Abstract
To examine the role of neuroplasticity in the pathology of psychiatric disorders, we measured cerebrospinal fluid (CSF) neuroplasticity-associated protein levels. Participants were 94 patients with schizophrenia, 68 with bipolar disorder (BD), 104 with major depressive disorder (MDD), and 118 healthy controls, matched for age, sex, and ethnicity (Japanese). A multiplex immunoassay (22-plex assay) was performed to measure CSF neuroplasticity-associated protein levels. Among 22 proteins, 11 were successfully measured in the assay. CSF amyloid precursor protein (APP) and glial cell-derived neurotrophic factor (GDNF) levels were significantly lower in patients with schizophrenia, and CSF APP and neural cell adhesion molecule (NCAM)-1 levels were significantly lower in patients with BD, than in healthy controls (all p < 0.05). Positive and Negative Syndrome Scale total, positive, and general scores were significantly and positively correlated with CSF hepatocyte growth factor (HGF) (p < 0.01) and S100 calcium-binding protein B (S100B) (p < 0.05) levels in patients with schizophrenia. Young mania-rating scale score was significantly and positively correlated with CSF S100B level in patients with BD (p < 0.05). Hamilton Depression Rating Scale, core, sleep, activity, somatic anxiety, and delusion subscale scores were significantly and positively correlated with CSF HGF level, while sleep subscale score was positively correlated with CSF S100B and VEGF receptor 2 levels in patients with MDD (p < 0.05). Our results suggest that CSF APP, GDNF, and NCAM-1 levels are associated with psychiatric disorders, and that CSF HGF, S100B, and VEGF receptor 2 levels are related to psychiatric symptoms.
Subject terms: Diagnostic markers, Molecular neuroscience
Introduction
Impaired neuroplasticity, including synaptic plasticity, has been suggested in the pathophysiology of major psychiatric disorders, such as schizophrenia1–3, bipolar disorder (BD)4, and major depressive disorder (MDD)5,6. Impaired neuroplasticity has been targeted for the treatment of BD4,7 and MDD6,8–11. The neuroplasticity hypothesis in psychiatric disorders has been supported by animal models12–14; however, there is a lack of empirical data in living human individuals supporting the hypothesis.
Postmortem brain studies reported decreased mRNA15 and increased protein16 levels of brain-derived neurotrophic factor (BDNF) in patients with psychiatric disorders. Concerning the living human brain, cerebrospinal fluid (CSF) is the optimal biomaterial to examine molecular status since CSF has been reported to well reflect the state of the central nervous system17,18. Some immunoassay studies measured CSF neuroplasticity-associated proteins, such as BDNF19,20, nerve growth factor (NGF)21, neurotrophin (NT)-322, and S100 calcium-binding protein B (S100B)23–27 levels in patients with major psychiatric disorders. Contrary to the previous studies cited above19,20, we could not detect mature BDNF protein in the CSF, although our western blotting assay found decreased CSF BDNF ‘pro-peptide’ levels in patients with MDD28. Furthermore, we measured CSF neural cell adhesion molecule (NCAM) level using an enzyme-linked immunosorbent assay (ELISA) and found decreased levels in psychiatric diseases, especially BD29. However, other neuroplasticity-associated proteins remain to be quantified in the CSF collected from patients with psychiatric disorders, which warrants further comprehensive immunoassays.
Although multiplex immunoassay was developed to measure multiple proteins simultaneously30, the technique has never been applied to measure CSF neuroplasticity-associated protein levels. Among many neuroplasticity-associated proteins, 22 were chosen since they could be investigated using commercially available products. This multiplex immunoassay study aimed to measure protein levels simultaneously in a relatively large sample of CSF collected from patients with major psychiatric disorders, or healthy controls to investigate the role of neuroplasticity in the pathology of psychiatric disorders. We tested the hypothesis that CSF neuroplasticity-associated protein levels would be reduced in patients with psychiatric disorders.
Materials and methods
Participants
Participants were 94 patients with schizophrenia (mean age: 40.5 ± 10.1 years, 56 males and 38 females), 68 with BD (43.6 ± 12.2 years, 33 males and 35 females), 104 with MDD (43.4 ± 11.0 years, 49 males and 55 females), and 118 healthy controls (42.4 ± 15.3 years, 66 males and 53 females) who were matched for age, sex, and ethnicity (Japanese). BD included both bipolar I and II disorders (n = 22 and 46). There were 83 patients with schizophrenia, 63 with BD, and 78 with MDD under any psychotropic medication. We used a total of 384 samples although their power value was not estimated based on pre-obtained effect size. All participants were recruited at the National Center of Neurology and Psychiatry (NCNP) by advertisement at the NCNP Hospital, on its website and in local free magazines. Participants were screened for psychiatric disorders by qualified psychiatrists by using the Japanese version of the Mini International Neuropsychiatric Interview (M.I.N.I.)31,32. Consensus diagnosis was determined according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition33, based on the information from the M.I.N.I., additional unstructured interviews and medical records, if available. The majority of patients were under psychotropic medication. Healthy controls had no history of contact with any psychiatric services. According to pre-established criteria, participants were excluded if they had a medical history of central nervous system diseases, severe head injury, substance abuse or mental retardation. After the study had been described, written informed consent was obtained from every participant. The study protocol was approved by the Ethics Committee at the NCNP and performed in accordance with the Declaration of Helsinki34.
Clinical assessments
The Japanese version of the Positive and Negative Syndrome Scale (PANSS) was used to evaluate symptom severity in patients with schizophrenia35,36. The Japanese version of the Young Mania Rating Scale (YMRS) was used to evaluate manic symptoms in patients with BD37. The Japanese version of the GRID 21-item version Hamilton Depression Rating Scale (HAMD-21) was used to assess depressive symptoms in patients with BD, and those with MDD38,39 and 6 subscale (core, sleep, activity, psychic anxiety, somatic anxiety, and delusion) scores were calculated as previously described40. These symptoms were assessed by qualified psychiatrists or research psychologists who were trained before the ratings by using the Japanese version of PANSS, YMRS, and HAMD-21 training manual and digital versatile disc. Daily doses of antipsychotics were converted to chlorpromazine-equivalent doses and those of antidepressants were converted to imipramine-equivalent doses according to published guidelines41. These medication statuses were recorded at the time of lumbar puncture.
Lumbar puncture
Lumber puncture was performed in the left lateral decubitus or sitting position during daytime (from 10:00 to 16:00). Smoking habits or fasting conditions were not controlled before the puncture. Each participant received local anaesthesia by lidocaine hydrochloride injection before puncture. CSF was withdrawn from the L3–L4 or L4–L5 interspace using an atraumatic pencil-point needle (Universe 22 or 23 G, 75 mm, Unisis Corp., Tokyo, Japan), collected in a low protein absorption tube (PROTEOSAVE SS, 15-mL Conicaltube, Sumitomo Bakelite Co., Tokyo, Japan) and immediately transferred to ice. The CSF was centrifuged (4000×g for 10 min) at 4 °C. The supernatant was divided into 0.5-mL aliquots and stored at −80 °C. Multiplex immunoassays were performed after a single melting and re-freezing of the sample for the preparation of 96-well plates.
Multiplex immunoassay
CSF protein level was measured by the MAGPIX CCD imaging system (Bio-Rad Laboratories, Inc.) using magnetic on-bead antibody for specific proteins (Human Magnetic Luminex Assay, R&D Inc.) based on the manufacturer’s instructions. A custom-made kit (LXSAHM-22) was used to measure neuroplasticity-associated proteins: 22-plex targeted for amyloid precursor protein (APP), BDNF, contactin-1, epidermal growth factor (EGF), ErB2, ErbB3, fibroblast growth factor (FGF) acidic, FGF basic, FGF-23, glial cell-derived neurotrophic factor (GDNF), hepatocyte growth factor (HGF), HGF receptor, NCAM-1, neuropilin-1, beta-NGF, NT-3, ROBO4, S100B, vascular endothelial growth factor (VEGF)-D, VEGF receptor 1, VEGF receptor 2, and VEGF receptor 3. CSF samples were diluted to 1:3 and a fivefold dilution series were used as standard samples (S1–7) according to the results of verification assay. The assay was performed using 384 single CSF samples to secure a large number after confirming that the mean intra- and inter-run coefficients of variance for proteins were less than 5% and 10% in the verification assay, respectively (intra-run: 1 set, maximum 3.5%, triplicate; inter-run: 1 set, maximum 8.7%, duplicate). The VIAFLO 96/384 system (INTEGRA Biosciences, Corp.) was used to apply samples and reagents into 96-well plates simultaneously. To adjust the inter-assay variations between 96-well plates, 8 independently selected CSF samples (i.e., 3, 3, and 2 samples of patients with schizophrenia, those with BD, and healthy controls, respectively) diluted to 1:3 and 2 standards were used as margin samples to fit measures of 4 plates to those of 1 standard plate that included 7 standard dilutions and 1 blank sample (each triplicate). Based on the measures of the margin samples, regression equations were calculated for each protein using two-dimensional scatter diagrams between the standard and other four plates for use in the inter-plate adjustment. Among the proteins assayed, the measurement results that satisfied the following criteria were deemed reliable: within the assay working range, less than 5.0% mean intra-run (7 standard and 1 blank sample [triplicate]) and inter-run (8 CSF and 2 standard samples [pentaplicate]) coefficients of variance, and strong Pearson’s correlation coefficients (r > 0.70) in the regression equations of inter-plate adjustment. According to the criteria, the results of 11 molecules (i.e., APP, contactin-1, ErbB3, GDNF, HGF, HGF receptor, NCAM-1, neuropilin-1, S100B, VEGF receptor 1, and VEGF receptor 2) were deemed reliable in the 22-plex assay. The assays that did not meet the criteria (i.e., those for BDNF, EGF, ErB2, FGF acidic, FGF basic, FGF-23, beta-NGF, NT-3, ROBO4, VEGF-D and VEGF receptor 3) were deemed unreliable. The 11 CSF protein levels were represented as pg or ng/ml.
Statistical analyses
Categorical and continuous variables were compared between three psychiatric diagnostic groups (schizophrenia, BD, and MDD) and the control group using the Chi-squared test and analysis of variance, respectively. We applied parametric tests for analyses of CSF neuroplasticity-associated proteins. CSF neuroplasticity-associated protein levels were compared between the 4 groups and drug-free and non-drug-free groups using multivariate analysis of covariance, controlling for age and sex, and the effect sizes were assessed with partial η2. Correlation of CSF neuroplasticity-associated protein levels with symptom scores was assessed using the Pearson’s partial correlation coefficient, controlling for age, sex, and drug use (only for patients), while correlation between CSF neuroplasticity-associated protein levels and clinical variables was assessed using the Pearson’s correlation coefficient (Student’s or Welch’s t test only for sex). The correlation matrix among CSF neuroplasticity-associated protein levels was assessed with the Pearson’s partial correlation coefficient, controlling for age, sex and drug use (only for patients). Sidak and Bonferroni corrections were applied for group comparisons (corrected p < 0.05) and correlation analyses (p < 0.05/11 = 0.0045), respectively. All statistical tests were two-tailed, and p < 0.05 was deemed significant. Statistical analyses were performed using the Statistical Package for the Social Sciences version 26.0 (IBM Japan, Ltd., Tokyo, Japan).
Results
The clinical characteristics of the participants are shown in Table 1. The distribution of age and sex showed no significant difference between any psychiatric diagnostic group or control group. However, body mass index and education level were significantly higher and lower, respectively, in patients with schizophrenia than in healthy controls (p = 0.001 and 0.002).
Table 1.
The clinical characteristics of the participants.
| Schizophrenia (n = 94) | Bipolar disorder (n = 68) | Major depressive disorder (n = 104) | Control (n = 118) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± standard deviation | Range | Mean ± standard deviation | Range | Mean ± standard deviation | Range | Mean ± standard deviation | Range | |
| Age (years) | 40.5 ± 10.1 | 18–65 | 43.6 ± 12.2 | 20–74 | 43.4 ± 11.0 | 18–71 | 42.4 ± 15.3 | 19–77 |
| Sex, male (%) | 56 (59.6) | 33 (48.5) | 49 (47.1) | 66 (55.9) | ||||
| Body mass index (kg/m2) | 24.7 ± 5.5 | 15.3–46.7 | 23.9 ± 4.8 | 13.9–35.6 | 22.3 ± 3.4 | 15.6–33.8 | 22.6 ± 3.4 | 15.8–32.5 |
| Education level (years) | 13.6 ± 2.7 | 9–22 | 14.8 ± 2.6 | 9–21 | 15.0 ± 2.5 | 10–26 | 14.9 ± 2.6 | 10–23 |
| Duration of illness (years) | 15.7 ± 9.6 | 2–47 | 10.4 ± 7.7 | 0–30 | 7.2 ± 7.6 | 0–38 | ||
| Chlorpromazine-equivalent dose (mg/day) | ||||||||
| Total | 950.7 ± 929.9 | 0–5195.5 | 175.0 ± 303.1 | 0–1409.1 | 70.0 ± 156.7 | 0–823.0 | ||
| Typical | 99.8 ± 387.1 | 0–2750.0 | 7.4 ± 25.9 | 0–150.0 | 6.3 ± 25.1 | 0–150.0 | ||
| Atypical | 850.8 ± 715.7 | 0–3645.5 | 171.3 ± 306.1 | 0–1409.1 | 63.7 ± 150.3 | 0–803.0 | ||
| Imipramine-equivalent dose (mg/day) | 21.4 ± 46.4 | 0–225.0 | 51.9 ± 99.1 | 0–456.3 | 164.0 ± 143.0 | 0–525.0 | ||
| Typical antipsychotic use, n (%) | 22 (23.4) | 6 (8.8) | 5 (4.8) | |||||
| Atypical antipsychotic use, n (%) | 60 (63.8) | 19 (27.9) | 13 (12.5) | |||||
| Antidepressant use, n (%) | 18 (19.1) | 17 (25.0) | 37 (35.2) | |||||
| Mood stabilizer use, n (%) | 10 (10.6) | 20 (29.4) | 7 (6.7) | |||||
| Antiparkinsonian use, n (%) | 31 (33.0) | 2 (2.9) | 2 (1.9) | |||||
| Minor tranquilizer use, n (%) | 47 (50.0) | 32 (47.1) | 39 (37.5) | |||||
| Drug free, n (%) | 11 (11.7) | 5 (7.3) | 26 (25.0) | |||||
| Positive and negative syndrome scale | ||||||||
| Total | 61.0 ± 16.1 | 33–115 | ||||||
| Positive | 14.2 ± 5.1 | 7–27 | ||||||
| Negative | 16.3 ± 5.2 | 7–28 | ||||||
| General | 30.5 ± 8.9 | 16–60 | ||||||
| Young mania-rating scale | 6.2 ± 7.5 | 0–33 | ||||||
| Hamilton depression rating scale | 11.4 ± 7.7 | 0–35 | 11.3 ± 9.2 | |||||
Drug free was counted if psychotropic medication was not used.
*First contact to psychiatric service.
Comparison between each psychiatric group and control group
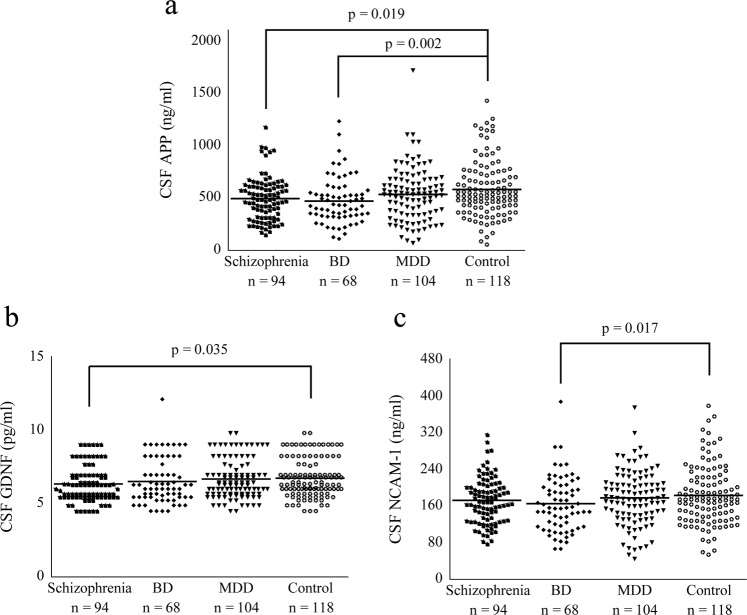
The comparisons of CSF neuroplasticity-associated protein levels between each psychiatric group and control group are shown in Table 2. CSF APP and GDNF levels were significantly lower in patients with schizophrenia {p = 0.019 (corrected p = 0.11) and 0.035 (corrected p = 0.21)}, while CSF APP and NCAM-1 levels were significantly lower in patients with BD {p = 0.002 (corrected p = 0.011) and 0.017 (corrected p = 0.097)}, than in healthy controls (Fig. 1). There was no molecule that showed a significant difference in CSF level between patients with MDD and controls.
Table 2.
The comparisons of cerebrospinal fluid neuroplasticity-associated protein levels between each psychiatric group and control group.
| Schizophrenia (n = 94) | Bipolar disorder (n = 68) | Major depressive disorder (n = 104) | Control (n = 118) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± standard deviation | vs. control | Mean ± standard deviation | vs. control | Mean ± standard deviation | vs. control | Mean ± standard deviation | Statistical comparison | |
| APP (ng/ml) | 498.5 ± 206.5 | p = 0.019 (0.11) | 475.9 ± 221.1 | p = 0.002 (0.011) | 537.3 ± 256.7 | p = 0.12 | 584.3 ± 266.0 | F(3, 378) = 3.78, p = 0.011, partial η2 = 0.029 |
| Contactin-1 (pg/ml) | 4272.5 ± 486.1 | p = 0.089 | 4270.9 ± 586.9 | p = 0.060 | 4367.2 ± 569.4 | p = 0.45 | 4419.1 ± 581.9 | F(3, 378) = 1.60, p = 0.19, partial η2 = 0.013 |
| ErbB3 (pg/ml) | 2539.1 ± 648.4 | p = 0.43 | 2545.0 ± 908.6 | p = 0.10 | 2649.2 ± 764.9 | p = 0.50 | 2682.9 ± 951.8 | F(3, 378) = 0.89, p = 0.45, partial η2 = 0.007 |
| GDNF (pg/ml) | 6.3 ± 1.2 | p = 0.035 (0.21) | 6.5 ± 1.5 | p = 0.27 | 6.7 ± 1.3 | p = 0.63 | 6.7 ± 1.3 | F(3, 378) = 1.65, p = 0.18, partial η2 = 0.013 |
| HGF (pg/ml) | 81.4 ± 18.5 | p = 0.38 | 86.8 ± 21.1 | p = 0.66 | 86.1 ± 20.7 | p = 0.78 | 84.8 ± 21.2 | F(3, 378) = 0.61, p = 0.61, partial η2 = 0.005 |
| HGF receptor (pg/ml) | 1105.8 ± 473.5 | p = 0.33 | 1030.8 ± 547.3 | p = 0.060 | 1197.5 ± 567.5 | p = 0.86 | 1187.6 ± 590.8 | F(3, 378) = 1.69, p = 0.17, partial η2 = 0.013 |
| NCAM-1 (ng/ml) | 172.4 ± 50.0 | p = 0.25 | 165.0 ± 59.9 | p = 0.017 (0.097) | 178.4 ± 59.0 | p = 0.39 | 184.0 ± 63.5 | F(3, 378) = 1.95, p = 0.12, partial η2 = 0.015 |
| Neuropilin-1 (pg/ml) | 3075.4 ± 857.0 | p = 0.57 | 2981.4 ± 1238.4 | p = 0.15 | 3189.6 ± 1182.0 | p = 0.96 | 3199.5 ± 1104.1 | F(3, 378) = 0.82, p = 0.48, partial η2 = 0.006 |
| S100B (pg/ml) | 818.3 ± 227.8 | p = 0.72 | 830.3 ± 287.4 | p = 0.98 | 819.6 ± 324.4 | p = 0.81 | 831.4 ± 290.1 | F(3, 378) = 0.60, p = 0.98, partial η2 = 0.000 |
| VEGF receptor 1 (pg/ml) | 37.2 ± 12.7 | p = 0.12 | 33.3 ± 15.2 | p = 0.51 | 33.2 ± 15.0 | p = 0.42 | 34.5 ± 15.1 | F(3, 378) = 2.08, p = 0.10, partial η2 = 0.016 |
| VEGF receptor 2 (pg/ml) | 664.7 ± 212.7 | p = 0.90 | 683.4 ± 287.3 | p = 0.67 | 674.2 ± 268.8 | p = 0.78 | 668.1 ± 223.2 | F(3, 378) = 0.07, p = 0.98, partial η2 = 0.001 |
APP amyloid precursor protein, GDNF glial cell line-derived neurotrophic factor, HGF hepatocyte growth factor, NCAM neural cell adhesion molecule, S100B S100 calcium-binding protein B, VEGF vascular endothelial growth factor.
Significant p values are shown in bold cases (right parentheses indicate corrected p values).
Fig. 1. Dot plots showing cerebrospinal fluid (CSF) amyloid precursor protein (APP), glial cell line-derived neurotrophic factor (GDNF), and neural cell adhesion molecule (NCAM)-1 levels in four diagnostic groups.
CSF APP level was significantly lower in patients with schizophrenia and those with BD than in healthy controls (a p < 0.05). CSF GDNF level in patients with schizophrenia and CSF NCAM-1 level in patients with BD levels were significantly lower than in healthy controls, respectively (b, c p < 0.05). Horizontal lines in the dot plots show mean values. BD bipolar disorder, MDD major depressive disorder.
Correlation between CSF neuroplasticity-associated protein levels and symptoms
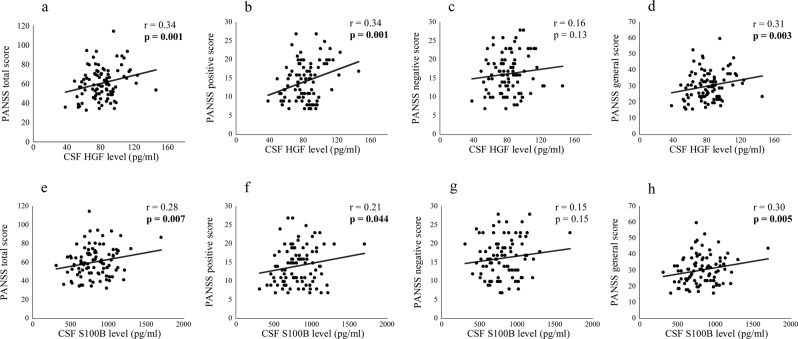
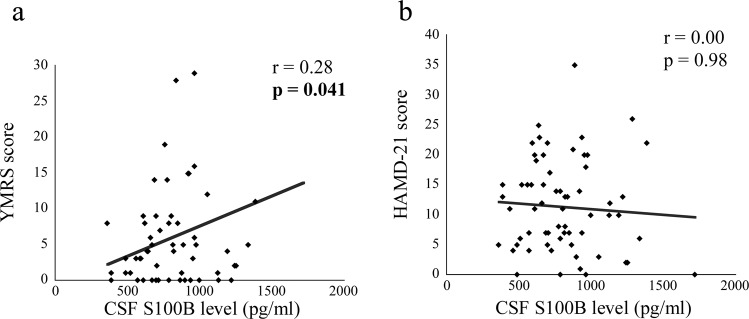
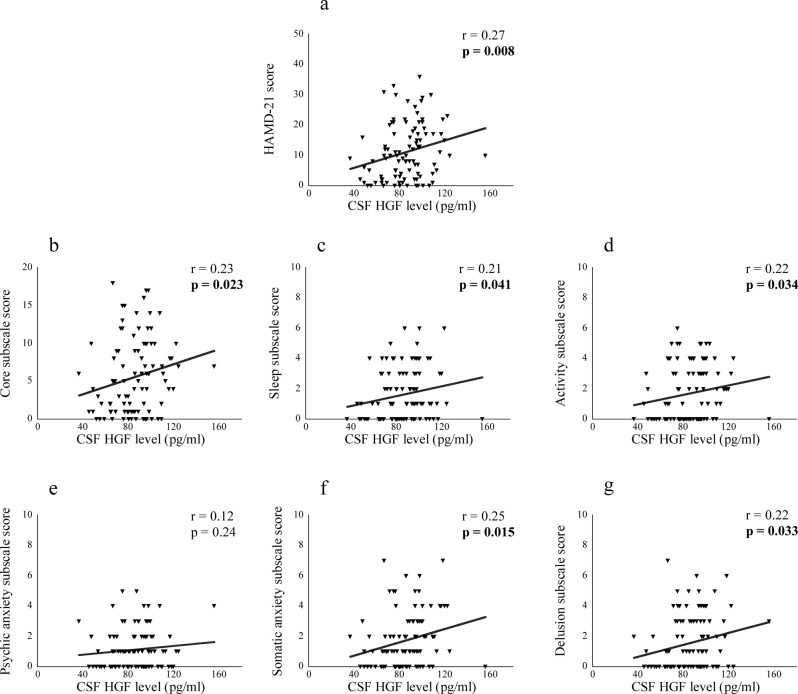
The correlations between CSF neuroplasticity-associated protein levels and symptom scores are shown in Table 3. PANSS total score was significantly and positively correlated with CSF HGF and S100B levels in patients with schizophrenia {r = 0.34, p = 0.001 (significant even after correction) and r = 0.28, p = 0.007}. Regarding the subscales, PANSS-positive and general scores were significantly and positively correlated with CSF HGF {r = 0.34, p = 0.001 (significant even after correction) and r = 0.31, p = 0.003 (significant even after correction)} and S100B (r = 0.21, p = 0.044 and r = 0.30, p = 0.005) levels {Fig. 2 (schizophrenia)}. YMRS score was significantly and positively correlated with CSF S100B score in patients with BD {r = 0.28, p = 0.041, Fig. 3 (BD)}. HAMD-21 and the subscale (core, sleep, activity, somatic anxiety, and delusion) scores were significantly and positively correlated with CSF HGF level in patients with MDD {r = 0.27, p = 0.008; r = 0.23, p = 0.023; r = 0.21, p = 0.041; r = 0.22, p = 0.034; r = 0.25, p = 0.015; r = 0.22, p = 0.030, Fig. 4 (MDD)}, while sleep score was significantly and positively correlated with CSF S100B and VEGF receptor 2 levels in patients with MDD {r = 0.22, p = 0.031 and r = 0.22, p = 0.028; Supplementary Fig. S1 (MDD)}.
Table 3.
The correlations between cerebrospinal fluid neuroplasticity-associated protein levels and symptom scores.
| Schizophrenia | Bipolar disorder | Major depressive disorder | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PANSS total | PANSS positive | PANSS negative | PANSS general | Young Mania Rating Scale | HAMD-21 | HAMD-21 | Core | Sleep | Activity | Psychic anxiety | Somatic anxiety | Delusion | ||||||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| APP | 0.06 | 0.55 | 0.12 | 0.27 | −0.02 | 0.88 | 0.06 | 0.60 | −0.03 | 0.85 | −0.14 | 0.28 | 0.07 | 0.51 | 0.06 | 0.59 | 0.06 | 0.57 | 0.03 | 0.75 | 0.01 | 0.96 | 0.06 | 0.59 | 0.04 | 0.71 |
| Contactin-1 | −0.01 | 0.90 | 0.06 | 0.59 | −0.08 | 0.47 | −0.01 | 0.92 | −0.01 | 0.92 | −0.08 | 0.53 | 0.08 | 0.46 | 0.06 | 0.59 | 0.08 | 0.43 | 0.00 | 0.98 | 0.00 | 0.96 | 0.08 | 0.43 | 0.08 | 0.43 |
| ErbB3 | 0.09 | 0.42 | 0.20 | 0.06 | −0.07 | 0.50 | 0.09 | 0.42 | −0.01 | 0.96 | −0.08 | 0.56 | 0.17 | 0.09 | 0.16 | 0.11 | 0.14 | 0.17 | 0.07 | 0.50 | 0.11 | 0.27 | 0.10 | 0.33 | 0.19 | 0.07 |
| GDNF | 0.03 | 0.76 | 0.19 | 0.07 | −0.11 | 0.30 | 0.01 | 0.89 | 0.13 | 0.33 | −0.07 | 0.59 | 0.15 | 0.15 | 0.18 | 0.08 | −0.03 | 0.79 | 0.13 | 0.21 | 0.16 | 0.13 | 0.11 | 0.28 | 0.16 | 0.11 |
| HGF | 0.34 | 0.001 | 0.34 | 0.001 | 0.16 | 0.13 | 0.31 | 0.003 | 0.00 | 1.00 | 0.02 | 0.86 | 0.27 | 0.008 | 0.23 | 0.023 | 0.21 | 0.041 | 0.22 | 0.034 | 0.12 | 0.24 | 0.25 | 0.015 | 0.22 | 0.030 |
| HGF receptor | 0.01 | 0.89 | 0.03 | 0.80 | −0.02 | 0.86 | 0.02 | 0.84 | −0.06 | 0.68 | −0.01 | 0.93 | 0.10 | 0.31 | 0.07 | 0.53 | 0.11 | 0.31 | 0.00 | 0.99 | 0.02 | 0.83 | 0.15 | 0.15 | 0.09 | 0.40 |
| NCAM-1 | −0.04 | 0.72 | 0.03 | 0.79 | −0.08 | 0.43 | −0.04 | 0.74 | 0.00 | 0.98 | −0.12 | 0.34 | 0.06 | 0.55 | 0.04 | 0.69 | 0.06 | 0.54 | 0.00 | 0.97 | 0.02 | 0.85 | 0.08 | 0.41 | 0.05 | 0.66 |
| Neuropilin-1 | −0.01 | 0.94 | 0.01 | 0.95 | 0.01 | 0.94 | −0.02 | 0.82 | 0.03 | 0.82 | −0.11 | 0.38 | 0.13 | 0.20 | 0.12 | 0.24 | 0.11 | 0.29 | 0.08 | 0.44 | 0.11 | 0.30 | 0.10 | 0.32 | 0.08 | 0.44 |
| S100B | 0.28 | 0.007 | 0.21 | 0.044 | 0.15 | 0.15 | 0.30 | 0.005 | 0.28 | 0.041 | 0.00 | 0.98 | 0.19 | 0.06 | 0.16 | 0.11 | 0.22 | 0.031 | 0.13 | 0.21 | 0.03 | 0.74 | 0.10 | 0.33 | 0.17 | 0.10 |
| VEGF receptor 1 | −0.11 | 0.31 | −0.09 | 0.40 | −0.06 | 0.58 | −0.11 | 0.31 | −0.02 | 0.89 | −0.02 | 0.88 | 0.06 | 0.55 | 0.04 | 0.73 | 0.16 | 0.12 | 0.00 | 0.98 | 0.08 | 0.42 | −0.02 | 0.87 | 0.02 | 0.84 |
| VEGF receptor 2 | 0.04 | 0.73 | 0.01 | 0.90 | 0.06 | 0.59 | 0.02 | 0.84 | 0.10 | 0.45 | −0.11 | 0.41 | 0.20 | 0.05 | 0.14 | 0.16 | 0.22 | 0.028 | 0.09 | 0.39 | 0.16 | 0.13 | 0.17 | 0.10 | 0.17 | 0.09 |
APP amyloid precursor protein, GDNF glial cell line-derived neurotrophic factor, HAMD-21 21-item version Hamilton Depression Rating Scale, HGF hepatocyte growth factor, NCAM neural cell adhesion molecule, PANSS positive and negative syndrome scale, S100B S100 calcium-binding protein B, VEGF vascular endothelial growth factor.
r, Pearson’s partial correlation cofficient (covariates: age, sex and drug use).
Significant p values are shown in bold cases (correctely significant cases are underlined, p < 0.0045).
Fig. 2. Dot plots showing correlation of cerebrospinal fluid (CSF) hepatocyte growth factor (HGF) and S100 calcium-binding protein B (S100B) levels with symptom scores in patients with schizophrenia.
Correlation of CSF HGF level with PANSS total (a), positive (b), and general scores (d p < 0.01). Correlation of CSF S100B level with PANSS total (e), positive (f), and general (h) scores (p < 0.05). PANSS positive and negative syndrome scale.
Fig. 3. Scatter plots showing correlation of cerebrospinal fluid (CSF) S100 calcium-binding protein B (S100B) level with symptom scores in patients with bipolar disorder.
Correlation of CSF S100B level with YMRS score (a p < 0.05). HAMD-21 21-item version Hamilton Depression Rating Scale, YMRS Young mania-rating scale.
Fig. 4. Scatter plots showing correlation of cerebrospinal fluid (CSF) hepatocyte growth factor (HGF) level with symptom scores in patients with major depressive disorder.
Correlation of CSF HGF level with HAMD-21 (a p < 0.01), core (b), sleep (c), activity (d), somatic anxiety (f), and delusion (g) subscale scores (p < 0.05). HAMD-21 21-item version Hamilton Depression Rating Scale.
Association between CSF neuroplasticity-associated protein levels and clinical variables
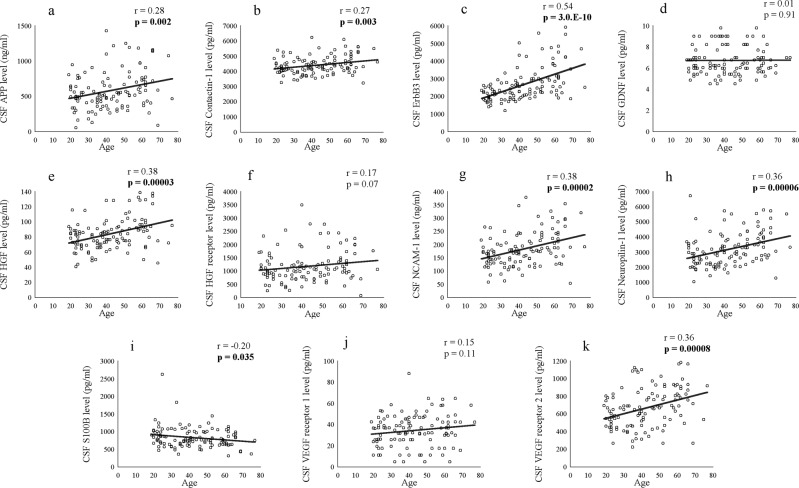
The comparisons of CSF neuroplasticity-associated protein levels between drug-free and non-drug-free patients are shown in Supplementary Tables S1 (schizophrenia), S2 (BD), and S3 (MDD). Except for a significantly higher CSF S100B level in drug-free patients with BD than in the non-drug-free patients (p = 0.021), there were no significant differences. The correlation between CSF neuroplasticity-associated protein levels and clinical variables is shown in Supplementary Tables S4 (schizophrenia), S5 (BD), S6 (MDD), and S7 (control). Notably, age was significantly and positively correlated with many CSF neuroplasticity-associated protein levels, especially in patients with BD, those with MDD, and healthy controls {all p < 0.05, Fig. 5 (control)}. The correlation matrices among CSF neuroplasticity-associated protein levels are shown in Supplementary Tables S8 (schizophrenia), S9 (BD), S10 (MDD), and S11 (control). As expected, many CSF neuroplasticity-associated protein levels significantly and positively correlated with each other in patients with schizophrenia, those with BD, those with MDD, and healthy controls (all p < 0.05). Except for a significant and negative correlation between CSF GDNF and VEGF receptor 1 levels in patients with schizophrenia (r = –0.26, p = 0.013), there were no proteins that showed a significant and negative correlation.
Fig. 5. Scatter plots showing correlation of age with cerebrospinal fluid (CSF) neuroplasticity-associated protein level in healthy controls.
Correlation of age with CSF amyloid precursor protein (APP, a), contactin-1 (b), ErbB3 (c), glial cell- derived neurotrophic factor (GDNF, d), hepatocyte growth factor (HGF, e), HGF receptor (f), neural cell adhesion molecule (NCAM)-1 (g), neuropilin-1 (h), S100 calcium-binding protein B (S100B, i), vascular endothelial growth factor (VEGF) receptor 1 (j), and VEGF receptor 2 (k) levels. Bold p values indicate significant cases (p < 0.05).
Discussion
CSF APP and GDNF levels were decreased in patients with schizophrenia, while CSF APP and NCAM-1 levels were decreased in patients with BD, compared with healthy controls. As for the symptoms, PANSS scores were positively correlated with CSF HGF and S100B levels in patients with schizophrenia, while YMRS and HAMD-21 (including subscale) scores were positively correlated with CSF S100B levels in patients with BD and CSF HGF, S100B, and VEGF receptor 2 levels in patients with MDD, respectively. These suggest that neuroplasticity-associated proteins may serve as state- and trait markers in the pathology of psychiatric disorders. Of note, the state-marker proteins promoted symptom severity, suggesting that their functions are inverse to the trait-maker proteins.
Decreased CSF APP level was observed in patients with schizophrenia and those with BD, which is consistent with previous studies reporting impaired neuroplasticity in patients with schizophrenia1–3 and those with BD4,7. APP is one of the transmembrane proteins42–44 related with neuroplasticity45. Hence, the neuroplasticity hypothesis for schizophrenia and BD suggested by animal models14 may be clinically supported by this CSF study. Among APP proteins, the soluble forms are considered measurable in the CSF; however, our multiplex immunoassay could not distinguish the α- and β-forms, secreted by α- and β-secretases, respectively42,44, although previous ELISA46 and multiplex immunoassay47 reported these forms separately. An ELISA study reported that the CSF-soluble form of APP α, rather than -β, was significantly reduced in 39 patients with BD compared with 71 healthy controls48. It is possible that the neurotropic (non-amyloidogenic) APP-α pathway might have been more reduced in our patients, as well as the neurotoxic (amyloidogenic) APP-β pathway43,45.
NCAM-1 is one of the cell adhesion molecules associated with neuroplasticity49. In our previous study29, we reported decreased CSF NCAM-1, particularly in BD, which is further supported by the present result that CSF NCAM-1 level was decreased, especially in patients with BD. As the aforementioned APP, soluble forms of NCAM-1 released from the cell membrane are considered measurable as previously described29. In addition, in a diagnostic group comparison, CSF GDNF level was decreased in patients with schizophrenia compared with healthy controls. To our knowledge, GDNF, a neurotropic factor50,51, has never been quantified using CSF samples from patients with psychiatric disorders. Although reports of GDNF are still scarce, glial dysfunction may be particularly related with the pathomechanisms of schizophrenia52–55.
It is very important that the directions of CSF neuroplasticity-associated protein- (including other non-significant proteins) level changes tend to be mostly downward, while we have the data that CSF total protein level was increased in patients with psychiatric disorders {schizophrenia: 42.4 ± 14.6 mg/dl (corrected p = 0.041), BD: 40.9 ± 15.6 mg/dl (corrected p = 0.38), MDD 42.3 ± 16.3 mg/dl (corrected p = 0.023) and control: 37.6 ± 14.6 mg/dl}. Furthermore, our prior study showed that CSF, a neuroplasticity-associated protein BDNF ‘pro-peptide’-level change, was also downward in patients with schizophrenia and those with MDD28. These support that neuroplasticity impairment is related with the pathology of psychiatric disorders.
CSF HGF level showed a positive correlation with PANSS total, positive, and general scores in patients with schizophrenia and HAMD-21, core, sleep, activity, somatic anxiety, and delusion subscale scores in patients with MDD, respectively. HGF is unable to permeate the blood–brain barrier (BBB), unless serious disruption occurs56, suggesting that CSF HGF level reflects the state of the BBB disruption in psychiatric disorders since the origin is dominantly peripheral57. Therefore, the observed positive correlation may imply the association of BBB disruption with symptoms in patients with schizophrenia and those with MD.
CSF S100B level showed a positive correlation with PANSS total, positive, and general scores in patients with schizophrenia. S100B has been implicated in the pathology of schizophrenia as a marker of astrocytic response and BBB dysfunction58,59. Therefore, the observed positive correlation suggests the association of astrocyte activation and BBB dysfunction with symptoms in patients with schizophrenia. CSF S100B level also shows a positive correlation with YMRS and sleep subscale scores, similarly suggesting the involvement of astrocyte activation and BBB dysfunction60,61 with manic symptoms in patients with BD and sleep disturbance in patients with MDD. However, as for the comparisons with healthy controls, this study showed that CSF S100B level was not altered in patients with psychiatric disorders, which is consistent with studies in 133 patients with BD26 and 31 with MDD24. However, this is inconsistent with studies reporting increased CSF S100B level in a relatively smaller number (n = 21 and 12) of patients with schizophrenia23,25 and 46 with MDD27.
In addition, CSF VEGF receptor 2 showed a positive correlation with sleep subscale score in patients with MDD. VEGF receptor 2 is a subtype of receptor involved in VEGF signaling62,63, suggesting that the function is associated with sleep disturbance in patients with MDD.
Inconsistent with previous ELISA studies regarding BDNF19,20, NGF21, and NT-322, our multiplex immunoassay concluded that CSF BDNF, beta-NGF, and NT-3 levels were lower than the assay’s working range. In contrast, CSF S100B level was able to be measured in this study and this is somewhat comparable to an electrochemiluminescence immunoassay24, although incomparable to immunoluminometric assays23,25,27 and an electrochemiluminescence immunoassay26, suggesting that the different immunoassays may have somehow influenced inconsistency regarding concentration.
Our study has the following limitations. First, the majority of patients (schizophrenia 88.3%, BD 92.7%, and MDD 75.0%) had been under psychiatric medication, although most CSF neuroplasticity-associated protein levels were not different between drug-free and non-drug-free patients. Second, CSF neuroplasticity-associated protein levels showed a positive correlation with age in all diagnostic groups, which was consistent with our study on NCAM-129. Ageing-related processes probably promoted the increased CSF neuroplasticity-associated protein levels; however, the mechanisms remain unclear. Third, relatively lower mean symptom (mean PANSS total, 61.0; YMRS, 6.2; and HAMD-21, 11.4 and 11.3) scores indicate that milder forms of the illness were overrepresented in our patients, which may have minimized the difference in CSF neuroplasticity-associated protein levels between the patients and controls, as well as their correlation with symptom scores. Fourth, the cross-sectional nature precludes determination of the causality. Fifth, a substantial proportion of ‘significant' results became non-significant after correcting for multiple testing; however, we chose to discuss our results based on nominal p values to avoid type 2 errors rather than to decrease the possibility of type 1 errors. Sixth, there may be other trait- or state biomarkers than those presented here, as all kinds of neuroplasticity-associated protein levels were not measured in this study. Finally, it cannot be determined whether the alteration of expression, processing or clearance of significant proteins (i.e., APP, GDNF, HGF, NCAM-1, S100B, and VEGF receptor 2) results in the association with psychiatric disorders. Postmortem brain studies may be useful to further elucidate the pathomechanisms as previously reported15,16. Furthermore, we would like to examine the relationship of CSF neuroplasticity-associated protein levels with brain structure and cognitive function in the future.
In conclusion, our data suggest the involvement of state- (i.e., HGF, S100B, and VEGF receptor 2) and trait (i.e., APP, GDNF, and NCAM-1) markers associated with neuroplasticity in the pathology of psychiatric disorders. Recovery from impaired neuroplasticity may be a treatment target in patients with these psychiatric disorders.
Supplementary information
Acknowledgements
S.H. designed and H.K. supervised the study. S.H., K.H., D.S., and M.O. determined the diagnoses and evaluated the symptoms. S.Y. helped the recruitment of participants from clinic patients. Y.Y., I.I., and J.M. conducted the psychological assessments. S.H., K.H., and D.S. executed the lumber puncture to collect CSF samples. K.H. selected and T.M. prepared the CSF sample set. R.M. created and maintained the database system. S.H. performed the laboratory experiments with the assistance of T.T. S.H. performed the statistical analyses and wrote the paper, which was revised and approved by all the authors.
We thank Mr. Masashi Hashimoto, Ms. Chie Kimizuka, Mr. Takahiro Tomizawa, Ms. Naoko Ishihara, Ms. Tomoko Kurashimo, Ms. Moeko Hiraishi, and Ms. Chiori Maeda for their assistance in recruitment, clinical assessments and lumber puncture of participants. We also thank Ms. Yuko Yamaoka, Ms. Ayumi Fujisawa, Ms. Izumi Sato, Ms. Misao Nakano, Ms. Yurika Adachi, and Ms. Megumi Tatsumi for their sample management. This paper was proofread by a scientific editor at Editage (Tokyo, Japan).
Funding
This work was supported by a Japan Society for the Promotion of Science KAKENHI (19K17076, S.H.), a grant from the SENSHIN Medical Research Foundation (S.H.), intramural Research Grants for Neurological and Psychiatric Disorders of NCNP (24-11 and 27-6, K.H. and H.K.), and Japan Agency for Medical Research and Development grants (16dk0307062h0001, 16ak0101044h0001, and 18dk0307081h0001, K.H. and H.K.). These funding sources were involved in no other aspect of the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shinsuke Hidese, Email: shidese@ncnp.go.jp.
Hiroshi Kunugi, Email: hkunugi@ncnp.go.jp.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-0843-5).
References
- 1.Chelini G, Pantazopoulos H, Durning P, Berretta S. The tetrapartite synapse: a key concept in the pathophysiology of schizophrenia. Eur. Psychiatry. 2018;50:60–69. doi: 10.1016/j.eurpsy.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Picker LJ, Morrens M, Chance SA, Boche D. Microglia and brain plasticity in acute psychosis and schizophrenia illness course: a meta-review. Front. Psychiatry. 2017;8:238. doi: 10.3389/fpsyt.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pocklington AJ, O’Donovan M, Owen MJ. The synapse in schizophrenia. Eur. J. Neurosci. 2014;39:1059–1067. doi: 10.1111/ejn.12489. [DOI] [PubMed] [Google Scholar]
- 4.Machado-Vieira R, Soeiro-De-Souza MG, Richards EM, Teixeira AL, Zarate CA., Jr. Multiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: developing treatments using an integrated translational approach. World J. Biol. Psychiatry. 2014;15:84–95. doi: 10.3109/15622975.2013.830775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsden WN. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;43:168–184. doi: 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Levy MJF, et al. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology. 2018;235:2195–2220. doi: 10.1007/s00213-018-4950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado-Vieira R. Lithium, stress, and resilience in bipolar disorder: deciphering this key homeostatic synaptic plasticity regulator. J. Affect. Disord. 2018;233:92–99. doi: 10.1016/j.jad.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Price JB, et al. Bioenergetics and synaptic plasticity as potential targets for individualizing treatment for depression. Neurosci. Biobehav. Rev. 2018;90:212–220. doi: 10.1016/j.neubiorev.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Umemori J., Winkel F., Didio G., Llach Pou M., Castren E. iPlasticity: induced juvenile-like plasticity in the adult brain as a mechanism of antidepressants. Psychiatry Clin. Neurosci. (2018). [DOI] [PMC free article] [PubMed]
- 10.Huang YJ, Lane HY, Lin CH. New treatment strategies of depression: based on mechanisms related to neuroplasticity. Neural Plast. 2017;2017:4605971. doi: 10.1155/2017/4605971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workman ER, Niere F, Raab-Graham KF. Engaging homeostatic plasticity to treat depression. Mol. Psychiatry. 2018;23:26–35. doi: 10.1038/mp.2017.225. [DOI] [PubMed] [Google Scholar]
- 12.Bartsch T, Wulff P. The hippocampus in aging and disease: from plasticity to vulnerability. Neuroscience. 2015;309:1–16. doi: 10.1016/j.neuroscience.2015.07.084. [DOI] [PubMed] [Google Scholar]
- 13.Monday HR, Younts TJ, Castillo PE. Long-term plasticity of neurotransmitter release: emerging mechanisms and contributions to brain function and disease. Annu. Rev. Neurosci. 2018;41:299–322. doi: 10.1146/annurev-neuro-080317-062155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagihara H, Takao K, Walton NM, Matsumoto M, Miyakawa T. Immature dentate gyrus: an endophenotype of neuropsychiatric disorders. Neural Plast. 2013;2013:318596. doi: 10.1155/2013/318596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J. Psychiatry Neurosci. 2011;36:195–203. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheldrick A, Camara S, Ilieva M, Riederer P, Michel TM. Brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3) levels in post-mortem brain tissue from patients with depression compared to healthy individuals - a proof of concept study. Eur. Psychiatry. 2017;46:65–71. doi: 10.1016/j.eurpsy.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Veening JG, Barendregt HP. The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospinal Fluid Res. 2010;7:1. doi: 10.1186/1743-8454-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head. Neck Dis. 2011;128:309–316. doi: 10.1016/j.anorl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Diniz BS, et al. Reduced cerebrospinal fluid levels of brain-derived neurotrophic factor is associated with cognitive impairment in late-life major depression. J. Gerontol. B Psychol. Sci. Soc. Sci. 2014;69:845–851. doi: 10.1093/geronb/gbu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pillai A, et al. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J. Neuropsychopharmacol. 2010;13:535–539. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- 21.Kale A, et al. Reduced cerebrospinal fluid and plasma nerve growth factor in drug-naive psychotic patients. Schizophr. Res. 2009;115:209–214. doi: 10.1016/j.schres.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Hock C, et al. Increased cerebrospinal fluid levels of neurotrophin 3 (NT-3) in elderly patients with major depression. Mol. Psychiatry. 2000;5:510–513. doi: 10.1038/sj.mp.4000743. [DOI] [PubMed] [Google Scholar]
- 23.Steiner J, Bielau H, Bernstein HG, Bogerts B, Wunderlich MT. Increased cerebrospinal fluid and serum levels of S100B in first-onset schizophrenia are not related to a degenerative release of glial fibrillar acidic protein, myelin basic protein and neurone-specific enolase from glia or neurones. J. Neurol. Neurosurg. Psychiatry. 2006;77:1284–1287. doi: 10.1136/jnnp.2006.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt FM, Mergl R, Stach B, Jahn I, Schonknecht P. Elevated levels of cerebrospinal fluid neuron-specific enolase (NSE), but not S100B in major depressive disorder. World J. Biol. Psychiatry. 2015;16:106–113. doi: 10.3109/15622975.2014.952776. [DOI] [PubMed] [Google Scholar]
- 25.Rothermundt M, et al. Glial cell dysfunction in schizophrenia indicated by increased S100B in the CSF. Mol. Psychiatry. 2004;9:897–899. doi: 10.1038/sj.mp.4001548. [DOI] [PubMed] [Google Scholar]
- 26.Jakobsson J, et al. Elevated concentrations of neurofilament light chain in the cerebrospinal fluid of bipolar disorder patients. Neuropsychopharmacology. 2014;39:2349–2356. doi: 10.1038/npp.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabe HJ, Ahrens N, Rose HJ, Kessler C, Freyberger HJ. Neurotrophic factor S100 beta in major depression. Neuropsychobiology. 2001;44:88–90. doi: 10.1159/000054922. [DOI] [PubMed] [Google Scholar]
- 28.Mizui T, et al. Cerebrospinal fluid BDNF pro-peptide levels in major depressive disorder and schizophrenia. J. Psychiatr. Res. 2019;113:190–198. doi: 10.1016/j.jpsychires.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Hidese S, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;76:12–18. doi: 10.1016/j.pnpbp.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Tighe PJ, Ryder RR, Todd I, Fairclough LC. ELISA in the multiplex era: potentials and pitfalls. Proteom. Clin. Appl. 2015;9:406–422. doi: 10.1002/prca.201400130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 32.Otsubo T, et al. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin. Neurosci. 2005;59:517–526. doi: 10.1111/j.1440-1819.2005.01408.x. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 34.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 35.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 36.Igarashi Y, et al. Interrater reliability of the Japanese version of the Positive and Negative Syndrome Scale and the appraisal of its training effect. Psychiatry Clin. Neurosci. 1998;52:467–470. doi: 10.1046/j.1440-1819.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- 37.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 38.Williams JB, et al. The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin. Psychopharmacol. 2008;23:120–129. doi: 10.1097/YIC.0b013e3282f948f5. [DOI] [PubMed] [Google Scholar]
- 39.Tabuse H, et al. The new GRID Hamilton Rating Scale for Depression demonstrates excellent inter-rater reliability for inexperienced and experienced raters before and after training. Psychiatry Res. 2007;153:61–67. doi: 10.1016/j.psychres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Serretti A, Lattuada E, Cusin C, Macciardi F, Smeraldi E. Analysis of depressive symptomatology in mood disorders. Depress. Anxiety. 1998;8:80–85. [PubMed] [Google Scholar]
- 41.Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin. Neurosci. 2015;69:440–447. doi: 10.1111/pcn.12275. [DOI] [PubMed] [Google Scholar]
- 42.Reinhard C, Hebert SS, De Strooper B. The amyloid-beta precursor protein: integrating structure with biological function. EMBO J. 2005;24:3996–4006. doi: 10.1038/sj.emboj.7600860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicolas M, Hassan BA. Amyloid precursor protein and neural development. Development. 2014;141:2543–2548. doi: 10.1242/dev.108712. [DOI] [PubMed] [Google Scholar]
- 44.Wild K, August A, Pietrzik CU, Kins S. Structure and synaptic function of metal binding to the amyloid precursor protein and its proteolytic fragments. Front. Mol. Neurosci. 2017;10:21. doi: 10.3389/fnmol.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner PR, O’Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 46.Araki W, et al. Re-evaluation of soluble APP-alpha and APP-beta in cerebrospinal fluid as potential biomarkers for early diagnosis of dementia disorders. Biomark. Res. 2017;5:28. doi: 10.1186/s40364-017-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabelle A, et al. Correlations between soluble alpha/beta forms of amyloid precursor protein and Abeta38, 40, and 42 in human cerebrospinal fluid. Brain Res. 2010;1357:175–183. doi: 10.1016/j.brainres.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Jakobsson J, et al. Altered concentrations of amyloid precursor protein metabolites in the cerebrospinal fluid of patients with bipolar disorder. Neuropsychopharmacology. 2013;38:664–672. doi: 10.1038/npp.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dityatev A, Bukalo O, Schachner M. Modulation of synaptic transmission and plasticity by cell adhesion and repulsion molecules. Neuron Glia Biol. 2008;4:197–209. doi: 10.1017/S1740925X09990111. [DOI] [PubMed] [Google Scholar]
- 50.Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev. Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- 51.Ghitza UE, et al. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci. Biobehav. Rev. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamuro K, Kimoto S, Rosen KM, Kishimoto T, Makinodan M. Potential primary roles of glial cells in the mechanisms of psychiatric disorders. Front. Cell Neurosci. 2015;9:154. doi: 10.3389/fncel.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Aleksic B, Ozaki N. Glia-related genes and their contribution to schizophrenia. Psychiatry Clin. Neurosci. 2015;69:448–461. doi: 10.1111/pcn.12290. [DOI] [PubMed] [Google Scholar]
- 54.Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr. Res. 2015;161:4–18. doi: 10.1016/j.schres.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog. Neurobiol. 2011;93:13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma S. Hepatocyte growth factor in synaptic plasticity and Alzheimer’s disease. Sci. World J. 2010;10:457–461. doi: 10.1100/tsw.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kern MA, et al. Concentrations of hepatocyte growth factor in cerebrospinal fluid under normal and different pathological conditions. Cytokine. 2001;14:170–176. doi: 10.1006/cyto.2001.0875. [DOI] [PubMed] [Google Scholar]
- 58.Yelmo-Cruz S, Morera-Fumero AL, Abreu-Gonzalez P. S100B and schizophrenia. Psychiatry Clin. Neurosci. 2013;67:67–75. doi: 10.1111/pcn.12024. [DOI] [PubMed] [Google Scholar]
- 59.Yarlagadda A, Hampe CS, Clayton AH. The blood brain barrier and the role of ratiometric molecular analysis in schizophrenia. Psychiatry. 2010;7:20–23. [PMC free article] [PubMed] [Google Scholar]
- 60.Patel JP, Frey BN. Disruption in the blood-brain barrier: the missing link between brain and body inflammation in bipolar disorder? Neural Plast. 2015;2015:708306. doi: 10.1155/2015/708306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J. Neuroinflamm. 2013;10:142. doi: 10.1186/1742-2094-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nowacka MM, Obuchowicz E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: a new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides. 2012;46:1–10. doi: 10.1016/j.npep.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Tillo M, Ruhrberg C, Mackenzie F. Emerging roles for semaphorins and VEGFs in synaptogenesis and synaptic plasticity. Cell Adhes. Migr. 2012;6:541–546. doi: 10.4161/cam.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.