16489. doi: 10.1021/jacs.7b10365. [DOI] [PubMed] [Google Scholar]
- 45.He C, Hu J, Wu Y, Ding H. Total syntheses of highly oxidized ent-kaurenoids pharicin A, pharicinin B, 7-O-acetylpseurata C, and pseurata C: A [5+2] cascade approach. J. Am. Chem. Soc. 2017;139:6098–6101. doi: 10.1021/jacs.7b02746. [DOI] [PubMed] [Google Scholar]
- 46.Ramachary DB, Anif Pasha M, Thirupathi G. Organocatalytic asymmetric formal [3+2] cycloaddition as a versatile platform to access methanobenzo[7]annulenes. Angew. Chem. Int. Ed. 2017;56:12930–12934. doi: 10.1002/anie.201706557. [DOI] [PubMed] [Google Scholar]
- 47.Zhao L, Li Z, Chang L, Xu J, Yao H, Wu X. Efficient construction of fused indolines with a 2-quaternary center via an intramolecular Heck reaction with a low catalyst loading. Org. Lett. 2012;14:2066–2069. doi: 10.1021/ol300584m. [DOI] [PubMed] [Google Scholar]
- 48.Su Y, Zhou H, Chen J, Xu J, Wu X, Lin A, Yao H. Solvent-controlled switchable C–H alkenylation of 4-aryl-1H-pyrrole-3-carboxylates: application to the total synthesis of (±)-rhazinilam. Org. Lett. 2014;16:4884–4887. doi: 10.1021/ol5023933. [DOI] [PubMed] [Google Scholar]
- 49.Yuan Z, Feng Z, Zeng Y, Zhao X, Lin A, Yao H. Palladium-catalyzed asymmetric intramolecular reductive Heck desymmetrization of cyclopentenes: access to chiral bicyclo [3.2.1] octanes. Angew. Chem. Int. Ed. 2019;58:2884–2888. doi: 10.1002/anie.201900059. [DOI] [PubMed] [Google Scholar]
- 50.Han C, Fu Z, Guo S, Fang X, Lin A, Yao H. Palladium-catalyzed remote 1,n-arylamination of unactivated terminal alkenes. ACS Catal. 2019;9:4196–4202. [Google Scholar]
- 51.Watson DA, Fan X, Buchwald SL. Carbonylation of aryl chlorides with oxygen nucleophiles at atmospheric pressure. Preparation of phenyl esters as acyl Transfer agents and the direct preparation of alkyl esters and carboxylic acids. J. Org. Chem. 2008;73:7096–7101. doi: 10.1021/jo800907e. [DOI] [PubMed] [Google Scholar]
- 52.Wu X-F, Neumann H, Beller M. Convenient carbonylation of aryl bromides with phenols to form aryl esters by applying a palladium/di-1-adamantyl-n-butylphosphine catalyst. ChemCatChem. 2010;2:509–513. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within the article and Supplementary Information files, and are also available from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Center (CCDC) under deposition numbers 1936197 (3aa) and 1936198 (7aa). These data could be obtained free of charge from The Cambridge Crystallographic Data Center via http://www.ccdc.cam.ac.uk/data_request/cif.